Abstract
Nitromethane is a known toxicant and suspected human carcinogen. Exposure to nitromethane in a representative sample of the civilian, non-institutionalized population in the United States ≥12 years old was assessed using 2007 – 2012 National Health and Nutritional Examination Survey (NHANES) data. Nitromethane was detected in all 8,000 human blood samples collected, of which 6,730 were used for analyses reported here. Sample-weighted median blood nitromethane was higher among exclusive combusted tobacco users (exclusive smokers; 774 ng/L) than non-users of tobacco products (625 ng/L). In stratified sample-weighted regression analysis, smoking 0.5 pack of cigarettes per day was associated with an increase in blood nitromethane by 150 ng/L, and secondhand smoke exposure (serum cotinine >0.05 ng/mL and <10 ng/mL) was associated with a 31.1 ng/L increase in blood nitromethane. Significant, but smaller increases in blood nitromethane were associated with certain dietary sources. At median consumption levels, blood nitromethane was associated with an increase of 7.55 ng/L (meat/poultry), 9.32 ng/L (grain products), and 14.5 ng/L (vegetables). This is the first assessment of the magnitude and relative source apportionment of nitromethane exposure in the U.S. population.
Keywords: nitromethane, human blood, tobacco, diet, exposure, NHANES
Introduction:
Nitromethane is a colorless, flammable, polar liquid commonly used in industry as a solvent for cyanoacrylate adhesives and as a stabilizer in halogenated solvents for vapor degreasing and dry cleaning.1 When mixed with methanol, nitromethane is used as a fuel source in professional drag racing and by hobbyists who operate radio-controlled cars and aircraft. Cigarette smoke contains nitromethane in the microgram range (0.9 – 6.5 µg/cigarette).2 Nitromethane is also detectable in exhaust fumes from diesel and gasoline engines.3 In vivo, nitromethane forms through the non-enzymatic dehalogenation of trihalonitromethanes (THNMs);4 the THNM chloropicrin is a food crop fumigant and can also form as a byproduct during water disinfection.5
Nitromethane enters the body via inhalation, ingestion, or dermal absorption. Blood nitromethane is a relatively short-lived exposure biomarker with a serum nitromethane half-life of 13.5 hrs6; controlled nitromethane exposure of non-human primates indicates that plasma nitromethane levels peak 0.3 – 6 hrs after exposure.7 Exposure to high concentrations of nitromethane can irritate the eyes, nose, and throat and damage the central nervous system, lungs, and liver.8 Nitromethane has been shown to be carcinogenic in animal studies and is classified as a possible human carcinogen (Group 2B).9, 10 The Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 100 ppm and an immediately dangerous to life or health concentration of 750 ppm.8 In one case study, two workers with prolonged dermal and inhalation exposure to nitromethane developed severe cases of peripheral neuropathy.11 Despite the potential health impact of nitromethane, exposure has not been characterized for a population-representative sample. Population-based biomonitoring data is useful for understanding the prevalence and magnitude of exposure, as well as the impact of regulatory change.12 We assessed blood nitromethane data from 8,000 participants 12 years of age and older as part of the 2007–2012 National Health and Nutrition Examination Survey (NHANES), and used sample-weighted multiple linear regression models to explore significant exposures.
Subjects and methods:
Study Design
NHANES is designed to assess the health and nutritional status of the US population by collecting questionnaire and physical examination data, as well as biologic samples, during two-year sampling cycles.13 Each participant is initially interviewed at home, completes several questionnaires, and undergoes physical examination at the NHANES Mobile Examination Center. A total of 8,000 participants 12 years of age and older had blood nitromethane data recorded during the 2007 – 2008, 2009 – 2010, and 2011 – 2012 NHANES cycles, of which a subset (see below) was used to obtain results reported herein.
Laboratory Method
In 2008, several of the authors in collaboration with other researchers published a method that quantified nitromethane in human blood with a limit of detection of 65 ng/L.4 Whole blood (3mL) was spiked with stable isotope-labeled nitromethane (13CH3NO2) internal standard into a 10mL vial before it was hermetically sealed with a Teflon-lined silicone septum. The vial headspace was sampled via solid-phase microextraction and analyzed using gas chromatography–high-resolution mass spectrometry. A 6890 Agilent GC (Palo Alto, CA) and a MAT95XP magnetic sector MS (Thermo Fisher Scientific Inc., Waltham, MA) were used. Xcalibur software (v1.3, Thermo Fisher Scientific Inc.) was used to process and quantitate nitromethane results. Reported results met the accuracy and precision specifications of the quality control/quality assurance program of the CDC National Center for Environmental Health, Division of Laboratory Sciences.14
Determination of Combusted Tobacco Use
Based on NHANES questionnaire data on recent tobacco use (NHANES dataset: SMQRTU), NHANES participants were excluded from analysis if they reported using smokeless tobacco or nicotine replacement therapy, as indicated by responding “yes” to question SMQ680 (tobacco or nicotine use within 5 days prior to NHANES physical examination) and “yes” to at least one of SMQ690D – SMQ690F (smokeless tobacco and nicotine delivery products). These participants were excluded because smokeless tobacco and nicotine replacement therapy are alternative sources of nicotine (and hence its metabolite cotinine), but whose use does not involve combustion. The remaining participants were then identified as exclusive users of combusted tobacco products (named “exclusive combusted tobacco users” or “exclusive smokers” in this report) if they responded “yes” to SMQ680 and “yes” to at least one of SMQ690A – SMQ690C (cigarettes, pipes, cigars). Participants were identified as non-users of tobacco products if they answered “no” to SMQ680 or were both missing a response to SMQ680 and had serum cotinine ≤10 ng/mL. The serum cotinine threshold of >10 ng/mL has been identified as consistent with active use of combusted tobacco products, and was used to stratify self-identified exclusive smokers and non-users in statistical analyses reported herein.15 Participants were excluded from analysis for use of smokeless tobacco and nicotine replacement therapy (N=244), for missing serum cotinine data (N=3), or for missing data for other variables used in regression models (N=1,023). This attrition left 6,730 study participants eligible for statistical analysis.
