Key Points
Question
What is the effect of a multicomponent home-based physical therapy intervention on the ability of older persons recovering from hip fracture to walk at a speed enabling them to carry out daily activities in the community?
Findings
In a randomized trial including 210 older adults who had experienced a hip fracture, receiving the intervention compared with an active control resulted in an ability to walk 300 m or more in 6 minutes after 16 weeks in 22.9% vs 17.8% of participants, respectively, a difference that was not statistically significant.
Meaning
This multicomponent intervention did not significantly improve community ambulation compared with an active control intervention.
Abstract
Importance
Disability persists after hip fracture in older persons. Current rehabilitation may not be sufficient to restore ability to walk in the community.
Objective
To compare a multicomponent home-based physical therapy intervention (training) with an active control on ability to walk in the community.
Design, Setting, and Participants
Parallel, 2-group randomized clinical trial conducted at 3 US clinical centers (Arcadia University, University of Connecticut Health Center, and University of Maryland, Baltimore). Randomization began on September 16, 2013, and ended on June 20, 2017; follow-up ended on October 17, 2017. Patients aged 60 years and older were enrolled after nonpathologic, minimal trauma hip fracture, if they were living in the community and walking without human assistance before the fracture, were assessed within 26 weeks of hospitalization, and were not able to walk during daily activities at the time of enrollment. A total of 210 participants were randomized and reassessed 16 and 40 weeks later.
Interventions
The training intervention (active treatment) (n = 105) included aerobic, strength, balance, and functional training. The active control group (n = 105) received transcutaneous electrical nerve stimulation and active range-of-motion exercises. Both groups received 2 to 3 home visits from a physical therapist weekly for 16 weeks; nutritional counseling; and daily vitamin D (2000 IU), calcium (600 mg), and multivitamins.
Main Outcomes and Measures
The primary outcome (community ambulation) was defined as walking 300 m or more in 6 minutes at 16 weeks after randomization. The study was designed to test a 1-sided hypothesis of superiority of training compared with active control.
Results
Among 210 randomized participants (mean age, 80.8 years; 161 women [76.7%]), 197 (93.8%) completed the trial (187 [89.0%] by completing the 6-minute walk test at 16 weeks and 10 [4.8%] by adjudication of the primary outcome). Among these, 22 of 96 training participants (22.9%) and 18 of 101 active control participants (17.8%) (difference, 5.1% [1-sided 97.5% CI, −∞ to 16.3%]; 1-sided P = .19) became community ambulators. Seventeen training participants (16.2%) and 15 control participants (14.3%) had 1 or more reportable adverse events during the intervention period. The most common reportable adverse events reported were falls (training: 6 [5.7%], control: 4 [3.8%]), femur/hip fracture (2 in each group), pneumonia (training: 2, control: 0), urinary tract infection (training: 2, control: 0), dehydration (training: 0, control: 2), and dyspnea (training: 0, control: 2).
Conclusions and Relevance
Among older adults with a hip fracture, a multicomponent home-based physical therapy intervention compared with an active control that included transcutaneous electrical nerve stimulation and active range-of-motion exercises did not result in a statistically significant improvement in the ability to walk 300 m or more in 6 minutes after 16 weeks.
Trial Registration
ClinicalTrials.gov Identifier: NCT01783704
This randomized clinical trial compares a multicomponent home-based physical therapy intervention with an active control on ability to walk in daily life among adults aged 60 years and older who had experienced a nonpathologic, minimal trauma hip fracture.
Introduction
The number of hip fractures in the United States has been projected to increase from 258 000 per year in 2010 to 458 000 per year by 2050.1,2 The number of hip fractures worldwide is projected to be 4.5 million per year by 2050.1,2,3 Most persons with a hip fracture experience difficulty walking after fracture.4,5 There is insufficient evidence regarding the effectiveness of exercise interventions to improve the ability to walk after hip fracture.6
Community ambulation (eg, ability to cross a street before the traffic light changes) requires endurance, dynamic balance, quadriceps strength, and sufficient walking speed.7 Prior exercise trials in patients with hip fracture reported positive outcomes.6,8,9,10 However, because control participants in most prior studies received usual care or only minimal staff contact, these studies were not able to discern the effect of treatment from that of intensive contact with well-trained professionals. Also, previous studies did not evaluate the effect of the intervention on community ambulation.
The Community Ambulation Project tested whether a home-based multicomponent 16-week intervention (training) that addressed specific walking-related abilities (endurance, balance, strength, and lower extremity function) was more successful in producing community ambulation (the ability to walk ≥300 m in 6 minutes) at 16 weeks after randomization than a nonspecific home-based multicomponent active control intervention that included active range-of-motion exercises and sensory-level transcutaneous electrical nerve stimulation (TENS). The study was designed to test a 1-sided hypothesis of superiority of training compared with active control.
Methods
Background
The trial protocol and statistical analysis plan are included in Supplement 1 and Supplement 2, respectively. The protocol was approved by the institutional review boards of the participating sites and an independent data and safety monitoring board. Participants provided written informed consent.
Participants
In this randomized, parallel, 2-group multicenter clinical trial, patients aged 60 years and older were assessed for eligibility and baseline performance within 26 weeks of hospitalization for a hip fracture at 3 sites (Arcadia University, University of Connecticut Health Center, and University of Maryland, Baltimore).
Inclusion Criteria
Inclusion criteria included a minimal trauma, closed, nonpathologic hip fracture with surgical repair; age 60 years and older; community-dwelling at time of fracture and randomization; ambulating without human assistance 2 months before the fracture; and walking less than 300 m during the 6-minute walk test at the time of randomization.11
Exclusion Criteria
Exclusion criteria included medical contraindications for exercise, low potential to benefit from the intervention, and practical impediments to participation.
Procedures
Eligible participants were randomized at baseline and reassessed at 16 weeks’ and 40 weeks’ follow-up. Randomization began on September 16, 2013, and ended on June 20, 2017; follow-up ended on October 17, 2017. To accelerate recruitment and conserve study resources, the protocol was modified after the study began to expand eligibility criteria twice (modified May 29, 2014, and December 12, 2014), reduce frequency of intervention visits from 3 to 2 times per week (modified December 12, 2014), allow make-up intervention visits (modified October 14, 2015), reduce target sample size from 300 to 210 (modified February 16, 2016), discontinue 40-week follow-up (modified July 12, 2016), and eliminate some outcomes (modified September 14, 2016). Telephone interviews were conducted every 4 weeks for adverse event monitoring. Participants were not blinded to treatment group.
