Abstract
BACKGROUND:
There is a need for safe and effective IBS treatments that provide immediate and sustained improvement of IBS symptoms, particularly among more severe patients. The aim was to assess long-term clinical response of cognitive behavioral therapy (CBT) with reference to IBS education.
METHODS:
A total of 436 Rome III-diagnosed IBS patients (80% F, M age = 41 years) were randomized to: 4 session home-based CBT (minimal contact (MC-CBT)), 10 session clinic-based CBT (standard (S-CBT)), or 4 session IBS education (EDU). Follow-up occurred at 2 weeks and 3, 6, 9, and 12 months following treatment completion. Treatment response was based a priori on the Clinical Global Improvement Scale (global IBS symptom improvement) and IBS Symptom Severity Scale (IBS-SSS).
RESULTS:
Post-treatment CGI gains were generally maintained by MC-CBT patients at quarterly intervals through 12-month follow-up with negligible decay. For MC-CBT and S-CBT, 39 and 33% of respondents maintained treatment response at every follow-up assessment. The corresponding percent for EDU was 19%, which was significantly lower (p < 0.05) than for the CBT groups. On the IBS-SSS, therapeutic gains also showed a pattern of maintenance with trends towards increased efficacy over time in all conditions, with the mean unit reductions between baseline and follows-up being approximately −76 at immediate and approximately −94 at 12 months (−50 = clinically significant).
CONCLUSIONS:
For treatment-refractory IBS patients, home-and clinic-based CBT resulted in substantial and enduring relief of multiple IBS symptoms that generally extended to 12-month post treatment.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common and challenging disorders confronting gastroenterologists. Symptoms include abdominal pain associated with altered bowel habits that are manifested in constipation and/or diarrhea. Generally speaking, the full range of IBS symptoms are unreliably treated with existing medical treatments whose effects are relatively modest and associated with side effects (1). Even when medical therapies are effective in reducing IBS symptoms, relapse rates (50%) after treatment withdrawal is high (2). The lack of a consistently effective medical treatment contributes to quality-of-life impairment that is among more severe IBS patients comparable to that of life-threatening diseases such as diabetes and hepatitis (3).
This state of affairs has led to the development of a number of psychological treatments for IBS. The class of psychotherapies for which there is most empirical support is cognitive behavior therapy (CBT). CBT is a skills-based treatment that is designed to normalize altered brain-gut interactions by targeting maladaptive information processing (e.g., cognitive biases) and behaviors (e.g., stress reactivity) that maintain IBS. While methodological issues complicated the interpretability of clinical trial data of the first generation of randomized clinical trials, more recent trials have incorporated quality indicators reflective of state-of-the-art trials for functional gastrointestinal (GI) disorders (4).
Recent data from one National Institutes of Health (NIH)-funded clinical trial comparing two “dosages” of CBT against IBS education found that a primarily home-based and clinic-based version of CBT delivered comparable outcomes on the primary endpoint of global IBS symptom improvement (5). Both versions of CBT outperformed an active IBS Education comparator on the primary outcome of global IBS symptom improvement at the end of acute treatment phase. Whether treatment gains of CBT persist long term is unknown. Ljótsson et al. (6) conducted a 1-year follow-up study of patients who had received internet-delivered CBT featuring exposure and mindfulness exercises. They found that treatment gains were maintained at 12-month follow-up, although the cross-over design of the study introduced bias due to confounding of carryover effects and direct treatment effects. Other researchers have failed to establish maintenance effects through 12-month follow-up (7). The purpose of the present study was to document immediate long-term effects of CBT for a recently completed randomized trial among refractory (i.e., those whose chronic, painful symptoms persist despite medical therapies and are a significant source of quality-of-life impairment (8)) IBS patients that assessed response to treatment at an immediate posttest, and at 3, 6, 9 and 12 months post treatment. We use two approaches to evaluate decay functions. First, we examine at the aggregate level the overall percent of responders or the group mean response to treatment changes as a function of time. Second, we conduct person-centered analyses to identify the decay patterns exhibited by individuals and document the relative frequency with which different decay functions occur post treatment. Such person-centered decay curves have not been reported in the literature and yield more detailed insights into the nature of sustained treatment effects once treatment is withdrawn.
METHODS
Description of the parent study
The present study is a secondary analysis of the Irritable Bowel Syndrome Outcome Study (IBSOS). Its rationale, experimental design, quality control procedures (e.g., methods for assuring treatment fidelity, therapist training), and short-term outcomes using per-protocol and intent-to-treat analyses are more fully detailed elsewhere (4) The IBSOS is a randomized controlled, parallel-group trial that allocated patients into one of three conditions at two sites (University at Buffalo, Northwestern University). Adults (18–70 years) suffering from IBS as defined by Rome III criteria (9) were included, if GI symptoms were at least moderately severe (i.e., occurred at least twice weekly and caused some self-reported interference in life domains such as work/school, social, household responsibilities). Patients were excluded if they presented evidence of current structural/biochemical abnormalities or other primary GI disease that better explained gastrointestinal symptoms; had been diagnosed with a malignancy other than localized basal or squamous cell carcinomas of the skin in the past 5 years; were undergoing IBS-targeted psychotherapy; could not commit to completing all scheduled follow-up visits; had an unstable extraintestinal condition or a major psychiatric disorder (e.g., depression with severe suicidality, psychotic disorder); reported an active GI infection within 2 weeks before evaluation; and used a gut-sensitive antibiotic during the 12 weeks prior to baseline assessment. Table 1 presents descriptive characteristics of the enrolled patients.
Table 1.