Statistical Analysis
Because NHANES participants are recruited through a multistage probability sample, it is necessary to account for this complex design to estimate variances properly and to produce unbiased, nationally representative statistics. Robust estimation may be accomplished by applying survey sample weights to each participant’s data and using Taylor series linearization to produce variance estimates. We used this estimation approach as implemented in the DESCRIPT subroutine of SUDAAN version 11.0.0 (Research Triangle Institute, Research Triangle Park, NC) called from the SAS statistical software application version 9.4 (SAS Institute, Cary, NC), as well as the SURVEYREG and SURVEYMEANS subroutines of SAS 9.4. Sample-weighted linear regression models stratified by tobacco use were fit to data from NHANES survey cycles 2007 – 2008, 2009 – 2010, and 2011 – 2012, where the dependent variable was blood nitromethane concentration (ng/L). Because the distribution of measurements was strongly right-skewed, which could have adversely affected hypothesis testing, the blood nitromethane concentration data was transformed with the natural log for regression analysis. We report slopes from these models along with their 95 percent confidence intervals and p-values. In addition, to facilitate interpretability, we report the slopes transformed to represent the absolute change in biomarker concentration associated with a unit-increase in the predictor , as adapted from Rodríguez-Barranco, et al., 201716: , where is the sample-weighted geometric mean of biomarker concentration. The tabulated regression results in Table 3 assume , so that represents the absolute change associated with a unit-increase in the predictor. The 95 percent confidence interval is: , where is the sample-weighted standard error of the slope. Both and its 95%CI are calculated at , which is reported in the caption accompanying the tabulated regression results. Since this geometric mean is treated as a fixed quantity, the width of the 95%CI may be slightly underestimated. In addition, at values different from the geometric mean, the value of and the width of its 95%CI will vary, owing to the log transformation of the dependent variable. Statistical significance was set to α ≤ 0.05.
Table 3.
Sample-weighted multiple linear regression model for blood nitromethane (ng/L), where tobacco smoke exposure is represented by measured serum cotinine (ng/mL). The geometric mean of blood nitromethane used for computing ΔY [95%CI] is 646 ng/L for non-users and 789 ng/L for exclusive smokers. NHANES 2007–2012 (N=6,730)
| Non-users (N=5,345) | Exclusive Smokers (N=1,385) | |||||
|---|---|---|---|---|---|---|
| Predictor | Slope [95%CI]a | p-Valuea | ΔY[95%CI]b | Slope [95%CI]a | p-Valuea | ΔY[95%CI]b |
| Serum cotinine (ng/mL) | 0.0166 [0.0056, 0.0276] | 0.0039 | 10.8 [3.81, 18.0] | 0.0005 [0.0003, 0.0007] | <.0001 | 0.402 [0.251, 0.552] |
| Sex | ||||||
| Female | 0.0626 [0.0372, 0.0880] | <.0001 | 41.7 [24.9, 58.9] | 0.0362 [−0.0253, 0.0976] | 0.2423 | 29.1 [−18.5, 79.6] |
| Male | Ref.c | . | Ref. | Ref. | . | Ref. |
| Age (years) | ||||||
| 12–19 | −0.0619 [−0.0974, −0.0264] | 0.0010 | −38.8 [−59.4, −17.4] | −0.0652 [−0.1313, 0.0008] | 0.0527 | −49.8 [−95.9, −0.663] |
| 20–39 | Ref. | . | Ref. | Ref. | . | Ref. |
