Abstract
Background
The aim of the present study was to investigate the morphologic features and mechanical properties of plantar fascia (PF), flexor hallucis brevis (FHB), flexor digitorum brevis (FDB), and abductor hallucis (AbH) muscles in individuals with halluks valgus (HV) and to compare the results with individuals without HV.
Methods
A total of 30 participants (27 female, 3 male) between the ages of 19–58 years with HV deformity and 30 individuals without HV (27 female, 3 male) between the ages of 20–58 years were included in the study. AbH, PF, FHB, and FDB thickness, cross-sectional area and stiffness were measured with an ultrasonography device. For stiffness measurements, Shear Wave Velocity (SWV) of the assessed soft tissues was calculated using a customized software program.
Results
Mean and standard deviation of SWV of PF, AbH, FHB, FDB, and thickness of PF, AbH, FHB, FDB, and cross-sectional area of AbH, FHB, FDB in individuals with HV were 7.6 ± 1.0 m/sec, 2.8 ± 0.3 m/sec, 2.6 ± 0.4 m/sec, and 3.4 ± 0.2 m/sec, 3.3 ± 0.5 mm, 11.4 ± 2.2 mm, 16.5 ± 1.9 mm, 8.9 ± 1.8 mm, 2.4 ± 0.5 cm2, 2.7 ± 0.5 cm2, 1.9 ± 0.6 cm2, respectively. Mean and standard deviation of SWV of PF, AbH, FHB, FDB and thickness of PF, AbH, FHB, FDB, and cross-sectional area of AbH, FHB, FDB in controls groups were 7.6 ± 1.2 m/sec, 2.3 ± 0.3 m/sec, 2.3 ± 0.4 m/sec, and 3.4 ± 0.4 m/sec, 3.3 ± 0.5 mm, 9.7 ± 2.1 mm, 14.5 ± 1.4 mm, 9.7 ± 1.2 mm, 2.1 ± 0.3 cm2, 2.3 ± 0.4 cm2, 2.1 ± 0.4 cm2, respectively. Individuals with HV had lower AbH and FHB thickness, cross-sectional area and SWV, however FDB thickness and cross-sectional area were higher in individuals with HV compared to that of individuals without HV (p < 0.05). PF thickness (p = 0.273), SWV of PF (p = 0.979) and FDB (p = 0.295) were similar in both groups.
Conclusion
Our results suggest that individuals with HV had lower AbH and FHB stiffness, however PF and FDB stiffness were similar in HV and control group. In addition, AbH and FHB thickness and cross-sectional area were lower in individuals with HV; however, FDB thickness and cross-sectional area were higher in individuals with HV compared to that of individuals without HV.
Level of Evidence
Level III, Diagnostic Study.
Keywords: Hallux valgus, Foot muscles, Stiffness, Muscle size, Ultrasonography, Sonoelestography
Introduction
Hallux valgus (HV) is one of the most common foot deformity in adults and is characterized with pronation of proximal phalanx, medial deviation of the first metatarsal and medial prominence of the first metatarsophalangeal joint.1 The prevalence for HV is reported to be 23% in adults aged 18 to 65 and is increased with ages and female sex.2 HV with high prevalence is an important health problem which may cause pain, cosmetic appearance concerns, and reduce in health-related quality of life.3, 4, 5
Mechanical properties of intrinsic foot muscles and plantar fascia (PF) may be related to the etiology of HV. Changes in foot intrinsic muscles and PF mechanical properties such as stiffness could affect first metatarsophalangeal joint position, because soft tissue stiffness is an important parameter for the regulation of human motion and control as well as for joint stability.6, 7, 8 In theory, decrease in stiffness foot intrinsic muscle and PF could cause a decrease resisting capacity against external loading, and this could cause a loss of stabilization of first metatarsophalangeal joint, and it may cause the HV. However, the relationship of changes in mechanical properties of the foot intrinsic muscles and PF with HV is not completely known. On the other hand, changes in morphological features of plantar fascia and foot intrinsic muscles may be another factor to be emerge the HV, because morphological features of muscles such as thickness and/or cross-sectional area are related to muscle function such as muscle strength.9 Decrease in muscle strength could cause an imbalance and/or instability of related joint.10, 11 Because of this reason, decrease in foot intrinsic muscle size may cause a loss of stabilization and/or a control deficit of first metatarsophalangeal joint, which may be related to emergence of HV. There exist a few studies on the morphological features of foot muscles and PF in individuals with HV. These studies reported that individuals with HV had lower thickness and cross-sectional area of abductor hallucis and flexor hallucis brevis compared to individuals without HV.12, 13, 14 Even though a few studies in the literature investigate the intrinsic foot muscles and PF thickness and cross-sectional area in individuals with HV, to our knowledge, there exists no study investigating the stiffness of intrinsic foot muscles and PF in individuals with HV. Identifying possible changes in intrinsic foot muscles and PF stiffness, thickness and cross-sectional area in individuals with HV may help to better understand the factors causing or resulting HV that may help inform better treatment options.
