Abstract
Objective
Although many studies have shown that the corpus callosum (CC) may play an important role in bipolar disorder (BD) and suicide, the pathophysiological mechanism of BD underlying suicidal behavior is still unclear. This study aimed to explore the relationship between the CC, and BD and suicidal ideation using diffusion tensor imaging (DTI).
Method
A total of 203 participants (47 BD patients with suicidal ideation, 59 with BD without suicidal ideation, and 97 healthy controls [HC]) underwent DTI scanning at a single site. We examined the white matter integrity of the CC in the three groups.
Results
A comparison among groups showed that BD patients with suicidal ideation had significant lower fractional anisotropy (FA) values than those of BD without suicidal ideation and HCs in the body and genu of the CC, and FA values of BD without suicidal ideation were significantly lower than those of HCs. However, in the splenium of corpus callosum, no difference was found between BD without suicidal ideation and HCs.
Conclusions
Our findings add to the evidence suggesting that the CC plays a key role in BD with suicidal ideation, especially with respect to the role of the genu and body of the CC subserving emotion regulation.
Keywords: Bipolar disorder, Suicidal ideation, Corpus callosum, Diffusion tensor imaging
Introduction
Suicide is a global public health problem that includes suicidal ideation, suicide attempts, and death by suicide. Suicidal ideation refers to thoughts, cognitions, and overt intent to kill oneself, which is of great significance in predicting suicidal behavior, as an early stage of suicide. Although people with suicidal ideation may not necessarily attempt suicide, suicidal ideation is still regarded as a risk factor [1]. Research has recognized that suicide is closely related to bipolar disorder (BD) [2]. An epidemiological study demonstrated that half of people who died of suicide had depression, and those with more serious mood disorders, such as BD, have a 20-fold increase in risk [3]. The risk of suicide among patients diagnosed with BD is as high as 6% over 20 years, while self-harm occurs in 30–40% [4].
Many magnetic resonance imaging (MRI) studies have been performed trying to reveal the underlying neuropathological mechanism of suicide in BD. Earlier studies focused on white matter (WM) abnormalities of BD patients with suicidal behavior [5–8]. In addition, the gray matter volume of BD who have suicidal behavior was shown to be decreased in the prefrontal cortex [9, 10], lingual gyrus, and right cuneus [11], and significantly increased in the right rostral anterior cingulate cortex [12]. A multimodal neuroimaging study [13] suggested that there are gray matter volume decreases, and structural and functional connectivity abnormalities in the ventral frontolimbic neural system in adolescents and young adults with BD who attempt suicide. Despite the many studies of suicide in patients with BD, a specific suicide-related biological basis of BD remains unclear, and few studies have focused on suicidal ideation, which is an early stage of suicidal behavior.
The corpus callosum (CC) is the largest interhemispheric commissure for higher cognitive functions associated with emotion, which involves sustaining attention [14], approach-related motivation and behavior [15], inhibition and excitation of the contralateral hemisphere [16], affective prosody [17], and memory [18]. Previous studies have linked alterations in the CC with the risk of suicide and the pathophysiology of BD. A review of diffusion tensor imaging (DTI) studies in BD suggested that regions underlying inter-hemispheric communication such as the CC may play a major role in the pathophysiology of bipolar disorder [19]. Moreover, a recent DTI study [20] also found that the genu and body of the CC are altered in non-suicide attempters with BD and the splenium is specifically altered in those who attempt suicide. Some studies of BD and suicide attempts failed to confirm the same findings [21, 22]; however, the authors explained that this might have been caused by the sample size, selection of the samples, medication, and state of the disease, among other factors. The close relationship between suicide risk in patients with BD and variation in the CC is, however, recognized by most researchers.
Therefore, we consequently inferred that the CC is the key to understand the specific region alteration in BD. To investigate the role of the CC in the mechanism of suicide in BD, we chose the CC as the region of interest to detect the WM integrity of the CC in BD patients with suicidal ideation compared with that in BD without suicidal ideation and healthy people. We hypothesized that the WM tracts of the BD patients with suicidal ideation in the CC would be decreased than those without suicidal ideation, especially in the anterior CC areas.
