Abstract
Background:
Among highly exposed populations, arsenic exposure in utero may be associated with decreased birth weight, however less is known about potential effects of arsenic exposure in urban communities without contaminated sources such as drinking water.
Objective:
Investigate the association of blood arsenic levels with birth weight-for-gestational age categories within a prospective birth cohort study.
Design/Methods:
We analyzed 730 mother-infant dyads within the Programming Research in Obesity, GRowth, Environment and Social Stressors (PROGRESS) cohort in Mexico City. Total arsenic was measured in maternal blood samples from the 2nd and 3rd trimesters, at delivery, as well as from infant umbilical cord blood samples. Multivariable, multinomial logistic regression models adjusting for maternal age at enrollment, pre-pregnancy body mass index, parity, infant sex, socioeconomic position, and prenatal environmental tobacco smoke exposure were used to calculate odds ratios of small-for-gestational age (<10th percentile, SGA) and large-for-gestational age (>90th percentile, LGA) compared to appropriate-for-gestational age (AGA) per unit increase of log-transformed arsenic.
Results:
Median (IQR) blood arsenic levels for maternal second trimester were 0.72 (0.33) μg/L, maternal third trimester 0.75 (0.41) μg/L, maternal at delivery 0.85 (0.70) μg/L, and infant cord 0.78 (0.65) μg/L. Maternal delivery and infant cord blood samples were most strongly correlated (spearman r = 0.65, p < 0.0001). Maternal arsenic levels at delivery were associated with significantly higher odds of both SGA (adj. OR=1.44, 95% CI: 1.08–1.93) and LGA (adj. OR=2.03, 95 % CI: 1.12–3.67) compared to AGA. Results were similar for cord blood. There were 130 SGA infants and 22 LGA infants. Earlier in pregnancy, there were no significant associations of arsenic and birth weight-for-gestational age. However, we observed non-significantly higher odds of LGA among women with higher arsenic levels in the 3rd trimester (adj. OR=1.46, 95% CI: 0.67–3.12).
Conclusion:
We found that in a Mexico City birth cohort, higher maternal blood arsenic levels at delivery were associated with higher odds of both SGA and LGA. However, sources and species of arsenic were not known and the number of LGA infants was small, limiting the interpretation of this finding and highlighting the importance of future large studies to incorporate arsenic speciation. If our findings were confirmed in studies that addressed these limitations, determining modifiable factors that could be mitigated, such as sources of arsenic exposure, may be important for optimizing fetal growth to improve long-term health of children.
Keywords: Arsenic, gestational age, birth weight, pregnancy
INTRODUCTION
Aberrant fetal growth can result in small-for-gestational age (SGA) and large-for-gestational age (LGA) infants that are at risk of both short and long-term morbidities and mortality (Das and Sysyn, 2004). SGA infants have an increased risk of hypoglycemia, perinatal asphyxia and hypothermia (Murki, 2014; Sharma et al., 2016) and have increased rates of cardiovascular disease, hypertension, and type 2 diabetes as adults (Barker, 1997; Barker et al., 1993). LGA infants have a heightened risk of complications during delivery (Najafian and Cheraghi, 2012) and increased rates of obesity and type 2 diabetes as adults (Ng et al., 2010; Schellong et al., 2012). Abnormal fetal growth results from a combination of genetic factors and environmental exposures including tobacco smoke, air pollution, and metals among others (Bobak, 2000; Windham et al., 2000; Zota et al., 2009). Many studies have investigated the effects of metal exposure on fetal growth, however, there is a lack of consensus regarding arsenic exposure in utero.
Arsenic is a naturally occurring element found at high levels in aquifers and groundwater in some areas around the globe (Smedley and Kinniburgh, 2002). In the environment, arsenic occurs in its inorganic and organic forms; inorganic arsenic is generally considered more toxic than organic because it is readily taken up by cells and more highly metabolized (Cohen et al., 2006). Sources of exposure to arsenic include drinking water, food such as seafood, rice, rice products, and juices, and, to a lesser extent, crops irrigated with contaminated water. Drinking water contains inorganic arsenic while arsenic found in seafood is predominantly in the organic form. Arsenic has been shown to readily cross the placental barrier in humans (Concha et al., 1998). Studies have shown that toxic metal exposure, including arsenic, may influence fetal growth resulting in higher infant mortality as well as chronic developmental and metabolic disorders among survivors (Pallotto and Kilbride, 2006; Resnik, 2002). While prenatal arsenic exposure has been associated with low birth weight in several studies (Claus Henn et al., 2016; Hopenhayn et al., 2003; Rahman et al., 2009), other studies have reported mixed findings, including an increase in birth length (Gilbert-Diamond et al., 2016) or no association (Kwok et al., 2006; Myers et al., 2010).
An understanding of the effects of chronic low-level arsenic exposure is also important due to ongoing exposure through water and food sources (Gilbert-Diamond et al., 2011; Jackson et al., 2012; Meacher et al., 2002). To clarify the relationship between low-level arsenic exposure and fetal growth, we analyzed data from a longitudinal birth cohort study in Mexico City. We investigated the associations of maternal blood arsenic levels during the second trimester, third trimester, and at delivery, as well as infant cord blood arsenic levels, with birth weight-for-gestational age. We hypothesized that increased blood arsenic levels would be associated with a higher risk of SGA.
METHODS
We analyzed data and samples from participants of a prospective birth cohort study, Programming Research in Obesity, GRowth, Environment and Social Stressors (PROGRESS) in Mexico City, which enrolled pregnant women between 2007 and 2011. Details of enrollment are published elsewhere (Braun et al., 2014; Burris et al., 2013). Briefly, all study participants received health insurance and prenatal care through the Mexican Social Security System (IMSS). Eligibility requirements included singleton pregnancy, less than 20 weeks gestation at the time of enrollment, expectation of living in Mexico City for the following three years, and no chronic medical conditions. The study was approved by institutional review boards at the participating institutions including Icahn School of Medicine (human subjects management #12–0075) and Instituto Nacional de Salud Pública (Project #560).