Baseline sociodemographic and clinical characteristics by treatment condition
| Characteristic | Overall (n = 436) | MC-CBT (n = 145) | S-CBT (n = 146) | EDU (n = 145) |
|---|---|---|---|---|
| Age, mean (SD) | 41.4 (14.8) | 40.9 (14.6) | 41.1 (14.4) | 42.2 (15.4) |
| Women, N (%) | 350 (80.3%) | 124 (85.5%) | 112 (76.7%) | 114 (79.2%) |
| Race/ethnicity, N (%) | ||||
| Non-Hispanic white | 390 (89.4%) | 133 (91.7%) | 128 (87.7%) | 129 (89.0%) |
| African-American | 28 (6.4%) | 8 (5.5%) | 9 (6.2%) | 11 (7.6%) |
| Other or missing | 18 (4.2%) | 4 (2.8%) | 9 (6.2%) | 5 (3.5%) |
| Marital status, N (%) | ||||
| Never married | 185 (42.4%) | 61 (44.1%) | 60 (41.1%) | 64 (44.1%) |
| Married | 185 (42.4%) | 68 (46.9%) | 58 (39.7%) | 59 (40.7%) |
| Separated/divorced | 57 (13.1%) | 11 (7.6%) | 26 (17.8%) | 20 (13.8%) |
| Widowed | 9 (2.1%) | 5 (3.4%) | 2 (1.4%) | 2 (1.4%) |
| Income ($), mean (SD) | 74.0 (54.2) | 77.9 (56.4) | 73.1 (52.2) | 71.3 (54.0) |
| Education, N (%) | ||||
| High school or less | 99 (22.7%) | 31 (21.4%) | 30 (20.5%) | 38 (26.2%) |
| Associate degree or vocational-technical | 65 (14.9%) | 25 (17.2%) | 22 (15.1%) | 18 (12.4%) |
| College degree | 142 (32.6%) | 54 (37.2%) | 41 (28.1%) | 47 (33.1%) |
| Postgraduate degree | 127 (29.1%) | 35 (24.1%) | 52 (35.6%) | 40 (27.6%) |
| Missing | 3 (0.7%) | 0 | 1 (0.7%) | 2 (1.4%) |
| Employment status, N (%) | ||||
| Employed full-or part-time | 277 (63.5%) | 92 (63.4%) | 91 (62.3%) | 94 (64.8%) |
| Unemployed | 109 (25.0%) | 38 (26.2%) | 40 (27.4%) | 31 (21.4%) |
| Homemaker | 13 (3.0%) | 4 (2.8%) | 5 (3.4%) | 4 (2.8%) |
| Retired | 33 (7.6%) | 9 (6.2%) | 9 (6.2%) | 15 (10.3%) |
| Missing | 4 (0.9%) | 2 (1.4%) | 1 (0.7%) | 1 (0.7%) |
| Predominant bowel type, N (%) | ||||
| Constipation | 130 (29.8%) | 43 (29.7%) | 40 (27.4%) | 47 (32.4%) |
| Diarrhea | 188 (43.1%) | 59 (40.7%) | 67 (45.9%) | 62 (42.8%) |
| Mixed | 98 (22.5%) | 33 (22.8%) | 35 (24.0%) | 30 (20.7%) |
| Undifferentiated | 20 (4.6%) | 10 (6.9%) | 4 (2.7%) | 6 (4.1%) |
| Years with IBS, mean (SD) | 17.1 (14.4) | 15.7 (13.3) | 17.7 (13.3) | 17.7 (16.4) |
| Received medical care for IBS (lifetime), N (%) | 328 (75.2%) | 107 (73.8%) | 116 (79.5%) | 105 (72.4%) |
| IBS treatment-naive, N (%) | 10 (2.2%) | 4 (2.6%) | 3 (1.9%) | 3 (1.9%) |
| Assessment scores, mean (SD) | ||||
| IBS Symptom Severity Scalea | 281.9 (72.1) | 278.0 (68.6) | 285.1 (76.7) | 282.4 (71.0) |
| Brief Symptom Inventory30,a | ||||
| Anxiety | 4.50 (4.50) | 4.22 (4.26) | 4.27 (4.41) | 5.02 (4.81) |
| Depression | 3.97 (4.29) | 4.07 (4.47) | 3.82 (4.33) | 4.03 (4.09) |
| Somatization | 4.22 (3.93) | 4.16 (4.31) | 4.00 (3.56) | 4.54 (3.91) |
| Global Severity Index | 12.7 (11.0) | 12.4 (11.6) | 12.1 (10.5) | 13.6 (10.8) |
| Medical comorbidities31,# | 4.6 (4.9) | 4.8 (5.2) | 4.3 (4.7) | 4.8 (5.0) |
| Psychiatric comorbidities32,# | 1.2 (1.6) | 1.1 (1.5) | 1.3 (1.7) | 1.2 (1.7) |
| Medication use for IBS symptoms (N, %) | 292 (67.0%) | 94 (64.8%) | 95 (65.1%) | 103 (71.0%) |
| Pain medication | 35 (8.0%) | 9 (6.2%) | 13 (8.9%) | 13 (9.0%) |
| Bowel medication | 271 (62.2%) | 86 (59.3%) | 87 (59.6%) | 98 (67.6%) |
| Multi-symptom medication | 20 (4.6%) | 6 (4.1%) | 7 (4.8%) | 7 (4.8%) |
| Psychiatric medication | 26 (6.0%) | 8 (5.5%) | 12 (8.2%) | 6 (4.1%) |
EDU, education condition; IBS, irritable bowel syndrome; MC-CBT, minimal contact-cognitive behavior therapy; S-CBT, standard-cognitive behavior therapy.
Higher scores indicate more severe symptoms; IBS-Symptom Severity Scale (SSS) ≥ 300 = severe.