Randomization
The study biostatistician (M.S.) designed the random allocation sequence, which was implemented using a secure web-based system. Staff, trained by the study’s clinical coordinating center, performed enrollment activities and communicated treatment assignments to participants. Treatments were assigned according to separate randomization schedules for each clinical site, in randomly ordered blocks of randomly selected sizes of 2, 4, 6, or 8, with equal numbers of participants assigned to each treatment within each block.
Interventions
For both treatment groups, physical therapists provided 60-minute in-home intervention visits on nonconsecutive days for 16 weeks. The first 75 participants received 3 visits per week during the first 8 weeks and 2 visits per week during the remaining 8 weeks (40 assigned visits). Participants randomized after the protocol change that reduced the number of visits per week received 2 visits per week (32 assigned visits).
The training intervention focused on lower extremity strength, endurance, balance, and function. Strength exercises were completed on the floor, targeting relevant muscles moving through locomotion-appropriate12 movements and ranges using a portable progressive resistance device (Shuttle MiniPress, Contemporary Design Company). Plantar flexion exercises also were performed. For strength training, the target volume was 3 sets of 8 repetitions per leg for each of 4 exercises (leg press, hip abduction, hip extension, and heel raises); target intensity was the load associated with completing the 8-repetition maximum on the MiniPress device. For plantar flexion exercises, target intensity was body weight. Target intensity was reassessed every 2 weeks. Endurance exercise began with a 2- to 3-minute warm-up, followed by outdoor ambulation (if able), indoor walking, or other upright activity. Target volume for endurance training was 20 minutes and target intensity was 50% of heart rate reserve; participants taking β-blocker medications used the 0-10 Rating of Perceived Exertion Scale with target intensity of 3 to 5.13
The active control intervention included 22 seated active range-of-motion exercises and sensory-level TENS unit application to lower extremity muscle groups. Active range-of-motion exercises focused on the neck, trunk, and upper and lower extremities and progressed from 3 to 20 repetitions per exercise; the volume target was 20 minutes or longer. TENS was applied bilaterally near the quadriceps, gluteal complex, and gastrocnemius muscles motor points. Frequency was set to 80 pulses/s, pulse duration to 50 μsec, and amplitude to produce a minimally detectable tingling sensation to each muscle complex for 7 minutes. Target volume for TENS was 21 minutes per session. These activities were selected because they are common physical therapy interventions but were not expected to have an effect on walking ability.
Participants in both groups received 2000 IU of vitamin D3, 600 mg of calcium, and a multivitamin daily for 40 weeks. A registered dietician provided nutritional counseling at baseline. Additional dietician consults were provided by telephone if participants lost 2% or more of weight over 4 weeks.
Visit adherence was defined as the number of completed therapist visits divided by the number of expected visits per participant. A participant was deemed not to have achieved an intervention target if any portion of the target was not achieved or if the expected visit was not performed. Treatment fidelity was assessed with written and performance-based physical therapist competency testing, quarterly in-home observations, participation of therapists in monthly conference telephone calls, and review of therapist logbooks. Heart rate was recorded during training sessions using Polar E600 heart rate monitors. Heart rates were reviewed monthly for physiologic response to the interventions.
At study initiation, physical therapists were randomly assigned to treatment groups; therapists hired later in the study were assigned to the group of the therapist being replaced. Therapists provided only 1 intervention and were not informed of the alternate treatment or of study outcomes.
Outcomes
Baseline assessments were performed at the clinical sites. Follow-up assessments were performed at the clinical sites or, if the participant was not able to come to the clinical site, at the participant’s home. The primary outcome, community ambulation at 16 weeks after randomization, was defined as walking 300 m or more in 6 minutes as assessed by the 6-minute walk test11 and represents the distance and velocity required to walk independently and without difficulty outside one’s residence. The 300-m distance approximates the distance from a parking space in a supermarket parking lot to the store and corresponds to a 0.8 m/s gait speed, which indicates the ability to walk outdoors to community locations.14,15
When the 6-minute walk test could not be administered at follow-up, a panel of 3 expert adjudicators evaluated whether the participant was a noncommunity ambulator based on death, illness incompatible with walking 300 m or more in 6 minutes, gait speed less than 0.6 m/s (from the Short Physical Performance Battery [SPPB] 4-m walk, if performed at the follow-up time at which the primary outcome was missing), or self- or proxy-reported severe walking limitation. Participants who neither completed a follow-up 6-minute walk test nor met any of these criteria were classified as “indeterminate” for the primary outcome.
Secondary outcomes included endurance (distance walked on the 6-minute walk test11; range ≥0; longer distance = greater endurance; small meaningful change, 20 m; substantial meaningful change, 50 m),16 dynamic balance (balance subscale of the SPPB17 plus 2 single-leg stands from the National Health and Aging Trends Study18; range, 0-90; higher score = better balance), isometric quadriceps strength on nonfractured side (lbs of force/lb of body weight19; range ≥0; higher value = greater strength), gait speed (m/s; 50-ft fast walk and 4-m usual walk; range ≥0; higher value = faster walking; minimal clinically important difference for 4-m walk, 0.1 m/s),20 and physical performance (modified Physical Performance Test [mPPT]8; range, 0-36; higher score = better performance; no minimal clinically important difference). Tertiary outcomes were lower extremity function (SPPB)17 (range, 0-12; higher score = better performance; minimal clinically important difference, 1.0)16 and increase of 50 m or more on 6-minute walk test from baseline to 16 weeks16 (yes/no). The following secondary and tertiary outcomes specified in the protocol are not reported in this article: cost-effectiveness, activities of daily living, quality of life, balance confidence, physical activity, depressive symptoms, cognitive status, and nutritional status.
The Center for Epidemiologic Studies Depression scale21 (range, 0-60; higher score = more depressive symptoms), modified Mini-Mental State Examination22 (range, 0-100; higher score = less cognitive impairment), and an interview version of the Mini Nutritional Assessment–Short Form23,24 (range, 0-14; higher score = better nutritional status) were measured at baseline to characterize the study groups. Assessors who measured outcomes were blinded to treatment group; participants were instructed not to discuss group assignments with assessors.