After participants provided written, informed consent, study staff collected demographic information including age, pre-pregnancy weight, tobacco smoke exposure, and parity through verbal questionnaires and interviews (Burris et al., 2015). Women self-reported pre-pregnancy weight and study staff measured their height at enrollment to calculate pre-pregnancy body mass index (BMI). Due to inconsistencies in self-reported pre-pregnancy weights and measured weights in the first visit, we fit a model including maternal height, age, measured weight at the first visit (and the duration of pregnancy at time of that weight), self-reported pre-pregnancy weight, socioeconomic position, and education to predict the “true” pre-pregnancy weight. We used 10-fold cross validation and then evaluated the performance of the model using the 87 women who had clinically measured weights within 20 days of the last menstrual period (LMP); the model performed well with a root mean squared error of 3.21 kg. Parity was determined based on the number of prior liveborn infants. Socioeconomic position was assessed using 13 questions related to the characteristics of the household and assets which classifies families into six socioeconomic levels; we collapsed these into three levels as we have in prior analyses (Carrasco, 2002; Sanders et al., 2018). Women self-reported smoking during pregnancy, and environmental tobacco smoke exposure was defined as a positive report of having household members who smoke inside or outside the home during the second trimester.
To investigate whether dietary intake might be a source of arsenic we explored data from the subset of women (n=139) who completed a one week recall food frequency questionnaire (FFQ) during pregnancy (n=119 in the third trimester, n=20 in the second trimester). The FFQ was relevant to the Mexican population and administered by qualified study staff (Hernández-Avila et al., 1998). The FFQ included 116 foods commonly consumed and had 10 frequency levels of consumption (Malin et al., 2018). We converted these values to grams/day for analysis and because the distribution was not normal we calculated Spearman correlation coefficients between intake and arsenic levels among the 120 women with both diet and arsenic data. We also categorized women into two groups, those who consumed seafood and those who did not to determine if the socioeconomic position of women and the birth weight-for-gestational age of infants differed by seafood intake. We analyzed foods that commonly contain arsenic: seafood (clams, tuna and sardines, fresh fish), rice, spinach, and juice (Schoof et al., 1999).
Gestational age was determined based on LMP and a standard physical examination to determine gestational age at birth that includes assessment of nipple formation, skin texture, ear form, breast size, plantar creases, scarf sign (flexibility at the shoulder), and head lag (Capurro et al., 1978). If the two assessments of gestational age differed by more than three weeks, the physical examination was used. Study staff recorded infant sex at delivery. Birth weight was obtained from the infant’s hospital chart. Birth weight-for-gestational age z-scores were calculated using the international reference developed by Fenton and Kim (Fenton and Kim, 2013). We categorized SGA as birth weight-for-gestational age below the 10th percentile, appropriate-for-gestational age (AGA) between the 10th and 90th percentiles, and LGA above the 90th percentile.
Blood was collected from women during the second trimester, third trimester, and from both women and infant cord at delivery. Blood was analyzed for total arsenic concentration at the Senator Frank R Lautenberg Environmental Health Science Laboratory at Icahn School of Medicine at Mount Sinai (New York, NY). Venous whole blood was collected in trace element free tubes, briefly refrigerated at 2–6°C, and subsequently frozen at −20°C until analyzed (Braun et al., 2014). For analysis, blood was thawed and then one milliliter of whole blood was digested in two milliliters of ultra-pure concentrated nitric acid for 48 hours at room temperature and then samples were further digested with one milliliter of 30% ultra-pure nitric acid for another 48 hours. Samples were then diluted to 10 ml with deionized water. All sample handling was performed in an ISO Class 5 laminar flow clean hood in the ISO Class 6 clean room. Digested samples were analyzed using external calibration using the Agilent 8800 ICP Triple Quad (ICP-QQQ) in MS/MS mode (ICP-MS). Arsenic was measured using an oxygen reaction gas to remove interferences. Tellurium was used as the internal standard to correct for the differences of sample introduction, ionization in the plasma and reaction rates in the reaction cell.
Quality control (QC) and quality assurance procedures included analyses of calibration verification standards, continuous calibration verification standards, procedural blanks, duplicates, spiked samples, Seronorm trace elements in whole blood L-3 (Billingstad, Norway), National Institute of Standard and Technology Standard Reference Material 1640a (trace elements in natural water, Gaithersburg, MD) and in-house blood (pooled digested blood sample) to monitor the accuracy, recovery rates and reproducibility of the procedure for each analytic batch. Lab recovery rates for QC standards and spiked samples with this method are 90 to 110% and precision (given as %RSD) is <5%. The limit of detection for this procedure is 0.2 μg/L. For analysis, when values were below this limit, we used the instrument-assigned value. No samples were below the limit of detection for the second trimester. One sample was below the limit of detection for the third trimester (0.17 μg/L). Four maternal samples were below the limit of detection at the time of delivery (range 0.04 – 0.18 μg/L). There were six infant cord blood samples below the limit of detection (range 0.04 – 0.19 μg/L). One outlying concentration of 314.2 μg/L from a woman at delivery was excluded because it was not credible, given that her level was much lower during the third trimester (0.23 μg/L) and in the concurrent infant cord blood (0.61 μg/L). Further evaluation of log-transformed maternal blood arsenic levels revealed that all values were within five standard deviations of the mean and thus were included in the analysis.
Statistical analyses included examination of correlations across time points of each of the blood arsenic levels as well as univariate and bivariate analyses of arsenic and participant characteristics. Given the positively skewed distribution of blood arsenic levels, we used natural log transformed values for all models. We performed unadjusted and adjusted multinomial logistic regression models to determine whether arsenic levels were associated with the odds of SGA or LGA compared to AGA. We chose a priori to adjust models for variables known to be associated with fetal growth in the literature, including maternal age at enrollment, pre-pregnancy BMI, socioeconomic position, prenatal environmental tobacco smoke exposure, parity and infant sex. We repeated the analysis excluding infants whose gestational age was reassigned with the physical examination assessment (n=28) to evaluate whether reassignment affected our findings. We also performed a sensitivity analysis excluding the 24 infants < 34 weeks of gestation and the two infants ≥ 42 weeks of gestation. Analyses were carried out using R (version: 3.5.1) and SAS 9.4 (Cary, NC).