Foot intrinsic muscle function such as strength or/and endurance cannot be measured dynamically because of their small size and limited accessibility, but ultrasonography can reliably assess foot intrinsic muscle morphological features such as their thickness and cross-sectional area.15 In addition, Shear-Wave Elatography (SWE), which is new reliably and valid imaging method based on ultrasonography, allow soft tissue mechanical properties such as stiffness to be measured.16, 17 Shear waves are a type of vibration waves which generate from the soft tissues in result of exposed acoustic waves at US examination. SWE method allow to be assessed the tissues stiffness by measuring the velocity of shear waves in tissues.18, 19 Furthermore, unlike grayscale ultrasonography, SWE method can be able to detect abnormalities in terms of alterations in shear wave velocity in assessed tissue, which provide to realize the pathological changed in soft tissue in early period of the disease.20
The purpose of the present study was to examine the stiffness, thickness and cross-sectional area of the PF as well as abductor hallucis (AbH), flexor hallucis brevis (FHB), and flexor digitorum brevis (FDB) muscles in individuals with HV. We hypothesized that the stiffness, thickness and cross-sectional area of the PF and AbH, FDB, and FHB muscles would be lower in individuals with HV compared with individuals without HV.
Materials and methods
Participants
A total of 30 participants (27 females and 3 males) between the ages of 19–58 years with hallux valgus deformity and 30 individuals without HV (27 females and 3 males) between the ages of 20–58 years were selected for the present study. Hallux valgus was assessed using the Manchester Scale, which is reported to be a reliable and valid tool in both clinical assessment and self-assessment of hallux valgus.21, 22, 23 The scale is graded 0 (no deformity) to grade 3 (severe deformity). Participant was observed in relaxed weight-bearing stance to determine the degree of hallux valgus deformity.23 Individuals with HV were classified according to the Manchester Scale; 17 subjects in the Grade 1, 8 in the Grade 2 and 5 in the Grade 3. Individuals were excluded from the study if they met any of the following exclusion criteria: (1), ankle or foot orthopedic injuries, such as plantar fasciitis, ligament injuries tendinopathy, bursitis, or ligament injuries, (2) a history of lower extremities surgery or major trauma, (3) rheumatic diseases, such as gout or rheumatoid arthritis, and (4) a systemic disease, such as diabetes and/or connective tissue disorders. Ethics approval was obtained from the Non-Invasive Clinical Research Ethics Board of the Faculty of Medicine and written consent was obtained from each participant.
