Abstract
Purpose:
Nigella sativa oil possesses a well-known ability to protect certain organs from oxidative, neoplastic, and inflammatory damage. This study investigated the potential chondroprotective effects of intraarticular injections of Nigella sativa oil in a rabbit osteoarthritis model.
Methods:
Osteoarthritis models were created by performing anterior cruciate ligament transections in 20 New Zealand rabbits. Rabbits were randomly divided into two groups of 10 and given intraarticular injections in their right knees weekly for 5 weeks, beginning in the third week post-operation. Injections given to the first group contained whole Nigella sativa oil, whereas the second group was injected with a saline solution. Knee joints were harvested 8 weeks after surgery. Knee joint surfaces were examined macroscopically, and medial femoral condyle sections were examined microscopically.
Results:
There was a statistically significant difference in the macroscopic grading results of the groups, with the Nigella sativa group having better results (p=0.001). The Nigella sativa group also received significantly better total Osteoarthritis Research Society International (OARSI) scores (p=0.035).
Conclusions:
Intraarticular administration of Nigella sativa oil has the potential to protect cartilage from degeneration in the early stages of osteoarthritis.
Keywords: Nigella sativa oil, Osteoarthritis, Intraarticular, ACLT, Rabbit
Introduction
Osteoarthritis (OA) is a chronic, degenerative joint disease characterized by cartilage destruction, joint space narrowing, osteophyte formation, and subchondral sclerosis. The damage to the articular cartilage causes progressive injury and loss of joint function[1]. This can cause severe morbidity and places an economic burden on society. The most commonly affected peripheral location is the knee joint[2]. The exact mechanism of OA is unknown, but the main factor in its development is thought to be cartilage damage due to chronic inflammatory processes triggered by proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α)[3,4].
Most OA treatments consist of symptom-modifying agents. The most commonly ordered medications are non-steroidal anti-inflammatory drugs (NSAIDs), which have the potential to increase cartilage destruction by inhibiting cartilage matrix synthesis[5]. The search continues for an effective drug that is capable of preventing, stabilizing, and reversing the development of OA with minimal side effects and low cost.
Recent medical trends have focused on natural alternatives over synthetic chemicals, and several natural nutrients may play an active role in preventing the development of OA when administered orally or intra-articularly[6-8]. Among these is Nigella sativa (NS), also known as black cumin, a member of the Ranunculaceae that has been safely consumed for thousands of years. Intraperitoneal and intra-articular applications have been demonstrated experimentally, in addition to its oral and topical usage[9,10]. The constituents of NS oil possess proven anti-neoplastic, anti-bacterial, bronchodilatory, hypotensive, hypolipidemic, antidiabetic, and hepatoprotective properties[11-13]. Anti-inflammatory effects for NS oil have been reported, and thymoquinone (TQ), an active metabolite of NS oil, has been shown to reduce the TNF-α and IL-1β levels in a rat model of arthritis[14,15]. In this study, we evaluated the chondroprotective effects of NS oil administered by intra-articular application in rabbits with knee osteoarthritis created by anterior cruciate ligament transection (ACLT).
Materials and methods
Animals
As permitted by the ethics committee, 20 mature (5-7 months old) male New Zealand white rabbits weighing 2.5-3.0 kg were provided by the Laboratory Animal Center of Abant Izzet Baysal University (Bolu, Turkey). The animals were housed in separate metal cages and allowed free access to solid food and distilled water under a natural light-dark cycle. This study was performed in accordance with the guidelines for animal research of the National Institutes of Health (NIH, Bethesda, MD, USA) and the 3R principles of the EU directive, and was approved by the Laboratory Animal Ethics Committee of Abant Izzet Baysal University (Bolu, Turkey; no. 2017/21).
Reagents
NS oil was produced by cold-pressing fresh seeds without the use of chemicals (Origo, Gaziantep, Turkey). The oil was sterilized by filtration through a 0.22-µm filter (Millipore, Bedford, MA, USA). The various components of NS oil are known to act synergistically. We therefore found it expedient to employ the whole oil of the seeds rather than isolated components, which have been frequently used in previous studies[11].
