Abstract
HIV testing remains below UNAIDS 90–90–90 goals in sub-Saharan Africa. The aim of this study was to understand gender-specific factors related to HIV testing in Kisarawe, Tanzania. Informed by Social Action Theory, we analyzed cross-sectional data from a population-based random sample using bivariate and multivariate logistic regression to identify the contextual, behavioral, and interpersonal factors associated with prior HIV testing – specifically, any prior testing and testing within the past year. Of 644 participants, 63.1% of men and 85.5% of women reported ever testing for HIV. Younger men and women (aged 18–25 years) had significantly lower odds of prior HIV testing compared with older participants. For men, low levels of anticipated stigma and having ever talked about HIV were both positively associated with any prior testing. Men who knew if a sexual partner had received an HIV test had almost three times the odds of receiving a recent HIV test compared to men with no knowledge of their partners’ testing status (aOR = 2.96, 95% CI: 1.22–7.17, p = 0.01). For women, knowing someone who is HIV-positive was associated with increased odds of any prior testing (aOR = 2.74, 95% CI: 1.24–6.07, p = 0.01). Gender-specific, proactive interventions are needed to increase testing uptake, especially for young people and men.
Keywords: Africa, women, men, HIV, diagnosis
Background
The first goal of the UNAIDS ‘90–90–90’ targets is to test and diagnose 90% of all people living with HIV (PLHIV) by 2020.1 Testing remains the only pathway to knowing one’s serostatus and accessing antiretroviral therapy (ART), which has both treatment and prevention benefits.2 Despite these targets, an estimated 46% of PLHIV globally remain unaware of their HIV status.3
In Tanzania, only 62% of women and 47% of men have ever taken an HIV test and received the results.4 Since 2000 Tanzania has enacted provider-initiated testing and counseling as part of prevention of mother to child transmission (PMTCT) programs, which routinely tests women for HIV during antenatal visits. Tanzania currently reports testing 85% of pregnant women for HIV during antenatal care.5 However, as in many countries in sub-Saharan Africa, men are not reached as frequently with HIV testing services, and as a result, more women are tested than men.
Gender inequities are key drivers of the HIV epidemic, as both biological and social factors increase risk for women.6 In Tanzania, traditional gender norms place women in a disadvantaged economic and social position,7 leading to exposure to violence,8 transactional sex,9 and lack of sexual decision-making power.10 Further related to this risk are conventional ideas of masculinities, which tie men’s concept of being a ‘strong man’ to sexual virility, dominance over women, and physical power.11 However, these same masculine ideals put men at a disadvantage for receiving HIV-related testing and treatment as they dissuade men from seeking care.12 As a result men in general are less likely to test for HIV13 and are less likely to be on ART compared to women.14
Factors related to HIV testing have been widely explored, but few studies use a gendered approach. Two quantitative studies from South Africa identified low testing rates among men and different motivations for testing between genders, with more women reporting testing due to nonvoluntary reasons, such as antenatal care.15,16 Additionally, several qualitative studies have identified significant gender differences in reasons for testing and testing behaviors across sub-Saharan Africa. A study from Uganda found that two competing ideas of masculinity – ‘reputation’ and ‘respectability’ – greatly influence men’s willingness to test, with testing conflicting with expectations that men should be strong, resilient, and not in need of assistance.17 Conversely, HIV testing aligned with men’s motivation to be a family-focused provider and protector.17 Another study from Lesotho found that men generally saw HIV testing as a service for women, not men, and that they could learn their HIV status ‘by proxy’ by assuming they have the same serostatus as their female partners.18 For women, studies suggest that rigid gender norms prevent women from discussing HIV or HIV testing with their sexual partners as this could be seen as an admission of infidelity and could lead to violence or other harms.18-20
Understanding differences in uptake of HIV testing between men and women could inform programs and generate ideas for developing gender-specific interventions to increase testing. This analysis sought to answer three questions about HIV testing uptake in Tanzania: (1) What reasons do men and women give for getting tested and not getting testing for HIV? (2) What contextual, behavioral, and interpersonal factors are related to any prior HIV testing? and (3) What recent risk behaviors and interpersonal factors are related to recent HIV testing?
Methods
Sampling methods and study population
We collected cross-sectional data in Kisarawe, Tanzania as part of the Triage Project, a phase II community-randomized trial assessing the effectiveness of a rural, community-based HIV prevention intervention. The Triage Project followed Project Accept, a multi-site HIV prevention trial conducted in a similar geographic area.21,22
A two-stage sampling strategy was used. First, we mapped all households in two communities using GPS coordinates and randomly selected a list of households to visit, generated in batches with all households per batch being visited to avoid bias. All household members were enumerated and an eligible member was randomly selected using a random assignment application on an electronic tablet. Eligibility criteria included living full-time in the household and being aged between 18 and 55 years. Interviews took place in a private location in or near the household and were conducted in Kiswahili by trained interviewers using Samsung Tab 2 tablets. Participants were tested for HIV using Determine and Unigold HIV 1/2 rapid tests and received pre- and posttest counseling. All participants provided written informed consent. The study was approved by institutional review boards at the Medical University of South Carolina and Muhimbili University of Health and Allied Sciences.