Ultrasonic examinations
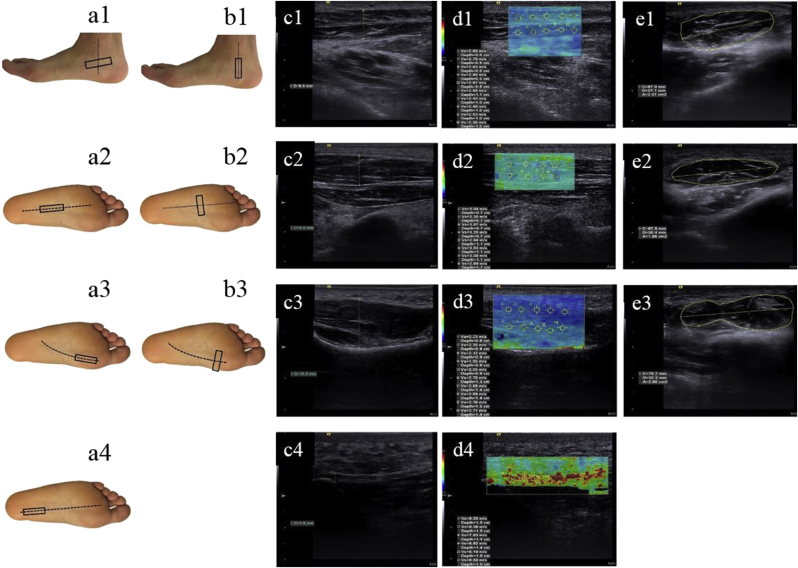
Ultrasonic examinations of the PF, AbH, FHB, and FDB were performed using an ultrasonography device (ACUSON S3000 Ultrasound System; Siemens Medical Solution, Mountain View, CA). The AbH stiffness, cross-sectional area and thickness measurements were performed when the subjects were in the side-lying position and the ankle was in the neutral position, so that the ultrasound probe had the best contact with the skin and with minimal pressure on the tissue. Stiffness, cross-sectional area and thickness measurements of the PF, FHB, and FDB were performed as the participants in the prone position with neutral ankle position and 90 degrees of knee flexion. Stiffness measurements of the PF were performed at the region between the calcaneal insertion of the plantar aponeurosis and 10 mm distal to the calcaneal insertion of the plantar aponeurosis. The origin of the PF on the medial tubercle of the calcaneus was used for PF thickness measurements. For the stiffness, cross-sectional area and thickness measurements of AbH, FHB, and FDB, the probe placed along the direction of the muscle fiber (Fig. 1). To avoid deformation on the assessed soft tissue that may increase the stiffness measurements and decrease thickness measurements, minimum pressure was applied on the probe to obtain a sufficient image quality and shear wave signals. For stiffness measurements, Shear Wave Velocity (SWV) of the assessed soft tissues was calculated using a customized software program (Virtual Touch Imaging and Quantification; Siemens Medical Solution). The thickness, cross-sectional area and stiffness of the selected tissues were calculated by taking the average of three successive measurements performed.
Fig. 1.
Probe location and ultrasound imaging measurements. (a) Probe position of the AbH (a1), FDB (a2), FHB (a3) and PF (a4) at thickness and stiffness measurements; (b) Probe position of the AbH (b1), FDB (b2) and FHB (b3) at cross-sectional area measurements; (c) 2-dimensional ultrasound image of the AbH (c1), FDB (c2), FHB (c3) and PF (c4) at thickness measurements; (d) color map images of the AbH (d1), FDB (d2), FHB (d3) and PF (d4) at stiffness measurements; (e) 2-dimensional ultrasound image of AbH (e1), FDB (e2) and FHB (e3) at cross-sectional area measurements. Regions of interest were placed, and corresponding shear wave velocity (m/sec) was recorded. Red-coded areas represent the highest stiffness, blue-coded areas represent the lowest stiffness, and green-coded areas represent intermediate stiffness. Plantar fascia, PF; abductor hallucis, AbH; flexor hallucis brevis, FHB; flexor digitorum brevis, FDB.
Statistical analysis
Statistical analyses were performed using a statistics software program (SPSS, Inc, an IBM Company, Chicago, IL). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk's test) to determine whether they were normally distributed. Demographic data and the assessed parameters were presented using mean and standard deviation (SD). Since the stiffness, cross-sectional area and thickness of the selected soft tissues were nonnormally distributed, the Mann–Whitney U test was used to compare these parameters between the groups. Correlation coefficients for relations between parameters and statistical significance were calculated using Spearman's test. Correlation analysis results were classified as follows: 0.00–0.20 (poor correlation), 0.21–0.40 (fair correlation), 0.41–0.60 (moderate correlation), 0.61–0.80 (strong correlation), and 0.81–1.00 (very strong correlation). A P value of less than 0.05 was considered to show a statistically significant result.
