Abstract
Spasticity is a sensorimotor disorder widely recognized as one of the features that contribute to patients’ disability. Transcutaneous electric neural stimulation (TENS/SES) has been adopted in spasticity rehabilitation as an alternative to pharmacological agents. Although previous studies have reported clinical benefits of TENS/SES in relieving spasticity, there is no clarity on how and whether this therapeutic modality affects specific neural circuitries. Thus, this systematic review aimed to verify the efficacy of TENS/SES in the control of spasticity and its consequences in spinal and corticospinal excitability. This study was carried out according to PRISMA recommendations using SCOPUS, PubMed, BVS, Google Scholar and BASE databases screening, which provided 483 references. Six additional records were found from other sources. All these records were submitted to a filtering process following the eligibility criteria, and 44 studies were selected for further analysis. Ten were replicas. Consequently, 34 studies were read in full with the aim of checking their eligibility criterion, which resulted in 10 manuscripts for qualitative synthesis. Even though they evaluated the effects of TENS/SES both at the spinal and/or corticospinal levels, the electrophysiological results seem to be inconsistent, corroborating the lack of agreement between them and with clinical outcomes.
Keywords: Spasticity, Transcutaneous Electrical Neural Stimulation, TENS, Transcranial Magnetic Stimulation, Hoffmann Reflex
Introduction
Spasticity is a sensorimotor disorder observed in different clinical conditions such as spinal cord injury, stroke, and multiple sclerosis. Clinically, it is described as “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motoneuron syndrome (UMNS)”[1]. Since spasticity is widely recognized as one of the features that contribute to the patients’ disability of the flexor and extensor muscles of upper and lower limbs, many different therapeutic approaches are suggested to alleviate it.
Pharmacological agents are among the first-line in the treatment of spasticity, even though some authors[2,3,6-9] highlight the side-effects of these drugs, which are not well tolerated by many patients. On the other hand, peripheral electrical stimulation is a modality of treatment that has gained increased attention, mainly in the last three decades, as an alternative for improving the motor performance of spastic patients[10-17].
Briefly, electrical stimulation applied at the sensory or motor threshold is suggested to modulate neuronal activity at the supra or medular levels with “positive” – or “negative” – effects in spasticity and therefore in motor performance. The efficacy of this modality on the improvement of spasticity depends on the parameters of stimulation such as pulse width, intensity, frequency, stimulus location, application time, and pulse morphology[18,19].
Among these methodological approaches, the transcutaneous electric neural stimulation (TENS) has been widely used to treat a variety of painful conditions, and also for treating spasticity. TENS is characterized by the placement of surface electrodes on the skin, and the intensity set at a comfortable level, without evoking any muscle contraction, which contrasts to the Functional Electrical Stimulation (FES). As an alternative to TENS, other authors have named such modality as somatosensory electrical stimulation (SES).
Many authors have reported significant clinical benefits of TENS/SES in neurorehabilitation. For instance, Conforto et al.[20-23], Sullivan et al.[24] and Wu et al.[25] mentioned improvements in the functional capacity of stroke patients after being submitted to this therapy. More straightly related to spasticity, Levin and Hui-Chan[26] and Karakoyun et al.[27] reported benefits of TENS in chronic spastic stroke patients; Sivaramakrishnan et al.[11] and Goulet et al.[28] observed positive effects in spastic spinal cord injured patients. In contrast, Garcia et al.[18] did not find any contribution of SES on chronic spastic stroke patients. It is interesting to note that these studies also evaluated the effects of TENS/SES both at the spinal and corticospinal levels with inconclusive results concerning the agreement between such measures and the level of spasticity. Even though previous systematic reviews also report some benefits of low-intensity electrical stimulation in relieving spasticity[29,30], there is no clarity on how and whether this therapeutic modality affects the cortical and/or spinal excitability concurrently.
In contrast to clinical scales, cortical and/or spinal excitability, which are obtained from transcranial magnetic stimulation (TMS) or transcutaneous electrical stimulation (TES), are measurements that contribute objectively to evaluate how external stimuli modulate the central nervous system[31,32]. Therefore, since there is a lack of information concerning the effects of TENS/SES in relieving spasticity, the present systematic review aimed to verify the efficacy of this electrotherapeutic approach in the control of this sensorimotor disorder and its consequences in cortical and/or spinal excitability.
Materials and methods
This systematic review was carried out according to PRISMA recommendations[33] using SCOPUS, PubMed, BVS (Biblioteca Virtual em Saúde – Virtual Library in Health), Google Scholar and BASE databases from May 7th, 2018 to May 11th, 2018, and covered all the documents available on these sources. We used the following indexing and text terms separated by boolean operators: “peripheral electrical stimulation” OR “somatosensory electrical stimulation” OR “SES” OR “transcutaneous electrical neural stimulation” OR “TENS” AND “spasticity” AND “motor evoked response” OR “Hoffmann reflex” OR “H-reflex”.