Theory
This analysis was informed by Social Action Theory, which emphasizes three interconnected domains driving health behavior: (1) structural context; (2) self-regulatory processes, including outcome expectancies and motivational appraisals that influence decisionmaking; and (3) social interaction.23 Social Action Theory has been recommended for use in HIV prevention as it recognizes the interplay between structural, social, and individual level factors that drive risk.24
Measures
Participants were asked a series of questions related to sexual risk behavior, HIV testing history, and HIV-related knowledge. Most survey items were adapted from NIMH Project Accept (HPTN-043) as these measures were used previously to analyze factors related to HIV testing.15,16,25 Participants who reported no prior HIV testing were asked about reasons they had never tested, and participants who reported prior testing were asked to provide reasons they had tested. The two primary outcomes used in this analysis were (1) ever testing for HIV and (2) testing for HIV within the past year (recent testing).
To assess factors related to ever testing for HIV, we chose variables based on a literature review related to goals, expectations, and motivational appraisals of HIV testing. Selected variables included: (1) anticipated stigma (‘Would you be hesitant to take an HIV test due to fear of people’s reaction if you tested positive for HIV?’), as this has been hypothesized to estimate the influence of stigma on HIV testing uptake26; (2) Having heard about ART, as this has been associated with HIV testing previously27; (3) knowing someone who is living with HIV as data suggest the lack of positive narratives of people living with HIV exacerbate fear of testing18; (4) discriminatory attitudes toward people living with HIV28,29; and (5) talking about HIV with others, as this has shown prior association with HIV testing.25 We assessed discriminatory attitudes using three questions asking whether someone would be comfortable buying vegetables from someone living with HIV, whether children should go to school with children living with HIV, and whether a teacher living with HIV should be allowed to teach. We assessed unidimensionality using exploratory factor analysis and internal consistency using Cronbach’s alpha (a = 0.79). The scale was dichotomized at the median.
Analysis
Reasons for testing/not testing were coded as a series of polytomous variables as participants could choose more than one response out of 18 predetermined response options. Separate Chi square analyses with a Bonferroni adjustment were run on each response to compare distributions between males and females. We calculated differences between key variables for males and females using a Pearson Chi square statistic.
To analyze factors related to prior HIV testing, we built a series of gender-stratified logistic regression models. We ran bivariate logistic regression for demographic and the behavioral/normative predictors outlined above. We retained all variables significant at p < 0.10 with prior HIV testing in multivariate models, in addition to controlling for key demographic variables, using backwards stepwise elimination. We restricted inclusion to sexually active participants.
To determine factors related to recent HIV testing, we examined recent behaviors and interpersonal factors among participants who reported having a recent main or regular partner. We ran bivariate logistic regression for factors related to recent sexual behavior (condom use and multiple partners within the past six months), participation in an HIV prevention event, and potential dyadic-level influence, including knowing whether a sexual partner has been tested for HIV. We ran multivariate models controlling for sociodemographic and behavioral variables previously identified. We also controlled for the village from which participants were sampled to account for geographical differences. Analyses were conducted using Stata (STATACORP, version 11, College Station, TX).
Results
Sample characteristics and HIV prevalence
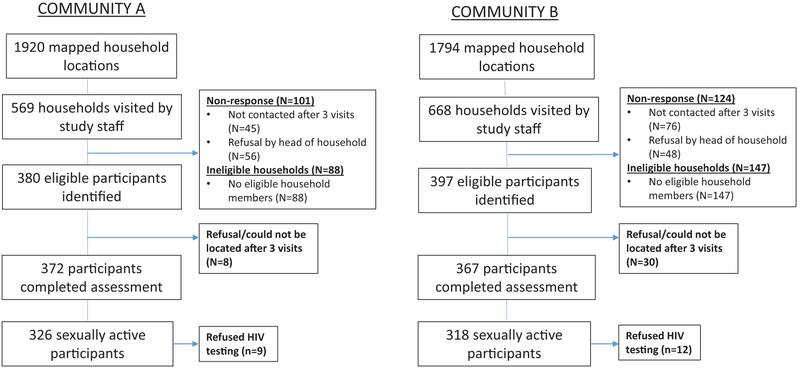
Figure 1 presents overall project sampling and recruitment. Of the 644 sexually active participants, 373 (58%) were female (Table 1). Overall significantly more women than men reported prior HIV testing (p < 0.01), as 63% of men and 86% of women reported prior testing. Fifty-six percent of women (n = 150/268) who reported prior testing were tested for a nonvoluntary reason, including due to pregnancy (being tested at an antenatal clinic). The overall HIV prevalence was 9.1% with no significant difference between genders. Twenty-one participants refused HIV testing (Figure 1). Over 68% of HIV-positive male participants were unaware of their serostatus at time of testing (n = 15/22). For females, 58.8% (n = 20/34) reported being previously unaware of their HIV-positive serostatus. The mean age for men was 37.7 and 34.8 years for women. Most participants were self-employed farmers or vendors (85.6%). Regarding education, 18% reported receiving no education, 70.5% received primary education, and 11.5% received a secondary education or higher.