Treatment administration
Standard-CBT (S-CBT (10)) involves 10 weekly, 60 min face-to-face sessions and emphasizes the provision of information regarding brain-gut interactions; self-monitoring of GI symptoms, their antecedents (i.e., triggers) and consequences; muscle relaxation to dampen physiological arousal and increase control over GI symptoms; worry control to challenge and dispute negatively skewed thinking patterns; flexible problem solving to aid in the deployment of more effective ways of managing realistic stressors; and relapse prevention training to maintain treatment gains. As a learning-based program, CBT assigns home exercises to facilitate acquisition of symptom self-management skills introduced in session through didactic instruction. Because minimal contact-CBT (MC-CBT (10)) requires only four clinic visits over the 10-week period, it relies more extensively on home study materials (11) to cover the same procedures that S-CBT introduces at each session. The education condition (EDU (4)) was equivalent to MC-CBT in time, attention, and the amount of home study materials received. EDU sessions were structured around education and support. Content included information about IBS, its clinical features, epidemiology, diagnostic criteria, medical tests, and treatment options as well as the role of stress, diet, and physical activity. Clinicians were prohibited from prescribing relevant behavior changes (e.g., stress management skills). To mimic receipt of the MC-CBT patient workbooks, EDU patients received a copy of IBS: Learn to Take Charge Of It (12) which emphasizes the “empowering” therapeutic value of patient education. All content referencing CBT strategies was extracted through a special printing of the book. As such, the EDU condition represents a viable treatment protocol in its own right and whose procedures did not overlap with those deemed critical to CBT for IBS. This design allowed rigorous evaluation of the incremental value of the technical features of CBT over and above the contribution of state-of-the-art educational protocols. It creates a much higher standard of comparison than designs that feature wait-list control or active controls with clinically inert activities. By emphasizing education and support, EDU incorporated lifestyle recommendations that are regarded as “of great importance in the management of patients with …IBS” (13) and featured in practice guidelines (14) and was therefore more clinically robust and ecologically valid than attention control conditions whose main goal is to control for nonspecific factors (e.g., attention, expectancy of improvement) (Figs 1 and 2).
Fig. 1.
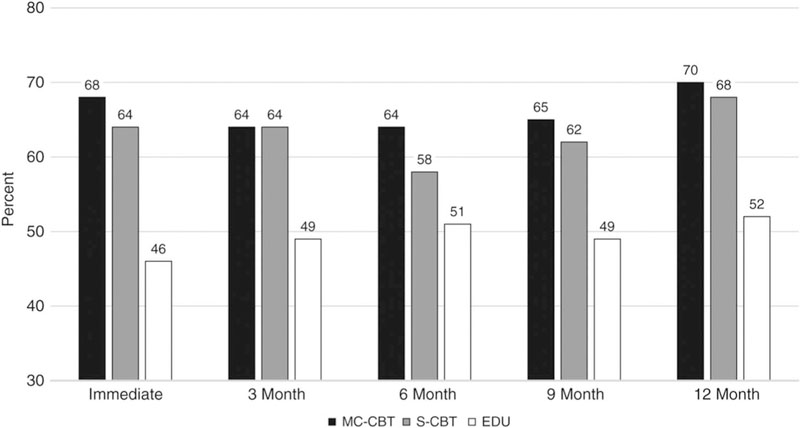
Percent responders as indexed by CGI: Longterm followup
Fig. 2.

Decay analyses over 12 months: Person-centered analyses
Outcome measure
Per Rome recommendations for clinical trials for functional gastrointestinal disorders (15), the primary endpoint was global IBS symptom improvement based on the IBS version (10) of the Clinical Global Impressions-Improvement Scale (CGI-I) (16): “Compared to how you felt prior to entering the study, how would you rate the IBS symptoms for which you sought treatment during the past week?” (1 = substantially worse, 2 = moderately worse 3, slightly worse, 4 = no change, 5 = slightly improved, 6 = moderately improved, 7 = substantially improved). Patients whose symptoms were rated per convention as “substantially improved” or “moderately improved” qualified as treatment responders. Secondary endpoint of symptom relief was the Irritable Bowel Syndrome Symptom Severity Scale (IBS-SSS) (17). The IBS-SSS is a 5-item instrument used to measure severity of abdominal pain, frequency of abdominal pain, severity of abdominal distension, dissatisfaction with bowel habits, and interference with quality of life on a 100-point scale. For four of the items, the scales are represented as continuous lines with endpoints 0 and 100%, with different descriptors at the endpoints and adverb qualifiers (e.g., “not very,” “quite”) strategically placed along the line. Respondents’ mark a point on the line reflecting the extremity of their judgment between the two endpoints and the proportional distance from zero is the score assigned for that scale (hence scores range from 0 to 100). The endpoints for the severity items are “no pain” and “very severe,” for satisfaction, the end-points are “not at all satisfied” and “very satisfied,” and for interference, they are “not at all interferes” to “completely interfere.” A final item asks the number of days out of 10 the patient experiences abdominal pain and the answer is multiplied by 10 to create a 0 to 100 metric. The items were summed and thus the total score could range from 0 to 500. The IBS-SSS was used as an endpoint of the clinical study from which secondary analyses were derived for this study. As noted, outcome measures were administered as part of assessment battery conducted at 2 weeks and 3, 6,9, and 12 months after the end of acute treatment phase.