The eligibility criteria were: (1) Electrical stimulation intensity applied from above the somatosensory to just below motor thresholds, i.e. without evoking any contraction and/or movements; (2) Subjects diagnosed with spasticity from any neurological disease; (3) Evaluation of spasticity level by means of qualitative (example: Ashworth scale) and/or quantitative (example: dynamometric systems) methods; (4) Adoption of methods of measurement of cortical and/or spinal excitability to evaluate TENS/SES effects; (5) Studies written in English. These studies were selected according to the titles and abstracts only by the present authors, the main limitation of this study. All the records had to meet the eligibility criteria. Full papers, abstracts, Master/Ph.D. theses as well as abstracts from conferences were considered for analysis. The studies that matched the criteria were subsequently examined for inclusion.
Results and discussion
The initial electronics search from SCOPUS (n=11), PubMed (n=8), BVS (n=61), Google Scholar (n=2) and BASE (n=401) databases provided 483 references. Six additional records were found from other sources. All these records were submitted to a filtering process following the eligibility criteria. After that, 44 studies were identified for further analysis. Since ten records were replicas, 34 studies were read in full with the purpose of checking each eligibility criteria, resulting in 10 manuscripts for qualitative synthesis (Figure 1).
Figure 1.
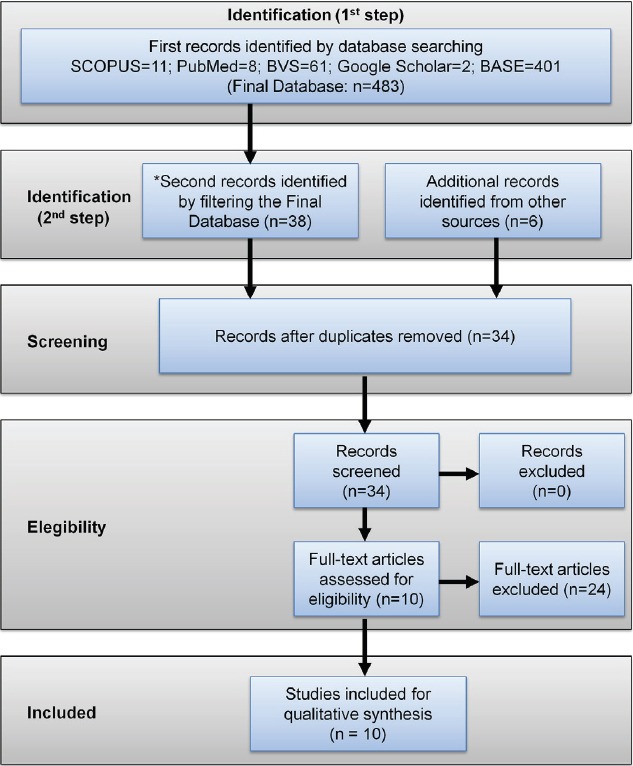
Diagram adopted from PRISMA recommendations illustrating the flow of information at each phase of this systematic review.
[Table 1] summarizes details of the process leading to ten studies that matched the inclusion criteria. The range date is from 1992 to 2018. Except the studies conducted by Joodaki et al.[34] and Ching-Chen et al.[37], which were respectively obtained in abstract and abstract proceedings forms, the remaining records were full articles published in scientific journals. Additionally, since the study conducted by van der Salm et al.[35] compared the effects of three different protocols of electrical stimulation, but based on two different intensities of stimulation (below, which is in accordance with the eligibility criterion “1”, and above the motor threshold), we decided to include their results reached only from TENS/SES applied below the motor threshold on review analysis.
Table 1.
Main characteristics of selected studies.
| Authors (Year) | Aim | Patients characteristics | Intervention | SES parameters | Number of sessions | Clinical and neurophysiological Evaluations | Results | Discussion and Conclusion |
|---|---|---|---|---|---|---|---|---|
| Levin and Hui-Chan (1992) | To investigate the effects of TENS over a period of 2–3 weeks on spasticity and their association with motor function and spinal excitability | 13 stroke patients divided in two groups (TENS: n=7; Placebo: n=6) | Electrodes applied over the common peroneal nerve, which supplied the muscles antagonistic to the spastic ones | Pulse width: 125 µs; Intensity: below the MT Frequency: 99 Hz Duration: 60’ | One single session/ weekday for 2–3 weeks | CSS, MVC for plantar and dorsi-flexion H-reflex, M-wave and H/M ratio, H-reflex during vibration, soleus stretch reflex (latency and onset angles) | ¯CSS (TENS group), MVC for plantar and dorsi-flexion H-reflex (N.S.), M-wave (N.S.) and H/M ratio (N.S.), ¯H-reflex during vibration, soleus stretch reflex (latency and onset angles) | TENS improved spasticity and dorsiflexion force after 2 weeks |
| Goulet et al. (1996) | To investigate the short-term effects of TENS on spasticity and its association with spinal excitability | 14 spinal cord injured survivors (13 ♂) ASIA scale: C-A | Electrodes applied over the common peroneal nerve, which supplied the muscles antagonistic to the spastic ones | Pulse width: 250 µs; Intensity: below the MT Frequency: 99 Hz Duration: 30’ | One single session of TENS | CSS (MAS, ATR and clonus) H-reflex, M-wave and and H/M ratio from triceps surae muscle | CSS (¯MAS and ¯ATR) H-reflex (N.S.), M-wave (N.S.) and H/M ratio (N.S.) | TENS improved spasticity but failed to modulate H-reflex in spinal cord-injured patients |
| Ching-Chen et al. (1998)* | To investigate the short- (10’) and long-term (24 h) effects of TENS on spasticity and its association with spinal excitability | 9 stroke survivors (8 ♂) and ↑8 months from the stroke onset | Electrodes applied over triceps surae muscle (active electrode on the muscle tendon junction and reference on distal end of achilles tendon) | Pulse width: 200 µs (bipolar); Intensity: below the MT Frequency: 20 Hz Duration: 20’ | One single session of TENS | MAS Fmax/Mmax ratio, H-reflex latency, H-reflex recovery curve before, 10’ and 24 h after TENS therapy | ¯MAS (10’) but N.S. at 24 h ¯Fmax/Mmax ratio (10’), ¯H-reflex recovery curve at 10’ but N.S. at 24 h | One single session of TENS was effective on improving spasticity although in the short-term |