Result
Both groups were similar in age (p = 0.976), height (p = 0.302), body mass (p = 0.940) and body mass index (p = 0.644). SWV of AbH (p < 0.001) and FHB (p = 0.002) were lower in HV group, but SWV of FDB (p = 0.295) and PF (p = 0.949) were similar in both groups. HV group had lower AbH (p = 0.002) and FHB (p < 0.001) thickness, whereas HV group had higher FDB thickness compared to control group (p = 0.027). Similarly, cross-sectional area of AbH (p = 0.003) and FHB (p = 0.001) were lower in HV group, but FDB cross-sectional area was higher in HV group compared to control group (p = 0.006) (Table 1).
Table 1.
Mean and standard deviation value for each parameter measured.
| Parameter | Control (n = 30) | Hallux valgus (n = 30) | P value |
|---|---|---|---|
| Age (years) | 36.5 ± 10.5 | 36.5 ± 12.4 | .976 |
| Height(m) | 1.63 ± 0.09 | 1.65 ± 0.08 | .302 |
| Body mass (kg) | 64.4 ± 12.9 | 64.4 ± 12.0 | .940 |
| BMI (kg/m2) | 24.0 ± 3.3 | 23.5 ± 3.2 | .644 |
| Gender (male/female) | 3/27 | 3/27 | |
| Thickness of selected tissues (mm) | |||
| Plantar fascia | 3.3 ± 0.5 | 3.3 ± 0.5 | .273 |
| Abductor hallucis | 11.4 ± 2.2 | 9.7 ± 2.1 | .002* |
| Flexor hallucis brevis | 16.5 ± 1.9 | 14.5 ± 1.4 | <.001* |
| Flexor digitorum brevis | 8.9 ± 1.8 | 9.7 ± 1.2 | .027* |
| Cross-sectional area of selected tissues (cm2) | |||
| Abductor hallucis | 2.4 ± 0.5 | 2.1 ± 0.3 | .003* |
| Flexor hallucis brevis | 2.7 ± 0.5 | 2.3 ± 0.4 | .001* |
| Flexor digitorum brevis | 1.9 ± 0.6 | 2.1 ± 0.4 | .006* |
| SWV of selected tissues (m/sec) | |||
| Plantar fascia | 7.6 ± 1.0 | 7.6 ± 1.2 | .949 |
| Abductor hallucis | 2.8 ± 0.3 | 2.3 ± 0.3 | <.001* |
| Flexor hallucis brevis | 2.6 ± 0.4 | 2.3 ± 0.4 | .002* |
| Flexor digitorum brevis | 3.4 ± 0.2 | 3.2 ± 0.4 | .295 |
* p < 0.05. Abbreviations: BMI, body mass index; SWV, Shear Wave Velocity.
The correlation analysis revealed that SWV of AbH muscle had a fair correlation with AbH thickness (r = 0.33, p = 0.013) and cross-sectional area (r = 0.29, p = 0.031). There are a strong correlation between AbH thickness and cross-sectional area (r = 0.65, p < 0.001). SWV of FHB had a fair correlation with FHB thickness (r = 0.27, p = 0.041) and cross-sectional area (r = 0.31, p = 0.011), but FHB thickness had a moderate correlated with FHB cross-sectional area (r = 0.58, p < 0.001). There are a strong correlation between FDB thickness and cross-sectional area (r = 0.68, p < 0.001), whereas FDB thickness and cross-sectional area did not correlate with SWV of FDB (r < 0.10, p > 0.05). PF thickness did not correlate with PF stiffness (r = −0.23, p = 0.088).