| 40–59 | 0.0388 [0.0014, 0.0762] | 0.0421 | 25.6 [1.52, 50.5] | 0.0143 [−0.0225, 0.0511] | 0.4383 | 11.4 [−16.8, 40.6] |
| ≥60 | 0.1480 [0.1038, 0.1922] | <.0001 | 103 [71.4, 136] | 0.1335 [0.0602, 0.2067] | 0.0006 | 113 [50.5, 179] |
| Race/Ethnicity | ||||||
| Mexican American | 0.1193 [0.0553, 0.1834] | 0.0005 | 81.9 [37.8, 129] | 0.0794 [0.0012, 0.1575] | 0.0466 | 65.2 [2.51, 133] |
| Non-Hispanic Black | 0.1422 [0.0978, 0.1866] | <.0001 | 98.7 [67.1, 132] | 0.0994 [0.0416, 0.1572] | 0.0011 | 82.4 [34.7, 133] |
| Non-Hispanic White | Ref. | . | Ref. | Ref. | . | Ref. |
| Other Race | 0.1523 [0.0985, 0.2061] | <.0001 | 106 [67.8, 147] | −0.0002 [−0.0548, 0.0544] | 0.9937 | −0.169 [−41.1, 43.0] |
| BMI | ||||||
| Healthy weight | Ref. | . | Ref. | Ref. | . | Ref. |
| Overweight/Obese | 0.0614 [0.0362, 0.0867] | <.0001 | 40.9 [24.2, 58.0] | 0.0711 [0.0339, 0.1084] | 0.0004 | 58.2 [28.0, 89.5] |
| Underweight | −0.0944 [−0.1843, −0.0046] | 0.0397 | −58.2 [−107, −4.39] | −0.0822 [−0.1894, 0.0251] | 0.1301 | −62.2 [−134, 17.9] |
| Poverty Status | ||||||
| No | Ref. | . | Ref. | Ref. | . | Ref. |
| Yes | −0.0372 [−0.0901, 0.0157] | 0.1638 | −23.6 [−54.9, 9.36] | 0.0341 [−0.0092, 0.0774] | 0.1204 | 27.4 [−6.42, 62.6] |
| NHANES Cycle | ||||||
| 2007–2008 | 0.0316 [−0.0365, 0.0997] | 0.3556 | 20.7 [−22.1, 66.5] | −0.0319 [−0.0903, 0.0266] | 0.2784 | −24.8 [−67.1, 20.1] |
| 2009–2010 | Ref. | . | Ref. | Ref. | . | Ref. |
| 2011–2012 | 0.0162 [−0.0382, 0.0706] | 0.5526 | 10.5 [−23.4, 46.3] | −0.0233 [−0.0821, 0.0355] | 0.4298 | −18.2 [−61.2, 27.3] |
| Fasting time (hrs) | −0.0011 [−0.0036, 0.0015] | 0.3996 | −0.694 [−2.29, 0.907] | −0.0008 [−0.0056, 0.0040] | 0.7361 | −0.638 [−4.32, 3.06] |
| Food group (kg/day) | ||||||
| Milk products | −0.0210 [−0.0732, 0.0312] | 0.4224 | 10.5 [−23.4, 46.3] | −0.0235 [−0.0991, 0.0521] | 0.5350 | −18.3 [−73.1, 40.7] |
| Meat, poultry | 0.0655 [0.0107, 0.1202] | 0.0201 | −13.4 [−44.8, 19.6] | 0.1353 [0.0284, 0.2421] | 0.0141 | 114 [24.9, 213] |
| Eggs | 0.0341 [−0.2005, 0.2688] | 0.7713 | 43.7 [7.84, 81.5] | −0.2002 [−0.3833, −0.0171] | 0.0328 | −143 [−249, −16.9] |
| Legumes, nuts, seeds | 0.2056 [0.0491, 0.3622] | 0.0111 | 22.4 [−114, 194] | 0.0834 [−0.2653, 0.4321] | 0.6330 | 68.6 [−179, 416] |
| Grain products | 0.0572 [0.0094, 0.1049] | 0.0199 | 147 [35.1, 278] | 0.0562 [−0.0284, 0.1408] | 0.1878 | 45.6 [−20.5, 117] |
| Fruits | 0.0389 [−0.0192, 0.0970] | 0.1846 | 38.0 [6.88, 70.6] | 0.0447 [−0.0373, 0.1267] | 0.2788 | 36.1 [−27.4, 105] |
| Vegetables | 0.2067 [0.1299, 0.2836] | <.0001 | 25.6 [−11.4, 64.7] | 0.0780 [−0.0229, 0.1788] | 0.1267 | 64.0 [−15.9, 152] |
| Fats, oils, salad dressings | 0.1314 [−0.2932, 0.5561] | 0.5367 | 148 [91.0, 210] | 0.6345 [−0.1907, 1.4596] | 0.1287 | 699 [−124, 2.54E+03] |
| Sugars, sweets, beverages | −0.0137 [−0.0239, −0.0036] | 0.0091 | 90.7 [−159, 469] | −0.0171 [−0.0337, −0.0006] | 0.0431 | −13.4 [−25.9, −0.758] |
| Cured meat | 0.3438 [−0.0125, 0.7001] | 0.0582 | −8.80 [−15.1, −2.46] | 0.4363 [−0.6751, 1.5478] | 0.4340 | 432 [−376, 2.82E+03] |
| Luncheon meat and hot dogs | −0.0133 [−0.2451, 0.2186] | 0.9090 | 265 [−2.37, 643] | 0.2733 [−0.0931, 0.6397] | 0.1403 | 248 [−63.6, 693] |
| Tap water | −0.0093 [−0.0231, 0.0045] | 0.1817 | −8.51 [−137, 153] | −0.0193 [−0.0422, 0.0036] | 0.0974 | −15.0 [−32.1, 2.43] |
The dependent variable, blood nitromethane (ng/L), was natural log-transformed for the regression model.