Analytic strategy
The purpose of the study was to characterize decay patterns in CBT treatment efficacy over time following successful completion of CBT treatment protocols. The appropriate analytic strategy for decay analysis is per protocol. Intent-to-treat (ITT) analyses are methodologically contraindicated for this question because they confound decay in treatment efficacy per se with dropping out of treatment (18) and the consequences thereof. Without follow-up assessments for treatment dropouts, an ITT approach introduces decay curve artifacts. To illustrate, one ITT strategy for estimating effectiveness is to conservatively assume no fundamental change in IBS symptoms for treatment drop-outs across time. Relative to decay curve analysis, this biases results characterizing decay curves towards stability. ITT analyses are appropriate for later-stage clinical trials to assess broader effectiveness, but less appropriate for gaining mechanistic insight surrounding decay patterns of efficacious treatments. Because the question of broad effectiveness differs from the question of decay in efficacy, they call for different analytic strategies. In the present study, basic conclusions using per-protocol analyses do not appreciably vary from those from ITT analyses because there was a small treatment dropout rate (9%), a rate that did not vary by condition.
The aggregate-level analyses to compare means or the percent of treatment responders at one time point versus another time point used single degree of freedom contrasts based on generalized estimating equations (GEE) with robust estimators for unknown dependence structures across time (implemented in geepack in R). For the dichotomous CGI outcome, these analyses used the GEE framework as applied to a modified linear probability model (19) and we then replicated the results using a logit function for sensitivity analyses. For person-centered analyses of the dichotomous CGI where we compared decay curve patterns between two conditions, we used standard single degree of freedom contrasts for comparing independent percentages, but in place of traditional chi square tests, we used significance tests based on Bayes confidence intervals (20). For the continuous IBS-SSS we identified clusters of individuals with similar decay patterns using k-means cluster analysis applied to the repeated measure data to derive Manhattan distance scores. The final cluster solution based on scree-tests of the percent of variance accounted for the Akaike information criterion, average sil-houette width indices, and conceptual meaningfulness (21,22). Missing data across time in all analyses were addressed using chained equation multiple imputation (23). We included non-white ethnicity, medication status (using IBS medication versus not), and site as covariates in all analyses to be consistent with the primary outcome analyses reported and justified elsewhere (5). The 95% confidence intervals are reported as margins of error using the half-widths of the interval.
RESULTS
Preliminary analyses
As noted, 9% of patients dropped out during treatment (no statistically significant percent differences between conditions). Dropout was unrelated to a range of demographic, psychological, and IBS-related variables measured at baseline, with one exception: an 8% treatment dropout rate for Whites versus a 22% rate for non-Whites (p < 0.05). Non-whites represented only 10% of the sample. This difference did not vary by treatment condition. Of the sample, 89% received a minimally sufficient dosage of their assigned treatment, defined a priori as completion of 8 of 10 for S-CBT sessions and 3 of 4 for MC-CBT and EDU. This percent did not vary significantly by condition. Cumulative attrition at the 3-month follow-up was 10.8%, at 6 months it was 12.9%, at 9 months it was 19.7% and at 12 months it was 18.4%. None of these rates varied significantly by condition. There were no statistically significant differences between those lost to attrition versus those retained on multiple demographic and clinical variables assessed at baseline, nor as a function of outcome variables at immediate posttest. The final sample sizes in the three groups at the immediate posttests for purposes of decay analyses of CGI were M-CBT = 128, S-CBT = 119, and EDU = 128. For the IBS-SSS, they were M-CBT = 125, S-CBT = 117, and EDU = 127.
CGI analyses
Aggregate analyses.
Collapsing across the three treatment conditions, the percent of treatment responders on the CGI was 59.3 ± 4.9% at the immediate follow-up, 59.6 ± 4.9%, at FU3, 57.8 ± 5.0%, at FU6, 58.8% ± 5.0 at FU9, and 62.2 ± 5.0 at FU12. There were no statistically significant differences among these percentages. Tests for a time-by-condition interaction yielded a non-significant result, suggesting the above aggregate-defined decay curves did not vary appreciably by treatment condition; response rates were durable to about the same degree in all three conditions.
Table 2 presents the percent of treatment responders at each posttest/follow-up as a function of treatment condition. The percent of responders in the MC-CBT condition was statistically significantly higher at each time point when compared with the EDU condition at that time point (with the one exception being a p value < 0.06 at FU6). In the MC-CBT condition, the percent of treatment responders was 68.2, 63.9, 63.7, 64.9, and 70.0% at immediate, FU3, FU6, FU9, and FU12, respectively; the corresponding percents for the EDU condition were 46.6, 49.4, 50.8, 48.9, and 51.7%. Thus, the effects for MC-CBT are large and durable at the aggregate level as compared with EDU (with the difference in treatment response between the two conditions being approximately 17% at each time point).
Table 2.
Percent responders as indexed on CGI
| Immediate | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|
| MC-CBT | 68.2a ± 8.1 | 63.9a ± 8.7 | 63.7b ± 8.8 | 64.9a ± 9.1 | 70.0a ± 8.6 |
| S-CBT | 64.7a ± 8.9 | 64.4a ± 9.3 | 57.9 ± 9.5 | 62.2b ± 9.7 | 68.4a ± 9.1 |
| EDU | 46.6 ± 8.8 | 49.4 ± 9.3 | 50.8 ± 9.6 | 48.9 ± 10.0 | 51.7 ± 10.0 |
CGI, Clinical Global Impressions -Improvement Scale; EDU, education condition; MC-CBT, minimal contact-cognitive behavior therapy; S-CBT, standard-cognitive behavior therapy.
Contrast with EDU condition within a column. i.e., at a given time point, is p < 0.05.
Contrast with EDU condition within a column is p < 0.06; sample sizes for MC-CBT, S-CBT, and EDU are 128, 119, and 128, respectively; percentages of sample sizes do not exactly equal whole numbers because of the chained equation imputation process.
Person-centered analyses.