Information on reportable adverse events, including serious or unexpected adverse events or injury that occurred under supervision by study staff, was obtained by telephone interview every 4 weeks and before each physical therapy visit and follow-up assessment.
Self-identified race and ethnicity were recorded in fixed categories to allow required reporting to the National Institutes of Health and institutional review boards.
Power and Sample Size
With 105 participants per group, the study was estimated to have 80% power to detect a 20–percentage point difference between groups on the primary outcome using a 1-sided 0.025-level test. This projection assumed that 16% of active control participants and 36% of training participants would achieve the outcome of community ambulation, accounted for the group sequential design (with 4 interim analyses), and accounted for indeterminate outcomes and nonadherence.25,26,27,28 The minimal detectable difference of 20 percentage points was based on the plausibility of observing a difference at least that large and on prior research suggesting that a difference of 50 m on the 6-minute walk test (corresponding to a difference of 19 percentage points) is a large clinically meaningful difference16 (section 15.6.1 in Supplement 1). A 1-sided hypothesis was assessed because the authors considered that the clinical implications would be the same whether training led to a worse outcome than the active control or if it had no effect. Reducing the sample size from 300 to 210 resulted in a decrease from 90% to 80% in power to test the primary study hypothesis.
Statistical Analyses
Participants were analyzed in the group to which they were randomly assigned; they were excluded from the primary analysis if their community ambulation status could not be ascertained by assessment or adjudication. Analyses were done using SAS software version 9.4 for Windows (SAS Institute Inc). The reported P value for analysis of the primary outcome is 1-sided. All other reported P values are 2-sided.
Primary Outcome
The 1-sided null hypothesis that training does not result in a higher proportion of community ambulators 16 weeks after randomization compared with active control was tested in 4 interim analyses (after 20%, 40%, 60%, and 80% of data were available) and 1 final analysis based on a Z statistic for the difference in proportions. Critical values for declaring statistical significance or futility at each analysis were based on a Hwang-Shih-Decani alpha-spending function to preserve an overall type I error rate of .025 (1-sided) using the R package “gsDesign” (R Project).29
Secondary and Tertiary Outcomes
The effect of training on quantitative measures at baseline, 16 weeks, and 40 weeks was assessed using longitudinal regression models fit by restricted maximum likelihood. The models included an unstructured variance/covariance matrix to account for within-person correlation. For longitudinal binary outcomes, group-specific proportions at each time point were estimated using logistic regression with random intercepts. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary and tertiary outcomes should be interpreted as exploratory.
Secondary Analyses
The differential effect of training on community ambulation in subgroups defined by clinical site, protocol version, and participant characteristics was examined (section 1.1.3 of the eAppendix in Supplement 3); P values were based on maximum likelihood estimates of interaction terms in a logistic regression model. To estimate delayed and sustained effects of training, community ambulation status at 40 weeks was compared with community ambulation status at 16 weeks using longitudinal regression and 3 × 3 contingency tables.
In prespecified sensitivity analyses, regression modeling was used to assess the effect of training on the primary outcome after adjusting for imbalances between groups in important baseline variables (section 1.1.2 of the eAppendix in Supplement 3) and the primary outcome was analyzed after including a random effect for physical therapists (section 1.5 of the eAppendix in Supplement 3). In post-hoc analyses, the primary, secondary, and tertiary outcomes were reanalyzed including a random effect for clinical site (section 1.6 of the eAppendix in Supplement 3); the primary outcome was analyzed using weighted estimating equations (upweighting subgroups that were more likely to have indeterminate outcomes) to adjust for potential biases due to indeterminate outcomes (section 1.10 of the eAppendix in Supplement 3); and the primary outcome was assessed after reclassifying participants who died or were too ill to walk 300 m or more in 6 minutes as “indeterminate” (section 1.9 of the eAppendix in Supplement 3). Details regarding these and other sensitivity analyses are in Supplement 3.
In another post-hoc analysis, heart rates during different components of the interventions were compared using 2-sample Wilcoxon tests for between-group comparisons or 1-sample Wilcoxon tests for within-group comparisons.
Results
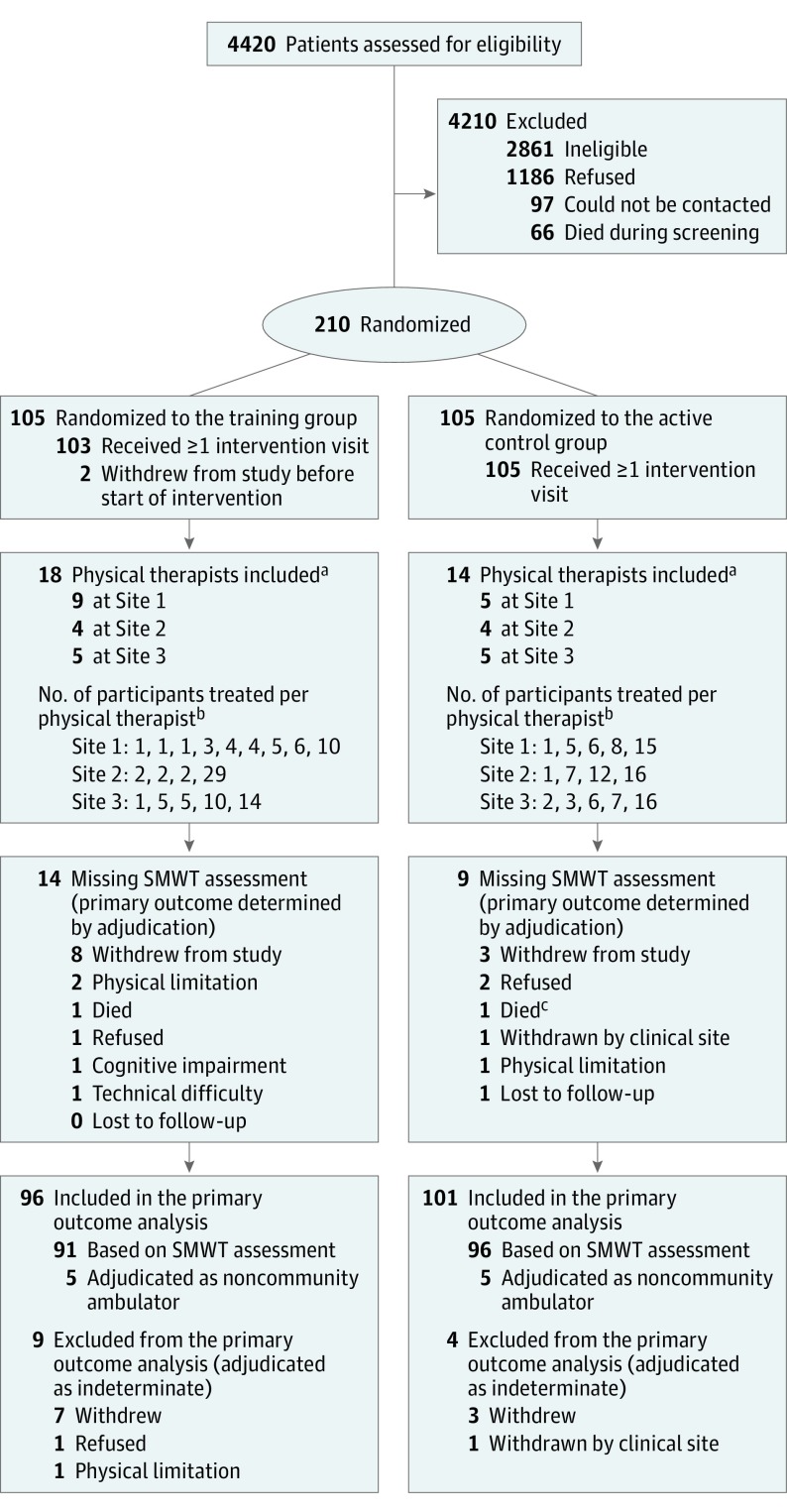
A total of 4420 patients with hip fracture were identified at 12 hospitals affiliated with the 3 clinical sites. Of 210 eligible and consenting participants, 105 were allocated to each treatment group (Figure). Groups were comparable on baseline characteristics (Table 1).