Surgery
The animals were sedated with an intramuscular injection of 10 mg/kg xylazine (Rompun, Bayer), and anesthetized with 50 mg/kg ketamine hydrochloride (Ketalar, Pfizer Warner Lambert) 5~10 minutes after the sedation onset. Maintenance of anesthesia was mediated by pinching the skin and fingers; additional ketamine hydrochloride was injected intramuscularly when necessary. Their right knees were shaved and disinfected with polyvinyl iodine (Betadine, Eczacıbaşı, Turkey), and a longitudinal incision was made in the skin from the superior pole of the patella to the tibial tuberosity. After a medial parapatellar arthrotomy, the anterior cruciate ligament was visualized and transected (ACLT) with a surgical blade (Figure 1). A positive anterior drawer test confirmed complete rupture of the ligament. After irrigation of the joint space with physiological saline solution, the medial retinaculum was repaired and the skin was closed separately. The animals were allowed unlimited postoperative activity within their cages. After the operation, the animals were numbered and then assigned to two equal groups of 10 each by simple random sampling using a random numbers table.
Figure 1.
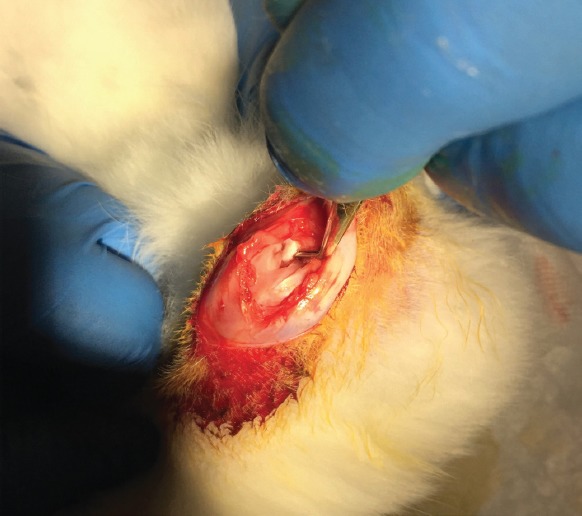
After a medial parapatellar arthrotomy, the anterior cruciate ligament was visualized and transected.
The NS group received intra-articular injections of 0.3 ml of NS oil once a week for 5 weeks, beginning 3 weeks after the operation. The control (C) group was given intra-articular injections of 0.3 ml of physiological saline solution on the same schedule. The injection volume of 0.3 ml was chosen based on the volumetric capacity of rabbit joints, as determined by previous studies[16]. The same investigator administered all injections, and no sedation was needed during the injection procedures. In the ninth week after the operation, the rabbits were given intramuscular anesthesia with xylazine and ketamine before being sacrificed with high-dose intravenous thiopental sodium (Pentothal, Abbott).
Morphological evaluation
The rabbits’ distal femurs and proximal tibiae were harvested after sacrifice. Each specimen’s articular cartilage was stained with India ink. A gross morphological assessment (GMA) was performed of the knee joints in a blinded fashion using the scoring system described by Meachim in 1972[17].
Histological evaluation
The distal femurs of the knee joints were placed in a 10% buffered formaldehyde solution followed by a buffered formic acid solution for decalcification. Sections of 5 µm were obtained from the samples and embedded in paraffin after processing. The sections were stained with hematoxylin-eosin and Safranin O. Histopathological findings were evaluated as described by Pritzker et al., calculating the Osteoarthritis Research Society International (OARSI) grades, stages, and scores[18]. Briefly, the OARSI grades are as follows: 0, normal; 1, intact surface; 2, surface discontinuity; 3, vertical fissures; 4, erosion; 5, denudation; and 6, deformation. The percentage of joint involvement (OARSI stage) was divided into four categories: 0, no OA activity seen; stage 1, <10%; stage 2, 10-25%; stage 3, 25-50%; and stage 4, >50%. The overall OARSI score was calculated for each case by multiplying the parameters of the histopathological grade and the percentage of the joint involvement. The histological examinations were performed in a blinded fashion.
Statistical analyses
Number Cruncher Statistical System 2007 Statistical Software (Utah, USA) was used for statistical analyses. In addition to descriptive statistics, a Mann-Whitney U-test was used to compare binary groups of variables without a normal distribution. A Spearman correlation test was used to determine relationships between variables, and a chi-square test was used to compare qualitative data. A p-value of less than 0.05 was considered statistically significant.