Figure 1. Study sampling and recruitment.
Reprinted from Kidney International Reports. 3(4), Ploth DW, Mbwambo JK, Fonner VA, et al. Prevalence of CKD, Diabetes, and Hypertension in Rural Tanzania, 905–915, Copyright (2018), with permission from Elsevier.
Table 1.
Sample characteristics (n = 644) disaggregated and compared by gender.
| Variable | Total N (%) |
Men (n=271) N (%) |
Women (n=373) N (%) |
p-value |
|---|---|---|---|---|
| Prior HIV testing | ||||
| No | 154 (23.9) | l00 (36.9) | 54 (14.5) | <0.01 |
| Yes | 490 (76.1) | 171 (63.1) | 319 (85.5) | |
| HIV test within the last 12 months | ||||
| No | 397 (61.7) | 193 (71.2) | 169 (45.3) | <0.01 |
| Yes | 247 (38.3) | 78 (28.8) | 204 (54.7) | |
| HIV status | ||||
| Negative | 558 (90.9) | 234 (91.4) | 324 (90.5) | 0.70 |
| Positive | 56 (9.12) | 22 (8.6) | 34 (9.5) | |
| Age (years) | ||||
| 18–25 | 132 (20.5) | 45 (16.6) | 87 (23.3) | <0.01 |
| 26–35 | 189 (29.4) | 67 (24.7) | 122 (32.7) | |
| 36–45 | 184 (28.6) | 93 (34.3) | 91 (24.4) | |
| 45+ | 139 (21.6) | 66 (24.4) | 73 (19.6) | |
| Education | ||||
| No education | 116 (18.0) | 39 (14.4) | 77 (20.6) | 0.12 |
| Primary | 454 (70.5) | 199 (73.4) | 255 (68.4) | |
| Secondary or above | 74 (11.5) | 33 (12.2) | 41 (11.0) | |
| Employment | ||||
| Self-employed | 551 (85.6) | 239 (88.2) | 312 (83.6) | <0.01 |
| Employed (salaried) | 37 (5.8) | 22 (8.1) | 12 (4.0) | |
| Not employed | 56 (8.7) | 10 (3.7) | 46 (12.3) | |
| Have a main sexual partner | ||||
| Yes | 356 (64.6) | 158 (66.7) | 198 (63.1) | |
| No | 195 (35.4) | 79 (33.3) | 116 (36.4) | 0.38 |
| Have heard about ART | ||||
| No | 211 (32.8) | 89 (32.8) | 211(32.7) | 0.52 |
| Yes | 433 (67.2) | 182 (67.2) | 251 (67.2) | |
| Condom use in past six months | ||||
| Never | 405 (73.4) | 162 (68.4) | 243 (77.9) | 0.04 |
| Sometimes | 102 (18.6) | 52 (21.9) | 50 (16.0) | |
| Always | 42 (7.6) | 23 (9.7) | 19 (6.1) |
ART: antiretroviral therapy.
Reasons for testing and not testing for HIV
Men and women reported similar reasons for not testing for HIV, with no statistical differences by gender (Tables 2 and 3). The most common reasons for not testing included ‘didn’t think I was at risk’ and ‘not important to me.’ Reasons reported for ever testing differed by gender with a significantly larger proportion of women (n = 150, 56%) reporting nonvoluntary testing (defined as testing related to pregnancy, military, or insurance reasons) as compared to men (χ2 p < 0.001). Other common reasons for prior testing included ‘wanting to know status’ and ‘not wanting to worry anymore.’
Table 2.
Reported reasons for HIV testing stratified by gender among those reporting prior HIV testing.
| Reason for testing | Male N(%) | Female N(%) | Total N(%) |
|---|---|---|---|
| Nonvoluntary test (pregnancy/tested at antenatal clinic, military, or insurance reasons) | 38 (22.1) | 150 (44.3) | 188 (36.8) |
| Recommended by healthcare provider | 15 (8.7) | 17 (5.0) | 32 (6.3) |
| Was sick/having symptoms of HIV/AIDS | 4 (2.3) | 4 (1.2) | 8 (1.6) |
| Sexual partner got tested | 1 (0.6) | 1 (0.3) | 2 (0.4) |
| Sexual partner got sick | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Sexual partner asked that I get tested | 2 (1.2) | 3 (0.9) | 5 (1.0) |
| Getting married | 3 (1.7) | 3 (0.9) | 6 (1.2) |
| Having children | 2 (1.2) | 5 (1.5) | 7 (1.4) |
| Wanted to know status | 71 (41.3) | 108 (31.9) | 179 (35.0) |
| Had risky behavior | 3 (1.7) | 0 (0.0) | 3 (0.6) |
| Sexual partner had risky behavior | 0 (0.0) | 4 (1.2) | 4 (0.8) |
| Didn’t want to worry anymore | 26 (15.1) | 41 (12.1) | 67 (13.1) |
| Tested at work program | 0 (0.0) | 2 (0.6) | 2 (0.4) |
| Other (donating blood, free test, easy access to test) | 7 (4.1) | 0 (0.0) | 7 (1.4) |
| Total | 172 | 339 | 511 |
Note: Based on total number of responses, not total number of participants (n = 149); most common reasons for testing are bolded.