ΔY is the expected change in nitromethane concentration in ng/L associated with a unit-increase in the predictor at the overall geometric mean, controlling for other predictors in the model.
Reference group
Sample-weighted regression models were stratified by combusted tobacco use, and the following variables were included as predictors: sex, age, race/ethnicity, poverty income ratio (PIR; ratio of self-reported family income to the U.S. Census poverty threshold), and fasting time (time elapsed since participant last ate or drank anything other than water and the time of specimen collection). Information for these potential confounders was self-reported. Age [year] was categorized into the following ranges: 12 – 19, 20 – 39, 40 – 59, and ≥60. Poverty level was determined by whether the ratio of a family’s income to poverty (INDFMPIR) was greater or less than the poverty threshold, which is represented by the ratio equaling unity.13 In addition, body mass index (BMI) from measurements at the physical examination was included as a predictor. Since standard definitions for underweight (BMI < 18.5 kg/m2), healthy weight (18.5 ≤ BMI < 25), and overweight/obese (BMI ≥ 25) apply to adults ≥20 y, participants younger than 20 y were classified based on their BMI percentile for their sex and age: below the 5th percentile (underweight), between the 5th and 85th percentile (healthy weight), and above the 85th percentile (overweight/obese).17 NHANES cycle was also included as a predictor.
Food consumption information was collected from participants using structured questionnaires administered by trained interviewers who used intensive elicitation techniques to translate a participant’s recollection of the type and amount of food consumed to a standardized numerical encoding and food mass. Dietary exposure was explored by assessing the mass NHANES participants consumed within each USDA (US Department of Agriculture) food group for the 24-hour period (midnight to midnight) preceding the in-person dietary recall interview conducted as part of the physical examination. Data for the 24-hour recall period are contained in the publicly available NHANES Individual Foods – First Day file (NHANES dataset: DR1IFF), which provides a record describing each food, water, or beverage consumed by the participant, including the mass reported consumed and eight-digit USDA food code. Standardized hierarchical food groups can be identified from the USDA code, where the first digit represents one of nine major food groups, and each subsequent digit represents subgroups of increasing specificity.18 The mass consumed in each food group was summed so that each participant was represented by a single record describing their dietary intake for the previous 24 hours. Each participant’s dietary intake was first apportioned over nine food groups: milk products; meat/poultry; eggs; legumes/nuts/seeds; grain products; fruits; vegetables; fats/oils/salad dressings; and sugars/sweets/beverages. In addition, we distinguished three subgroups: cured meats, luncheon meats and hot dogs, and tap water. The cured meats food group was constructed using the search term “cured” in the USDA What We Eat In America search tool and selecting all food codes referring to meats.19 The luncheon meat and hot dog food group was constructed by searching the term “luncheon” and selecting all meat food codes and searching “hot dog.” The tap water food group was constructed by searching “tap water.” To avoid double counting, the mass consumed in each subgroup was subtracted from the mass consumed in their respective food group. The USDA food codes and logic for apportioning dietary intake are detailed in Supplemental Table S1.
Serum cotinine was used as a continuous predictor to represent tobacco smoke exposure for both exclusive combusted tobacco users and non-users of tobacco products. Cotinine is a highly specific metabolite of nicotine, the primary addictive agent in tobacco and tobacco smoke, and is thereby present in the blood serum of tobacco smokers. Likewise, since tobacco smoke exposure among non-users of tobacco products is attributable to inhalation of secondhand tobacco smoke (SHS), this exposure can be quantified with serum cotinine. In addition, to provide an alternative representation of tobacco smoke exposure, we ran a regression model where exposure among exclusive smokers was represented by the self-reported average number of cigarettes smoked per day (CPD) over the five days preceding the NHANES physical exam. This CPD regression model was sample-weighted, unstratified, and comprised the same predictors as in the stratified models, except that exposure was classified as ≤0.05 ng/mL serum cotinine (non-exposed to tobacco smoke); >0.05 – ≤10 ng/mL (presumptively exposed to second-hand tobacco smoke); 1 – 10 cigarettes per day (CPD; 0.5 pack), 11 – 20 (1 pack), and >20 (>1 pack), where the reference category was non-exposed participants. The non-exposed category was defined at ≤0.05 ng/mL serum cotinine, which was its LOD in the 1999 – 2000 NHANES cycle, and although this improved in 2001 to 0.015 ng/mL, we use 0.05 ng/mL to permit historical comparison of serum cotinine results.20 The analytic dataset for the CPD model comprised the same participants as in the stratified models, but excluded participants who could not be assigned to a CPD category (N=266), leaving 6,464 participants.
Results:
Nitromethane was detected in all 8,000 blood samples from the 2007–2012 NHANES cycles. Measurements ranged from 195 to 5,830 ng/L. Table 1 displays demographic distributions for non-users (N=5,345) and exclusive smokers (N=1,385) eligible for statistical analysis. Sample-weighted medians with 25th and 75th percentiles for blood nitromethane among non-users and exclusive smokers are detailed in Table 2. The sample-weighted median nitromethane for exclusive smokers (774 ng/L) was higher than non-users (625 ng/L). Sample-weighted geometric means and additional percentiles for blood nitromethane stratified by non-users and exclusive smokers are available in Supplemental Tables S2 and S3.