Results
Two rabbits from the NS group died after the first surgical operation. No adverse reaction to surgery was observed in any other rabbits from either group during the experimental period. GMA of the femoral condyles and tibial plateaus after staining with India ink revealed normal joint surfaces (Grade 1; Figure 2A) in three of eight rabbits (37.5%) and minimal fibrillation (Grade 2) in the remaining five rabbits (62.5%) in the NS group. In the control (C) group, we observed minimal fibrillation (Grade 2) in one rabbit (10%), overt fibrillation (Grade 3) in five rabbits (50%), and bone exposures (Grade 4) in four rabbits (40%; Figure 2B). There was a statistically significant difference in the macroscopic grading results of the two groups (p=0.001; Figure 3, Table 1).
Figure 2.
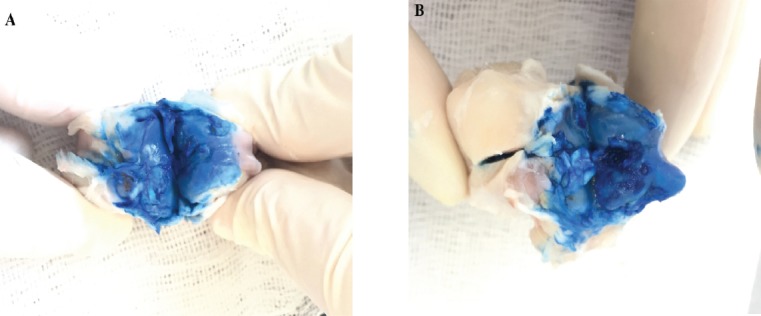
Examples of gross morphological assessments of femoral condyles after staining with India ink. A. Minimal fibrillation (Grade 2), seen in five of eight rabbits (62.5%) in the NS group. B. Areas of bone exposure (Grade 4), seen in four of 10 rabbits (40%) in the saline group.
Figure 3.
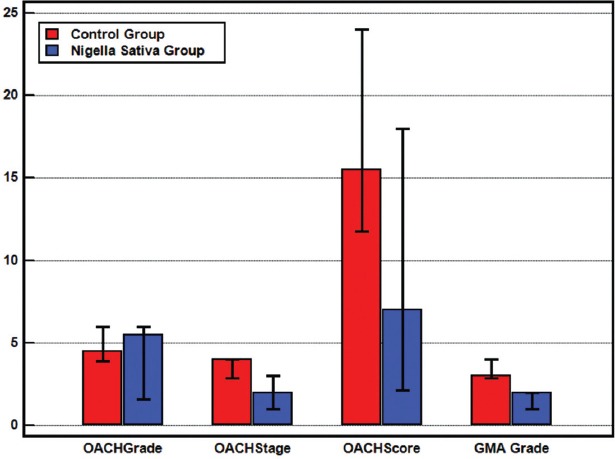
Median OARSI grades, stages, scores, and GMA grades.
Table 1.
The mean (±SD) and Median (IQR) results of the OACH grade, stage, score and GMA grades of the groups.
| Control Group | Nigella Sativa Group | p | ||
|---|---|---|---|---|
| OACH Grade | Mean±SD | 4,60±0,97 | 4,63±1,85 | 0,581 |
| Median (IQR) | 4,50 (4-5,25) | 5,50 (3,25-6) | ||
| OACH Stage | Mean±SD | 3,5±0,71 | 1,88±0,84 | 0,002 |
| Median (IQR) | 4 (3-4) | 2 (1-2,75) | ||
| OACH Score | Mean±SD | 16,10±5,04 | 9,25±6,21 | 0,035 |
| Median (IQR) | 15,5 (12-21) | 7 (5,25-16,5) | ||
| GMA Grade | Mean±SD | 3,30±0,68 | 1,63±0,52 | 0,001 |
| Median (IQR) | 3 (3-4) | 2 (1-2) | ||
OACH: Osteoarthritis Cartilage Histopathology, GMA: Gross Morphological Assessment, SD: Standard deviation, IQR: Interquartile range.