Table 3.
Reported reasons for not testing for HIV stratified by gender among those with no prior history of HIV testing.
| Reason for not testing | Male N(%) | Female N(%) | Total N(%) |
|---|---|---|---|
| Don’t think I am at risk | 30 (28.0) | 19 (30.6) | 49 (29.0) |
| Nervous to get results | 4 (3.7) | 4 (6.5) | 8 (4.7) |
| Don’t know where to get tested | 4 (3.7) | 0 (0.0) | 4 (2.4) |
| Worried people would think I was sick | 3 (2.8) | 3 (4.8) | 6 (3.6) |
| Test too expensive | 0 (0.0) | 1 (1.6) | 1 (0.6) |
| Don’t have time or opportunity | 12 (11.2) | 11 (17.7) | 23 (13.6) |
| Testing site too far from home | 5 (4.7) | 1 (1.6) | 6 (3.6) |
| Can’t leave work to get tested | 2 (1.9) | 0 (0.0) | 2 (1.2) |
| Results take too long | 2 (1.9) | 0 (0.0) | 2 (1.2) |
| Didn’t ever think of getting test | 14 (13.1) | 8 (12.9) | 22 (13.0) |
| Not important to me | 30 (28.0) | 15 (24.2) | 45 (26.6) |
| Doubt confidentiality of test results | 1 (0.9) | 0 (0.0) | 1 (0.6) |
| Total | 107 | 62 | 169 |
Note: Based on total number of responses, not total number of participants (n = 149); most common reasons for testing are bolded.
Associations of prior testing and contextual factors
HIV status was not significantly associated with prior testing for either men or women. For men, prior HIV testing was associated with age and education in bivariate analysis. In multivariate analysis, men aged 26–35 had over three times the odds of reporting prior testing compared to men aged 18–25 (aOR = 3.56, 955 CI: 1.34–9.45, p < 0.01). For women, age was significantly associated with prior testing in bivariate and multivariate regression, with young women (18–25 years) and older women (aged 46 years and above) reporting less prior testing than women aged 26–45 years. In multivariate regression, women aged 25–36 had over six times the odds of prior testing as compared to younger women (aged 18–25) (aOR = 6.03, 95% CI: 1.57–23.16, p < 0.01). Results are presented in Table 4.
Table 4.
Correlates of prior HIV testing among sexually active participants (n = 644) using bivariate and multivariate logistic regression.
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Prior HIV test N (%) |
Unadjusted OR (95% CI) |
P value | Adjusted ORa (95% CI) |
P value | Prior HIV test N (%) |
Unadjusted OR (95% CI) |
P value | Adjusted ORa (95% CI) |
P value |
| Sociodemographic and contextual variables | ||||||||||
| Age (years) | ||||||||||
| 18–25 | 26 (57.8) | 1.00 | 1.00 | 73 (83.9) | 1.00 | 1.00 | ||||
| 26–35 | 52 (77.6) | 2.53 (1.11–5.78) | 0.03 | 3.56 (1.34–9.45) | 0.01 | 119 (97.5) | 7.61 (2.11–27.4) | <0.01 | 6.03 (1.57–23.16) | <0.01 |
| 36–45 | 61 (65.6) | 1.39 (0.67–2.89) | 0.37 | 1.61 (0.62–4.19) | 0.33 | 81 (89.0) | 1.55 (0.65–3.71) | 0.32 | 1.09 (0.39–3.00) | 0.87 |
| 45+ | 32 (48.5) | 0.69 (0.32–1.47) | 0.34 | 0.77 (0.28–2.14) | 0.62 | 46 (63.0) | 0.33 (0.16–0.68) | <0.01 | 0.21 (0.08–0.56) | <0.01 |
| Marital status | ||||||||||
| Single | 48 (57.1) | Ref | 1.00 | 82 (85.4) | Ref | 1.00 | ||||
| Ever married | 123 (65.8) | 1.44 (0.85–2.44) | 0.17 | 1.93 (0.97–3.83) | 0.06 | 237 (85.6) | 1.01 (0.52–1.95) | 0.97 | 1.30 (0.55–3.05) | 0.55 |
| Education | ||||||||||
| No education | 19 (48.7) | Ref | 1.00 | 63 (81.8) | Ref | 1.00 | ||||
| Primary | 130 (65.3) | 1.98 (0.99–3.96) | 0.05 | 1.70 (0.77–3.77) | 0.19 | 222 (87.1) | 1.49 (0.75–2.96) | 0.25 | 1.31 (0.59–2.93) | |
| Secondary or higher | 22 (66.7) | 2.11 (0.81–5.49) | 0.13 | 2.30 (0.76–6.92) | 0.14 | 34 (82.9) | 1.08 (0.40–2.93) | 0.88 | 0.52 (0.15–1.78) | 0.30 |
| Ethnic group | ||||||||||
| Other | 66 (62.9) | Ref | 1.00 | 100 (87.7) | Ref | 1.00 | ||||