| Joodaki et al. (2001)** | To investigate the effects of TENS on spinal excitability | 10 healthy subjects and 3 spastic stroke patients | Electrodes applied over the common peroneal nerve, which supplied the muscles antagonistic to the spastic ones | Pulse width: 250 µs; Intensity: below the MT Frequency: 99 Hz Duration: 30’ | One single session of TENS | Latencies and amplitudes of H-reflex and F-wave, and H/M and F/M ratios from soleus muscle | Latencies and ¯amplitudes of H-reflex and F-wave, and H/M and F/M ratios in both groups | One single session of TENS was effective on improving spasticity although in the short-term (10’) |
| Aydin et al. (2005) | To determine the short- and long-term effects of TENS on spasticity and compare with baclofen treatment | 21 spinal cord injury patients divided in two groups (baclofen: n=10, 10♀; TENS: n=11, 5♀) and 20 control subjects Asia score: D-A | Electrodes applied over tibial nerve, bilaterally, to involve gastrocnemius muscles | Pulse width: 100 ms (?) (biphasic); Intensity: below the MT Frequency: 100 Hz Duration: 15’ | 15 sessions for 15 days | SFS, ATR, clonus AS, FIM H-reflex, M-wave and H/M ratio from gastrocnemius muscle | ¯SFS, ¯AS, FIM Baclofen: ¯H-reflex amplitude and H/M amplitude (N. S.) TENS: ¯H-reflex amplitude and H/M amplitude (N. S.) | The efficacy of TENS on spasticity depends on repeated sessions |
| van der Salm et al. (2006) | To compare the effects of TENS applied of 3 different methods on spasticity and their association with spinal excitability | 10 spinal cord injury patients (8 ♂) and ↑6 months from the injury onset Spasticity level: 1–3 (MAS) | Electrodes on (1) tibialis anterior (antagonist), (2) triceps surae (agonist), and (3) dermatome (lateral side of the foot – S1) | Pulse width: (1, 2) 100 and (3) 300 µs; Intensity: (1, 2) above and below (3) the MT Frequency: 30 Hz Duration: 45’ | One single session of TENS for each protocol and in different days | MAS, Clonus Score, Stretch Reflex H-reflex, M-wave and H/M ratio from soleus muscle | ¯(2) MAS, Clonus Score (N.S.), ¯(1) Stretch Reflex (N.S.) H-reflex, (N.S.) M-wave and (N.S.) H/M ratio from soleus muscle | TENS applied over triceps surae (spastic muscles), even above MT, seemed to be effective according to MAS in contrast to TENS and applied over S1 dermatome (3) but below the MT |
| Martins et al. (2012) | To evaluate the effects of TENS and cryotherapy on spasticity and electrophysiological measures | 16 stroke survivors (6 ♂) and ↑6 months from the stroke onset Spasticity level: x̄ =1.93 (AS) | Electrodes on S1 and S2 dermatomes (gastrocnemius and soleus muscles) | Pulse width: 60 µs; Intensity: below the MT Frequency: 100 Hz Duration: 30’ | One single session for each therapy (TENS and cryotherapy) | MVC (+EMG) from tibialis anterior muscle H-reflex, H-reflex latency, M-wave and H/M ratio from soleus muscle | MAS, MVC (+EMG) from tibialis anterior muscle (N. S.) ¯H/M ratio | TENS lead to a lower reflex excitability |
| Karakoyun et al. (2015) | To evaluate the effects of TENS on spasticity and electrophysiological measures | Stroke survivors (15 ♂; 12♀) and ↑6 months from the stroke onset and control subjects (n=24) not paired by age Spasticity level: 1 (AS) | Electrodes on the tibialis nerve, between the muscle tendon and the medial malleolus | Pulse width: 100 ms (?); Intensity: below the MT Frequency: 50 Hz Duration: 30’ | One single session of TENS | AS, Brunnstrom stage H-reflex, M-wave and H/M ratio (slopes and amplitudes) from triceps surae muscles | ¯AS ¯M amplitude, H-reflex amplitude and slope, H/M amplitude and slope after TENS in patients | TENS lead to an improvement on spasticity and spinal reflexes |
| Garcia et al. (2016) | To evaluate the effects of TENS frequencies (3, 30, 150 and 300 Hz) in cortical and spinal excitability | Stroke survivors (4 ♂; 1♀) and ↑6 months from the stroke onset And control subjects (n=5) not paired by age Spasticity level: 1+ (MAS) | Cathode (proximal) and anode over the whole extension of the forearm flexor muscles | Pulse width: 500 µs; Intensity: just below the MT and kept during whole session Duration: 30’ | One single session at each TENS frequency although stroke patients were only submitted to 3 Hz | MAS and isokinetic passive wrist torque measurement TMS and H-reflex flexor (ipsi and contralateral) and extensor carpi radialis, and abductor pollicis brevis | No significant statistical differences for corticospinal excitability, H-reflex or passive wrist torque | None of frequencies were able to lead to carry over any effect in the central nervous system or spasticity |