The mean OARSI grade of the NS group was 4.63±1.85 and that of the C group was 4.6±0.97. The difference between the groups was not significant (p=0.581) (Figures 4 and 5). There was, however, a statistically significant difference (p=0.002) in the OARSI stages of the two groups, with the mean stages of the NS and C groups being 1.5±0.84 and 3.5±0.71, respectively. The mean OARSI scores of the NS and C groups were 9.25±6.21 and 16.1±5.04, respectively, and this difference was significant (p=0.035; Figure 3, Table 1). There was also a statistically significant positive correlation between the GMA scores and the OARSI stages of each group (p=0.027).
Figure 4.
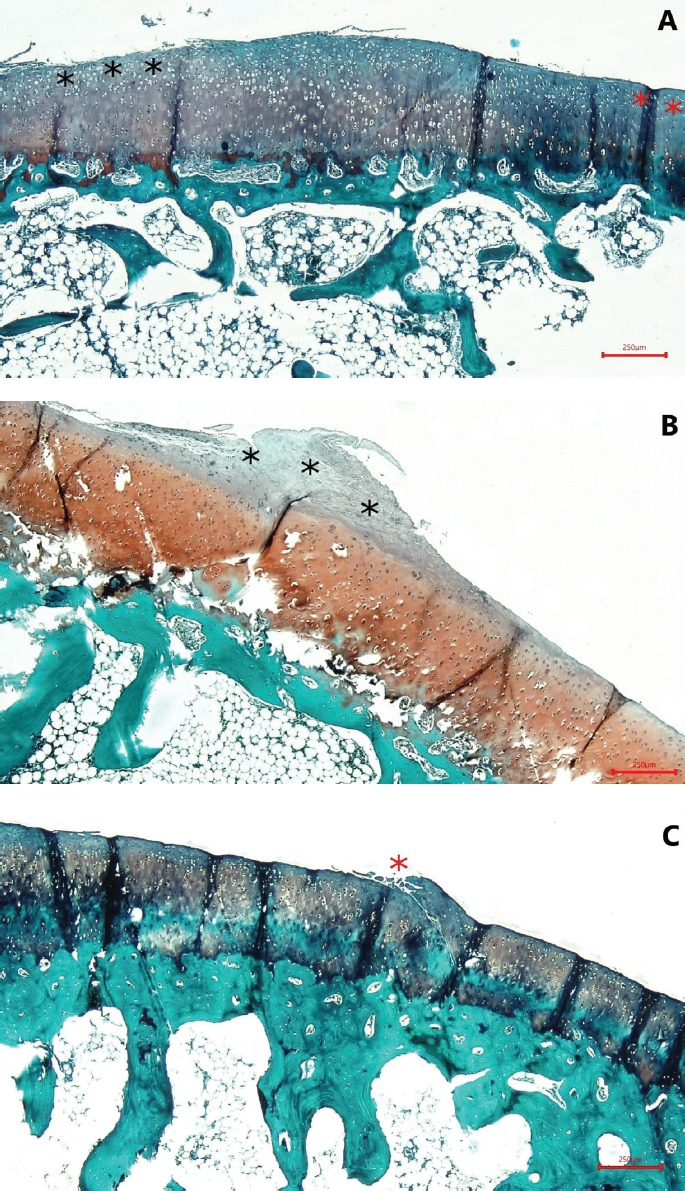
A. OARSI grade 1; focal abrasion (black asterisk) and normal zone (red asterisk). B. OARSI grade 2; fibrillation into the superficial cartilage zone (black asterisk). C. OARSI grade 3; focal vertical fissure formation (red asterisk) (Safranin O, ×40).
Figure 5.