| Zaramo | 105 63.2 | 1.02 (0.61–1.69) | 0.95 | 1.38 (0.74–2.56) | 0.31 | 219 (84.6) | 0.77 (0.40–1.47) | 0.43 | 0.90 (0.42–1.95) | 0.79 |
| HIV status | ||||||||||
| Negative | 149 (63.9) | Ref | 278 (85.8) | Ref | ||||||
| Positive | 14 (63.6) | 1.00 (0.40–2.48) | 0.99 | 30 (88.24) | 1.24 (0.42–3.69) | 0.70 | ||||
| Attitudes, beliefs, and behavior variables | ||||||||||
| Fear of being tested | ||||||||||
| No | 162 (64.5) | Ref | 1.00 | 301 (85.5) | Ref | |||||
| Yes | 9 (45.0) | 0.44 (0.18–1.13) | 0.09 | 0.31 (0.11–0.86) | 0.03 | 15 (88.2) | 1.27 (0.28–5.72) | 0.76 | ||
| Know about ART | ||||||||||
| No | 51 (57.3) | Ref | 99 (81.2) | Ref | ||||||
| Yes | 120 (65.9) | 1.44 (0.86–2.43) | 0.17 | 220 (87.6) | 1.65 (0.91–2.97) | 0.10 | ||||
| Know HIV+ person | ||||||||||
| No | 91 (59.1) | Ref | 194 (90.0) | Ref | ||||||
| Yes | 78 (69.0) | 1.54 (0.92–2.57) | 0.10 | 120 (90.9) | 1.91 (0.96–3.80) | 0.07 | 2.74 (1.24–6.07) | 0.01 | ||
| Discriminatory attitudes | ||||||||||
| Low | 110 (64.0) | 1.0 | 206 (87.3) | 1.00 | ||||||
| High | 61 (61.2) | 0.90 (0.54–1.51) | 0.70 | 111 (82.2) | 0.67 (0.38–1.21) | 0.19 | ||||
| Ever talk about HIV | ||||||||||
| No | 40 (52.0) | Ref | 1.00 | 111 (82.2) | Ref | |||||
| Yes | 131 (67.5) | 1.92 (1.12–3.29) | 0.02 | 2.28 (1.20–4.31) | 0.01 | 207 (87.7) | 1.54 (0.86–2.78) | 0.15 | ||
| Lifetime alcohol use | ||||||||||
| No | 84 (71.8) | Ref | 1.00 | 225 (87.6) | Ref | |||||
| Yes | 87 (56.5) | 0.51 (0.31–0.85) | 0.01 | 0.56 (0.29–1.02) | 0.06 | 94 (81.0) | 0.61 (0.33–1.10) | 0.10 | ||
ART: antiretroviral therapy.
Adjusted for all variables listed in addition to village.
Associations of prior testing and behavioral, beliefs, and normative factors
For men, bivariate analysis indicated that prior testing was associated with no reported lifetime alcohol use and low anticipated stigma (i.e. little to no fear of testing due to people’s reaction of a positive result). In multivariate analysis, lifetime alcohol use and fear of being tested were associated with lower odds of prior HIV testing (aOR = 0.56, 95% CI: 0.29–1.02 and aOR = 0.31, 95% CI: 0.10–0.86, respectively). Ever talking about HIV was associated with higher odds of prior HIV testing (aOR = 2.28, 95% CI: 1.20–4.31, p = 0.01). For women, odds of prior testing were positively associated with having heard of ART and knowing someone who is living with HIV in bivariate analysis. In multivariate analysis, knowing someone who is living with HIV remained a significant predictor of prior testing (aOR = 2.74, 95% CI: 1.24–6.07, p = 0.01).
Associations between recent HIV testing and behaviors, social interaction, and partner influence
This analysis was restricted to 356 participants who reported having a recent main or regular sexual partner (Table 5). In bivariate analysis, no recent sexual behavior variables, including frequency of condom use and recent number of sexual partners, were significantly associated with recent HIV testing for either gender. In multivariate analysis, men who reported knowing that their partner had tested for HIV had over twice the odds of reporting recent testing as compared to men who did not know (aOR= 2.96, 95% CI: 1.22–7.17, p = 0.01). For women, knowing whether their partner had received an HIV test was correlated with recent testing in bivariate analysis (p < 0.10) but not in multivariate analysis.
Table 5.
Bivariate and multivariate logistic regression model of factors related to recent HIV testing (within the past 12 months) for sexually active men and women reporting a main or regular partner in the past six months (n = 356).