| Peres et al. (2018) | To evaluate the effects of TENS at 3 Hz in the corticospinal excitability | Control group (n=5; 3 ♂) and stroke survivors (5 ♂) and ↑6 months from the stroke onset, paired by age Spasticity level: 1-1+ (MAS) | Cathode (proximal) and anode near the wrist, both over the forearm flexor muscles | Pulse width: 500 µs (monopolar); Intensity: just below the MT and kept during whole session Duration: 30’ Frequency: 3 Hz | One single session over the forearm spastic muscles and the dominant side for the control | MAS and isokinetic passive wrist torque measurement TMS from flexor and extensor carpi radialis muscles from the spastic (patients) and dominant side (control group) | Significant statistical differences (increase and decrease) for corticospinal excitability in 3 patients but no agreement with passive wrist torque, which also varies widely | Inconsistent SES effects in corticospinal excitability and wrist passive torque |
These studies were obtained in abstract and abstract proceedings forms, respectively. FAC: Functional Ambulation Categories; AS: Ashworth Scale; MAS: Modified Ashworth Scale; FIM: Functional Independence Measure; H-reflex: Hoffmann reflex; H/M: H-reflex/M wave ratio; MT: Motor threshold; TMS: Transcranial magnetic stimulation; SFS: spasm frequency scale; CSS: Clinical spasticity scores (Achilles tendon jerks, clonus and passive resistance to movement); ATR: Achilles tendon reflex; MVC: Maximal voluntary contraction; ASIA Scale: American Spinal Injury Association Impairment Scale; N.S.: Not statistically significant; ↓ parameter decreased; ↑ parameter increased
Population and intervention
According to our criteria, the selected studies covered only stroke (n=7) and spinal cord injured (n=3) patients. It is interesting to note that eight studies evaluated TENS/SES effects in lower leg muscles[26-28,34-38] in both groups of patients while the other two concerned the forearm flexors[18,39]. Five[26-28,34,38] from the whole applied TENS/SES over two nerves (common peroneal and tibialis), which supply the lower leg muscles. The other five[18,35,36,37,39] applied TENS/SES over spastic muscles and dermatome region. In these last cases, TENS/SES seemed not to be effective in relieving spasticity.
Ching-Chen et al.[37] succeed in relieving spasticity by applying an active electrode on the tendon junction of triceps surae muscle and the reference on the distal end of Achilles tendon, a remarkable protocol. We must also highlight the lack of studies giving attention to the spastic muscles concerning TENS/SES application and H-reflex from the forearm, presumably due to the misunderstanding that this neurophysiological measure can only be accurately recorded from soleus muscle[40]. The contrary seems to be true for corticospinal data recording since the lower limb representation in the primary motor cortex (M1) is much smaller and deep in the longitudinal cerebral fissure than that of the upper limb, thus offering methodological constraints to obtain motor evoked responses from TMS application.
Electrical stimulation: parameters of stimulation
There seem to be a consensus that[18,19,41,42] specific parameters of SES lead to different neurophysiological responses. Therefore, the variety of parameters presented by these studies could lead to different clinical effects and neurophysiological responses. For example, some studies have applied TENS/SES over muscles (agonists – spastic ones – or their antagonists) or the nerve supply of spastic muscles as well as previously mentioned. According to this review, the stimulation seemed to be more effective in alleviating spasticity when applied over the nerves that supply spastic muscles in comparison to other locations such as dermatomes – five out of the ten studies[26-28,34,38] positioned surface electrodes over these nerves and achieved positive results in relieving spasticity in the short- and long-term, although the H-reflex did not seem to be modulated in 3 out of 5 cases. Thus, TENS/SES applied over the nerve supply of spastic muscles seemed to relieve spasticity but failed to modulate the H-reflex in stroke and spinal cord-injured patients. These findings may suggest that stimulating nerve supplies can be suitable for the treatment of spasticity by TENS/SES.
We may hypothesize that delivering TENS/SES circumscribed to a restricted spot may be more selective in recruiting sensory nerves from a target muscle or muscle group than other skin locations such as the dermatome level, which may lead to lower recruitment of somatosensory receptors recruitment due to electrical current spread. However, these studies reported different effects on the H-reflex. Three of them[26,34,38] reported a decrease in H-reflex, in contrast to Karakoyun et al.[27] and Goulet et al.[28], which observed either an increase or no effect in H-reflex, respectively. Interestingly, only one study[37] succeeded on improving spasticity by applying TENS/SES on a different skin location than in nerve supply, even though van der Salm et al.[35] reached a positive clinical result applying electrical stimulation over the spastic muscle, but above the motor threshold, which is not recommended by other authors[43]. Moreover, Veldman et al.[42] argue that TENS/SES applied to a motor nerve might lead to more consistent responses in corticospinal excitability when compared to stimulating a motor point.