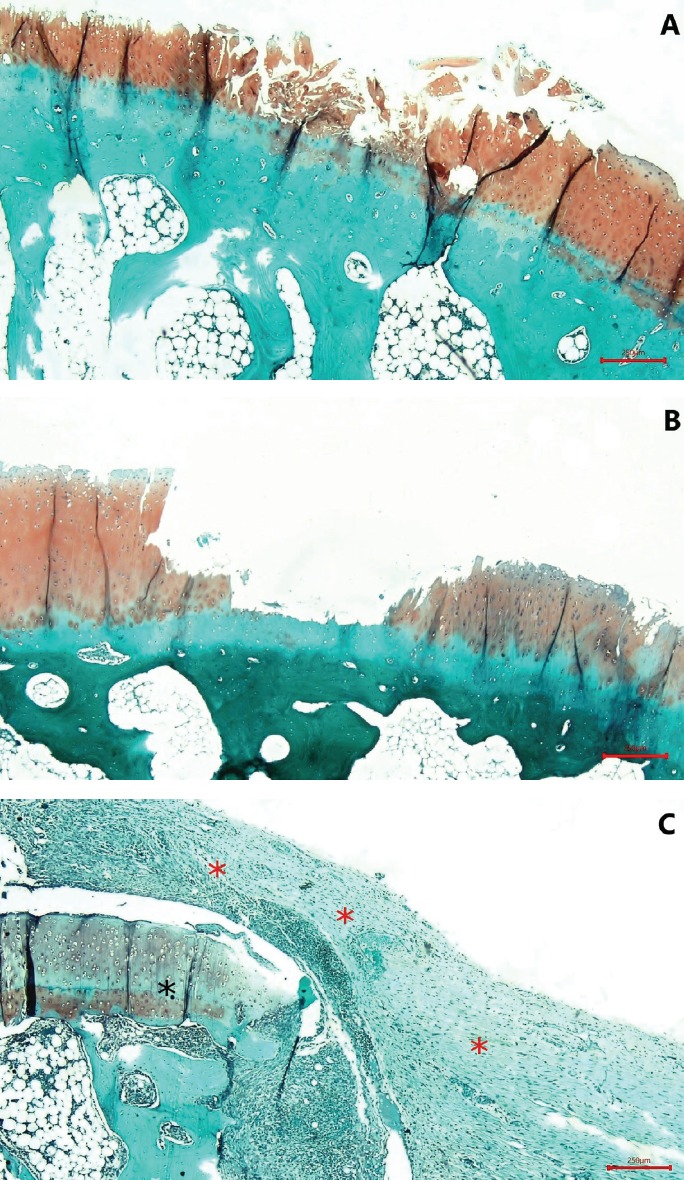
A. OARSI grade 4; erosion and loss of matrix in fissured domains. B. OARSI grade 5; complete erosion of un-mineralized cartilage and denudation. C. Changing contour of the articular surface due to micro-fracture, repair, and bone remodeling; deformation and fibroblast proliferation (red asterisk) compared with the normal zone (black asterisk) (Safranin O, ×40).
Discussion
This study investigated, for the first time, the chondroprotective effects of NS oil administered by intra-articular application in a rabbit knee OA model. The results showed that the use of NS oil has a significant protective effect on articular cartilage. The nutritional components of NS include vitamins, carbohydrates, mineral elements, fats, and proteins containing eight or nine essential amino acids. The fixed oil of NS (32-40%) contains beta-sitosterol, cycloeucalenol, cycloartenol, sterol esters, sterol glucosides, and various unsaturated fatty acids, including arachidonic, eicosadienoic, linoleic, linolenic, oleic, palmitoleic, palmitic, stearic, and myristic acids[19-21]. The volatile oil (0.4-0.45%) contains saturated fatty acids, nigellone (the only component of the carbonyl fraction of the oil), TQ, thymohydroquinone, dithymoquinone, thymol, carvacrol, α- and β-pinene, d-limonene, d-citronellol, p-cymene, t-anethole, 4-terpineol, and longifolene[19,20,22]. Studies have shown that the major component of the essential oil is TQ, which has well known antioxidative, anti-inflammatory, and anticancer activities[14,23-26].
Creating an OA model by performing ACLT in rabbit knees is a well-established technique[27]. Cartilage degeneration caused by unstable joints can be observed microscopically 2 weeks after the ACLT, and the progressive gradual postoperative changes that occur in the articular cartilage of ACLT rabbits resemble the progression of human OA[28,29]. The osteoarthritic changes in rabbit knees usually occur in two distinct phases: an initial degenerative phase (0-8 weeks), followed by a regeneration phase (8-16 weeks)[29]. Based on this typical progression, we began administering intra-articular injections 3 weeks after the ACLT surgery and continued for 5 weeks (through the end of the degenerative phase).
The treatment of OA with intra-articular injections is popular worldwide. Analgesics, NSAIDs, steroids, and hyaluronic acid (HA) are commonly administered by injection[30]. Other studies have demonstrated the chondroprotective effects of N-acetylglucosamine and vitamin E in OA[16,31]. In addition to these genetically engineered materials, some intra-articularly administered elementary nutrients were also found to be effective for OA treatment[32]. In our study, natural NS oil was used; it is relatively easy to use and inexpensive.