RESULTS
Demographics of the 730 mother-infant pairs from the PROGRESS study included in the primary analysis are presented in Table 1. Mean maternal age at enrollment was 28 years and ranged from 18 to 44 years. The average birth weight was 3.0 kg and ranged from 0.6 kg to 4.6 kg; the average gestational age was 38 weeks and ranged from 24 weeks to 43 weeks. There were 130 SGA infants (18%) and 22 LGA infants (3%). Half of the women (51%) were within the lowest socioeconomic position category and one third (29.8%) reported tobacco smoke exposure in the home. Four women reported smoking during pregnancy.
Table 1.
Characteristics of PROGRESS birth cohort participants (Mexican mother-infant pairs) with arsenic levels from maternal blood samples at delivery, n=730, Mexico City.
| Mean | SD | Range | |
| Maternal age (years) | 27.8 | 5.4 | 18, 44 |
| Pre-pregnancy BMI (kg/m2) | 26.4 | 4.2 | 17.1, 43.5 |
| Birth weight (kg) | 3.05 | 0.48 | 0.63, 4.63 |
| Gestational age (weeks) | 38.3 | 1.9 | 24, 43 |
| Birth weight-for-gestational age z score | −0.46 | 0.94 | −5.7, 3.2 |
| n | % | ||
| Small-for-gestational age (SGA) | 130 | 17.8 | |
| Large-for-gestational age (LGA) | 22 | 3.0 | |
| Nulliparous | 326 | 44.7 | |
| Male infant | 393 | 53.8 | |
| Socioeconomic position | |||
| Low | 375 | 51.4 | |
| Medium | 268 | 36.7 | |
| High | 87 | 11.9 | |
| Environmental tobacco smoke exposure^ | 217 | 29.8 |
Missing environmental tobacco smoke exposure data for 3 participants.
BMI, body mass index
Median (IQR) blood arsenic levels were: 0.72 (0.33) μg/L for maternal second trimester, 0.75 (0.41) μg/L for maternal third trimester, 0.85 (0.70) μg/L for maternal at delivery, and 0.78 (0.65) μg/L for infant cord blood. For the 730 mother-infant pairs with maternal blood arsenic levels at delivery, levels were also available for 693 (95%) in the second trimester, 596 (92%) in the third trimester, and 487 (67%) with infant cord blood. The only significant difference we found between women with and without blood arsenic level at delivery information was that more women were exposed to environmental tobacco smoke in the group without arsenic level information (Supplemental Table 1). Maternal blood arsenic levels at delivery were correlated with infant cord blood arsenic levels (spearman r = 0.65, p < 0.0001). Maternal blood arsenic levels at delivery were also weakly correlated with levels at the second trimester (r = 0.23, p < 0.0001) and third trimester (r = 0.22, p < 0.0001) (Supplemental Table 2).
Dietary intake data are presented in Supplemental Table 3. Maternal blood arsenic levels at delivery were weakly correlated with seafood (Spearman r = 0.25, p < 0.01) and fresh fish (Spearman r = 0.20, p < 0.05) consumption during pregnancy (Supplemental Table 4). We did not detect significant differences between maternal arsenic levels at delivery among women who reported seafood consumption (median (IQR): 0.83 (0.58) μg/L) compared to women with no seafood consumption (median (IQR): 0.67 (0.31) μg/L) (t-test of log-transformed arsenic values p= 0.25).
Maternal blood arsenic levels at delivery stratified by participant characteristics are presented in Table 2. Only socioeconomic position was associated with arsenic levels (p= 0.02). Median (IQR) maternal blood arsenic levels at delivery for low, medium and high socioeconomic position were 0.86 (0.67) μg/L, 0.80 (0.60) μg/L and 0.99 (1.12) μg/L, respectively. Socioeconomic position was not associated with birth weight-for-gestational age categories (Supplemental Table 5). We did not detect an association between socioeconomic position and seafood consumption (p= 0.15, data not shown). Nor did we detect an association between seafood consumption and birth weight-for-gestational age categories in the subset of participants who filled out the FFQ (p= 0.40) (Supplemental Table 6).
Table 2.
Maternal arsenic levels (μg/L) across participant characteristics.
| Median | IQR | Range | p-value^ | |
|---|---|---|---|---|
| Maternal age (years) | ||||
| < 25 | 0.82 | 0.69 | (0.22, 12.04) | 0.54 |
| 25 – <35 | 0.85 | 0.76 | (0.04, 19.39) | |
| >= 35 | 0.80 | 0.60 | (0.07, 8.59) | |
| Pre-pregnancy BMI (kg/m2) | ||||
| < 25 | 0.83 | 0.69 | (0.18, 19.39) | 0.46 |
| 25 – <30 | 0.87 | 0.71 | (0.04, 7.73) | |
| >= 30 | 0.80 | 0.67 | (0.07, 10.72) | |
| Parity | 0.79 | |||
| 0 | 0.81 | 0.72 | (0.07, 12.04) | |
| > 0 | 0.86 | 0.69 | (0.04, 19.39) | |
| Infant sex | ||||
| Male | 0.86 | 0.63 | (0.04, 19.39) | 0.88 |
| Female | 0.81 | 0.77 | (0.18, 8.70) | |
| Socioeconomic position | ||||
| Low | 0.86 | 0.67 | (0.40, 10.51) | 0.02 |
| Medium | 0.80 | 0.60 | (0.07, 19.39) | |
| High | 0.99 | 1.12 | (0.22, 12.04) | |
| Environmental tobacco smoke | ||||
| No smokers in the home | 0.85 | 0.69 | (0.07, 19.39) | 0.91 |
| At least 1 smoker in the home | 0.82 | 0.73 | (0.04, 10.51) |
p value is from ANOVA or t-test of the log-transformed values.