Person-centered analyses classified patients into groups based on the type of decay curve they exhibited across time between the immediate posttest and FU12. We identified 10 a priori patterns of change that are of substantive interest (see Table 3). Each pattern is signified by a 0 at a given point in time if the person was not a responder at that time and a 1 if the person was a responder at that time. For the 5 post-treatment time periods, a person with the pattern 0,0,0,0,0,0 was a non-responder at each time period. A person with the pattern 1, 1, 1, 1, 1 was a responder at each time period. A person with the pattern 0,1,1,1,1 was a non-responder at the immediate posttest, but a persistent responder at the subsequent periods, i.e., it took extra time for the treatment to have its effect.
Table 3.
Decay patterns for CGI responder status
| Decay pattern | Total sample | MC-CBT | S-CBT | EDU |
|---|---|---|---|---|
| 0, 0, 0, 0, 0 | 16.1% | 14.6% | 10.7% | 22.4% |
| 0, 1, 1, 1, 1 | 4.9% | 7.7% | 3.3% | 3.7% |
| 0, 0, 1, 1, 1 | 1.8% | 0.0% | 1.6% | 3.7% |
| 0, 0, 0, 1, 1 | 1.0% | 1.5% | 0.0% | 1.5% |
| 0, 0, 0, 0, 1 | 2.6% | 3.8% | 2.5% | 1.5% |
| 1,0, 0,0,0 | 2.8% | 0.8% | 2.5% | 5.2% |
| 1, 1,0, 0,0 | 2.6% | 2.3% | 4.9% | 0.7% |
| 1, 1, 1,0,0 | 1.0% | 1.5% | 0.8% | 0.7% |
| 1, 1, 1, 1,0 | 2.6% | 0.8% | 1.6% | 5.2% |
| 1, 1, 1, 1, 1 | 30.1% | 39.2% | 32.8% | 18.7% |
| Other | 34.5% | 27.1% | 39.3% | 36.5% |
Sample sizes for MC-CBT, S-CBT, and EDU are 128,119, and 128, respectively; percentages of sample sizes do not exactly equal whole numbers because of the chained equation imputation process.
CGI, Clinical Global Impressions -Improvement Scale; EDU, education condition; MC-CBT, minimal contact-cognitive behavior therapy; S-CBT, standard-cognitive behavior therapy.
For the pattern of full responders (1, 1, 1, 1, 1), there was a statistically significant difference between the percent of such patients in the MC-CBT condition (39.2%) and the EDU condition (18.7%), difference = 20.5 ± 10.7%, p < 0.01, and this was also true when comparing the S-CBT condition (32.8%) to the EDU condition, difference = 14.1 ± 10.7%, p < 0.01. For the pattern of complete non-response (0, 0, 0, 0, 0, 0) there was a trend towards between-group differences for the percent of such patients in the MC-CBT condition (14.6%) and the EDU condition (22.4%), difference = −7.8 ± 9.2%, p < 0.10. For the S-CBT condition for this pattern (10.7%), the percentage difference was −11.7 ± 8.7%, p < 0.05. For the two CBT conditions pooled, the percentage difference was − 10.1 ± 8.2%, p < 0.05.
We also calculated the percent ofindividuals in each condition who achieved responder status in a majority of the 5 time periods, i.e., for at least 3 of the 5 follow-up periods. In the MC-CBT condition, the percent was 68.5 ± 8.0%, for the S-CBT condition it was 65.6 ± 8.6%, and in the EDU condition it was 51.5 ± 8.1%. The difference in the percentages for both CBT conditions separately were statistically significantly different from the EDU condition (for MC-CBT, difference = 17.0 ± 12.0%,p < 0.01 and for S-CBT condition, difference = 14.1 ± 11.9%, p < 0.05).
IBS-SSS analyses
Aggregate analyses.
Collapsing across all three conditions, the mean change was − 85.8 ± 9.7 at the immediate follow-up, − 99.7 ± 9.3 at FU3, −108.3 ± 9.9 at FU6, −103.8 ± 9.5 at FU9, and − 112.1 ± 9.5 at FU12. These represent sizeable decreases in IBS-SSS relative to the baseline, with each differing significantly from baseline (p < 0.05) in a more improved direction using the accepted 50-unit change standard of clinical improvement. A test for a time by condition interaction yielded a non-significant result, suggesting the above aggregate defined decay curves did not vary meaningfully by treatment condition. Table 4 presents the mean change in IBS-SSS scores relative to the baseline score for each condition and each time period. The average within-condition SD for change in IBS-SSS scores was 97.8. This yields Cohen effect size values for change in IBS-SSS scores of approximately − 0.89 at the immediate follow-up, −1.03 at FU3, − 1.11 at FU6, − 1.06 at FU9, and − 1.15 at FU12, all representing large effects. None of the means were statistically significant from one another, either across time or by condition within time.
Table 4.
Mean change from baseline for the IBS-SSS
| Immediate | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|
| MC-CBT | −89.2 ±17.0 | −106.3 ± 17.7 | −113.1 ± 17.9 | −111.2 ± 17.7 | −120.1 ± 17.9 |
| S-CBT | −80.8 ±17.0 | −103.60 ± 16.5 | −105.4 ± 17.5 | −104.6 ± 17.0 | −112.9 ± 18.4 |
| EDU | −84.3 ±14.7 | −88.9 ± 14.6 | −99.8 ± 15.2 | −94.4 ±17.1 | −103.4 ± 18.6 |
None of the contrasts within a column, i.e., at a given time point, were statistically significant; all changes at a given time and in a given condition are statistically significantly more negative than −50. For example, at FU12 in the MC-CBTcondition, the average change from baseline was −120.1, which is statistically significantly greater than −50; sample sizes for the three groups are MC-CBT = 125, S-CBT = 117 and EDU = 127.