Figure. Enrollment, Randomization, and Follow-up of Participants.
SMWT indicates 6-minute walk test.
aA physical therapist was considered to be part of a given site if the therapist was assigned to at least 50% of the expected visits for at least 1 participant at that site.
bA given participant was considered to have been treated by a given physical therapist if the therapist was assigned to at least 50% of expected visits for that participant. If no single therapist was assigned to at least 50% of the participant’s expected intervention visits, the therapist who had been assigned to the largest number of visits was considered to have treated the participant.
cAn additional participant in the active control group died after the 16-week assessment window.
Table 1. Baseline Characteristics by Treatment Group.
| Characteristic | Training Group (n = 105) | Active Control Group (n = 105) |
|---|---|---|
| Demographic | ||
| Age, mean (SD), y | 80.3 (8.0) | 81.2 (8.8) |
| Sex, No. (%) | ||
| Women | 80 (76.2) | 81 (77.1) |
| Men | 25 (23.8) | 24 (22.9) |
| Race, No. (%) | ||
| White | 101 (96.2) | 101 (96.2) |
| Black or African American | 2 (1.9) | 3 (2.9) |
| >1 Race | 2 (1.9) | 1 (1.0) |
| Hispanic or Latino | 2 (1.9) | 0 |
| Clinical | ||
| Type of hip fracture, No. (%) | ||
| Closed pertrochanteric fracture | 52 (49.5) | 56 (53.3) |
| Closed fracture of femoral neck | 51 (48.6) | 47 (44.8) |
| Closed fracture, unspecified part | 2 (1.9) | 2 (1.9) |
| Body mass index, mean (SD)a | 25.3 (4.9) | 26.1 (4.1) |
| Body mass index, No. (%)a | ||
| <20 | 13 (12.4) | 4 (3.8) |
| 20-<30 | 74 (70.5) | 83 (79.0) |
| ≥30 | 18 (17.1) | 18 (17.1) |
| Resting heart rate, mean (SD), beats/min | 71.6 (12.4) | 69.8 (11.8) |
| Interval between hospitalization for hip fracture and randomization, median (IQR), wk | 13.6 (10.7-17.9) | 14.0 (11.0-18.0) |
| MNA-SF score, mean (SD)b | 9.0 (2.1) | 9.4 (1.9) |
| CES-D score, mean (SD)c | 10.4 (7.7) | 9.5 (7.4) |
| 3MS score, mean (SD)d | 91.3 (6.9) | 90.9 (6.6) |
| Comorbidities, No. (%) | ||
| Cardiac diseasee | 20 (19.0) | 23 (21.9) |
| Diabetes (type 1 or 2) | 16 (15.2) | 22 (21.0) |
| Pulmonary diseasef | 14 (13.3) | 17 (16.2) |
| History of stroke or transient ischemic attack | 11 (10.5) | 13 (12.4) |
| Physical Performance | ||
| Distance walked in 6 min, mean (SD), mg | 188.4 (56.5) | 185.4 (54.5) |
| SPPB score, mean (SD)h | 5.6 (2.2) | 5.6 (2.4) |
| mPPT score, mean (SD)i | 18.2 (6.8) | 17.5 (7.3) |
| NHATS balance score, mean (SD), sj | 25.1 (12.3) | 24.4 (9.8) |
| Gait speed, 50-ft fast walk, mean (SD), m/sk | 0.7 (0.2) | 0.7 (0.2) |
| Gait speed, 4-m usual walk, mean (SD), m/sl | 0.6 (0.2) | 0.6 (0.2) |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depression scale; IQR, interquartile range; MNA-SF, Mini Nutritional Assessment–Short-Form; mPPT, modified Physical Performance Test; NHATS, National Health and Aging Trends Study; SPPB, Short Physical Performance Battery; 3MS, modified Mini-Mental State Examination.
Calculated as weight in kilograms divided by height in meters squared.
MNA-SF: range, 0-14, with higher scores indicating better nutritional status; score of 8-11 indicates risk for malnutrition.24
CES-D: range, 0-60, with higher scores indicating more depressive symptoms; score ≥16 indicates risk for clinical depression.30
3MS: range, 0-100, with higher scores indicating less cognitive impairment; 92 is mean score for community-dwelling independent older adults.31
Cardiac disease was defined as the self-reported presence of 1 or more of the following: angina, congestive heart failure, history of myocardial infarction, history of stent, or history of coronary artery bypass graft.
Pulmonary disease was defined as the self-reported presence of 1 or more of the following: asthma, chronic obstructive pulmonary disease, acquired respiratory distress syndrome, or emphysema.