With regard to other parameters of stimulation, the studies which applied a narrow pulse width (≤125 µs[26]) and high frequencies (≥99 Hz[18,36,38]) were able to relieve spasticity accompanied by a decrease on H-reflex in comparison to those that used large pulse widths (250 µs[28]) and lower frequencies (50 Hz[27]), although observing distinct H-reflex responses (no change and an increase, respectively). Besides, it is important to highlight that Goulet et al.[28] studied spinal cord injured patients, which reinforce the existence of different mechanisms in the physiopathology of spasticity when compared to chronic stroke patients. The other authors varied more widely in the TENS/SES parameters adopted. Most reports applied TENS/SES pulse widths between 100 and 500 µs, although Karakoyun et al.[27] and Aydin et al.[38] reported, curiously, positive clinical and H-reflex modulation results with a pulse width set at 100 ms and frequencies at 50 and 100 Hz, respectively. However, it sounds utterly meaningless since, in both reports, the maximal frequency of stimulation could be only set at a maximum of 10 Hz due to this pulse width (100 ms). Therefore, we conjecture that these authors intended to mention “100 µs” instead of “100 ms” in their manuscript.
Concerning frequency, TENS/SES was applied with frequencies varying from 3 to 100 Hz. According to Levin and Hui-Chan[26] and Aydin et al.[38], high frequencies of stimulation should be preferred as they recruit larger diameter afferents. Besides, some studies have been addressed to evaluate whether the frequency of stimulation can switch particular neural circuits in spastic stroke patients[18,19]. While Garcia et al.[18] reported that TENS/SES set at 3 Hz did not relieve spasticity nor lead to any evoked cortical or spinal modulation, Koyama et al.[19] suggested that high frequencies (≥200 Hz) of stimulation can lead in turn to different responses on healthy subjects and stroke patients even though they stimulated above the motor threshold. Even so, Veldman et al.[42] reinforce the lack of studies that compare TENS/SES effects at different frequencies to determine how they can modulate spinal and/or supraspinal neural circuitries and their carry-over effects on motor performance.
There also seems to be no agreement about the time spent during stimulation. The minimum time spent on TENS/SES therapy was 15’[38] while the maximum was 60’[26]. Both studies reported improvement in spasticity and motor performance of spinal cord and chronic stroke patients, but Levin and Hui-Chan[26] did not observe any impact on H-reflex. Even so, they agreed that the maintenance of short-term effects of TENS/SES in spasticity depends on the frequency of sessions.
This study intended to review manuscripts that have applied TENS/SES intensities below the motor threshold. All the present studies determined the intensity of stimulation based on subjective parameters such as “volunteers’ reports”, for example. According to Veldman et al.[42], small step adjustments on the intensity of stimulation from the sensory to the motor levels can induce different modulation responses on the corticospinal pathway. They suggest that TENS/SES at the perceptual threshold cannot even lead to modulatory responses. Therefore, the lack of a reliable and objective method to better set the stimulation intensity can contribute negatively to the comparison of protocols adopted by these studies and their results as well.
Spasticity evaluation
Spasticity was evaluated by different qualitative and quantitative methodological approaches. Most authors used the Ashworth Scale[44] or its modified version[45], which is in agreement with the clinical routine, while other evaluated spasticity using functional scales, muscle force, and isokinetic dynamometers. Therapists usually assess spasticity using qualitative passive resistance measures. Even though widely accepted, these measures may be misleading into scoring the degree of a spastic muscle or muscle group, mainly due to their subjectivity, low resolution (i.e., very few scores) and, therefore, low reliability. These scales can mask particular evidence of TENS/SES effects mainly in chronic spastic patients who present increased muscle stiffness and normal stretch reflex[18,39], reinforcing the need of evaluating spasticity objectively, i.e. by dynamometers capable of detecting minimal changes in passive resistance, thus eliminating subjective aspects of the evaluation and offering higher resolution in measurements.
Cortical and spinal excitability
There seems to be no consensus on evaluating spasticity quantitatively[18,27,39]. Concerning the proposed mechanisms underlying this sensorimotor disorder (increase excitability in alpha motorneurons and fusimotor neurons due to loss supraspinal drives), H-reflex, F-wave, and motor potentials (MEP) evoked by TMS have been used to evaluate the central effects of TENS/SES. However, the electrophysiological results of TENS/SES in the treatment of spasticity are inconsistent in the literature, corroborating the lack of agreement between electrophysiological and clinical results[38].
Burke et al.[4] and Burke[40] highlighted the challenges of evaluating the excitability of a reflex circuit during movement and at rest. Additionally, these authors reinforce that even though some spinal circuits are altered, they are not straightforwardly related to the level of spasticity. It suggests therefore that the H-reflex may not offer suitable evidence of TENS/SES effects in spasticity. Accordingly, H-reflex and other measures (F-response and H/M ratio, for example) were herein inconsistent with the results obtained from spasticity assessments, which is corroborated by Levin and Hui-Chan[46]. Therefore, one may suppose that, based on the physiopathology of spasticity known currently, an increase in such measure may reflect an improvement in this sensorimotor disorder even in short-term.