EDU, education condition; FU, follow-up; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale; MC-CBT, minimal contact-cognitive behavior therapy; S-CBT, standard-cognitive behavior therapy.
Person-centered analyses.
Table 5 presents the results of the k-means cluster analyses that identified four types of decay curves for IBS-SSS. Each group defining a decay curve shows a different response to the initial treatment and then tends to retain that response across all of the subsequent follow-ups, i.e., there is durability for whatever the initial response to treatment was. Group 4, which comprises nonresponders, constitutes about 17% of the sample. Their IBS-SSS change scores consistently fell short of 50-unit change that is regarded as clinically significant. Group 3, which are patients with optimal response to treatment, account for 11% of the sample. Their IBS-SSS change scores exceeded 50-point difference by a factor of 4 to 5 on average. Group 2, representing about 43% of the sample, are patients who had a favorable response to treatment. Their pre-post reduction on the IBS-SSS was above 50 units across the 5 follow-up periods but not as dramatic as in other treatment responder groups. Group 1 (about 28% of the sample) had a very favorable response to treatment. Pre-post IBS-SSS difference exceeded 50 points signifying clinical improvement by factor of 3. There were no meaningful differences in the proportion of patients in the respective groups as a function of treatment condition.
Table 5.
Decay patterns for mean change from baseline for IBS-SSS
| Decay pattern |
% Sample |
Immediate | FU3 | FU6 | FU9 | FU12 |
|---|---|---|---|---|---|---|
| Group 1 | 28.5% | −139.5 | −148.9 | −162.2 | −152.0 | −167.6 |
| Group 2 | 43.3% | −61.8 | −78.6 | −84.1 | −78.8 | −81.7 |
| Group 3 | 10.9%- | 221.2 | −239.8 | −269.5 | −279.7 | −281.6 |
| Group 4 | 17.4% | 27.4 | 16.4 | 21.1 | 23.5 | 9.13 |
Total N is 369; percentages of N = 369 do not exactly equal whole numbers because of the chained equation imputation process for longitudinal missing data
FU, follow-up; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale.
DISCUSSION
This study sought to characterize decay curves and treatment response durability among a relatively large sample of CBT-treated IBS patients relative to an active comparator that emphasized support and education. At an aggregate level, rates of IBS symptom improvement superiority (as measured by the CGI) in the two CBT groups over the EDU group persisted at quarterly intervals through 12-month follow-up, a superiority of about 17% in treatment responders. The percent of patients showing symptom improvement at 12-month follow-up in the two CBT conditions was 69%, which is comparable to the proportion of responders at immediate follow-up (66%). The proportion of responders at intermediate follow-up periods in the two CBT conditions did not fall below 57%. These data suggest that CBT, whether delivered face to face or primarily home based, has a sustained aggregate effect that unlike medications or dietary therapies (2) persists well after treatment completion. In no instance, did the proportion of responders of either S-CBT or EDU exceed that of MC-CBT. These data extend our earlier work (10) with an independent sample of IBS patients that CBT was associated with symptom improvement 3 months after treatment withdrawal.
An innovative aspect of this study was the use of both aggregate and person-centered analyses to characterize decay curves of CBT delivered in two dosages. Conventional approaches to characterizing long-term treatment effects use aggregated data that pool responses at a given time period of patients assigned to a given condition. Maintenance effects are inferred by the consistency in aggregate treatment response at each time point. This approach yielded comparable results at extended follow-up to the results achieved immediately upon completion of therapy, suggesting stable aggregate response patterns. However, intra-individual variability from one follow-up to another is obscured with such aggregate-level analyses. Symptom improvement among patients may fluctuate such that responder status may differ from one follow-up to the next even though the overall responder status of the condition to which respondents are assigned remains roughly the same. A patient who improves at the immediate post-treatment assessment may report decay at the next follow-up period. A different patient may report sustained improvement across multiple follow-ups. A third patient may have delayed response such that improvement becomes evident after the end of the immediate follow-up. These represent important differences that capture more nuanced aspects of the efficacy profile of a treatment. A person-centered approach offers a more fine-grained perspective on the quality of response at the patient level that aggregate level data obscure. We compared individually defined patterns of therapeutic gain across time as a function of treatment condition. In this regard, CBT tends to outperform EDU. For example, for the CGI, the pattern of consistent full responders showing treatment maintenance at each time period was higher for the two CBT groups (MC-CBT = 39.2%, S-CBT = 32.8%) than EDU (18.7%). For profiles showing positive responder status for at least 3 of the 5 follow-up sessions, the percent in the CBT conditions was approximately 67% as compared to only 51% in the EDU condition, suggesting a more durable treatment response for CBT-treated patients. For the pattern of complete non-response across every time period, only 10.1% of the pooled CBT groups showed such a response pattern as compared to 22.4% in the EDU condition, a result that trended towards significant (p < 0.10). Considered multivariately, these data suggest CBT patients are more likely to achieve symptom improvement and maintain gains long term but less likely to show consistent treatment non-response than EDU patients.
A more complex picture emerged from the IBS-SSS analyses. Patients assigned to all three conditions reported significant reductions in IBS symptom severity at immediate follow-up which extended through quarterly follow-up periods for the ensuing 12 months. The overall average unit reduction in IBS-SSS at all time periods well exceeded the 50-point standard traditionally used to gauge clinical improvement (17). The similarity in efficacy of CBT and EDU on mean IB-SSS suggests that both conditions have therapeutic benefits that, to some extent, operate through shared mechanisms. It is unlikely that positive effects are merely a placebo response. Placebo responses typically decrease after treatment is withdrawn and are accompanied by a return of symptoms. This did not occur. In the case of both CBT and EDU, therapeutic benefit as measured by both IBS symptom improvement and symptom reduction was sustained after treatment withdrawal. One possibility is that in comparison to treamtents based on a curative biomedical model, both CBT and EDU provide patients with an explanatory model of IBS that corrects misinformation about and misinterpretations of GI symptoms as well as the illness experience of IBS. Corrective information about IBS (its causes, time course, controllability, etc.)—whether delivered in CBT through structured cognitive restructuring exercises or in the EDU through provision of patient education—may reduce perceptions of unpredictability that are believed to moderate the pathogenic effects of stress on brain-gut interactions.