Method
Subjects
One hundred and six patients with BD and 97 healthy controls (HCs), aged 15–50 years, were involved in this study. Written informed consent was obtained from all participants in the study, and this study was approved by the Ethics Committee of the first affiliated Hospital of China Medical University. All patients were recruited from the inpatient department of the Shenyang Mental Health Center and the outpatient clinic of the Department of Psychiatry of the First Affiliated Hospital of China Medical University, Shenyang, China. The subjects were considered to have “BD” if they met the diagnostic criteria of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Axis I disorders for those ≥ 18 years old and the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version in those < 18 years old. The exclusion criteria were (1) no history of other Axis I mental disorders; (2) no history of major somatic disease, especially diseases that may be related to changes in brain tissue, such as hypertension or diabetes; (3) no history of abnormalities of the nervous system, including major head trauma, epilepsy, cerebrovascular disease, brain tumors, or neurodegenerative diseases; and (4) no contraindications for MRI.
HCs were recruited through advertisements and matched the BD group for age, sex, and number of years in education. No healthy participants had a history of suicide attempts or suicidal ideation, current Axis I disorder, or history of Axis I disorders in their first-degree relatives according to a detailed family history; the other exclusion criteria were the same as the participants with BD.
Clinical assessment
Suicidal ideation severity was assessed using the 19-item Beck Scale for Suicide Ideation [23]. Based on their scores on the Beck Scale for Suicide Ideation, we divided all patients with BD into a BD with suicidal ideation group and BD without suicidal ideation group and excluded any healthy participants who had suicidal ideation.
The severity of clinical symptoms in all participants was evaluated using the 17-item Hamilton Depression Rating Scale (HAMD_17), Hamilton Anxiety Scale (HAMA), and Young Manic Rating Scale (YMRS). Detailed demographic and clinical characteristics of participants are outlined in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Characteristic | BD with suicidal ideation (N = 47) | BD without suicidal ideation (N = 59) | HC (N = 97) | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F/t | p | |
| Age | 26.53 | 8.02 | 25.15 | 7.69 | 24.32 | 8.35 | 1.189 | 0.307 |
| Education, years | 13.40 | 2.92 | 12.19 | 2.83 | 12.92 | 2.49 | 2.796 | 0.063 |
| Duration, month | 43.54 | 49.14 | 42.03 | 53.39 | NA | NA | 0.134 | 0.894 |
| HAMD-17 | N = 45 | N = 58 | 0.449 | 0.655 | ||||
| 11.51 | 10.34 | 10.66 | 9.00 | NA | NA | |||
| HAMA | N = 45 | N = 53 | 1.363 | 0.176 | ||||
| 11.69 | 10.91 | 9.04 | 8.33 | NA | NA | |||
| YMRS | N = 46 | N = 56 | 0.203 | 0.840 | ||||
| 6.04 | 8.96 | 5.70 | 8.29 | NA | NA | |||
| Characteristic | BD with suicidal ideation (N = 47) | BD without suicidal ideation (N = 59) | HC (N = 97) | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | χ 2 | p | |
| Sex, male | 16 | 34.04 | 26 | 44.07 | 47 | 48.45 | 2.672 | 0.263 |
| Medications, yes | 37 | 78.72 | 36 | 61.02 | NA | NA | 3.825 | 0.050 |
| First episode, yes | 19 | 40.43 | 31 | 52.54 | NA | NA | 1.765 | 0.184 |
| Mood state at scan | NA | NA | 3.006 | 0.222 | ||||
| Euthymic | 10 | 21.28 | 7 | 11.87 | NA | NA | ||
| Depressed | 19 | 40.43 | 33 | 55.93 | NA | NA | ||
| Stable | 18 | 38.29 | 19 | 32.20 | NA | NA | ||
Data are presented as n (%) or mean (standard deviation)
BD bipolar disorder, HC healthy control, N/A not applicable, HAMD_17 17-item Hamilton Depression Rating Scale, HAMA Hamilton Anxiety Scale, YMRS Young Manic Rating Scale
Image acquisition
All participants consented to a DTI scanning session using a GE Sigma HDX 3.0-T superconductive magnetic resonance scanner with a standard 8-channel head coil at the First Affiliated Hospital of China Medical University, Shenyang, China. The scanning parameters were as follows: TR/TE = 17,000/85.4 ms, image matrix = 120 × 120, FOV = 240 × 240 mm2, 65 contiguous slices of 2 mm without gap, 25 noncollinear directions (b = 1000 s/mm2), together with axial acquisition without diffusion weighting (b = 0), and voxel size = 2.0 mm3.