Previous studies have largely used the active metabolite of NS oil (TQ) instead of whole NS oil in the treatment of OA. However, the different components of NS oil act synergistically, and experiments using the complete oil may be more likely to produce results[11]. In a recent clinical trial, topical application of NS oil was shown to provide effective pain relief in patients with knee OA, and the authors recommended the use of NS oil as a safe supplement for the elderly[33]. In our study, we used Nigella sativa oil as a whole rather than the isolated TQ component.
Inflammation plays a major role in OA development, and studies have shown that IL-1β is the main determinant of this inflammatory response[34]. There are few studies in the English literature about the chondroprotective effects of TQ in OA. Chen et al. showed that TQ downregulated matrix metalloproteinase (MMP) expression in vitro and reduced cartilage degradation in vivo in an experimental rabbit OA model[9]. In 2015, in an in vitro study, Wang et al. showed that TQ suppressed IL-1β-induced prostaglandin E2 (PGE2), nitric oxide (NO), and MMP synthesis in chondrocytes, inhibiting inflammation and the degenerative process[35]. The results of these two studies suggested that TQ as a promising therapeutic agent for the treatment of OA. In parallel, in our study, the use of whole NS oil instead of TQ was found to protect cartilage from the degenerative process in the early stages of OA.
In this study, although the OARSI grades of the rabbits that were given intra-articular injections of NS oil did not differ significantly from those of the control group, the OARSI stages, scores and GMA results were significantly better. The use of whole NS oil as an intra-articular injection can provide a significant protective effect to articular cartilage, making it an attractive alternative to its active metabolite TQ, the production of which requires considerably greater cost and effort than the production of NS oil.
To our knowledge, this study is the first animal experiment to evaluate the possible chondroprotective effects of whole NS oil, a natural product, administered by intra-articular injection. Despite the importance of this study, it had some limitations. No blood was drawn from the rabbits due to investigational difficulties, and an evaluation of parameters such as markers of cartilage degradation could have provided additional information. This study may set an example for future studies designed to compare the effectiveness of NS oil and its combination with other agents, for example HA and N-acetylglucosamine, in the treatment of OA.
Conclusion
This study verified that the local administration of NS oil into the joint may provide a promising new lead in the search for effective osteoarthritis treatments.
Footnotes
This research was supported by grants from the Scientific Research Projects Unit of Duzce University, Duzce, Turkey (2017.4.2.667). The authors declare no conflict of interest.
Edited by: G. Lyritis
References
- 1.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354(8):841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Neogi T. Osteoarthritis:is it a disease of cartilage or of bone? Arthritis & Rheumatism:Official Journal of the American College of Rheumatology. 2004;50(2):341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 3.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 4.Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingle JT. The effects of NSAID on the matrix of human articular cartilages. Zeitschrift für Rheumatologie. 1999;58(3):125–129. doi: 10.1007/s003930050161. [DOI] [PubMed] [Google Scholar]
- 6.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 7.Shen CL, Hong KJ, Kim SW. Effects of ginger (Zingiber officinale Rosc.) on decreasing the production of inflammatory mediators in sow osteoarthrotic cartilage explants. J Med Food. 2003;6(4):323–328. doi: 10.1089/109662003772519877. [DOI] [PubMed] [Google Scholar]
- 8.Piscoya J, Rodriguez Z, Bustamante SA, et al. Efficacy and safety of freeze-dried cat's claw in osteoarthritis of the knee:mechanisms of action of the species Uncaria guianensis. Inflamm Res. 2001;50(9):442–448. doi: 10.1007/PL00000268. [DOI] [PubMed] [Google Scholar]
- 9.Chen WP, Tang JL, Bao JP, et al. Thymoquinone inhibits matrix metalloproteinase expression in rabbit chondrocytes and cartilage in experimental osteoarthritis. Experimental Biology and Medicine. 2010;235(12):1425–1431. doi: 10.1258/ebm.2010.010174. [DOI] [PubMed] [Google Scholar]