Discussion
The purpose of the present study was to investigate the changes in morphologic features and mechanical properties of intrinsic foot muscles and PF in individuals with HV. As far as our knowledge, this study is first study which investigate the changes in stiffness of foot intrinsic muscles and PF in individuals with HV. We hypothesized that the stiffness of PF and intrinsic foot muscles would be lower in individuals with HV. Contrary to our hypothesis, our results suggest that stiffness of PF and FDB do not related to HV. In line with our hypothesis, our results show that stiffness of AbH and FHB muscles are lower in individuals with HV compared with those without HV. Decrease in stiffness of AbH and FHB muscles can cause a decrease in resisting capacity of these muscles which lead to hallux in extension and adduction. Decrease in stiffness of AbH and FHB muscles in individuals with HV may be arise from a few reason. First, our correlation analysis results suggest that the lower stiffness of AbH and FHB was related to lower muscle size of AbH and FHB, but this relationship was low, and it affected a small population of assessed individuals. On the other hand, decrease in stiffness of AbH and FHB muscles may be related to the histological and morphologic changes in these muscles. It was reported that histological abnormal, increase infiltration of fat and collagen and loss of muscle fiber in AbH and FHB in individuals with HV.14, 24 It is well established that morphological and histological changes in muscle structure could affect muscle stiffness.25, 26
In line with our hypothesis, we found that the AbH and FHB thickness and cross-sectional area were lower in individuals with HV compared to control group. Similar to our results, previous studies reported that individuals with HV had lower AbH and FHB cross-sectional area and thickness compared to healthy control.12, 13, 14 The results of the present study show that muscle strength of AbH and FHB muscles decrease in individuals with HV, because muscle size is an important determinant of muscle strength.9 On the other hand, it is unclear whether decrease in AbH and FHB muscle strength lead to HV deformity, or whether the HV deformity leads to decrease AbH and FHB muscle strength. For example, decrease in AbH muscle strength may cause a decrease resisting capacity against adductor muscle which may lead to adduction of the first metatarsophalangeal joint. On the other hand, the decrease in AbH muscle strength may be related to mechanical changes in the event of HV. HV deformity can cause malalignment of the bones, increase the distance between origin and insertion of AbH muscle and the insertion of the adductor hallucis moves toward the medio-plantar side.1, 27 Alternatively, tendon of FHB is displayed laterally and tendon of extensor hallucis longus is displayed towards the fibula in case of HV.1 Changes of these tendon direction and/or mechanical changes may decrease the capacity of AbH and FHB muscles in individuals with HV and these changes may chance muscle function and strength of AbH and FHB muscles. Further studies are necessary to investigate the effect of increase muscle size on the big toe position that may have more clinical implications to in the management of HV.
Contrary to our hypothesis, our results show that FDB cross-sectional area was higher in individuals with HV and PF thickness was similar in both groups. In contrast with our results, Lobo et al13 reported that PF thickness is increased in individuals with HV, while FDB thickness and cross-sectional area are similar in with and without HV. Mickle et al12 found that FDB thickness and cross-sectional area were similar in individuals with HV compared with those without HV. Increase in FDB thickness and cross-sectional area may be a compensation or adaptation against mechanical changes in foot and decreased intrinsic muscle strength associated with HV. Mechanical changes in foot and/or decreased intrinsic muscle strength may increase the loading on FDB muscle and this may cause hypertrophy on FDB muscle.
Our results suggest that decrease in stiffness and size of AbH and FHB muscles could cause a decrease in toe strength and stabilization and it may cause HV. Strengthening the intrinsic foot muscle may be able to prevent or delay the progression of HV, because strengthening the intrinsic muscles is effective to increase toe strength and stabilization as well as physical performance.28 In addition, strengthening exercise may cause an increase in stiffness of foot intrinsic muscle as well as an increase in muscle strength. There is no study that investigated the strengthening programs on muscle stiffness, however muscle stiffness is related to muscle strength.29 Furthermore, muscle strengthening programs could decrease the fatty infiltration in muscle and it could chance muscle fiber type,30, 31 which may cause an increase in muscle stiffness.25, 26 Because of these reasons, strengthening program for the foot intrinsic muscle may be a treatment option for individuals with HV. Further investigation is warranted and required, which investigate the effect of intrinsic foot muscle strengthening programs in muscle stiffness and toe position in individuals with HV.
This study has several limitations. Firstly, stiffness, thickness and cross-sectional area measurements were not performed by a researcher blinded to the groups. However, standardized instructions used in stiffness and thickness measurements to minimize the bias. Secondly, the study included only young or middle age individuals. In different populations (geriatric, athletes … etc.), effects of HV might be different on selected tissues stiffness, thickness and cross-sectional area. Finally, in the present study, changes in morphological and mechanical properties of foot intrinsic muscles and PF in individuals with HV were evaluated in comparison with individuals without HV. Further studies are necessary to improve knowledge about changes in intrinsic foot muscles and PF in patients with different various severity levels of HV that may have more clinical implications to in the management of HV.