Table 1.
Sample-weighted demographic distribution of exclusive smokers and non-users in NHANES 2007 – 2012 ≥ 12 years-old (N = 6,730)a
| Demographic Category | Exclusive Smokers | Non-users | |||
|---|---|---|---|---|---|
| Nb | % (SEc) | Nb | % (SEc) | ||
| NHANES Cycle | 2007 – 2008 | 500 | 7.47 (0.79) | 1,864 | 27.03 (1.35) |
| 2009 – 2010 | 514 | 7.01 (0.57) | 1,993 | 25.47 (1.21) | |
| 2011 – 2012 | 371 | 6.63 (0.63) | 1,488 | 26.39 (1.62) | |
| Age (years) | 12–19 | 112 | 1.10 (0.13) | 1,045 | 11.31 (0.56) |
| 20–39 | 545 | 8.87 (0.50) | 1,393 | 23.76 (1.10) | |
| 40–59 | 472 | 8.35 (0.58) | 1,322 | 25.09 (0.97) | |
| ≥60 | 256 | 2.78 (0.18) | 1,585 | 18.74 (0.86) | |
| Sex | Male | 826 | 12.21 (0.65) | 2,505 | 36.09 (0.74) |
| Female | 559 | 8.89 (0.48) | 2,840 | 42.81 (0.64) | |
| Race/Ethnicity | Non-Hispanic White | 725 | 15.33 (1.09) | 2,264 | 53.68 (1.68) |
| Non-Hispanic Black | 349 | 2.89 (0.30) | 1,078 | 8.27 (0.94) | |
| Mexican American | 141 | 1.21 (0.20) | 1,008 | 7.37 (1.02) | |
| Other Race | 170 | 1.67 (0.23) | 995 | 9.56 (0.82) | |
| BMI | Underweight | 45 | 0.62 (0.14) | 70 | 0.87 (0.17) |
| Healthy weight | 475 | 7.27 (0.47) | 1,724 | 26.18 (1.08) | |
| Overweight/Obese | 865 | 13.22 (0.72) | 3,551 | 51.85 (1.02) | |
| Poverty status | No | 926 | 15.81 (0.78) | 4,252 | 68.77 (1.07) |
| Yes | 459 | 5.29 (0.43) | 1,093 | 10.12 (0.87) | |
Same data as in stratified serum cotinine regression models.
Sample size, unweighted.
Standard error.
Table 2.
Nitromethane sample-weighted medians (ng/L) with 25th and 75th percentiles for exclusive smokers and non-users, NHANES 2007–2012 (N=6,730)a
| Demographic Category | Median (ng/L) [25th, 75th Percentiles], exclusive smokers |
Median (ng/L) [25th, 75th Percentiles], non-users |
|
|---|---|---|---|
| All | 774 [633, 955] |
625 [502, 797] |
|
| NHANES Cycle | 2007 – 2008 | 763 [628, 954] |
627 [511, 815] |
| 2009 – 2010 | 788 [645, 954] |
615 [495, 793] |
|
| 2011 – 2012 | 779 [624, 958] |
631 [509, 781] |
|
| Age (years) | 12–19 | 668 [559, 829] |
556 [459, 709] |
| 20–39 | 750 [631, 927] |
619 [501, 761] |
|
| 40–59 | 781 [630, 945] |
631 [508, 812] |
|
| ≥60 | 885 [696, 1150] |
669 [535, 885] |
|
| Sex | Male | 773 [628, 951] |
614 [497, 773] |
| Female | 774 [645, 956] |
635 [510, 814] |
|
| Race/Ethnicity | Non-Hispanic White | 772 [627, 954] |
610 [494, 759] |
| Non-Hispanic Black | 853 [683, 1040] |
673 [535, 870] |
|
| Mexican American | 749 [634, 946] |
649 [519, 836] |
|
| Other Race | 729 [632, 900] |
692 [544, 884] |
|
| BMI | Underweight | 736 [613, 834] |
551 [467, 679] |
| Healthy weight | 745 [608, 918] |
588 [473, 754] |
|
| Overweight/Obese | 790 [647, 965] |
639 [523, 812] |
|
| Poverty status | No | 773 [632, 948] |
628 [508, 793] |
| Yes | 785 [635, 988] |
601 [478, 818] |
|
Same data as in stratified cotinine regression models.
Table 3 presents results from sample-weighted multivariable regression analyses. Blood nitromethane was significantly and positively associated with serum cotinine among non-users (ΔY=10.8 ng/L per cotinine ng/mL) and exclusive smokers (ΔY=0.402 ng/L per cotinine ng/mL), adjusted for sex, age, race/ethnicity, BMI, poverty status, fasting time, NHANES cycle, and diet. Among non-users, nitromethane was positively associated with consuming meat/poultry (ΔY=43.7 ng/L per kg/day consumed), legumes/nuts/seeds (ΔY=147 ng/L per kg/day), grain products (ΔY=38.0 ng/L per kg/day), and vegetables (ΔY=148 ng/L per kg/day), while negatively associated with consuming sweets/beverages/sugars (ΔY=−8.80 ng/L per kg/day). Among exclusive smokers, blood nitromethane was positively associated with eating meat/poultry (ΔY=114 ng/L per kg/day consumed), while negatively associated with consuming eggs (ΔY=−143 ng/L per kg/day) and sweets/ beverages/sugars (ΔY=−13.4 ng/L per kg/day).