Distance walked in 6 minutes: range ≥0, with longer distance indicating greater endurance; 233 m is mean value for community-dwelling older adults with mild to moderate mobility limitations.16
SPPB: range, 0-12, with higher scores indicating better performance; score <7 indicates high risk of subsequent mobility disability.17
mPPT: range, 0-36, with higher scores indicating better performance; score of 17-24 indicates moderate frailty.32
NHATS: range, 0-90, with higher scores indicating better balance; score of 20-29 indicates ability to hold tandem stance for <10 seconds.33
Gait speed, 50-ft fast walk: range ≥0, with higher values indicating faster walking; 1.06 m/s is the median speed needed to navigate a crosswalk.15
Gait speed, 4-m usual walk: range ≥0, with higher values indicating faster walking; speed of 0.43-0.58 m/s indicates high risk of subsequent mobility disability.17
Primary Outcome
The primary outcome was measured by the 6-minute walk test for 91 training participants and 96 active control participants. Of the 14 training participants who did not perform the 16-week 6-minute walk test, 5 were classified as noncommunity ambulators by adjudication and 9 were excluded from the primary analysis. Of the 9 active control participants who did not perform the 16-week 6-minute walk test, 5 were classified as noncommunity ambulators by adjudication and 4 were excluded from the primary analysis.
At the 16-week follow-up, 22 of 96 training participants (22.9%) and 18 of 101 active control participants (17.8%) (difference, 5.1% [1-sided 97.5% CI, −∞ to 16.3%]; 1-sided P = .19) met criteria for community ambulation (Table 2). There were no statistically significant differences between the groups with respect to community ambulation in any of the prespecified subgroups (eFigure 1 in Supplement 3). At 1 clinical site, results were unfavorable to training. At the other 2 sites, results were favorable to training (P for interaction = .051) (eTable 2 in Supplement 3).
Table 2. Study Outcomes by Treatment Groupa.
| Outcomes at 16-Week Follow-Up | ||||
|---|---|---|---|---|
| Training (n = 105) | Active Control (n = 105) | Differenceb (Training Minus Control) (1-Sided 97.5% CI) | P Valuec | |
| No. of Community Ambulators/No. Assessed or Adjudicated (%) | No. of Community Ambulators/No. Assessed or Adjudicated (%) | |||
| Primary outcome | ||||
| Community ambulation | 22/96 (22.9) | 18/101 (17.8) | 5.1% (−∞ to 16.3) | .19 |
| Mean (SD) [No. Assessed] | Mean (SD) [No. Assessed] | Differenced (Training Minus Control) (95% CI) | ||
| Secondary outcomes | ||||
| Distance walked in 6 min, m | 242.4 (83.7) [91] | 233.1 (83.1) [96] | 8.7 (−9.5 to 26.8) | .35 |
| mPPT score | 20.6 (7.4) [91] | 20.9 (7.8) [97] | −0.8 (−2.1, 0.6) | .27 |
| NHATS balance score, s | 26.1 (12.3) [91] | 26.6 (12.0) [97] | −0.9 (−3.8 to 1.9) | .52 |
| Gait speed, 50-ft fast walk, m/s | 0.81 (0.25) [89] | 0.81 (0.27) [93] | −0.02 (−0.08 to 0.04) | .47 |
| Gait speed, 4-m usual walk, m/s | 0.73 (0.23) [89] | 0.74 (0.22) [95] | −0.02 (−0.07 to 0.03) | .43 |
| Quadriceps muscle strength, lb of force per lb of body weight | 0.28 (0.11) [67] | 0.29 (0.12) [67] | −0.01 (−0.04 to 0.01) | .19 |
| Tertiary outcomes | ||||
| SPPB score | 6.7 (2.7) [91] | 7.1 (3.0) [95] | −0.3 (−0.9 to 0.3) | .34 |
| No. With Increase/No. Assessed (%) | No. With Increase/No. Assessed (%) | Differenceb (Training Minus Control) (95% CI) | ||
| Increase of ≥50 m in distance walked in 6 min relative to baseline | 44/91 (48.4) | 40/96 (41.7) | 6.7% (−7.6 to 20.9) | .36 |
| Outcomes at 40-Week Follow-up | ||||
| Training (n = 76)e | Active Control (n = 76)e | |||
| No. of Community Ambulators/No. Assessed or Adjudicated (%) | No. of Community Ambulators/No. Assessed or Adjudicated (%) | Differenceb (Training Minus Control) (95% CI) | P Valuec | |
| Secondary outcomes | ||||
| Community ambulation | 15/69 (21.7) | 14/65 (21.5) | 0.2% (−14.2 to 13.8) | .98 |
| Mean (SD) [No. Assessed] | Mean (SD) [No. Assessed] | Differenced (Training Minus Control) (95% CI) | ||
| Distance walked in 6 min, m | 239.2 (93.7) [60] | 241.6 (95.6) [59] | −6.3 (−30.0 to 17.3) | .60 |
| mPPT score | 19.7 (8.7) [64] | 19.8 (8.7) [61] | −0.1 (−2.1 to 1.9) | .90 |
| NHATS balance score, s | 23.8 (12.7) [65] | 25.2 (13.6) [62] | −1.6 (−4.8 to 1.7) | .34 |
| Gait speed, 50-ft fast walk, m/s | 0.82 (0.27) [62] | 0.80 (0.29) [59] | −0.01 (−0.08 to 0.06) | .82 |
| Gait speed, 4-m usual walk, m/s | 0.77 (0.28) [62] | 0.74 (0.26) [59] | 0.02 (−0.04 to 0.09) | .48 |
| Quadriceps muscle strength, lb of force per lb of body weight | 0.27 (0.10) [55] | 0.30 (0.14) [57] | −0.03 (−0.06 to −0.004) | .03 |
| Tertiary outcomes | ||||
| SPPB score | 6.7 (3.1) [63] | 6.8 (3.1) [60] | −0.0 (−0.8 to 0.7) | .89 |
| No. With Increase/No. Assessed (%) | No. With Increase/No. Assessed (%) | Differenceb (Training Minus Control) (95% CI) | ||
| Increase of ≥50 m in distance walked in 6 min relative to baseline | 30/60 (50.0) | 29/59 (49.2) | 0.8 (−17.2 to 18.9) | .93 |
Abbreviations: mPPT, Modified Physical Performance Test; NHATS, National Health and Aging Trends Study; SPPB, Short Physical Performance Battery.