Conclusion
In conclusion, we found that PF stiffness and FDB stiffness were similar in individuals with and without HV, while individuals with HV had lower AbH and FHB stiffness compared to that of individuals without HV. AbH and FHB thickness and cross-sectional area were lower in individuals with HV; however, FDB thickness and cross-sectional area were higher in individuals with HV compared to that of individuals without HV. In addition, our correlation analysis results suggest that lower stiffness of AbH and FHB was related to lower muscle size of these muscles. Our results indicate that decrease in muscle size and stiffness of AbH and FHB might be an important cause to decrease resisting capacity of these muscle, which may lead to hallux valgus.
Ethical committee approval
Required permission was obtained from the Non-Invasive Clinical Research Ethics Board of Faculty of Medicine, Hacettepe University with the decision dated 15 January 2018 and numbered GO 18/60-30.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Serkan Taş, Email: serkntas@gmail.com.
Alp Çetin, Email: alpcetin68@gmail.com.
References
- 1.Perera A., Mason L., Stephens M. The pathogenesis of hallux valgus. J Bone Joint Surg Am. 2011;93(17):1650–1661. doi: 10.2106/JBJS.H.01630. [DOI] [PubMed] [Google Scholar]
- 2.Nix S., Smith M., Vicenzino B. Prevalence of hallux valgus in the general population: a systematic review and meta-analysis. J Foot Ankle Res. 2010;3:21. doi: 10.1186/1757-1146-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez D.L., Callejo Gonzalez L., Losa Iglesias M.E. Quality of life impact related to foot health in a sample of older people with hallux valgus. Aging Dis. 2016;7(1):45–52. doi: 10.14336/AD.2015.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menz H.B., Roddy E., Thomas E., Croft P.R. Impact of hallux valgus severity on general and foot-specific health-related quality of life. Arthritis Care Res (Hoboken.) 2011;63(3):396–404. doi: 10.1002/acr.20396. [DOI] [PubMed] [Google Scholar]
- 5.Abhishek A., Roddy E., Zhang W., Doherty M. Are hallux valgus and big toe pain associated with impaired quality of life? A cross-sectional study. Osteoarthritis Cartilage. 2010;18(7):923–926. doi: 10.1016/j.joca.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Riemann B.L., Lephart S.M. The sensorimotor system, Part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37(1):80–84. PMCID: PMC164312. [PMC free article] [PubMed] [Google Scholar]
- 7.Potvin J.R., Brown S.H. An equation to calculate individual muscle contributions to joint stability. J Biomech. 2005;38(5):973–980. doi: 10.1016/j.jbiomech.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto N., Hirata K., Kimura N., Miyamoto-Mikami E. Contributions of hamstring stiffness to straight-leg-raise and sit-and-reach test scores. Int J Sports Med. 2018;39(2):110–114. doi: 10.1055/s-0043-117411. [DOI] [PubMed] [Google Scholar]
- 9.Kent-Braun J.A., Ng A.V. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol (1985) 1999;87(1):22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- 10.McKeon P.O., Hertel J., Bramble D., Davis I. The foot core system: a new paradigm for understanding intrinsic foot muscle function. Br J Sports Med. 2015;49(5):290. doi: 10.1136/bjsports-2013-092690. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn T., Guskiewicz K.M., Petschauer M.A., Prentice W.E. Balance and joint stability: the relative contributions of proprioception and muscular strength. J Sport Rehabil. 2000;9(4):315–328. [Google Scholar]
- 12.Mickle K.J., Nester C.J. Morphology of the toe flexor muscles in older people with toe deformities. Arthritis Care Res (Hoboken). 2018;70(6):902–907. doi: 10.1002/acr.23348. [DOI] [PubMed] [Google Scholar]