Image processing
Brain images were processed using the Pipeline for Analyzing braiN Diffusion imAges (PANDA) (http://www.nitrc.org/projects/panda), which is a MATLAB toolbox for the pipeline processing of diffusion MRI [24]. Processing proceeded as follows: first, the voxel-wise diffusion tensor matrix was calculated for each subject in the native space. Next, diagonalization was performed to yield three pairs of eigenvalues and eigenvectors. Based on the three eigenvalues, FA values were computed on a voxel-by-voxel basis. Specifically, the FA image of each subject was nonlinearly registered to the FMRIB58_FA template in a standard Montreal Neurological Institute (MNI) space via spatial normalization (voxel size: 1 mm × 1 mm × 1 mm). The mean of all aligned FA images was then calculated. Finally, FA images were smoothed with a 6-mm full width at half maximum Gaussian filter.
Region of interest
To compare the structural changes in BD patients with and without suicidal ideation, we selected the CC as the region of interest (ROI). The CC mask was defined according to JHU ICBM-DTI-81 WM labels atlas [25] contained in MRIcron, which included the genu, body, and splenium of the CC.
Statistical analysis
We compared the demographic data of BD patients with and without suicidal ideation and HCs using Student’s t-tests, one-way analyses of variance (ANOVA), and Chi-square tests. All statistical analyses were conducted using IBM SPSS statistical software for Windows, version 22.0 (Armonk, NY, USA). Categorical variables are described using frequencies and proportions. Continuous variables are presented as the mean ± standard deviation.
Further, to investigate the main effect of group, we performed an ANOVA among BD with suicidal ideation group, BD without suicidal ideation groups and HC group using the CC mask. Statistical significance was determined at level of p < 0.001 after Gaussian Random Field (GRF) correction, corresponding to a threshold of 20. All statistical analyses were performed using the Statistical Parametric Mapping (SPM) software (SPM8; The Wellcome Department of Cognitive Neurology) and Data Processing and Analysis for Brain Imaging software (DPABI, DPABI_V1.2_141101, http://rfmri.org/dpabi), and age, sex, and years of education as covariates.
In post hoc analyses, FA values were extracted for each cluster with significant differences among the three groups, and groups then be compared pair-wise. Significance level was set at p < 0.05 (least significant difference test; LSD).
Results
Clinical results
Table 1 describes the demographic and clinical data of the three groups. No significant differences in age (F = 1.189, p = 0.30; < 18 years), sex (χ2 = 2.672, p = 0.263), or years of education (F = 2.796, p = 0.063) were found among groups. Among our samples, 35 participants younger than 18 years have been included into our study (BD with suicidal ideation group: n = 4, 0.09%; BD without suicidal ideation group: n = 10, 0.17%; HC group: n = 21, 0.22%). In our samples, there were no significant differences between the BD with suicidal ideation group and BD without suicidal ideation group in the duration of disease, medications, type of first episode, mood state at scan, and HAMD, HAMA, and YMRS scores.
Group differences within DTI images
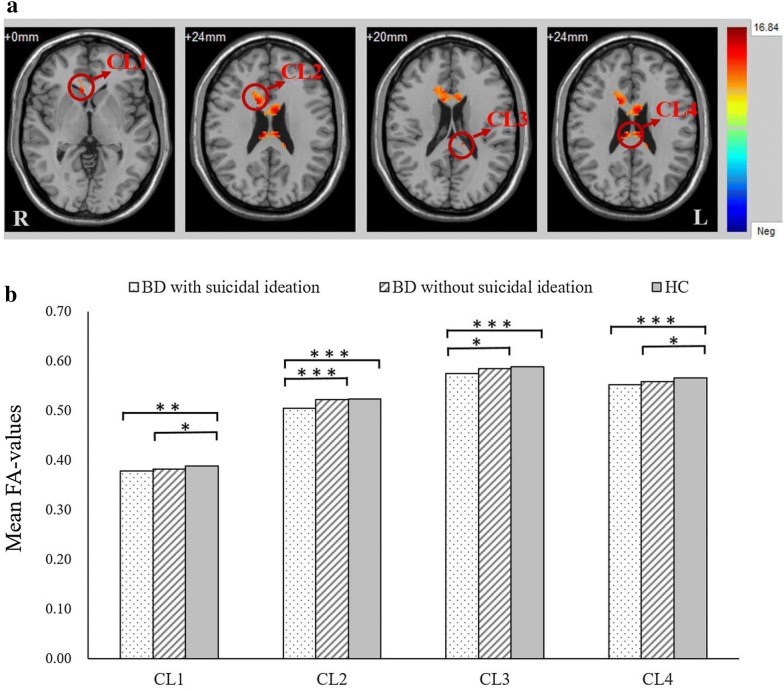
In our samples, the inter-group comparisons demonstrated significant differences in four clusters within the body, splenium, and genu of the CC (p < 0.001, GRF corrected). In particular, the patients with BD had lower FA values than HCs in all four clusters, a difference which was common to both BD with and without suicidal ideation groups. Post hoc analysis revealed that the BD with and without suicidal ideation groups had lower FA values in the body and genu of the CC (CL 1 and CL 4) than HCs (p < 0.05, LSD test), but there was no significant difference between the BD with and without suicidal ideation groups (p > 0.05, LSD test). The FA values of the BD with suicidal ideation group were much lower than those of the BD without suicide ideation group and HCs in the body, splenium, and genu of the CC (CL 2 and CL 3), but there was no significant difference (p > 0.05, LSD test) between the BD without suicidal ideation group and HCs (Fig. 1 and Table 2).