Among non-users and exclusive smokers, Table 3 shows that blood nitromethane was higher among females, although this was only significant among non-users. The rank-order of differences in nitromethane increased with age and BMI, although statistically significant differences with their respective reference group varied between non-users and exclusive smokers. Mexican Americans and Non-Hispanic Blacks had significantly higher blood nitromethane than Non-Hispanic Whites for both non-users and exclusive smokers, while Other Race non-users had significantly higher blood nitromethane.
Figure 1 displays the least-square means of blood nitromethane for exposure categories in the sample-weighted, unstratified CPD regression model (N=6,464). At successively higher exposure levels, Figure 1 demonstrates significantly greater blood nitromethane compared to the ≤0.05 ng/mL serum cotinine (non-exposed) reference group. Blood nitromethane among non-users exposed to SHS was significantly higher by 31.1 ng/L than non-exposed (≤0.05 ng/mL serum cotinine) non-users, adjusted for sex, age, race/ethnicity, BMI, poverty status, fasting time, NHANES cycle, and diet. Smoking 1–10 CPD (0.5 pack) among exclusive combusted tobacco users was associated with a larger increase of 150 ng/L blood nitromethane over non-exposed non-users, with greater increases through successively higher CPD levels. Quantitative results from the CPD regression model are reported in Supplemental Table S4.
Figure 1.
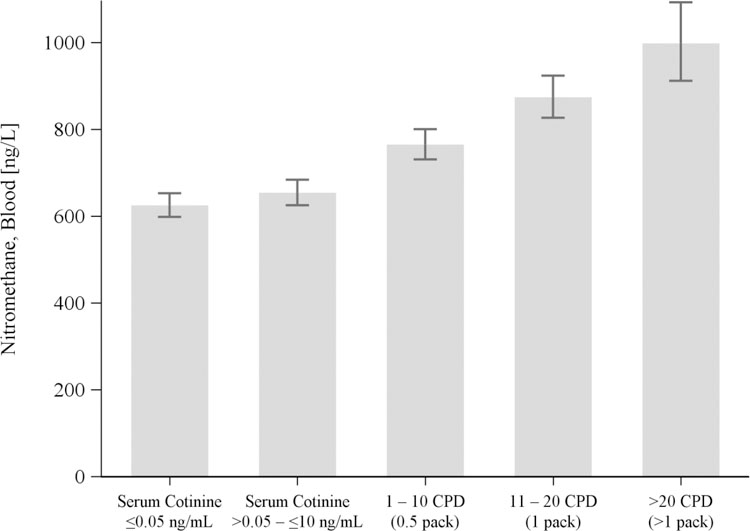
Least-square means (95% CI) for blood nitromethane (ng/L) increases with tobacco smoke exposure, represented by serum cotinine (non-users) and self-reported cigarettes per day (CPD; exclusive smokers), and controlling for other predictors in the model (Supplemental Table S4). NHANES 2007–2012 (N=6,464).
Table 4 shows the United States median mass consumed of food groups that were statistically significant contributors to blood nitromethane in the CPD model: meat/poultry; grain products; and vegetables. All three food groups were significant contributors in the stratified non-user model, while only meat/poultry was significant in the exclusive smoker model. The sample-weighted median mass consumed and its respective CPD regression slope can be applied to the to yield an estimate of the nitromethane exposure from the most likely amount consumed in the United States population, adjusted for sex, age, race/ethnicity, BMI, poverty status, fasting time, NHANES cycle, and diet. In decreasing order of exposure, median consumption of vegetables would be associated with an increase in blood nitromethane of 14.5 ng/L; grain products with an increase of 9.32 ng/L nitromethane; and meat/poultry with an increase of 7.55 ng/L nitromethane. Other select percentiles of consumption of food groups are listed in Supplemental Table S5.
Table 4.
Increase in blood nitromethane (ng/L) associated with median daily consumption of food group (kg/day). The geometric mean of blood nitromethane used for computing ΔY [95%CI] is 672 ng/L. NHANES 2007 – 2012 (N=6,464).
| Food Group | SE[β]a | Median Consumed [95% CI]b (kg/day) | ΔY [95% CI]c (ng/L) |
|---|---|---|---|
| Meat, poultry | 0.0246 | 0.140 [0.133, 0.146] | 7.55 [2.97, 12.2] |
| Grain products | 0.0212 | 0.259 [0.246, 0.271] | 9.32 [2.05, 16.7] |
| Vegetables | 0.0342 | 0.115 [0.108, 0.123] | 14.5 [9.21, 19.8] |
Sample-weighted standard error of the regression slope for food group used to compute 95%CI of ΔY.