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Unadjusted OR (95% CI) |
P value | Adjusted ORa (95% CI) |
P value | Unadjusted OR (95% CI) |
P value | Adjusted OR (95% CI) |
P value |
| Condom use | ||||||||
| Never | 1.00 | |||||||
| Sometimes | 1.01 (0.45–2.27) | 0.97 | 1.41 (0.68–2.90) | 0.35 | ||||
| Always | 0.71 (0.21-2.41) | 0.58 | 1.78 (0.52–6.10) | 0.36 | ||||
| Number of sex partners | ||||||||
| 0–1 | 1.00 | 1.00 | ||||||
| >1 | 0.73 (0.36–1.50) | 0.40 | 0.90 (0.39–2.1) | 0.81 | ||||
| Know partner tested for HIV | ||||||||
| No | 1.00 | 1.00 | 1.00 | |||||
| Yes | 2.64 (1.35–5.18) | <0.01 | 2.96 (1.22–7.17) | 0.01 | 1.85 (0.91–3.78) | 0.09 | ||
| Living situation | ||||||||
| With partner | 1.49 (0.89–2.50) | 1.10 (0.61–1.97) | ||||||
| Other | 1.00 | 0.13 | 1.00 | 0.75 | ||||
| Recent talk about HIV | ||||||||
| None | 1.00 | 1.00 | ||||||
| At least one | 1.16 (0.61–2.23) | 0.65 | 1.33 (0.74–2.37) | 0.34 | ||||
| Recent participation in HIV activities | ||||||||
| No | 1.00 | 0.04 | 1.00 | |||||
| Yes | 2.10 (1.04–4.26) | 1.73 (0.86–3.45) | 0.12 | |||||
Adjusted for village, age, education, ethnic group, alcohol use, fear of testing, and talking about HIV.
Discussion
Our results indicate that factors related to HIV testing uptake differ between men and women. Uptake of testing was significantly lower for men than women, which is consistent with prior findings from Tanzania,30 as well as with similar findings in the region that men get tested less, both due to HIV testing’s incongruence with traditional gender norms17,31,32 and men’s limited exposure to routine testing. Uptake of testing was also low for young adults aged 18–25 years, similar to results from a global review that found most adolescents and young people are unaware of their HIV status.33 This finding is especially concerning as young women are particularly vulnerable to HIV infection.34 HIV testing interventions targeting high-risk young adults are urgently needed. Our results suggest that young adults who are sexually active but not yet accessing routine services (i.e. antenatal care) are a high-risk population that has been overlooked as far as designing and implementing tailored HIV testing strategies. A recent systematic review identified several potential interventions to help increase testing among young adults, including using mobile technology, such as sending text messages to at-risk young people to encourage testing, setting up nontraditional HIV testing venues, utilizing self-testing, and offering testing during community events.35 One youth-focused door-to-door testing campaign in Zambia and South Africa found the home-based approach increased testing from approximately 28 to 89% among youth who accepted the intervention.36 However, many of the strategies reviewed focused on adolescents and not young adults.
Regarding reasons for testing and not testing for HIV, findings between men and women were similar except that women overwhelmingly reported being tested due to attending antenatal services, reflecting testing offered through PMTCT programs.37 Common reasons for not testing suggested that there are not significant structural barriers to testing, such testing being too expensive or inaccessible. Most people reported not testing either because they felt they were not at risk or that testing was not important to them. In other words, HIV testing was simply not a priority in peoples’ lives. This finding suggests that more proactive, convenient HIV testing strategies are needed.
Regarding women, over half of female participants reported being tested for HIV as part of antenatal care. We hypothesize that because so many women were tested during routine antenatal care, the blanket testing strategy obscured associations between social and behavioral factors with HIV testing. It is noteworthy that knowing someone who is HIV-positive was the only psychological factor significantly associated with prior testing for women. A systematic review of factors associated with prior HIV testing found that most studies including the measure of knowing someone who is HIV-positive also found a positive correlation with prior testing, although results were not stratified by gender.38
For men, ever talking about HIV was related to prior lifetime history of testing. These findings are consistent with results from Project Accept, which found that having prior HIV-related conversations was significantly associated with testing across sites.25 For men the fear of testing HIV-positive might be rooted in gender norms, as testing HIV-positive could show weakness, embarrassment, or damage to sexual prowess.17,18,39 The inverse association between lifetime alcohol use and prior testing, which was marginally significant for men, warrants further study. Given the indirect association between alcohol and HIV infection, targeting testing interventions to men who frequent drinking establishments could increase HIV testing among this high-risk group, and a review of interventions within alcohol-serving establishments found that offering onsite HIV testing was feasible and acceptable.40
When assessing factors related to recent testing, dyadic-level variables showed significant correlation for men and women, which speaks to the importance of partner communication and relationship contexts in regards to testing. This finding supports use of the Social Action Theory as it emphasizes the importance of social interdependence in facilitating or impeding a partner’s motivation for and action to enact a health behavior change.23 Knowing whether a sexual partner had been tested for HIV was significantly correlated with recent testing for men but not women. A theoretical framework emphasizing the importance of dyadic interactions in HIV prevention recognizes the phenomenon of ‘reciprocal influence’ where a partner can significantly influence an individual’s decision to engage in a health behavior regardless of his/her own personal norms, attitudes, and beliefs.41 These findings highlight the potential for interventions seeking to motivate members of couples to recruit their partners for testing. Such interventions include: (1) male involvement in PMTCT,42,43 (2) partner notification strategies,44,45 (3) using sexual partners to distribute self-test kits to partners who otherwise would not test,46,47 and (4) increasing acceptance and availability of couples’ testing and counseling.48
Engaging in high-risk sexual practices, including multiple partners and condomless sex, was not associated with recent testing for either gender. This corroborates findings from a study conducted in Tanzania that found no association between risk perception and HIV testing,49 suggesting people either do not perceive themselves at risk or believe they are at risk but choose not to test.50 Therefore, communication campaigns aiming to increase HIV testing uptake might be ineffective if messages focus on linking people’s sexual history and sexual behaviors to testing.