BMI, body mass index
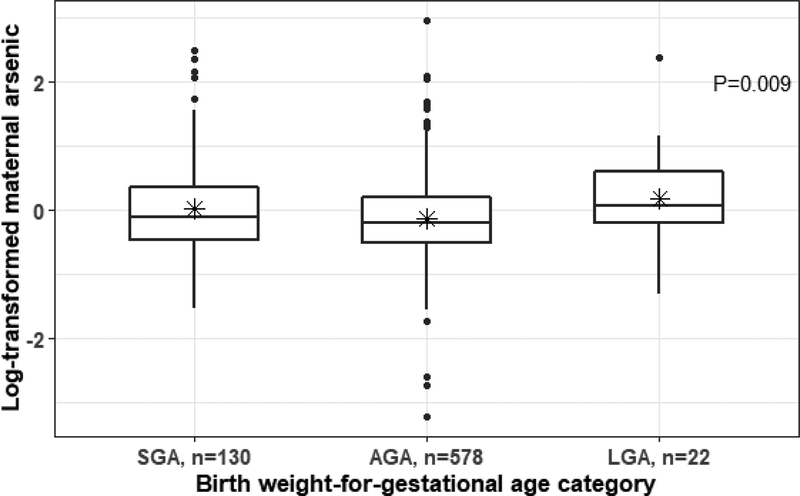
Maternal blood arsenic levels at delivery were significantly associated with birth weight-for-gestational age categories; specifically, mothers of SGA and LGA infants had higher arsenic levels than women who delivered AGA infants (Figure 1). In unadjusted models, per increment of log-transformed maternal blood arsenic levels at delivery, there were elevated odds of SGA (OR=1.40; 95% CI: 1.06–1.86) and LGA (OR=1.90; 95% CI: 1.08–3.34) compared with AGA (Table 3). In models adjusting for maternal age, BMI, socioeconomic position, environmental tobacco smoke exposure, parity and infant sex, maternal blood arsenic levels at delivery remained significantly associated with increased odds of SGA and LGA compared to AGA. Per increment of log-transformed maternal blood arsenic levels at delivery, we observed higher odds of SGA (OR=1.44; 95% CI: 1.08–1.93) and LGA (OR=2.03; 95% CI: 1.12–3.67). In models excluding the 28 infants whose gestational age was reassigned due to physical examination assessment that differed by more than three weeks from the LMP, results were similar. There were higher odds of SGA (OR=1.40, 95% CI: 1.04–1.89) and LGA (OR=2.02, 95% CI: 1.12–3.64). Our results were similar when we restricted the 699 infants that were ≥ 34 weeks and < 42 weeks of gestation (adjusted OR for SGA: 1.37 (1.02, 1.86); adjusted OR for LGA: 1.99 (1.08, 3.67)).
Figure 1.
Log-transformed maternal blood arsenic levels at delivery (μg/L prior to log transformation) stratified by birth weight-for-gestational age categories. P value is from ANOVA. * = mean; horizontal line= median. SGA, small-for-gestational age; AGA, appropriate-for-gestational age; LGA, large-for-gestational age
Table 3.
Odds ratios per unit of log-transformed maternal blood arsenic level and birth weight-for-gestational age categories in the PROGRESS cohort, Mexico City.
| SGA§ | LGA§ | |||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| 2nd trimester blood arsenic | ||||
| Unadjusted (n= 157 SGA, 718 AGA, 25 LGA) | 0.86 | (0.57–1.29) | 1.00 | (0.41–2.42) |
| Adjusted^ (n= 155 SGA, 714 AGA, 24 LGA) | 0.83 | (0.55–1.25) | 1.18 | (0.49–2.84) |
| 3rd trimester blood arsenic | ||||
| Unadjusted (n= 129 SGA, 599 AGA, 22 LGA) | 1.01 | (0.70–1.47) | 1.42 | (0.67–3.04) |
| Adjusted^ (n= 128 SGA, 595 AGA, 21 LGA) | 1.04 | (0.72–1.51) | 1.46 | (0.67–3.12) |
| Maternal blood arsenic at delivery | ||||
| Unadjusted (n= 130 SGA, 578 AGA, 22 LGA) | 1.40 | (1.06–1.86) | 1.90 | (1.08–3.34) |
| Adjusted^ (n= 129 SGA, 576 AGA, 21 LGA) | 1.44 | (1.08–1.93) | 2.03 | (1.12–3.67) |
| Infant cord blood arsenic | ||||
| Unadjusted (n= 95 SGA, 402 AGA, 14 LGA) | 1.30 | (0.95–1.78) | 1.93 | (0.99–3.78) |
| Adjusted^ (n=94 SGA, 402 AGA, 14 LGA) | 1.32 | (0.96–1.82) | 2.01 | (0.98–4.11) |
Multinomial regression with AGA as reference group.
Adjusted for maternal age, body mass index, socioeconomic position, environmental tobacco smoke, parity and infant sex.
SGA, small-for-gestational age; AGA, appropriate-for-gestational age; LGA, large-for-gestational age.
Although not significant, we did observe similar results between infant cord blood arsenic levels and odds of SGA (OR=1.32; 95% CI: 0.96–1.82) and LGA (OR=2.01; 95% CI: 0.98–4.11), respectively. We did not detect significant associations between second or third trimester arsenic levels and birth weight-for-gestational age categories (Table 3). However, we did observe a trend toward higher odds of LGA with higher third trimester levels (adj. OR=1.46, 95% CI: 0.67–3.12).
DISCUSSION
We found that higher blood arsenic levels were associated with increased odds of both SGA and LGA, however, our study was limited by lack of arsenic speciation and small numbers of LGA infants. If future studies without such limitations confirm our findings, determining modifiable factors that can be mitigated, such as arsenic exposure, may be important for optimizing fetal growth to improve long-term health of children.