Fig. 1.
CL 1: Genu of corpus callosum, CL 2: Body of corpus callosum/genu of corpus callosum, CL 3: Splenium of corpus callosum, CL 4: Body of corpus callosum. a Significant differences in fractional anisotropy values among BD patients with suicidal ideation, BD patients without suicidal ideation, and healthy controls. Significant at p < 0.001 after GRF correction. b Post hoc analysis of fractional anisotropy values with significant differences among the three groups. ***p < 0.001 level, **p < 0.01 level, LSD test
Table 2.
Comparison of FA values in the corpus callosum among BD patients with suicidal ideation, BD patients without suicidal ideation and healthy controls
| Index | WM labelsa | Cluster size | Montreal neurological institute coordinates | F* | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Cluster 1 | Genu of corpus callosum | 21 | 8 | 22 | 0 | 12.72 |
| Cluster 2 | Body of corpus callosum | 642 | 14 | 12 | 24 | 13.56 |
| Genu of corpus callosum | ||||||
| Cluster 3 | Splenium of corpus callosum | 22 | − 12 | − 34 | 20 | 11.07 |
| Cluster 4 | Body of corpus callosum | 100 | 10 | − 26 | 24 | 16.84 |
* Significant at p < 0.001 corrected by GRF correction
aWM labels are provided in accordance with the ICBM-DTI-81 White Matter Labels Atlas
Discussion
Our results show that BD patients with suicidal ideation have significantly lower FA values in the CC than BD patients without suicidal ideation and HCs. Indeed, the FA values of BD patients with suicidal ideation were lower than those of HCs. In accordance with the law of diminishing returns, comparing the FA values found that BD patients with suicidal ideation have the most severe white matter damage, followed by BD patients without suicidal ideation, which suggests that the presence of suicidal ideation is related to this change.
The CC connects the left and right cerebral hemispheres and enables interhemispheric communication. Also, the genu and anterior CC are thought to be relevant in the regulation of emotion [26]. Our finding that the reduction in FA in the CC associated with BD was in the body and genu of the CC is consistent with previous studies. For instance, a meta-analysis [27] revealed that BD show reduced callosal areas in volume in comparison with healthy volunteers. Furthermore, a reduction in the anterior CC (including the genu, posterior body, and isthmus) area, was previously reported [28, 29], in addition to a reduction in the central CC area [30]. Accordingly, we thought that reduced callosal areas may lead to altered interhemispheric communication, participant in the pathophysiological mechanism of BD.
Moreover, we found a significant reduction of FA values in the whole CC in the BD with suicidal ideation group compared to those in the BD without suicidal ideation group and HCs. No significant differences were observed between the BD without suicidal ideation group and HCs, which suggests an association between the CC and suicidal ideation. It has been proposed that abnormalities in hemispheric connectivity are associated with suicidal behavior [30] and that the CC is believed to play a more important role in interhemispheric connections. Studies of suicide have directly highlighted that FA value reductions in the CC are associated with suicidal behaviors in agreement with our findings. A additional study have also linked decreased CC areas to elderly and other populations who have suicidal behavior [31, 32]. These studies support our findings that the observed reduced FA values in the CC may reveal the structural brain alterations underlying suicidal ideation.