Sample-weighted median daily amount of food group consumed.
Expected change in nitromethane concentration associated with consuming the median amount of food group at the overall geometric mean, controlling for predictors in the CPD regression model.
Discussion
Our analysis indicates that tobacco smoke is the predominant source of nitromethane exposure for the US population. Exclusive tobacco smokers had higher median blood nitromethane than non-users, and nitromethane increased significantly with serum cotinine for both non-users of tobacco products and exclusive smokers, controlling for potential confounders. Least-square means of blood nitromethane (Figure 1) also demonstrated consistent elevations as tobacco smoke exposure increased from non-exposed participants to SHS-exposed non-users, then continuing through escalating frequency of daily cigarette smoking frequency among exclusive smokers.
We also found that certain dietary sources significantly contributed to nitromethane exposure. Among both non-users and exclusive smokers, consuming meat/poultry was associated with higher blood nitromethane. Nitromethane can form during high temperature cooking of meat/poultry, which produces reactive nitrogen species that subsequently nitrate biomolecules to form nitromethane.4, 21, 22. Among non-users, consuming legumes/nuts/seeds, grains products, and vegetables was associated with higher blood nitromethane. This may be attributable to residues of chloropicrin, a volatile fumigant used as an insecticide to protect food crops, which may degrade in situ to form nitromethane.23, 24 Chloropicrin has also been shown to dehalogenate to form nitromethane when added to blood in vitro.4
In the CPD model, smoking one-half pack of cigarettes per day was associated with an increase in blood nitromethane by 150 ng/L, and exposure to SHS among non-users was associated with an increase of 31.1 ng/L compared with non-exposed non-users. These increases are larger than increases associated with dietary sources, where the greatest increase of 14.5 ng/L was associated with the median dietary consumption of vegetables (Table 4). While diet is a potential exposure source, the “time of food fasting” was not significant for any models; previous modeling of other diet-driven and short-lived exposure biomarkers (e.g. perchlorate) find that “time of food fasting” predicts decreased biomarker levels.25, 26
Drinking water was also investigated as a possible source of nitromethane exposure. Trihalonitromethanes can be formed as a by-product of drinking water disinfection, and subsequently form nitromethane in vivo.27,28 THNMs are not widely prevalent in drinking water, however, and are observed in the low parts-per-billion range.27,28 An individual drinking two liters of water per day may ingest up to about 20 ng THNMs, but these would dehalogenate in vivo to form only a few nanograms of nitromethane. We found that self-reported tap water consumption was not a significant predictor of blood nitromethane.
Contributions to exposure from tobacco smoke and diet notwithstanding, neither our data nor our models account for all potential routes of nitromethane. Air pollution is known to contain nitromethane from sources such as vehicle emissions and photochemical smog3, 29, but air monitoring data for NHANES participants is not directly accessible. Occupational exposures are also possible, and although the number of workers who handle nitromethane is a small proportion of the US population30, occupational information is sparse for NHANES participants. In addition, nitromethane may be formed endogenously. Macrophage activation, for example, is known to produce reactive nitrating species that can react with biomolecules to form nitromethane.4,31 Additionally, oxidative metabolism is known to produce oxygen radicals that could similarly react with nitrogenous biomolecules to form nitromethane.31 Oxygen radicals and oxidative damage increase with age, so our observation that nitromethane increased with age may be attributable to age-dependent increases in oxidative damage.32
Supplementary Material
Acknowledgements:
The authors would like to acknowledge and thank Dr. David M. Chambers of the CDC for his valuable input and discussions of data for this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Markofsky SB, Nitro Compounds, Aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA: 2000. [Google Scholar]
- 2.Sampson MM; Chambers DM; Pazo DY; Moliere F; Blount BC; Watson CH, Simultaneous analysis of 22 volatile organic compounds in cigarette smoke using gas sampling bags for high-throughput solid-phase microextraction. Anal Chem 2014, 86, (14), 7088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekimoto K, I. S., Tanimoto H, Fushimi A, Fujitani Y, Sato K, Yamada H, Characterization of nitromethane emission from automotive exhaust. Atmospheric Environment 2013, 81, 523–531. [Google Scholar]
- 4.Alwis KU; Blount BC; Silva LK; Smith MM; Loose KH, Method for quantifying nitromethane in blood as a potential biomarker of halonitromethane exposure. Environ Sci Technol 2008, 42, (7), 2522–7. [DOI] [PubMed] [Google Scholar]
- 5.Plewa MJ; Wagner ED; Jazwierska P; Richardson SD; Chen PH; McKague AB, Halonitromethane drinking water disinfection byproducts: chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ Sci Technol 2004, 38, (1), 62–8. [DOI] [PubMed] [Google Scholar]
- 6.Murphy CM; Devlin JJ; Beuhler MC; Cheifetz P; Maynard S; Schwartz MD; Kacinko S, Detection of ingested nitromethane and reliable creatinine assessment using multiple common analytical methods. Clin Toxicol (Phila) 2018, 56, (4), 237–244. [DOI] [PubMed] [Google Scholar]
- 7.EPA. Skin absorption and metabolism/toxicokinetic study of 14C—nitromethane in female rhesus monkeys. 1990.