Moreover, Karakoyun et al.[27] observed a decrease in spasticity with a parallel increase of H-reflex amplitude, which contrasts to other four studies[34,36-38] that reported a decline in this parameter. Aydin et al.[38] reinforce that H-reflex may not explain the underlying mechanisms of spasticity since supramedular circuitries contribute to its physiopathology, which must also be carefully taken into account. Thus, there seems to be no consensus and clarity on how to link and interpret the H-reflex to the level of spasticity.
On the other hand, Burke et al.[4] and Trompetto et al.[5] also discuss the importance of taking into account the abnormal supraspinal drives on the motoneuronal pool. They propose that the homosynaptic depression between the Ia afferent and the motoneuron, which is under supraspinal control from the dorsal, medial and vestibulospinal tracts, plays an essential role in fine-tuning the stretch reflex. This fine-tuning can be disrupted by central lesions and consequently leading to spasticity. Therefore, we may hypothesize that the corticospinal excitability evaluated by means TMS would provide additional information concerning the underlying mechanisms of spinal and supraspinal spasticity and TENS/SES influences in this sensorimotor disease.
TENS/SES and other therapeutic interventions on spasticity
According to our findings, only two studies[36,38] compared TENS/SES with other therapeutic approaches to treat spasticity. Aydin et al.[36] evaluated the effects of TENS/SES and oral baclofen from two groups composed of spinal cord injury spastic patients. TENS/SES electrodes were applied over the tibial nerve, bilaterally, to involve gastrocnemius muscles. They reported similar effects of both therapies on H-reflex, M-wave and H/M ratio from gastrocnemius muscle, and functional measures as well. In turn, Martins et al.[36] compared TENS/SES and cryotherapy effects on triceps surae muscle of chronic spastic stroke patients. They were all submitted to both therapies although separately and in different days. The authors reported benefits to treat spasticity from TENS/SES in contrast to cryotherapy. Their findings were supported by H-reflex, M-wave, and Hmax/Mmax ratio. Even though we found only two studies that compared TENS/SES with other therapeutic approaches, their results support this intervention as a promising alternative in the treatment of spasticity.
Potential TENS/SES parameters at a glance
Even though it would be hasty to configure what must be the best parameters of stimulation, the present review suggests that TENS/SES applied for more than 20’, and set at ~100 µs (pulse width) and ~100 Hz (frequency) may lead to a short-term improvement in spasticity, which deserves to be investigated. Nonetheless, Garcia et al.[18] and Veldman et al.[42] pointed out that a fine-tuning on stimulation intensity may lead to different excitability responses at the cortical and spinal levels, as previously highlighted.
Conclusion
Spasticity is a sensorimotor disorder characterized by an abnormal increase in muscle tone that impairs the performance of daily living activities. Several authors have advocated the use of electrical stimulation as a therapeutic tool for the treatment of spasticity, mainly due to non-significant side effects[47]. Some of them have reported benefits from the electrical stimulation applied below the motor threshold (TENS or SES). Nonetheless, since the physiopathology of spasticity is still unclear (some suggest reduced spinal inhibitory reflexes), those who intend to apply TENS/SES also fail onto switching the key cortical and spinal circuitries that can result on some improvement of this sensorimotor disorder. Thus, we seem to be dealing with “trial-and-error” approaches and very few evidence that can lead us to a consensus toward parameters of stimulation, mainly due to the lack of studies, that can maximize the efficacy of TENS/SES as well as on evaluating spasticity quantitatively. Besides, we also support that the use of TENS/SES by the patients themselves at home may provide some advantages in contrast to FES, which offers a higher level of complexity in the regulation of parameters and, therefore, in use without the continuous supervision of a clinician.