- 10.Tayman C, Cekmez F, Kafa IM, et al. Protective effects of Nigella sativa oil in hyperoxia-induced lung injury. Archivos de Bronconeumología (English Edition) 2013;49(1):15–21. doi: 10.1016/j.arbres.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytotherapy Research. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Irmaileh BE, Afifi FU. Herbal medicine in Jordan with special emphasis on commonly used herbs. J Ethnopharmacol. 2003;89(2-3):193–197. doi: 10.1016/s0378-8741(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 13.Randhawa MA, Al-Ghamdi MS. A review of the pharmaco-therapeutic effects of Nigella sativa. Pak J Med Res. 2002;41(2):77–83. [Google Scholar]
- 14.El Mezayen R, El Gazzar M, Nicolls MR, et al. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol Lett. 2006;106(1):72–81. doi: 10.1016/j.imlet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Tekeoglu I, Dogan A, Ediz L, et al. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21(9):895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 16.Ozkan FU, Ozkan K, Ramadan S, et al. Chondroprotective Effect of N-Acetylglucosamine and Hyaluronate in Early Stages of Osteoarthritis. Bulletin of the NYU hospital for joint diseases. 2009;67(4):352–327. [PubMed] [Google Scholar]
- 17.Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972;31(6):457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology:grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Tembhurne SV, Feroz S, More B, et al. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J Med Plants Res. 2014;8(3):166–167. [Google Scholar]
- 20.Ahmad A, Husain A, Mujeeb M, et al. A review on therapeutic potential of Nigella sativa:A miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menounos P, Staphylakis K, and Gegiou D. The sterols of Nigella sativa seed oil. Phytochemistry. 1986;25(3):761–763. [Google Scholar]
- 22.Enomoto S, Asano R, Iwahori Y, et al. Hematological studies on black cumin oil from the seeds of Nigella sativa L. Biol Pharm Bull. 2001;24(3):307–310. doi: 10.1248/bpb.24.307. [DOI] [PubMed] [Google Scholar]
- 23.Burits M, and Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy research. 2000;14(5):323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Houghton PJ, Zarka R, de las Heras B, et al. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61(01):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 25.El-Dakhakhny M, Madi NJ, Lembert N, et al. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81(2):161–164. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 26.Yi T, Cho SG, Yi Z, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7(7):1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka M, Coutts RD, Amiel D, et al. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4(2):87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- 28.Hulth A, Lindverg L, and Telhag H. Experimental osteoarthritis in rabbits. Acta Orthop Scand. 1970;41(5):522–530. doi: 10.3109/17453677008991540. [DOI] [PubMed] [Google Scholar]
- 29.Papaioannou NA, Krallis N, Triantafillopoulos IK, et al. Optimal timing of research after anterior cruciate ligament resection in rabbits. Contemp Top Lab Anim Sci. 2004;43(6):22–27. [PubMed] [Google Scholar]
- 30.Uthman I, Raynauld JP, Haraoui B. Intra-articular therapy in osteoarthritis. Postgrad Med J. 2003;79(934):449–453. doi: 10.1136/pmj.79.934.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozkan FU, Uzer G, Türkmen I, et al. Intra-articular hyaluronate, tenoxicam and vitamin E in a rat model of osteoarthritis:evaluation and comparison of chondroprotective efficacy. Int J Clin Exp Med. 2015;8(1):1018–1026. [PMC free article] [PubMed] [Google Scholar]
- 32.Park YS, Lim SW, Lee IH, et al. Intra-articular injection of a nutritive mixture solution protects articular cartilage from osteoarthritic progression induced by anterior cruciate ligament transection in mature rabbits:a randomized controlled trial. Arthritis Res Ther. 2007;9(1):R8. doi: 10.1186/ar2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kooshki A, Forouzan R, Rakhshani MH, et al. Effect of topical application of Nigella sativa oil and oral acetaminophen on pain in elderly with knee osteoarthritis:a crossover clinical trial. Electronic physician. 2016;8(11):3193–3197. doi: 10.19082/3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandell LJ, Xing X, Franz C, et al. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis and Cartilage. 2008;16(2):1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Qiao J, Zhao X, et al. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation. 2015;38(6):2235–2241. doi: 10.1007/s10753-015-0206-1. [DOI] [PubMed] [Google Scholar]