See footnotes to Table 1 for range, direction and clinical meaning of scores.
Differences, confidence intervals, and P values for binary variables were based on differences in proportions and large-sample methods (ie, χ2 tests).
The P value for the primary outcome (community ambulation at 16 weeks) is reported as 1-sided. All others are reported as 2-sided.
Differences, 95% CIs, and P values for quantitative variables were based on longitudinal regression models, allowing different variances and covariances at and between each time point and fit by maximum likelihood.
The number of participants assessed was lower at the 40-week follow-up than at the 16-week follow-up because of a protocol change that eliminated the 40-week follow-up for the 58 participants who were randomized after approval of the protocol change. The number of participants assessed for quadriceps muscle strength was lower than for other measures at the 16-week and 40-week follow-ups because of a protocol change that eliminated several measures from follow-up assessments for participants who were randomized after approval of the protocol change.
Three cases of unblinding of assessment staff were reported (2 at 16 weeks and 1 at 40 weeks; all in the training group).
Secondary Analyses
There were no statistically significant differences between the groups in any secondary or tertiary outcomes at 16- or 40-week follow-up except for quadriceps muscle strength at 40 weeks, which was greater for active control than for training (difference, −0.03 lbs force/lb body weight [95% CI, −0.06 to −0.004]; P = .03). In both groups, there were statistically significant improvements (P < .001) from baseline to the 16- and 40-week assessments in 6-minute walk distance, SPPB, mPPT, and gait speed (eTable 6 in Supplement 3). There was no evidence of differences between the groups regarding delayed or sustained effects of the training intervention (eTables 4 and 5 in Supplement 3). Using weighted estimating equations to account for potential biases due to missing outcomes, the estimated difference in the proportion of community ambulators was 5.4% (1-sided 97.5% CI, −∞ to 17.0%) (section 1.10 of the eAppendix in Supplement 3). Similarly, results of other sensitivity analyses did not substantially differ from the primary results (eTables 1, 3, and 7-11 in Supplement 3).
Intervention Visit Adherence and Treatment Fidelity
The mean (SD) percentage of expected intervention visits completed per participant was 78.3% (27.6%) in the training group and 87.0% (14.3%) in the active control group; 72.4% of training participants and 81.0% of active control participants completed at least 80% of expected intervention visits. Table 3 shows the distribution of participants according to achievement of intervention targets. For strength and endurance components of training, the volume target was reached in more visits than the intensity target. The number of visits in which volume targets were achieved was lower for training than active control.
Table 3. Achievement of Intervention Targets (Secondary Outcome) by Treatment Group.
| Intervention Targets | No. (%) Participants by No. of Intervention Visits in Which Intervention Target Was Achieveda | ||||
|---|---|---|---|---|---|
| 0-8 Visits | 9-16 Visits | 17-24 Visits | 25-38 Visitsb | Total | |
| Training Group | |||||
| Strength component | |||||
| Volume: perform 3 sets of 8 repetitions for each of 4 exercises for each leg (192 repetitions) | 19 (18.1) | 8 (7.6) | 11 (10.5) | 67 (63.8) | 105 (100.0) |
| Intensity: perform all 192 repetitions through the full range of motion at the prescribed load | 25 (23.8) | 17 (16.2) | 27 (25.7) | 36 (34.3) | 105 (100.0) |
| Endurance component | |||||
| Volume: perform at least 20 min of walking or other upright activity | 16 (15.2) | 5 (4.8) | 15 (14.3) | 69 (65.7) | 105 (100.0) |
| Intensity: perform at least 20 min of walking or other upright activity while maintaining a score of 3-5 on the Borg Scale13 or 50% of heart rate reserve | 24 (22.9) | 14 (13.3) | 23 (21.9) | 44 (41.9) | 105 (100.0) |
| Active Control Group | |||||
| Perform at least 20 min of active range-of-motion exercises | 5 (4.8) | 6 (5.7) | 20 (19.0) | 74 (70.5) | 105 (100.0) |
| Receive at least 21 min of TENS, 7 min to each of 3 muscle groups (bilaterally) | 5 (4.8) | 3 (2.9) | 9 (8.6) | 88 (83.8) | 105 (100.0) |
Abbreviation: TENS, transcutaneous electrical nerve stimulation.
Participants were deemed not to have achieved the intervention target if the expected visit was not completed or if the visit was completed but the target was not achieved. There were 40 expected visits among participants randomized under the original protocol (n = 39 training, 36 active control). There were 32 expected visits among participants randomized under the revised protocol (n = 66 training, 69 active control).
Visits 1 and 2 were excluded from the calculation of the number of intervention visits in which the intervention targets were achieved because the first 2 visits were introductory sessions and had different content and expectations relative to later visits.
In a post-hoc analysis of the endurance portion of training visits, the median of participants’ mean heart rates across all visits at which heart rate was measured (excluding the first 2 visits) was 95.1 beats/min (interquartile range [IQR], 83.8-106.1; n = 97). This was significantly higher than the median heart rate during the strength component of training visits (83.0 beats/min; IQR, 74.2-91.2; n = 98) or the active range-of-motion or TENS components of active control visits (76.3 beats/min; IQR, 69.3-83.1; n = 102 and 72.2 beats/min; IQR, 66.2-78.2; n = 102, respectively).
Adverse Events
Seventeen participants in the training group (16.2%) and 15 in the active control group (14.3%) had 1 or more reportable adverse events during the intervention period (Table 4). The number of reportable adverse events per participant and the severity and relatedness of the events were similar between the 2 groups. The most commonly reported adverse events were falls (training: 6 [5.7%], control: 4 [3.8%]), femur/hip fracture (2 in each group), pneumonia (training: 2, control: 0), urinary tract infection (training: 2, control: 0), dehydration (training: 0, control: 2), and dyspnea (training: 0, control: 2). There were 3 deaths (1 during the intervention period [training group], 1 after the intervention period but before the end of the 16-week assessment window [active control group], and 1 after the 16-week assessment window [active control group]).