Arsenic exposure from drinking water is a global issue; with the most serious health effects in highly exposed populations such as those in Bangladesh and India (Petrusevski et al., 2007). Arsenic is also found globally in foods such as rice, flour, grape juice and spinach (Schoof et al., 1999). The mechanisms by which arsenic may affect fetal growth are unknown, but since arsenic may readily be transferred across the placenta to the fetus (Concha et al., 1998; Guan et al., 2012), several pathways have been proposed. Altered expression of AQP9 and ENPP2, genes involved in arsenic transport and regulation of angiogenesis, respectively (Fei et al., 2013), could lead to impaired fetal growth by dysregulation of adipose tissue growth. Infant cord blood leptin levels (Gossai et al., 2015) have been shown to be associated with prenatal arsenic levels; leptin is a hormone strongly associated with fetal growth (Karakosta et al., 2011; Mantzoros et al., 2009) by regulation of energy homeostasis and metabolism. Impaired immune function (Ahmed et al., 2011) and activation of pathways involving inflammation, cell proliferation, stress and apoptosis (Fry et al., 2007) could also account for the association of blood arsenic level with SGA. Mechanisms connecting the association of maternal blood arsenic levels with LGA are less clear. Studies have linked arsenic exposure to an increased risk of impaired glucose tolerance during pregnancy (Ettinger et al., 2009) and type 2 diabetes in adults (Navas-Acien et al., 2008). This could contribute to an association between maternal arsenic exposure and increased risk of LGA because it has been shown that maternal hyperglycemia increases fetal insulin, stimulating growth (Silverman et al., 1991). We do not have data on impaired glucose tolerance during pregnancy in our study so could not evaluate potential confounding or effect modification by this variable. Our results suggest that more than one mechanism is operational in this study as both LGA and SGA were associated with arsenic exposure. Unmeasured factors such as complex mixtures of chemicals, or genetic susceptibility may be driving which of the mechanisms is more active at the individual level.
To our knowledge, our study is the largest to date that investigates fetal growth and arsenic exposure using blood arsenic levels. However, previous studies have examined blood arsenic levels and birth outcomes (Claus Henn et al., 2016; Guan et al., 2012; Hu et al., 2015; Luo et al., 2017; Remy et al., 2014; Zhong et al., 2019), and others have done the same with arsenic levels measured in urine (Gilbert-Diamond et al., 2016; Laine Jessica E. et al., 2015; Rahman et al., 2009) and drinking water (Hopenhayn et al., 2003; Kwok et al., 2006; Myers et al., 2010). Our finding of an association between arsenic exposure and SGA largely agrees with those of prior studies using blood arsenic levels. In a prospective birth cohort study of 622 mother-infant pairs in Oklahoma, Claus Henn et al. found maternal blood arsenic level was negatively associated with fetal growth (Claus Henn et al., 2016). Similarly, a study in Belgium showed that increased cord blood arsenic levels were associated with reduced birth weight and a higher risk of SGA (Remy et al., 2014). In a cross-sectional study of 125 mother-infant pairs in Dailan, China, Guan et al. found the same negative relationship between maternal blood arsenic levels and birth weight and length (Guan et al., 2012). Of note, unlike our study, none of these studies found higher odds of LGA associated with arsenic exposure. Nonetheless, if replicated, our finding is important because LGA is also associated with adverse health outcomes including birth trauma and metabolic disturbances. One explanation for the association of higher arsenic level and higher risk of LGA could be increased dietary intake of food, especially food containing arsenic such as rice, which could also lead to increased fetal growth. In this study, we only had access to dietary intakes for a small subset of women during pregnancy (n=139) and further work would be required to determine if dietary sources were responsible for the association between arsenic and LGA.
With respect to studies of urine arsenic levels (Gilbert-Diamond et al., 2016; Laine Jessica E. et al., 2015; Rahman et al., 2009) and well water arsenic levels (Hopenhayn et al., 2003; Kwok et al., 2006; Myers et al., 2010), results are mixed. A study of 1,578 mother-infant pairs in Bangladesh (Rahman et al., 2009) found a negative association between arsenic exposure and birth weight when restricted to women with low-levels of urinary arsenic (<100 μg/L in urine) but did not detect associations among women with high levels (≥100 μg/L in urine). A study of 200 mother-infant pairs in Mexico (Laine Jessica E. et al., 2015) found that inefficient metabolism of inorganic arsenic, as shown by high urinary proportions of monomethylated arsenicals and high ratios of monomethylated to dimethylated arsenicals, were associated with lower birth weight. A study of 706 mother-infant pairs exposed to low levels of arsenic in New Hampshire (Gilbert-Diamond et al., 2016) found that increased total urinary arsenic concentration was associated with lower ponderal index for infants with overweight/obese mothers, decreased birth weight in infant females with overweight/obese mothers, and increased birth length in infant males. Studies that relied on drinking water arsenic levels in varying regions found associations between arsenic exposure and birth defects (Kwok et al., 2006) and reduced birth weight (Hopenhayn et al., 2003), although another study in Inner Mongolia, China found no association (Myers et al., 2010).
Compared to highly exposed populations, our study had low blood arsenic levels (median maternal levels across time points: 0.72–0.85 μg/L; median infant cord blood: 0.78 μg/L). However, our levels are comparable to prior studies in the United States and Europe including the aforementioned study in Oklahoma (median maternal blood: 1.4 μg/L; median infant cord blood: 2.4 μg/L) (Claus Henn et al., 2016), North Carolina (mean maternal blood: 0.44 μg/L) (Sanders et al., 2012) and Belgium (mean infant cord blood: 0.56 μg/L) (Remy et al., 2014). This suggests that our findings of low-level chronic arsenic exposure are relevant to populations with similar arsenic exposure. Further, while our levels were lower than other areas in the world, our findings with respect to impaired fetal growth were consistent with results observed in Bangladesh (Rahman et al., 2009) and Chile (Hopenhayn et al., 2003), as well as China (median maternal blood: 3.7 μg/L; median infant cord blood: 5.3 μg/L) (Guan et al., 2012), suggesting that arsenic may play a role in fetal growth across the exposure spectrum.