Strengths and limitations
We used cross-sectional data based on self-report, so causality cannot be inferred, and social desirability bias may have influenced results. For the model assessing factors relating to recent HIV testing, we do not know the sequence of reported behaviors and partner-level influence, so behaviors could have influenced testing or vice versa. Additionally, we did not assess whether participants had previously received HIV testing as an individual or together with their partner. For variables related to knowing whether a sexual partner has been tested, the context in which the dyadic communication occurred is unknown. We also did not assess upstream factors that affect and shape gender norms and expectations, such as economic and socio-cultural factors. Study strengths include using a population-based random sample and theory-informed, gender-stratified analysis.
Conclusions
HIV testing is a gendered experience in Tanzania. Women are often tested as a result of receiving antenatal care, and men who have tested are more likely to have had some social interaction related to HIV, such as talking about HIV or knowing if their partner tested. The immense need to better target HIV testing to those who are HIV-positive or at high risk of infection is evident in our finding that over 50% of HIV-positive participants were unaware of their serostatus. Gender-specific interventions to increase testing are warranted. In the immediate future, interventions are needed to increase HIV testing, particularly for people who are at high risk of being or becoming HIV infected, while ensuring interventions do not increase harm or compromise human rights. In the long term, interventions are needed to address gender inequities and promote healthy relationships to encourage safer, more equitable health decision-making.
Acknowledgments
We thank Basant Singh and all Triage Project staff, including Weston Ndomba, Rosemary Ntimizi, Wilson Shoo, and Isihaka Hamidu. We also thank the communities that partnered with us in conducting this research and all study participants for their contributions.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Triage Project was sponsored by the US National Institute of Mental Health, R01MH095869-01A1. The study on which this manuscript is based was funded by the U.S. National Institute of Mental Health (NIMH), R01MH095869-01A1.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Joint United Nations Programme on HIV/AIDS 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS, 2014. [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. AIDS by the numbers. Geneva: UNAIDS, 2016. [Google Scholar]
- 4.Tanzania Commission for AIDS (TACAIDS). Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of Chief Government Statistician(OCGS), ICF International. Tanzania HIV/AIDS and Malaria Indicator Survey 2011–12. Dar es Salaam: TACAIDS, ZAC, NBS, OCGS, and ICF International, 2013. [Google Scholar]
- 5.Tanzania Commission for AIDS (TACAIDS). Global AIDS response country progress report. Dar es Salaam: UNAIDS, WHO, Ministry of Health and Social Welfare, 2014. [Google Scholar]
- 6.Greig A, Peacock D, Jewkes R, et al. Gender and AIDS: time to act. AIDS 2008; 22: S35–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojcicki JM. Socioeconomic status as a risk factor for HIV infection in women in East, Central and Southern Africa: a systematic review. J Biosoc Sci 2005; 37: 1–36. [DOI] [PubMed] [Google Scholar]
- 8.Dunkle KL, Jewkes RK, Brown HC, et al. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. The Lancet 2004; 363: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 9.Maganja RK, Maman S, Groves A, et al. Skinning the goat and pulling the load: transactional sex among youth in Dar es Salaam, Tanzania. AIDS Care 2007; 19: 974–981. [DOI] [PubMed] [Google Scholar]
- 10.Tsai AC and Subramanian S. Proximate context of gender-unequal norms and women’s HIV risk in sub-Saharan Africa. AIDS 2012; 26: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell R Masculinities. Berkeley: University of California Press, 2005. [Google Scholar]
- 12.Mills EJ, Beyrer C, Birungi J, et al. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med 2012; 9: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermeyer CM, Sankara A, Bastien V, et al. Gender and HIV testing in Burkina Faso: an exploratory study. Soc Sci Med 2009; 69: 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health 2007; 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight L, McGrath N, van Rooyen H, et al. Characteristics of sexually experienced HIV testers aged 18 to 32 in rural South Africa: baseline results from a community-based trial, NIMH Project Accept (HPTN 043). BMC Public Health 2014; 14: 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesh KK, Madiba P, De Bruyn G, et al. Who gets tested for HIV in a South African urban township? Implications for test and treat and gender-based prevention interventions. J Acquir Immune Defic Syndr 2011; 56: 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siu GE, Wight D and Seeley JA. Masculinity, social context and HIV testing: an ethnographic study of men in Busia district, rural eastern Uganda. BMC Public Health 2014; 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiCarlo AL, Mantell JE, Remien RH, et al. ‘Men usually say that HIV testing is for women’: gender dynamics and perceptions of HIV testing in Lesotho. Cult Health Sex 2014; 16: 867–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker L, Pettifor A, Maman S, et al. Concerns about partner infidelity are a barrier to adoption of HIV-prevention strategies among young South African couples. Cult Health Sex 2014; 16: 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musheke M, Ntalasha H, Gari S, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in sub-Saharan Africa. BMC Public Health 2013; 13: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Global Health 2014; 2: e267–e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis 2011; 11: 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewart CK. Social action theory for a public health psychology. Am Psychol 1991; 46: 931–946. [DOI] [PubMed] [Google Scholar]