Table 4. Participants With Reportable Adverse Events During the Intervention Period by Treatment Group.
| Adverse Event | No. (%) | |
|---|---|---|
| Training Group (n = 105) | Active Control Group (n = 105) | |
| ≥1 Reportable adverse eventa | 17 (16.2) | 15 (14.3) |
| No. of reportable adverse events per participant | ||
| 1 | 13 (12.4) | 14 (13.3) |
| 2 | 3 (2.9) | 1 (1.0) |
| ≥3 | 1 (1.0) | 0 |
| Severityb | ||
| Mild or moderate | 11 (10.5) | 10 (9.5) |
| Severe or life-threatening | 5 (4.8) | 5 (4.8) |
| Fatal | 1 (1.0) | 0c |
| Relatednessd | ||
| Not related | 15 (14.3) | 11 (10.5) |
| Possibly related | 0 | 3 (2.9)e |
| Definitely related | 2 (1.9)f | 0 |
| Unknown | 0 | 1 (1.0) |
| Most frequent preferred terms describing eventsg | ||
| Fall | 6 (5.7) | 4 (3.8) |
| Femur/hip fracture | 2 (1.9) | 2 (1.9) |
| Pneumonia | 2 (1.9) | 0 |
| Urinary tract infection | 2 (1.9) | 0 |
| Dehydration | 0 | 2 (1.9) |
| Dyspnea | 0 | 2 (1.9) |
An adverse event was considered reportable if it occurred while the participant was under the supervision of study staff or if it resulted in (1) hospitalization (or prolongation of an existing hospitalization), (2) a life-threatening condition, (3) death, (4) permanent or significant disability or incapacity, or (5) medical or surgical intervention. The intervention period was defined as the first 16 weeks after randomization or the last intervention visit, whichever was later.
Participants who had multiple reportable adverse events of different severity were classified according to the most severe reportable adverse event.
Two active control participants died but are not included in this Table because they died after the intervention period. Neither death was deemed to be related to the intervention.
Participants who had multiple reportable adverse events of different relatedness were classified according to the most significant relatedness status, with “unknown” being considered the least significant, “not related” being considered more significant, “possibly related” being next most significant, and “definitely related” being the most significant.
Of the 3 participants whose reportable adverse event was judged to be possibly related to being in the study, 1 fell backward in a parking lot and suffered a head wound, 1 fell and was hospitalized for a femur fracture, and 1 experienced a transient alteration of awareness during an intervention visit.
Of the 2 participants whose reportable adverse event was judged to be definitely related to being in the study, 1 fell during an intervention visit and was hospitalized for a femur fracture and 1 fell without injury during an intervention visit.
The percentage is the number of participants with at least 1 reportable adverse event described by the preferred term divided by the number of randomized participants. If a participant had more than 1 reportable adverse event with the same preferred term, the participant was only counted once. Because some reportable adverse events were described by multiple preferred terms, the total number of preferred terms is greater than the total number of reportable adverse events.
Discussion
Among older adults with a hip fracture, a multicomponent home-based physical therapy intervention designed to improve endurance, strength, balance, and function did not result in a statistically significant improvement in participants’ ability to walk 300 m or more in 6 minutes after 16 weeks as compared with an active control intervention that included TENS and active range-of-motion exercises. The difference between the groups in proportions of participants achieving this outcome was not statistically significant.
A strength of this study was the inclusion of an active control intervention, allowing the effect of the training intervention to be isolated from the effect of receiving regular contact with a physical therapist. Although the active control intervention was designed to include activities that do not directly affect walking ability, it may have had a benefit beyond attention. During active control visits, participants performed up to 20 repetitions of each of 22 active range-of-motion exercises. Movement velocity is an independent predictor of function34 and the volume and speed of exercise movements may have enhanced lower extremity power and walking ability in active control participants. In addition, physical therapists were engaged actively with study participants, providing motivation and positive reinforcement throughout the exercise sessions; the resulting therapeutic alliance may have contributed to improved walking ability in the active control group.35,36 Both groups had clinically meaningful improvements from baseline to 16 weeks and from baseline to 40 weeks for 3 of the secondary/tertiary outcomes (6-minute walk distance, SPPB, and gait speed16,20,37). While the observed changes may reflect natural recovery after hip fracture, changes in usual care control groups in other clinical trials of patients with hip fracture9,10 were smaller than those observed in this study, suggesting that in this trial, both study interventions provided greater benefits than usual post–hip fracture care.
Heterogeneity of response to progressive strength training in older adults is well established.38 It is possible that the dose of exercise received by some participants in the training group was not sufficient. Although in post hoc analyses the physiologic response to the intervention (as measured by heart rate) was greater for the training than the active control group and adherence in both groups was greater than reported adherence to exercise in older adults,39,40 visit adherence was lower in training than active control, which may have contributed to the smaller than hypothesized between-group differences. Future studies should address the influence of modifiable factors, such as exercise dose, adherence, behavioral and environmental factors, and body composition, on response to training in persons with hip fracture.
Limitations
This study has several limitations. First, the proportion of screened patients who were enrolled was low, limiting generalization to all patients with hip fracture. Second, missing data were not likely to have been missing at random, potentially biasing results. Third, due to the nature of the interventions, it was not possible to blind participants to treatment. Because there were only 3 instances in which participants revealed their treatment group to assessment staff, results are unlikely to have been biased by assessors’ knowledge of treatment assignment. Fourth, because the outcome measures were partly effort dependent, participants’ knowledge of treatment assignment could have affected their performance. To reduce bias, participants were informed that they would be randomized to either a specific or a nonspecific physical therapy intervention. Fifth, the decision (made midstudy) to eliminate the 40-week follow-up resulted in availability of data on longer-term effects of the intervention for only 134 (63.8%) of the 210 participants. Sixth, although delivering the intervention in the participants’ homes removed an important barrier to participation, illness and hospitalization may have been barriers to completion of the protocol. Seventh, the study was powered to detect a large and clinically meaningful difference but did not have sufficient power to detect smaller meaningful differences, particularly with the reduced sample size. Based on findings reported here, differences favorable to the training intervention smaller than the upper limit of the confidence interval (16.3%) cannot be excluded.
Conclusions
Among older adults with a hip fracture, a multicomponent home-based physical therapy intervention compared with an active control that included TENS and active range-of-motion exercises did not result in a statistically significant improvement in the ability to walk 300 m or more in 6 minutes after 16 weeks.