Author contributions
MACG and CDV: Conception, design, and analysis and interpretation of the literature; they have participated in drafting of the manuscript and critical revision of the manuscript for important intellectual content; final approval of the version to be submitted.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This work is part of the ABRAÇO Initiative for the Brachial Plexus Injury (http://abraco.numec.prp.usp.br/) of the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)’s Research, Innovation and Dissemination Center for Neuromathematics (grant 2013/07699-0, http://neuromat.numec.prp.usp.br/). It was also supported by the Financiadora de estudos e projetos (FINEP), the Conselho Nacional de Pesquisa CNPq) (grants 306817/2014-4 and 309560/2017-9) and the Fundação de Amparo à Pesquisa do Rio de Janeiro FAPERJ (grants E-26/111.655/ 2012, E26/010.002902/2014 and E-26/010.002474/2016).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Lance JW. Feldman RG, Young RR, Koella WP, editors. Spasticity:Disordered Motor Control. Symposium synopsis. 1980:485–494. [Google Scholar]
- 2.Dietz V, Sinkjær T. Spastic movement disorder:impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6(8):725–33. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee A, Chakravarty A. Spasticity Mechanisms –for the Clinician. Front Neurol. 2010;1:149. doi: 10.3389/fneur.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke D, Wissel J, Donnan GA. Pathophysiology of spasticity in stroke. Neurology. 2013;80(3 Suppl.2):S20–6. doi: 10.1212/WNL.0b013e31827624a7. [DOI] [PubMed] [Google Scholar]
- 5.Trompetto C, Marinelli L, Mori L, Pelosin E, Currà A, Molfetta L, Abbruzzese G. Pathophysiology of spasticity:Implications for neurorehabilitation. Biomed Res Int. 2014;2014:354906. doi: 10.1155/2014/354906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brashear A, Gordon MF, Kassicieh VD, Marciniak C, Do M, Lee CH, Jenkins S, Turkel C and Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347(6):395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 7.Chang E, Ghosh N, Yanni D, Lee S, Alexandru D, Mozaffar T. A Review of Spasticity Treatments:Pharmacological and Interventional Approaches. Crit Rev Phys Rehabil Med. 2013;25(1-2):11–22. doi: 10.1615/CritRevPhysRehabilMed.2013007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin LP. Advances in pharmacotherapies for movement disorders in children:current limitations and future progress. Curr Opin Pediatr. 2017;29(6):652–664. doi: 10.1097/MOP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 9.Naro A, Leo A, Casella C, Buda A, Crespantini A, Porcari B, Carioti L, Billeri L, Bramanti A, Bramanti P, Calabrò RS. Breakthroughs in the spasticity management:Are non-pharmacological treatments the future? J Clin Neurosci. 2017;39:16–27. doi: 10.1016/j.jocn.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Sharif F, Ghulam S, Malik AN, Saeed Q. Effectiveness of Functional Electrical Stimulation (FES) versus Conventional Electrical Stimulation in Gait Rehabilitation of Patients with Stroke. J Coll Physicians Surg Pak. 2017;27(11):703–706. [PubMed] [Google Scholar]
- 11.Sivaramakrishnan A, Solomon JM, Manikandan NJ. Comparison of transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES) for spasticity in spinal cord injury - A pilot randomized cross-over trial. J Spinal Cord Med. 2018;41(4):397–406. doi: 10.1080/10790268.2017.1390930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carda S, Biasiucci A, Maesani A, Ionta S, Moncharmont J, Clarke S, Murray MM, Millán JDR. Electrically Assisted Movement Therapy in Chronic Stroke Patients With Severe Upper Limb Paresis:A Pilot, Single-Blind, Randomized Crossover Study. Arch Phys Med Rehabil. 2017;98(8):1628–1635.e2. doi: 10.1016/j.apmr.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Jung KS, In TS, Cho HY. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke:A randomized controlled study. Gait Posture. 2017;54:183–187. doi: 10.1016/j.gaitpost.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Meng F, Xu MY, Yue SW. Full-movement neuromuscular electrical stimulation improves plantar flexor spasticity and ankle active dorsiflexion in stroke patients:a randomized controlled study. Clin Rehabil. 2016;30(6):577–86. doi: 10.1177/0269215515597048. [DOI] [PubMed] [Google Scholar]
- 15.Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil. 2008;22(7):627–34. doi: 10.1177/0269215507084648. [DOI] [PubMed] [Google Scholar]
- 16.Sommerfelt K, Markestad T, Berg K, Saetesdal I. Therapeutic electrical stimulation in cerebral palsy:a randomized, controlled, crossover trial. Dev Med Child Neurol. 2001;43(9):609–13. doi: 10.1017/s0012162201001104. [DOI] [PubMed] [Google Scholar]
- 17.Carmick J. Managing equinus in children with cerebral palsy:electrical stimulation to strengthen the triceps surae muscle. Dev Med Child Neurol. 1995;37(11):965–75. doi: 10.1111/j.1469-8749.1995.tb11951.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia MAC, Catunda JMY, Souza MN, Fontana AP, Sperandei S, Vargas CD. Is the Frequency in Somatosensory Electrical Stimulation the Key Parameter in Modulating the Corticospinal Excitability of Healthy Volunteers and Stroke Patients with Spasticity? Neural Plast. 2016;2016:3034963. doi: 10.1155/2016/3034963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyama S, Tanabe S, Takeda K, Sakurai H, Kanada Y. Modulation of spinal inhibitory reflexes depends on the frequency of transcutaneous electrical nerve stimulation in spastic stroke survivors. Somatosens Mot Res. 2016;33(1):8–15. doi: 10.3109/08990220.2016.1142436. [DOI] [PubMed] [Google Scholar]