- 24.Traube DE, Holloway IW and Smith L. Theory development for HIV behavioral health: empirical validation of behavior health models specific to HIV risk. AIDS Care 2011; 23: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriksen ES, Hlubinka D, Chariyalertsak S, et al. Keep talking about it: HIV/AIDS-related communication and prior HIV testing in Tanzania, Zimbabwe, South Africa, and Thailand. AIDS Behav 2009; 13: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stangl AL, Brady L and Franz K. Measuring HIV stigma and discrimination. Washington, DC: International Center for Research on Women, 2012. [Google Scholar]
- 27.Phakathi Z, Van Rooyen H, Fritz K, et al. The influence of antiretroviral treatment on willingness to test: a qualitative study in rural KwaZulu-Natal, South Africa. Afr J AIDS Res 2011; 10: 173–180. [DOI] [PubMed] [Google Scholar]
- 28.Pettifor A, MacPhail C, Suchindran S, et al. Factors associated with HIV testing among public sector clinic attendees in Johannesburg, South Africa. AIDS Behav 2010; 14: 913–921. [DOI] [PubMed] [Google Scholar]
- 29.Young SD, Hlavka Z, Modiba P, et al. HIV-related stigma, social norms and HIV testing in Soweto and Vulindlela, South Africa: NIMH Project Accept (HPTN 043). J Acquir Immune Defic Syndr 2010; 55: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isingo R, Wringe A, Todd J, et al. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Trop Med Int Health 2012; 17: e15–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacPherson EE, Richards E, Namakhoma I, et al. Gender equity and sexual and reproductive health in Eastern and Southern Africa: a critical overview of the literature. Glob Health Action 2014; 7: 23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skovdal M, Campbell C, Madanhire C, et al. Masculinity as a barrier to men’s use of HIV services in Zimbabwe. Global Health 2011; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr 2014; 66: S144–S153. [DOI] [PubMed] [Google Scholar]
- 34.Dellar RC, Dlamini S and Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015; 18: 19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanoni BC, Elliott RJ, Neilan AM, et al. Screening for HIV and linkage to care in adolescents: insights from a systematic review of recent interventions in high- versus low- and middle-income settings. Adolesc Health Med Ther 2018; 9: 211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanaube K, Schaap A, Chaila MJ, et al. Community intervention improves knowledge of HIV status of adolescents in Zambia: findings from HPTN 071-PopART for youth study. AIDS 2017; 31: S221–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrams EJ, Simonds RJ, Modi S, et al. PEPFAR scale-up of pediatric HIV services: innovations, achievements, and challenges. J Acquir Immune Defic Syndr 2012; 60: S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evangeli M, Pady K and Wroe AL. Which psychological factors are related to HIV testing? A quantitative systematic review of global studies. AIDS Behav 2016; 20: 880–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matovu JK, Wanyenze RK, Wabwire-Mangen F, et al. “Men are always scared to test with their partners … it is like taking them to the Police”: motivations for and barriers to couples’ HIV counselling and testing in Rakai, Uganda: a qualitative study. J Int AIDS Soc 2014; 17: 19160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalichman SC. Social and structural HIV prevention in alcohol-serving establishments: review of international interventions across populations. Alcohol Res Health 2010; 33: 184–194. [PMC free article] [PubMed] [Google Scholar]
- 41.Karney BR, Hops H, Redding CA, et al. A framework for incorporating dyads in models of HIV-prevention. AIDS Behav 2010; 14: 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morfaw F, Mbuagbaw L, Thabane L, et al. Male involvement in prevention programs of mother to child transmission of HIV: a systematic review to identify barriers and facilitators. Syst Rev 2013; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyondo AL, Choko AT, Chimwaza AF, et al. Invitation cards during pregnancy enhance male partner involvement in prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV) in Blantyre, Malawi: a randomized controlled open label trial. PLoS One 2015; 10: e0119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown LB, Miller WC, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr 2011; 56: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henley C, Forgwei G, Welty T, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis 2013; 40: 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CC and Corbett EL. HIV self-testing to scale up couples and partner testing. Lancet HIV 2016; 3(6): e243–4. [DOI] [PubMed] [Google Scholar]
- 47.Thirumurthy H, Masters SH, Mavedzenge SN, et al. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV 2016; 3(6): e266–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medley A, Baggaley R, Bachanas P, et al. Maximizing the impact of HIV prevention efforts: interventions for couples. AIDS Care 2013; 25: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman MR, Massey M, Tsang SW, et al. An assessment of HIV testing in Tanzania to inform future strategies and interventions. AIDS Care 2014; 27: 213–217. [DOI] [PubMed] [Google Scholar]
- 50.Ntsepe Y, Simbayi LC, Shisana O, et al. Perceptions about the acceptability and prevalence of HIV testing and factors influencing them in different communities in South Africa. Sahara J 2014; 11: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]