Our findings should be interpreted in the context of our study’s limitations. The association of arsenic exposure and higher odds of LGA may be spurious due to low numbers of LGA infants (n=22), and thus requires replication. We did not have access to urine, nor did we have information regarding arsenic speciation for this pregnancy cohort. Since the half-life of blood arsenic is short (Pomroy et al., 1980), urine is often preferred so as to not miss associations (Type II error). However, blood arsenic would not be expected to produce a false positive association (Type I error). If indeed organic arsenic, thought to be less toxic (Shi et al., 2004), was associated with fetal growth, then the association between arsenic and birth weight-for-gestational age may be more likely to be due to confounding variables such as dietary intake than due to a causal effect of arsenic. However, in the small subset of women who did complete the FFQ during pregnancy, seafood consumption was weakly associated with arsenic, but it was not associated with birth weight-for-gestational age categories, making confounding less likely. The use of blood as a biomarker of arsenic exposure may be imperfect due to the lack of speciation characterization (Hughes, 2006) and its short half-life (Pomroy et al., 1980), thereby increasing the risk of misclassification. Indeed, one possible explanation for the lack of correlation of blood arsenic levels across time points observed in our study from the second trimester, third trimester, and at delivery could be the short half-life of arsenic in blood. However, it is reassuring that maternal and infant cord blood arsenic levels at delivery were more highly correlated (spearman r = 0.65, p < 0.0001), supporting that blood arsenic is readily transferred from mother to fetus. We measured total blood arsenic, which includes inorganic and organic species and does not distinguish between metabolized forms that may vary in their toxicity. The metabolic pathway of inorganic arsenic involves sequential reduction followed by oxidative methylation, the intermediates in this pathway could result in variable biological actions (Thomas et al., 2001). Although blood arsenic concentration measures only total arsenic, new studies suggest that forms of organic and inorganic arsenic previously thought to be nontoxic may undergo biotransformation resulting in a more toxic compound (Molin et al., 2015).
We did not have access to data regarding sources of arsenic exposure. Although some foods are known to be a common source of arsenic exposure, we were unable to include foods as primary covariates in our analysis because only 139 women completed the FFQ during pregnancy (20 SGA, 116 AGA, 3 LGA). Additionally, interpretation of this finding was also limited by the low number of LGA infants among women with FFQ data, its short one week recall and its completion by women only once during their second or third trimester. This may not be indicative of each woman’s diet throughout pregnancy or close to delivery, the time point which we compared to blood arsenic levels. Another limitation to our study is our use of LMP recall as a gestational age assessment due to the lack of ultrasound data on all subjects in our cohort which could result in inaccurate gestational ages. However, we confirmed gestational age with a physical examination assessment and our findings were unchanged in a sensitivity analysis excluding infants whose gestational age was reassigned based on physical examination.
Our study also has several strengths. This is a large longitudinal study with 730 mother-infant pairs within the well characterized, established PROGRESS cohort. By performing our study in PROGRESS, we leveraged the resources of a rich cohort with prospectively collected variables including four measurements of blood arsenic levels at three time points. Our finding of a stronger association between maternal blood arsenic levels and birth outcomes compared to cord blood arsenic levels is consistent with other studies that also considered both biomarkers (Claus Henn et al., 2016; Guan et al., 2012; Xu et al., 2011), indicating that maternal blood arsenic may be a more informative biomarker of perinatal exposure (and thus fetal exposure) than cord blood arsenic. Alternatively, we may have simply been underpowered to detect an association between cord blood arsenic and birth weight-for-gestational age categories because fewer infants had cord blood available for analysis. This is an ethnically and socioeconomically homogeneous cohort of Mexican women and infants; therefore, we expect little residual or unmeasured confounding and high internal validity.
In conclusion, we found arsenic exposure to be associated with abnormal fetal growth in a Mexico City cohort of mothers and infants. While limited by lack of arsenic speciation, small numbers of LGA infants, and the possibility of confounding by dietary intake, this study contributes to the growing body of literature suggesting a contribution of arsenic exposure to adverse birth outcomes. If replicated, our finding that blood arsenic level may be associated with higher odds of both SGA and LGA has important implications because both can result in adverse health outcomes for infants and mothers including hypoglycemia, perinatal asphyxia, poor neurodevelopmental outcomes, obesity, and labor complications. Our results support further study into the importance of reducing maternal exposure to arsenic as well as investigation into the mechanisms and impact of maternal arsenic exposure on fetal growth.
Supplementary Material
Highlights:
Higher maternal blood arsenic at delivery was associated with small-for-gestational age (SGA) and large-for-gestational age (LGA).
Arsenic earlier in pregnancy was not significantly associated with SGA or LGA.
Findings should be interpreted with caution given lack of arsenic speciation and small numbers of LGA infants.
Acknowledgements:
We would like to acknowledge the PROGRESS study participants and field staff for their generosity and tireless efforts. The authors thank the American British Cowdray Hospital for providing part of the research facilities used for this research.
Funding sources: NIH: K23 K23ES022242; R00ES027508; R01ES013744; R01ES014930; P30ES023515; R24ES028522; R25ES021649; P30ES013508.