It is also possible that treatment response across time relates to the demands of participation in a research trial and not simply technical aspects of the treatment participants received. These include participants’ knowledge that they are part of a clinical trial with clearly stated circumscribed therapeutic goals featuring “state of the art” treatments, regularly scheduled follow-up visits, encouraging staff, experienced clinicians trained to a high level of competence, and monitored using detailed treatment protocols (analogous to a Hawthorne effect whereby people change or modify an aspect of their behavior by virtue of being observed in a test, trial, or study independent of variation in experimental conditions to which they are exposed). We are reluctant to think that such nonspecific effects explain treatment effects. First, any results due to systematic bias arising from research participation would not explain the differential response to the CGI for CBT-and EDU-treated patients because it should operate the same in all conditions. If responses were due to Hawthorne-like phenomena, then we would not expect between-group differences on the primary outcome measure. In fact, both versions of CBT outperformed EDU on the primary outcome measure of global symptom improvement (CGI).
Between-group comparisons within a given time period give some perspectives on treatment effects over and above Hawthorne-like phenomena because such effects should operate comparably in all three treatment conditions. The consistent better performance of the CBT conditions over the EDU condition for the primary outcome measure (CGI-Improvement) across all follow-up periods is, thus, important. Within a given CBT treatment condition, it is virtually impossible to know how much of a treatment effect is due to CBT per se because the practice of CBT is confounded with the monitoring that is central to the data collection process of clinical trials that feature patient-reported outcomes (although adaptations of the classic Solomon four group design could allow one to tease out testing/monitoring effects). The overarching goal of CBT for IBS is psychophysiological self-regulation. The capacity to self-regulate depends on an ability to monitor and record some aspect of one’s own behavior and evaluate it relative to some standard. Patients then make appropriate changes, are reinforced for these changes, compare again, and adjust their behavior until goals (e.g., IBS symptom relief) are met (24). This feedback-loop of self-regulation depends on a person being able to monitor his or her behavior. Whether the goal is glucose control, smoking cessation, weight loss, or, as is the case of the present study, improvement of GI symptoms, people can only regulate themselves if they attend to what they are trying to change. The version of CBT developed for this study (10) has a strong emphasis on self-monitoring which we believe helps explains the magnitude and persistence of its effects relative to other less robust behavioral regimens featured in the literature. Self-monitoring disrupts the automaticity of sequence of thoughts, feelings, somatic sensations, and behaviors that if maladaptive can undermine selfcontrol (25). Through regular self-monitoring, patients learn that symptoms do not “come out of the blue” but are part of a behavior chain that is predictable and to an important extent controllable. These cognitive changes (i.e., increased sense of control) set the stage for adoption of more adaptive symptom self-management skills. It is possible that the decay of small subset of CBT-treated patients is due to lack of persistence with monitoring.
In terms of limitations, because our questions about decay required per-protocol (as opposed to intent-to-treat) analyses, one could argue that our analyses overstate treatment response. However, because of low (and seemingly random) dropout rates across conditions, one would not expect differences between results for the two forms of analysis. Results do not necessarily extend beyond our sample even though we intentionally established relatively broad participant eligibility criteria to increase the external validity of outcomes. Despite these efforts our sample represented a relatively homogeneous demographic profile and results may not generalize to more diverse populations elsewhere. The differential patterning ofresults for the CGI versus the IBS-SSS may reflect the nature of the measures per se. Unlike the CGI which requires patients to make a global judgment of improvement of both sensory and defecatory symptoms, the IBS-SSS heavily weighs pain (26). In this respect, the IBS-SSS may understate improvement in bowel symptoms that follows decease in the severity of symptom decrease. This is one reason we adopted the GGI as a priori primary outcome measure for the IBSOS. While patient-reported data are the recommended source of information for gauging therapeutic benefit for IBS treatments (27), they are vulnerable to bias, although any bias should operate uniformally across conditions. Further, in previous research we found that symptom improvement was similar when assessed by physicians who were not vulnerable to self-report bias (5).
In sum, whether home or clinic based, CBT resulted in substantial relief of IBS symptoms across multiple efficacy endpoints (global symptom improvement, reduced IBS symptom severity) over an extended period of time for treatment-refractory IBS patients. A greater proportion of CBT-treated patients had a more complete long-term response than EDU patients in that they reported symptomatic improvement at each of the 5 follow-up periods out to 12 months post treatment. Conversely, a greater proportion of complete non responders was represented in EDU relative to CBT. What biological and/or behavioral factors account for this difference can inform the development of more robust disease management approaches for a condition that is not adequately treated through conventional medical options. While previous research indicates that a significant proportion of patients respond rapidly to CBT (28) and maintain these gains through follow-up, a sizable (8%) proportion of MC-CBT-treated patients have a somewhat delayed treatment response. They do not register as treatment responders immediately after treatment but first reported clinical improvement at the 3-month follow-up and at every subsequent one through the 12-month follow-up period. It is notable the greatest proportion of delayed responders were MC-CBT patients who received only 4 h of therapist contact. These patients may require more time to achieve skill proficiency than S-CBT patients who had the advantage of working with a clinician 1 h a week over 10 weeks. This explanation is consistent with the findings of self-administered CBT for headache that documented a time lag of 6 months before full treatment response (improvement in headache activity and in analgesic medication use) was evident (29). At the very least, our data suggest that the immediate efficacy value of novel therapeutic agents emerging from a RCT may not necessarily correspond with its “real world” therapeutic benefit once the study is completed for about 10% of patients. Indeed, the proportion of patients who have a delayed response is comparable to the proportion of patients whose gains decay at each follow-up. Understanding the underlying mechanisms that account for these differential trajectories is an important area for future investigation.