Trial Protocol
Statistical Analysis Plan
eAppendix. Supplementary Analyses
eReferences
Data Sharing Statement
References
- 1.Stevens JA, Rudd RA. The impact of decreasing US hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728. doi: 10.1007/s00198-013-2375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CA, Starr AZ, Nunley JA. Analysis of past secular trends of hip fractures and predicted number in the future 2010-2050. J Orthop Trauma. 2012;26(2):117-122. doi: 10.1097/BOT.0b013e318219c61a [DOI] [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407-413. doi: 10.1007/PL00004148 [DOI] [PubMed] [Google Scholar]
- 4.Dyer SM, Crotty M, Fairhall N, et al. ; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group . A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16(1):158. doi: 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507. doi: 10.1093/gerona/55.9.M498 [DOI] [PubMed] [Google Scholar]
- 6.Handoll HH, Sherrington C. Mobilisation strategies after hip fracture surgery in adults. Cochrane Database Syst Rev. 2007;23(1):CD001704. [DOI] [PubMed] [Google Scholar]
- 7.Lee KB, Lim SH, Ko EH, Kim YS, Lee KS, Hwang BY. Factors related to community ambulation in patients with chronic stroke. Top Stroke Rehabil. 2015;22(1):63-71. doi: 10.1179/1074935714Z.0000000001 [DOI] [PubMed] [Google Scholar]
- 8.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292(7):837-846. doi: 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 9.Salpakoski A, Törmäkangas T, Edgren J, et al. Effects of a multicomponent home-based physical rehabilitation program on mobility recovery after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(5):361-368. doi: 10.1016/j.jamda.2013.12.083 [DOI] [PubMed] [Google Scholar]
- 10.Latham NK, Harris BA, Bean JF, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311(7):700-708. doi: 10.1001/jama.2014.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 12.Winter DA, Eng JJ, Ishac MG, Craik RL, Oatis CA. A review of kinetic parameters in human walking In: Gait Analysis: Theory and Application. St Louis, MO: Mosby; 1995:252-270. [Google Scholar]
- 13.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90-93. [PubMed] [Google Scholar]
- 14.Langlois JA, Keyl PM, Guralnik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87(3):393-397. doi: 10.2105/AJPH.87.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities: a clinical report. Phys Ther. 1988;68(9):1371-1373. doi: 10.1093/ptj/68.9.1371 [DOI] [PubMed] [Google Scholar]
- 16.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556-561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman VA, Kasper JD, Cornman JC, et al. Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci. 2011;66(9):1013-1021. doi: 10.1093/gerona/glr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauer K, Specht N, Schuler M, Bärtsch P, Oster P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age Ageing. 2002;31(1):49-57. doi: 10.1093/ageing/31.1.49 [DOI] [PubMed] [Google Scholar]
- 20.Palombaro KM, Craik RL, Mangione KK, Tomlinson JD. Determining meaningful changes in gait speed after hip fracture. Phys Ther. 2006;86(6):809-816. [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 22.Tombaugh T, McDowell I, Kristjansson B, Hubley A. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assess. 1996;8(1):48-59. doi: 10.1037/1040-3590.8.1.48 [DOI] [Google Scholar]
- 23.Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(1, pt 2):S59-S65. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser MJ, Bauer JM, Ramsch C, et al. ; MNA-International Group . Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi: 10.1007/s12603-009-0214-7 [DOI] [PubMed] [Google Scholar]
- 25.Fay MP, Halloran ME, Follmann DA. Accounting for variability in sample size estimation with applications to nonadherence and estimation of variance and effect size. Biometrics. 2007;63(2):465-474. doi: 10.1111/j.1541-0420.2006.00703.x [DOI] [PubMed] [Google Scholar]
- 26.Shardell MD, El-Kamary SS. Calculating sample size for studies with expected all-or-none nonadherence and selection bias. Biometrics. 2009;65(2):635-639. doi: 10.1111/j.1541-0420.2008.01114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 28.Hwang IK, Shih WJ, De Cani JS. Group sequential designs using a family of type I error probability spending functions. Stat Med. 1990;9(12):1439-1445. doi: 10.1002/sim.4780091207 [DOI] [PubMed] [Google Scholar]
- 29.Package 'gsDesign': Group Sequential Design [computer program]. Version 3.0-1. https://cran.r-project.org/web/packages/gsDesign/gsDesign.pdf. Vienna, Austria: R Project; 2016.
- 30.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277-287. doi: 10.1037/0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- 31.Jones TG, Schinka JA, Vanderploeg RD, Small BJ, Graves AB, Mortimer JA. 3MS normative data for the elderly. Arch Clin Neuropsychol. 2002;17(2):171-177. [PubMed] [Google Scholar]
- 32.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55(6):M350-M355. doi: 10.1093/gerona/55.6.M350 [DOI] [PubMed] [Google Scholar]
- 33.Orwig D, Mangione KK, Baumgarten M, et al. Improving community ambulation after hip fracture: protocol for a randomised, controlled trial. J Physiother. 2017;63(1):45-46. doi: 10.1016/j.jphys.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayers SP, Guralnik JM, Thombs LA, Fielding RA. Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc. 2005;53(3):467-471. doi: 10.1111/j.1532-5415.2005.53166.x [DOI] [PubMed] [Google Scholar]
- 35.Babatunde F, MacDermid J, MacIntyre N. Characteristics of therapeutic alliance in musculoskeletal physiotherapy and occupational therapy practice: a scoping review of the literature. BMC Health Serv Res. 2017;17(1):375. doi: 10.1186/s12913-017-2311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resnick B, Orwig D, Yu-Yahiro J, et al. Testing the effectiveness of the exercise plus program in older women post-hip fracture. Ann Behav Med. 2007;34(1):67-76. doi: 10.1007/BF02879922 [DOI] [PubMed] [Google Scholar]
- 37.Alley DE, Hicks GE, Shardell M, et al. Meaningful improvement in gait speed in hip fracture recovery. J Am Geriatr Soc. 2011;59(9):1650-1657. doi: 10.1111/j.1532-5415.2011.03560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The importance of resistance exercise training to combat neuromuscular aging. Physiology (Bethesda). 2019;34(2):112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picorelli AM, Pereira LS, Pereira DS, Felício D, Sherrington C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. 2014;60(3):151-156. doi: 10.1016/j.jphys.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 40.Osho O, Owoeye O, Armijo-Olivo S. Adherence and attrition in fall prevention exercise programs for community-dwelling older adults: a systematic review and meta-analysis. J Aging Phys Act. 2018;26(2):304-326. doi: 10.1123/japa.2016-0326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. Supplementary Analyses
eReferences
Data Sharing Statement