The IRBs of the participating institutions approved this study: Icahn School of Medicine human subject’s management #12–00751 and Instituto Nacional de Salud Pública project #560
Abbreviations:
- SGA
small for gestational age
- AGA
appropriate for gestational age
- LGA
large for gestational age
- LMP
last menstrual period
- PROGRESS
Programing Research in Obesity
- GRowth
Environmental and Social Stressors
- QC
quality control
- RSD
relative standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References:
- Ahmed S, Khoda SM, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekström E-C, Vahter M, Raqib R, 2011. Arsenic-Associated Oxidative Stress, Inflammation, and Immune Disruption in Human Placenta and Cord Blood. Environ. Health Perspect. 119, 258–264. 10.1289/ehp.1002086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, 1997. Fetal nutrition and cardiovascular disease in later life. Br. Med. Bull 53, 96–108. 10.1093/oxfordjournals.bmb.a011609 [DOI] [PubMed] [Google Scholar]
- Barker DJP, Godfrey KM, Gluckman PD, Harding JE, Owens JA, Robinson JS, 1993. Fetal nutrition and cardiovascular disease in adult life. The Lancet, Originally published as Volume 1, Issue 8850 341, 938–941. 10.1016/0140-6736(93)91224-A [DOI] [PubMed] [Google Scholar]
- Bobak M, 2000. Outdoor air pollution, low birth weight, and prematurity. Environ. Health Perspect. 108, 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo y Ortiz M, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM, 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ. Health 13, 50 10.1186/1476-069X-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Baccarelli AA, Byun H-M, Cantoral A, Just AC, Pantic I, Solano-Gonzalez M, Svensson K, Tamayo y Ortiz M, Zhao Y, Wright RO, Téllez-Rojo MM, 2015. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics 10, 913–921. 10.1080/15592294.2015.1078963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Braun JM, Byun H-M, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM, 2013. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 5, 271–281. 10.2217/epi.13.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R, 1978. A simplified method for diagnosis of gestational age in the newborn infant. J. Pediatr 93, 120–122. 10.1016/S0022-3476(78)80621-0 [DOI] [PubMed] [Google Scholar]
- Carrasco AV, 2002. The amai system of classifying households by socio-economic level: The experience of mexico and its comparison with brazil and argentina. ESOMAR. [Google Scholar]
- Claus Henn B, Ettinger Adrienne S, Hopkins Marianne R, Jim Rebecca, Amarasiriwardena Chitra, Christiani David C., Coull Brent A., Bellinger David C., Wright Robert O., 2016. Prenatal Arsenic Exposure and Birth Outcomes among a Population Residing near a Mining-Related Superfund Site. Environ. Health Perspect. 124, 1308–1315. 10.1289/ehp.1510070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD, 2006. Methylated Arsenicals: The Implications of Metabolism and Carcinogenicity Studies in Rodents to Human Risk Assessment. Crit. Rev. Toxicol. Boca Raton 36, 99–133. [DOI] [PubMed] [Google Scholar]
- Concha G, Vogler G, Lezcano D, Nermell B, Vahter M, 1998. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. Off. J. Soc. Toxicol 44, 185–190. 10.1006/toxs.1998.2486 [DOI] [PubMed] [Google Scholar]
- Das U (“Shonu”) G, Sysyn GD, 2004. Abnormal fetal growth: intrauterine growth retardation, small for gestational age, large for gestational age. Pediatr. Clin. North Am., Common Issues and Concerns in the Newborn Nursery, Part I 51, 639–654. 10.1016/j.pcl.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H, Wright RO, 2009. Maternal Arsenic Exposure and Impaired Glucose Tolerance during Pregnancy. Environ. Health Perspect. 117, 1059–1064. 10.1289/ehp0800533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, Marsit CJ, Karagas MR, Robbins DJ, 2013. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environ. Health 12, 58 10.1186/1476-069X-12-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Kim JH, 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD, 2007. Activation of Inflammation/NF-κB Signaling in Infants Born to Arsenic-Exposed Mothers. PLoS Genet. 3 10.1371/journal.pgen.0030207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR, 2011. Rice consumption contributes to arsenic exposure in US women. Proc. Natl. Acad. Sci 201109127. 10.1073/pnas.1109127108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR, 2016. Relation between in Utero Arsenic Exposure and Birth Outcomes in a Cohort of Mothers and Their Newborns from New Hampshire. Environ. Health Perspect. 124 10.1289/ehp.1510065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossai A, Lesseur C, Farzan S, Marsit C, Karagas MR, Gilbert-Diamond D, 2015. ASSOCIATION BETWEEN MATERNAL URINARY ARSENIC SPECIES AND INFANT CORD BLOOD LEPTIN LEVELS IN A NEW HAMPSHIRE PREGNANCY COHORT. Environ. Res 136, 180–186. 10.1016/j.envres.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Piao F, Zhang X, Li X, Li Q, Xu L, Kitamura F, Yokoyama K, 2012. Prenatal Exposure to Arsenic and Its Effects on Fetal Development in the General Population of Dalian. Biol. Trace Elem. Res 149, 10–15. 10.1007/s12011-012-9396-7 [DOI] [PubMed] [Google Scholar]
- Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, Willett W, 1998. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Pública México 40, 133–140. 10.1590/S0036-36341998000200005 [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, Hertz-Picciotto I, 2003. Arsenic Exposure from Drinking Water and Birth Weight. Epidemiology 14, 593 10.1097/01.ede.0000072104.65240.69 [DOI] [PubMed] [Google Scholar]
- Hu X, Zheng T, Cheng Y, Holford T, Lin S, Leaderer B, Qiu J, Bassig BA, Shi K, Zhang Y, Niu J, Zhu Y, Li Y, Guo H, Chen Q, Zhang J, Xu S, Jin Y, 2015. Distributions of Heavy Metals in Maternal and Cord Blood and the Association with Infant Birth Weight in China. J. Reprod. Med 60, 21–29. [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, 2006. Biomarkers of Exposure: A Case Study with Inorganic Arsenic. Environ. Health Perspect. 114, 1790–1796. 10.1289/ehp.9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL, 2012. Arsenic, Organic Foods, and Brown Rice Syrup. Environ. Health Perspect. 120, 623–626. 10.1289/ehp.1104619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M, 2011. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatr. Perinat. Epidemiol 25, 150–163. 10.1111/j.1365-3016.2010.01163.x [DOI] [PubMed] [Google Scholar]
- Kwok RK, Kaufmann RB, Jakariya M, 2006. Arsenic in drinking-water and reproductive health outcomes: a study of participants in the Bangladesh Integrated Nutrition Programme. J. Health Popul. Nutr 24, 190–205. [PubMed] [Google Scholar]