- 8.OSHA, Chemical Sampling Information, Nitromethane 2004. Retrieved June 8, 2017 from: https://www.osha.gov/dts/chemicalsampling/data/CH_257700.html
- 9.NTP Toxicology and Carcinogenesis Studies of Nitromethane (CAS No. 75–52-5) in F344/N Rats and B6C3F1 Mice (Inhalation Studies). National Toxicology Program technical report series 1997, 461, 1–289. [PubMed] [Google Scholar]
- 10.Nitromethane. IARC Monogr Eval Carcinog Risks Hum 2000, 77, 487–501. [PMC free article] [PubMed] [Google Scholar]
- 11.Page EH; Pajeau AK; Arnold TC; Fincher AR; Goddard MJ, Peripheral neuropathy in workers exposed to nitromethane. Am J Ind Med 2001, 40, (1), 107–13. [DOI] [PubMed] [Google Scholar]
- 12.Pirkle JL; Sampson EJ; Needham LL; Patterson DG; Ashley DL, Using biological monitoring to assess human exposure to priority toxicants. Environ Health Perspect 1995, 103 Suppl 3, 45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHANES. National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/index.htm 2017.
- 14.Caudill SP; Schleicher RL; Pirkle JL, Multi-rule quality control for the age-related eye disease study. Stat Med 2008, 27, (20), 4094–106. [DOI] [PubMed] [Google Scholar]
- 15.Pirkle JL; Flegal KM; Bernert JT; Brody DJ; Etzel RA; Maurer KR, Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 1996, 275, (16), 1233–40. [PubMed] [Google Scholar]
- 16.Rodriguez-Barranco M; Tobias A; Redondo D; Molina-Portillo E; Sanchez MJ, Standardizing effect size from linear regression models with log-transformed variables for meta-analysis. BMC Med Res Methodol 2017, 17, (1), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHANES. Body Mass Index (BMI) (https://www.cdc.gov/healthyweight/assessing/bmi/). 2015.
- 18.Ahuja JK; Moshfegh AJ; Holden JM; Harris E, USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr 2013, 143, (2), 241S–9S. [DOI] [PubMed] [Google Scholar]
- 19.Agriculture Research Service, USDA. What’s In The Foods You Eat Search Tool, 2013–2014. Available at: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/whats-in-the-foods-you-eat-emsearch-toolem/
- 20.Pirkle JL; Bernert JT; Caudill SP; Sosnoff CS; Pechacek TF, Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect 2006, 114, (6), 853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamat JP, Peroxynitrite: a potent oxidizing and nitrating agent. Indian J Exp Biol 2006, 44, (6), 436–47. [PubMed] [Google Scholar]
- 22.Soladoye OP; Juárez ML; Aalhus JL; Shand P; Estévez M, Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Comprehensive Reviews in Food Science and Food Safety 2015, 14, (2), 106–122. [DOI] [PubMed] [Google Scholar]
- 23.Daft J, Determining multifumigants in whole grains and legumes, milled and low-fat grain products, spices, citrus fruit, and beverages. J Assoc Off Anal Chem 1987, 70, (4), 734–9. [PubMed] [Google Scholar]
- 24.Desaeger JA; Csinos AS, Root-knot nematode management in double-cropped plasticulture vegetables. J Nematol 2006, 38, (1), 59–67. [PMC free article] [PubMed] [Google Scholar]
- 25.Blount BC; Valentin-Blasini L; Osterloh JD; Mauldin JP; Pirkle JL, Perchlorate exposure of the US Population, 2001–2002. J Expo Sci Environ Epidemiol 2007, 17, (4), 400–7. [DOI] [PubMed] [Google Scholar]
- 26.Aylward LL; Hays SM; Smolders R; Koch HM; Cocker J; Jones K; Warren N; Levy L; Bevan R, Sources of variability in biomarker concentrations. J Toxicol Environ Health B Crit Rev 2014, 17, (1), 45–61. [DOI] [PubMed] [Google Scholar]
- 27.Hu J; Song H; Addison JW; Karanfil T, Halonitromethane formation potentials in drinking waters. Water Res 2010, 44, (1), 105–14. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg HS; Krasner SW; Richardson SD; Thruston AD Jr., The Occurrence of Disinfection By-Products (DBPs) of Health Concern in Drinking Water: Results of a Nationwide DBP Occurrence Study. U.S. Environmental Protection Agency 2002.
- 29.Lin JK; Chen KJ; Liu GY; Chu YR; Lin-Shiau SY, Nitration and hydroxylation of aromatic amino acid and guanine by the air pollutant peroxyacetyl nitrate. Chem Biol Interact 2000, 127, (3), 219–36. [DOI] [PubMed] [Google Scholar]
- 30.National Occupational Exposure Survey (1981–1983). National Institute for Occupational Safety and Health Available at: https://web.archive.org/web20110716084755/http:/www.cdc.gov/noes/
- 31.Pacher P; Beckman JS; Liaudet L, Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007, 87, (1), 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ames BN, Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun 1989, 7, (3–6), 121–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