In addition, the results indicate that the reduction in FA values in the body and genu of the CC was closely related to both the pathology of BD and the existence of suicidal ideation, while decreased FA values in the splenium of the CC may be a trait of BD. A previous study that also used the CC as the ROI [21] found that suicidal BD patients with higher impulsivity had a smaller anterior genu area, which supports our finding that an atrophied anterior CC region in patients with BD may lead to suicidal behaviors, even if they only currently exhibit suicidal ideation. However, there was a study [33] that did not explore the differences in the CC between suicide attempters and non-attempters that raised the idea that central parts of the CC may be a biomarker of bipolarity, which may be because the sample contained two diagnoses, BD and MDD, and is inconsistent with the single population of this study. Interestingly, a similar study in female suicide attempters in France was performed [20] and reached a conclusion diametrically opposed to ours. Therefore, we may need to further study the brain structure alterations of female Chinese BD patients with suicidal behavior or include more samples to verify the results. Generally, we identified that there are certainly some connections between the atrophied CC region and BD with suicidal behavior, especially related to the anterior CC. This led us to make the deduction that the reason for suicidal ideation in BD may be that CC atrophy leads to impaired higher cognitive function associated with emotions, such as the regulation of emotion.
Most of these studies focused on damage to the cerebral circuitry in patients with BD who attempted suicide; however, little attention was paid to BD with suicidal ideation. We preliminarily focused on suicidal ideation with the idea that it is the first step to suicide [34] and found that BD patients with suicidal ideation have lower FA values in the body and genu of the CC. This suggests that impairments in the CC exist in the early stages of suicide risk.
Certainly, the limitations of this study should also be considered. Firstly, there were a small number of subjects included in this study. Thus, the characteristics of the CC in BD patients with suicidal behavior still need to be validated in a larger sample. Secondly, our sample size was not sufficient to analyze differences between patients with type I and type II BD; later studies should examine the relationship with BD type to produce more convincing results. Another limitation was medication is hardly to deal with. Although many studies showed no significant effects of medications on FA, a few studies suggested that those patients with BD treated with a mood stabilizer (versus not taking mood stabilizers) [35] or lithium (versus not taking lithium) [36, 37] may contribute to increased FA values in WM tracts. In the present study, we were not sure how medication affect those DTI measurements and how differ in WM tracts among groups with different drug abuse merits. In the future, we need to recruit more nonmedicated patients with BD to explore this problem (Additional file 1: Table S1).
In summary, this study proved that brain alterations in the body and genu of the CC are associated with suicidal ideation in BD, while changes in the splenium of the CC may be a pathological trait of BD. Moreover, our study provided preliminary imaging evidence for the early identification and prevention of suicide risk in BD and presented a good example for further study of functional and structural features in BD with suicidal ideation and attempts. In the future, large samples and longitudinal studies are needed to identify the specific imaging markers associated with suicidal risk in BD. Additional studies of the role of the corpus callosum in suicidal behavior in BD are also warranted.
Supplementary information
Additional file 1: Table S1. The medication status of the two BD groups.
Acknowledgements
The authors appreciate the study participants for their cooperation and the First Affiliated Hospital of China Medical University for its active support in the project.
Abbreviations
- CC
corpus callosum
- BD
bipolar disorder
- DTI
diffusion tensor imaging
- HC
health controls
- FA
fractional anisotropy
- MRI
magnetic resonance imaging
- WM
white matter
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- HAMD_17
the 17-item Hamilton Depression Rating Scale
- HAMA
Hamilton Anxiety Scale
- YMRS
Young Manic Rating Scale
- PANDA
the Pipeline for Analyzing braiN Diffusion imAges
- MNI
Montreal Neurological Institute
- ROI
region of interest
- ANOVA
analyses of variance
- SPM
Statistical Parametric Mapping Software
- DPABI
Data Processing and Analysis for Brain Imaging software
- GRF
Gaussian Random Field
- LSD
least significant difference test
Authors’ contributions
RZ conceived, designed and coordinated the study, and drafted the manuscript. SW support data collection and contributed to drafting the manuscript. MC and XJ collected data. YT and FW support data collection and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (81701336 to Shengnan Wei, 81571311, 81071099 and 81271499 to Yanqing Tang, 81725005, 81571331 to Fei Wang), National Key Research and Development Program (2016YFC1306900 to Yanqing Tang), Liaoning Education Foundation (L2015591 to Shengnan Wei), Liaoning Education Foundation (Pandeng Scholar, Fei Wang), National Key Research and Development Program (2016YFC0904300 to Fei Wang).