Study Highlights.
WHAT IS KNOWN
 IBS Is common, costly, and unsatisfactorily treated with medical therapies.
IBS Is common, costly, and unsatisfactorily treated with medical therapies.
 Despites its established short -term benefits, the long-term benefits of CBT are unknown.
Despites its established short -term benefits, the long-term benefits of CBT are unknown.
WHAT IS NEW HERE
 IBS symptom improvement in CBT-treated patients are maintained through 12 months.
IBS symptom improvement in CBT-treated patients are maintained through 12 months.
 CBT shows less decay than IBS education over 12 months.
CBT shows less decay than IBS education over 12 months.
ACKNOWLEDGEMENTS
NIDDK scientists (Drs. Frank Hamilton and Patricia Robuck) contributed to the design and conduct of the study, which included the collection and management of data. The project scientist from the NIDDK served as a member of the IBSOS steering committee. The decision to publish was made by the IBSOS steering committee in consultation with all authors, with no restrictions imposed by the sponsor. As a coauthor, the NIDDK scientist contributed to the interpretation of the data and the preparation, review, and approval of the manuscript.
Guarantor of the article: Jeffrey M. Lackner, PsyD, and James Jaccard, PhD.
Financial support: Research reported in this manuscript was supported by the NIH/NIDDK Grant 77738 (to JL). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
CONFLICTS OF INTEREST
Potential competing interests: None reported.
REFERENCES
- 1.Shah E, Kim S, Chong K, et al. Evaluation ofharm in the pharmacotherapy of irritable bowel syndrome. Am J Med. 2012;125:381–93. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Chey WY, Heath AT, et al. Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2004;99:2195–203. [DOI] [PubMed] [Google Scholar]
- 3.Enck P, Aziz Q, Barbara G. et al. Irritable bowel syndrome. Nat Rev Dis Prim. 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lackner JM, Keefer L, Jaccard J, et al. The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self-versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33:1293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lackner JM, Jaccard J, Keefer L, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018;155:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljótsson B, Hedman E, Andersson E, et al. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol. 2011;106:1481. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy T, Jones R, Darnley S, et al. Cognitive behaviour therapy in addition to antispasmodic treatment for irritable bowel syndrome in primary care: randomised controlled trial. Br Med J. 2005;331:435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376:2566–78. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Corazziari E, et al. Rome The functional gastrointestinal disorders: diagnosis, pathophysiology and treatment: a multinational consensus. 2 ed McLean: Degnon Associates; 2006. [Google Scholar]
- 10.Lackner JM, Jaccard J, Krasner SS, et al. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lackner JM, Holroyd KA. Breaking the bonds of IBS: A step-by-step guide. 2nd ed Buffalo: University at Buffalo, SUNY; 2010. [Google Scholar]
- 12.Gordon D IBS: Learn to take charge ofit. NewYork: Barnes & Noble; 2004. [Google Scholar]
- 13.Ringstrom G, Storsrud S, Posserud I, et al. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:420–8. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Moayyedi P, Lacy BE, et al. American College ofGastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2. [DOI] [PubMed] [Google Scholar]
- 15.Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. [DOI] [PubMed] [Google Scholar]
- 16.Klein KB. Assessment of treatment outcome in the functional gastrointestinal disorders In: Corazziari E, editor. Approach to the patient with chronic gastrointestinal disorders. Milano: Messaggi; 1999. [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 18.Feinman RD. Intention-to-treat. What is the question? Nutrition & Metabolism 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angrist JaPJ. Mostly harmless econometrics: an empiricist’s companion. Princeton: Princeton University Press; 2009. [Google Scholar]
- 20.Agresti A Introduction to categorical data analysis. New York: Wiley; 2007. [Google Scholar]
- 21.Marshall A, Altman D, Holder R. et al. Combining estimates ofinterest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaccard J Complex statistics made accessible: Cluster analysis. Miami: Applied Scientific Analysis; 2018. [Google Scholar]
- 23.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. [DOI] [PubMed] [Google Scholar]
- 24.Carver CS, Scheier MF. Attention and self-regulation: a control-theory approach to human behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- 25.Wilson GT. Rapid response to cognitive behavior therapy. Clin Psychol: Sci Pract. 1999;6:289–92. [Google Scholar]
- 26.Lackner JM, Jaccard J, Baum C, et al. Patient-reported outcomes for irritable bowel syndrome are associated with patients’ severity ratings of gastrointestinal symptoms and psychological factors. Clin Gastroenterol Hepatol. 2011;9:957–64.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food and Drug Administration. Guidance for Industry Irritable Bowel Syndrome — Clinical Evaluation of Products for Treatment. In: U.S. Department of Health and Human Services, ed, 2010. [Google Scholar]
- 28.Lackner JM, Gudleski GD, Keefer L. et al. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holroyd KA, O’Donnell FJ, Stensland M, et al. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial [see comment]. JAMA. 2001;285:2208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derogatis LR. Brief Symptom Inventory (BSI) 18. Minneapolis: National Computer System, 2000. [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 32.Lackner JM, Ma CX, Keefer L, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]