- Laine Jessica E, Bailey Kathryn A, Rubio-Andrade Marisela, Olshan Andrew F., Smeester Lisa, Drobná Zuzana, Herring Amy H., Stýblo Miroslav, García-Vargas Gonzalo G., Fry Rebecca C., 2015. Maternal Arsenic Exposure, Arsenic Methylation Efficiency, and Birth Outcomes in the Biomarkers of Exposure to ARsenic (BEAR) Pregnancy Cohort in Mexico. Environ. Health Perspect. 123, 186–192. 10.1289/ehp.1307476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, McCullough LE, Tzeng J-Y, Darrah T, Vengosh A, Maguire RL, Maity A, Samuel-Hodge C, Murphy SK, Mendez MA, Hoyo C, 2017. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 17, 354 10.1186/s12889-017-4225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin AJ, Busgang SA, Cantoral AJ, Svensson K, Orjuela MA, Pantic I, Schnaas L, Oken E, Baccarelli AA, Téllez-Rojo MM, Wright RO, Gennings C, 2018. Quality of Prenatal and Childhood Diet Predicts Neurodevelopmental Outcomes among Children in Mexico City. Nutrients 10, 1093 10.3390/nu10081093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW, 2009. Cord Blood Leptin and Adiponectin as Predictors of Adiposity in Children at 3 Years of Age: A Prospective Cohort Study. Pediatrics 123, 682–689. 10.1542/peds.2008-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacher DM, Menzel DB, Dillencourt MD, Bic LF, Schoof RA, Yost LJ, Eickhoff JC, Farr CH, 2002. Estimation of Multimedia Inorganic Arsenic Intake in the U.S. Population. Hum. Ecol. Risk Assess. Int. J 8, 1697–1721. 10.1080/20028091057565 [DOI] [Google Scholar]
- Molin M, Ulven SM, Meltzer HM, Alexander J, 2015. Arsenic in the human food chain, biotransformation and toxicology – Review focusing on seafood arsenic. J. Trace Elem. Med. Biol., Special Section: 10th Nordic Symposium on Trace Elements in Human Health and Disease, edited by Jan Aaseth 31, 249–259. 10.1016/j.jtemb.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Murki S, 2014. Intrauterine Growth Retardation - A Review Article. J. Neonatal Biol 03 10.4172/2167-0897.1000135 [DOI] [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, Mendola P, 2010. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. J. Epidemiol. Community Health 64, 325–329. 10.1136/jech.2008.084392 [DOI] [PubMed] [Google Scholar]
- Najafian M, Cheraghi M, 2012. Occurrence of Fetal Macrosomia Rate and Its Maternal and Neonatal Complications: A 5-Year Cohort Study. ISRN Obstet. Gynecol. 2012. 10.5402/2012/353791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E, 2008. Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. JAMA 300, 814–822. 10.1001/jama.300.7.814 [DOI] [PubMed] [Google Scholar]
- Ng S-K, Olog A, Spinks AB, Cameron CM, Searle J, McClure RJ, 2010. Risk factors and obstetric complications of large for gestational age births with adjustments for community effects: results from a new cohort study. BMC Public Health 10, 460 10.1186/1471-2458-10-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotto EK, Kilbride HW, 2006. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol 49, 257–269. [DOI] [PubMed] [Google Scholar]
- Petrusevski B, Sharma S, Schippers J, Shordt K, 2007. Arsenic in Drinking Water.
- Pomroy C, Charbonneau SM, McCullough RS, Tam GKH, 1980. Human retention studies with 74As. Toxicol. Appl. Pharmacol 53, 550–556. 10.1016/0041-008X(80)90368-3 [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, Persson L-Å, Ekström E-C, 2009. Arsenic Exposure During Pregnancy and Size at Birth: A Prospective Cohort Study in Bangladesh. Am. J. Epidemiol 169, 304–312. 10.1093/aje/kwn332 [DOI] [PubMed] [Google Scholar]
- Remy S, Govarts E, Bruckers L, Paulussen M, Wens B, Hond ED, Nelen V, Baeyens W, van Larebeke N, Loots I, Sioen I, Schoeters G, 2014. Expression of the sFLT1 Gene in Cord Blood Cells Is Associated to Maternal Arsenic Exposure and Decreased Birth Weight. PLoS ONE 9 10.1371/journal.pone.0092677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik R, 2002. Intrauterine growth restriction. Obstet. Gynecol 99, 490–496. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Flood K, Chiang S, Herring AH, Wolf L, Fry RC, 2012. Towards Prenatal Biomonitoring in North Carolina: Assessing Arsenic, Cadmium, Mercury, and Lead Levels in Pregnant Women. PLOS ONE 7, e31354 10.1371/journal.pone.0031354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, Svensson K, Gennings C, Burris HH, Oken E, Amarasiriwardena C, Basnet P, Pizano-Zarate ML, Schnaas L, Tamayo-Ortiz M, Baccarelli AA, Satlin LM, Wright RO, Tellez-Rojo MM, 2018. Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ. Int 120, 464–471. 10.1016/j.envint.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellong K, Schulz S, Harder T, Plagemann A, 2012. Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS ONE 7 10.1371/journal.pone.0047776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, Menzel DB, 1999. A Market Basket Survey of Inorganic Arsenic in Food. Food Chem. Toxicol 37, 839–846. 10.1016/S0278-6915(99)00073-3 [DOI] [PubMed] [Google Scholar]
- Sharma D, Shastri S, Sharma P, 2016. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 10, 67–83. 10.4137/CMPed.S40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Shi X, Liu KJ, 2004. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem 255, 67–78. 10.1023/B:MCBI.0000007262.26044.e8 [DOI] [PubMed] [Google Scholar]
- Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE, 1991. Long-Term Prospective Evaluation of Offspring of Diabetic Mothers. Diabetes 40, 121–125. 10.2337/diab.40.2.S121 [DOI] [PubMed] [Google Scholar]
- Smedley PL, Kinniburgh DG, 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem 17, 517–568. 10.1016/S0883-2927(02)00018-5 [DOI] [Google Scholar]
- Thomas DJ, Styblo M, Lin S, 2001. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol 176, 127–144. 10.1006/taap.2001.9258 [DOI] [PubMed] [Google Scholar]
- Windham GC, Hopkins B, Fenster L, Swan SH, 2000. Prenatal Active or Passive Tobacco Smoke Exposure and the Risk of Preterm Delivery or Low Birth Weight. Epidemiology 11, 427–433. [DOI] [PubMed] [Google Scholar]
- Xu L, Yokoyama K, Tian Y, Piao F-Y, Kitamura F, Kida H, Wang P, 2011. Decrease in birth weight and gestational age by arsenic among the newborn in Shanghai, China. Nihon Koshu Eisei Zasshi Jpn. J. Public Health 58, 89–95. [PubMed] [Google Scholar]
- Zhong Q, Cui Y, Wu H, Niu Q, Lu X, Wang L, Huang F, 2019. Association of maternal arsenic exposure with birth size: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol 69, 129–136. 10.1016/j.etap.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO, 2009. Maternal Blood Manganese Levels and Infant Birth Weight. Epidemiol. Camb. Mass 20, 367–373. 10.1097/EDE.0b013e31819b93c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.