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional. And, the study was approved by the Medical Science Research Ethics Committee of the First Affiliated Hospital of China Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ran Zhang, Email: ran_1994@yeah.net.
Xiaowei Jiang, Email: jxw024@163.com.
Miao Chang, Email: changmiao618@163.com.
Shengnan Wei, Phone: 8624-83283405, Email: weisn_1982@126.com.
Yanqing Tang, Phone: 8624-83283405, Email: yanqingtang@163.com.
Fei Wang, Phone: 8624-83283405, Email: fei.wang@cmu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12991-019-0243-5.
References
- 1.Gliatto MF, Rai AK. Evaluation and treatment of patients with suicidal ideation. Am Fam Physician. 1999;59(6):1500–1506. [PubMed] [Google Scholar]
- 2.Baldessarini RJ, Faedda GL. Antidepressant-associated mood-switching and transition from unipolar major depression to bipolar disorder: a review. J Affect Disord. 2013;148(1):129–135. doi: 10.1016/j.jad.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Kutcher S, Chehil S. Suicide risk management: a manual for health professionals. New York: Wiley; 2007. [Google Scholar]
- 4.Anderson IM, Haddad PM, Scott J. Bipolar disorder. Bmj. 2012;345(345):e8508. doi: 10.1136/bmj.e8508. [DOI] [PubMed] [Google Scholar]
- 5.Pompili M, Ehrlich S, De PE, Mann JJ, Innamorati M, Cittadini A, Montagna B, Iliceto P, Romano A, Amore M. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257(8):494–499. doi: 10.1007/s00406-007-0755-x. [DOI] [PubMed] [Google Scholar]
- 6.Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, Serafini G, Rigucci S, Romano A, Tamburello A, et al. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1501–1507. doi: 10.1016/j.pnpbp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Pompili M, Serafini G, Innamorati M, Serra G, Forte A, Lester D, Ducci G, Girardi P, Tatarelli R. White matter hyperintensities, suicide risk and late-onset affective disorders: an overview of the current literature. La Clinica terapeutica. 2010;161(6):555–563. [PubMed] [Google Scholar]
- 8.Serafini G, Pompili M, Innamorati M, Fusarpoli P, Akiskal HS, Rihmer Z, Lester D, Romano A, de Oliveira IR, Strusi L. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord. 2011;129(1):47–55. doi: 10.1016/j.jad.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Lijffijt M, Rourke ED, Swann AC, Zuntasoares GB, Soares JC. Illness-course modulates suicidality-related prefrontal gray matter reduction in women with bipolar disorder. Acta Psychiatr Scand. 2014;130(5):374–387. doi: 10.1111/acps.12314. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F, Riccaboni R, Poletti S, Radaelli D, Locatelli C, Lorenzi C, Pirovano A, Smeraldi E, Colombo C. The serotonin transporter genotype modulates the relationship between early stress and adult suicidality in bipolar disorder. Bipolar Disord. 2014;16(8):857–866. doi: 10.1111/bdi.12250. [DOI] [PubMed] [Google Scholar]
- 11.Giakoumatos CI, Tandon N, Shah J, Mathew IT, Brady RO, Clementz BA, Pearlson GD, Thaker GK, Tamminga CA, Sweeney JA. Are structural brain abnormalities associated with suicidal behavior inpatients with psychotic disorders? J Psychiatr Res. 2013;47(10):1389–1395. doi: 10.1016/j.jpsychires.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dgg D, Mcl N, Albuquerque MR, Turecki G, Ding Y, de SouzaDuran FL, Busatto G, Correa H. Structural brain abnormalities in patients with type I bipolar disorder and suicidal behavior. Psychiatry Res. 2017;265:9. doi: 10.1016/j.pscychresns.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Johnston JA, Wang F, Liu J, Blond BN, Wallace A, Liu J, Spencer L, Cox Lippard ET, Purves KL, Landerosweisenberger A. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry. 2017;174(7):667–675. doi: 10.1176/appi.ajp.2016.15050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rueckert L, Levy J. Further evidence that the callosum is involved in sustaining attention. Neuropsychologia. 1996;34(9):927–935. doi: 10.1016/0028-3932(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 15.Schutter DJ, Harmon-Jones E. The corpus callosum: a commissural road to anger and aggression. Neurosci Biobehav Rev. 2013;37(10):2481–2488. doi: 10.1016/j.neubiorev.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15(2):59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 17.Paul LK, Van LD, Schieffer B, Dietrich R, Brown WS. Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain Lang. 2003;85(2):313–324. doi: 10.1016/S0093-934X(03)00062-2. [DOI] [PubMed] [Google Scholar]