- 13.Lobo C.C., Marin A.G., Sanz D.R. Ultrasound evaluation of intrinsic plantar muscles and fascia in hallux valgus: a case-control study. Medicine (Baltimore) 2016;95(45):e5243. doi: 10.1097/MD.0000000000005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart S., Ellis R., Heath M., Rome K. Ultrasonic evaluation of the abductor hallucis muscle in hallux valgus: a cross-sectional observational study. BMC Musculoskelet Disord. 2013;14:45. doi: 10.1186/1471-2474-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crofts G., Angin S., Mickle K.J., Hill S., Nester C.J. Reliability of ultrasound for measurement of selected foot structures. Gait Posture. 2014;39(1):35–39. doi: 10.1016/j.gaitpost.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Tas S., Onur M.R., Yilmaz S., Soylu A.R., Korkusuz F. Shear wave elastography is a reliable and repeatable method for measuring the elastic modulus of the rectus femoris muscle and patellar tendon. J Ultrasound Med. 2017;36(3):565–570. doi: 10.7863/ultra.16.03032. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto N., Hirata K., Kanehisa H., Yoshitake Y. Validity of measurement of shear modulus by ultrasound shear wave elastography in human pennate muscle. PLoS one. 2015;10(4):e0124311. doi: 10.1371/journal.pone.0124311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bercoff J., Tanter M., Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(4):396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 19.Shiina T., Nightingale K.R., Palmeri M.L. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Szczepanek-Parulska E., Wolinski K., Stangierski A. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PloS one. 2013;8(11):e81532. doi: 10.1371/journal.pone.0081532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menz H.B., Fotoohabadi M.R., Wee E., Spink M.J. Validity of self-assessment of hallux valgus using the Manchester scale. BMC Musculoskelet Disord. 2010;11:215. doi: 10.1186/1471-2474-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menz H.B., Munteanu S.E. Radiographic validation of the Manchester scale for the classification of hallux valgus deformity. Rheumatology (Oxford) 2005;44(8):1061–1066. doi: 10.1093/rheumatology/keh687. [DOI] [PubMed] [Google Scholar]
- 23.Garrow A.P., Papageorgiou A., Silman A.J., Thomas E., Jayson M.I., Macfarlane G.J. The grading of hallux valgus: the manchester scale. J Am Podiatr Med Assoc. 2001;91(2):74–78. doi: 10.7547/87507315-91-2-74. PMID: 11266481. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmeyer P., Cox J.N., Blanc Y., Meyer J.M., Taillard W. Muscle in hallux valgus. Clin Orthop Relat Res. 1988;232:112–118. PMID: 3383479. [PubMed] [Google Scholar]
- 25.Schleip R., Naylor I.L., Ursu D. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med Hypotheses. 2006;66(1):66–71. doi: 10.1016/j.mehy.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 26.de Bruin M., Smeulders M.J., Kreulen M., Huijing P.A., Jaspers R.T. Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PloS one. 2014;9(6):e101038. doi: 10.1371/journal.pone.0101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arinci Incel N., Genc H., Erdem H.R., Yorgancioglu Z.R. Muscle imbalance in hallux valgus: an electromyographic study. Am J Phys Med Rehabil. 2003;82(5):345–349. doi: 10.1097/01.PHM.0000064718.24109.26. [DOI] [PubMed] [Google Scholar]
- 28.Mickle K.J., Caputi P., Potter J.M., Steele J.R. Efficacy of a progressive resistance exercise program to increase toe flexor strength in older people. Clin Biomech (Bristol, Avon) 2016;40:14–19. doi: 10.1016/j.clinbiomech.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Secomb J.L., Lundgren L.E., Farley O.R., Tran T.T., Nimphius S., Sheppard J.M. Relationships between lower-body muscle structure and lower-body strength, power, and muscle-tendon complex stiffness. J Strength Cond Res. 2015;29(8):2221–2228. doi: 10.1519/JSC.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster B.H., Chomentowski P., Ward B.K. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105(5):1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson C.G.R., Dickinson A.L., Ringel S.P. Skeletal muscle fiber area alterations in two opposing modes of resistance-exercise training in the same individual. Eur J Appl Physiol Occup Physioly. 1990;61(1–2):37–41. doi: 10.1007/BF00236691. PMID: 2149702. [DOI] [PubMed] [Google Scholar]