- 20.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51(1):122–5. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 21.Conforto AB, Cohen LG, Santos RLD, Scaff M, Marie SKN. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254(3):333–9. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- 22.Conforto AB, Santos RL, Farias SN, Marie SKN, Mangini N, Cohen LG. Effects of Somatosensory Stimulation on the Excitability of the Unaffected Hemisphere in Chronic Stroke Patients. Clinics (Sao Paulo) 2008;63(6):735–40. doi: 10.1590/S1807-59322008000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SK, Baltieri SC, Scaff M, Cohen LG. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24(3):263–72. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan JE, Hurley D, Hedman LD. Afferent stimulation provided by glove electrode during task-specific arm exercise following stroke. Clin Rehabil. 2012;26(11):1010–1020. doi: 10.1177/0269215512442915. [DOI] [PubMed] [Google Scholar]
- 25.Wu CW, Seo HJ, Cohen LG. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Arch Phys Med Rehabil. 2006;87(3):351–7. doi: 10.1016/j.apmr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Levin MF, Hui-Chan CW. Relief of hemiparetic spasticity by TENS is associated with improvement in reflex and voluntary motor functions. Eletroencephalogr Clin Neurophysiol. 1992;85(2):131–42. doi: 10.1016/0168-5597(92)90079-q. [DOI] [PubMed] [Google Scholar]
- 27.Karakoyun A, Boyraz I, Gunduz R, Karamercan A, Ozgirgin N. Electrophysiological and clinical evaluation of the effects of transcutaneous electrical nerve stimulation on the spasticity in the hemiplegic stroke patients. J Phys Ther Sci. 2015;27(11):3407–11. doi: 10.1589/jpts.27.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulet C, Arsenault AB, Bourbonnais D, Laramée MT, Lepage Y. Effects of transcutaneous electrical nerve stimulation on H-reflex and spinal spasticity. Scand J Rehabil Med. 1996;28(3):169–76. [PubMed] [Google Scholar]
- 29.Kwong PW, Ng GY, Chung RC, Ng SS. Transcutaneous electrical nerve stimulation improves walking capacity and reduces spasticity in stroke survivors:a systematic review and meta-analysis. Clin Rehabil. 2017;269215517745349 doi: 10.1177/0269215517745349. [DOI] [PubMed] [Google Scholar]
- 30.Mills PB, Dossa F. Transcutaneous Electrical Nerve Stimulation for Management of Limb Spasticity:A Systematic Review. Am J Phys Med Rehabil. 2016;95(4):309–18. doi: 10.1097/PHM.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 31.Abbruzzese G, Trompetto C. Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol. 2002;19(4):307–21. doi: 10.1097/00004691-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Misiaszik JE. The H-reflex as a tool in neurophysiology:its limitations and uses understanding nervous system function. Muscle Nerve. 2003;28(2):144–60. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, TetzlaffJ J, Altman DG PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses:The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joodaki MR, Olyaei GR, Bagheri H. The effects of electrical nerve stimulation of the lower extremity on H-reflex and F-wave parameters. Electromyogr Clin Neurophysiol. 2001;41(1):23–8. [PubMed] [Google Scholar]
- 35.van der Salm A, Veltink PH, IJzerman MJ, Groothuis-Oudshoorn KC, Nene AV, Hermens HJ. Comparison of electric stimulation methods for reduction of triceps surae spasticity in spinal cord injury. Arch Phys Med Rehabil. 2006;87(2):222–8. doi: 10.1016/j.apmr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Martins FL, Carvalho LC, Silva CC, Brasileiro JS, Souza TO, Lindquist ARR. Immediate effects of TENS and cryotherapy in the reflex excitability and voluntary activity in hemiparetic subjects:a randomized crossover trial. Rev Bras Fisioter. 2012;16(4):337–44. doi: 10.1590/s1413-35552012005000032. [DOI] [PubMed] [Google Scholar]
- 37.Ching-Chen S, Chen Y, Handa Y. Proceedings of the 3th Annual Conference of IFESS and INS. Luzern, Switzerland: 1998. The therapeutic effect of surface electrical stimulation on spasticity. [Google Scholar]
- 38.Aydin G, Tomruk S, Keles I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity:clinical and electrophysiologic comparison. Am J Phys Med Rehabil. 2005;84(8):584–92. doi: 10.1097/01.phm.0000171173.86312.69. [DOI] [PubMed] [Google Scholar]
- 39.Peres ASC, Souza VH, Catunda JMY, Mazzeto-Betti KC, Santos-Pontelli TEG, Vargas CD, Baffa O, Araújo DB, Pontes-Neto OM, Leite JP, Garcia MAC. Can somatosensory electrical stimulation relieve spasticity in post-stroke patients?A TMS pilot study. Biomed Tech (Berl) 2018;63(4):501–6. doi: 10.1515/bmt-2016-0162. [DOI] [PubMed] [Google Scholar]
- 40.Burke D. Clinical uses of H reflexes of upper and lower limb muscles. Clin Neurophysiol Pract. 2016;1:9–17. doi: 10.1016/j.cnp.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chipchase LS, Schabrun SM, Hodges PW. Corticospinal excitability is dependent on the parameters of peripheral electric stimulation:a preliminary study. Arch Phys Med Rehabil. 2011;92(9):1423–30. doi: 10.1016/j.apmr.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Veldman MP, Maffiuletti NA, Hallett M, Zijdewind I, Hortobágyi T. Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans. Neurosci Biobehav Rev. 2014;47:22–35. doi: 10.1016/j.neubiorev.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Garcia MAC. DSc [dissertation] Rio de Janeiro (RJ), Brazil: Federal University of Rio de Janeiro; 2011. Acute modulatory effect of the somatosensory electrical stimulation applied at different frequencies in the corticospinal excitability. [Google Scholar]
- 44.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–2. [PubMed] [Google Scholar]
- 45.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 46.Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J Neurol. 1993;240(2):63–71. doi: 10.1007/BF00858718. [DOI] [PubMed] [Google Scholar]
- 47.Kilgore KL. New Frontiers in the Management of UMN Syndrome. UMN Management and Research, AAP Course. 2003 [Google Scholar]