- 18.Erickson RL, Paul LK, Brown WS. Verbal learning and memory in agenesis of the corpus callosum. Neuropsychologia. 2014;60:121. doi: 10.1016/j.neuropsychologia.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37(Pt 5):1096. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- 20.Cyprien F, Champfleur NMD, Deverdun J, Olié E, Bars EL, Bonafé A, Mura T, Jollant F, Courtet P, Artero S. Corpus callosum integrity is affected by mood disorders and also by the suicide attempt history: a diffusion tensor imaging study. J Affect Disord. 2016;206:115–124. doi: 10.1016/j.jad.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Nielsen N, Nicoletti MA, Hatch JP, Monkul ES, Watanabe Y, Zuntasoares GB, Nery FG, Soares JC. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci Lett. 2010;469(1):75–80. doi: 10.1016/j.neulet.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasar AS, Monkul ES, Sassi RB. MRI study of corpus callosum in children and adolescents with bipolar disorder. Psychiatry Res Neuroimaging. 2015;146(1):83–85. doi: 10.1016/j.pscychresns.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Kovacs M, Weissman A. Assessment of suicidal ideation: the scale for suicide ideation. J Consult Clin Psychol. 1979;47(2):343–352. doi: 10.1037/0022-006X.47.2.343. [DOI] [PubMed] [Google Scholar]
- 24.Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7(42):42. doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Rushton A. Gray’s anatomy: the anatomical basis of clinical practice. Int J Ther Rehabil. 2006;13(5):237. [Google Scholar]
- 27.Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118(5):357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 28.Brambilla P, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biol Psychiat. 2003;54(11):1294–1297. doi: 10.1016/S0006-3223(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd AJ, Ali HE, Nesbitt D, Moore PB, Young AH, Ferrier IN. Corpus callosum changes in euthymic bipolar affective disorder. Br J Psychiatry J Ment Sci. 2014;204(2):129–136. doi: 10.1192/bjp.bp.112.123687. [DOI] [PubMed] [Google Scholar]
- 30.Sarrazin S, d’Albis MA, McDonald C, Linke J, Wessa M, Phillips M, Delavest M, Emsell L, Versace A, Almeida J, et al. Corpus callosum area in patients with bipolar disorder with and without psychotic features: an international multicentre study. J Psychiatry Neurosci. 2015;40(5):352–359. doi: 10.1503/jpn.140262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nau JY. Suicidal behavior associated with atrophied corpus callosum in the elderly. Revue Medicale Suisse. 2011;7(295):1114–1115. [PubMed] [Google Scholar]
- 32.Cyprien F, Courtet P, Malafosse A, Maller J, Meslin C, Bonafé A, Bars EL, Champfleur NMD, Ritchie K, Artero S. Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiat. 2011;70(4):320–326. doi: 10.1016/j.biopsych.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Gifuni AJ, Olie E, Ding Y, Cyprien F, le Bars E, Bonafe A, Courtet P, Jollant F. Corpus callosum volumes in bipolar disorders and suicidal vulnerability. Psychiatry Res Neuroimaging. 2017;262:47–54. doi: 10.1016/j.pscychresns.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Serafini G, Solano P, Amore M. Suicidal ideation: a comprehensive overview. New York: Nova Science Publishers Inc.; 2015. [Google Scholar]
- 35.Amelia V, Almeida JRC, Stefanie H, Walsh ND, Massimiliano N, Klein CR, Kupfer DJ, Phillips ML. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65(9):1041. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sussmann J, Lymer K, Mckirdy J, Moorhead W, Maniega SM, Job D, Hall J, Bastin M, Johnstone E, Lawrie S. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;98(1):6. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 37.Macritchie KA, Lloyd AM. White matter microstructural abnormalities in euthymic bipolar disorder. Br J Psychiatry. 2010;196(1):52–58. doi: 10.1192/bjp.bp.108.058586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The medication status of the two BD groups.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.