Key Points
Question
Is dialysis facility ownership associated with access to kidney transplantation?
Findings
In this retrospective cohort study that included 1 478 564 patients with end-stage kidney disease treated at 6511 US dialysis facilities from 2000-2016, patients receiving dialysis at for-profit facilities vs nonprofit facilities had significantly lower 5-year cumulative incidence rates for placement on the deceased donor kidney transplantation waiting list (−13.2%), receipt of a living donor kidney transplant (−2.3%), and receipt of a deceased donor kidney transplant (−4.3%).
Meaning
Receiving dialysis at for-profit facilities in the United States was associated with lower kidney transplantation rates.
Abstract
Importance
For-profit (vs nonprofit) dialysis facilities have historically had lower kidney transplantation rates, but it is unknown if the pattern holds for living donor and deceased donor kidney transplantation, varies by facility ownership, or has persisted over time in a nationally representative population.
Objective
To determine the association between dialysis facility ownership and placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, or receipt of a deceased donor kidney transplant.
Design, Setting, and Participants
Retrospective cohort study that included 1 478 564 patients treated at 6511 US dialysis facilities. Adult patients with incident end-stage kidney disease from the US Renal Data System (2000-2016) were linked with facility ownership (Dialysis Facility Compare) and characteristics (Dialysis Facility Report).
Exposures
The primary exposure was dialysis facility ownership, which was categorized as nonprofit small chains, nonprofit independent facilities, for-profit large chains (>1000 facilities), for-profit small chains (<1000 facilities), and for-profit independent facilities.
Main Outcomes and Measures
Access to kidney transplantation was defined as time from initiation of dialysis to placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, or receipt of a deceased donor kidney transplant. Cumulative incidence differences and multivariable Cox models assessed the association between dialysis facility ownership and each outcome.
Results
Among 1 478 564 patients, the median age was 66 years (interquartile range, 55-76 years), with 55.3% male, and 28.1% non-Hispanic black patients. Eighty-seven percent of patients received care at a for-profit dialysis facility. A total of 109 030 patients (7.4%) received care at 435 nonprofit small chain facilities; 78 287 (5.3%) at 324 nonprofit independent facilities; 483 988 (32.7%) at 2239 facilities of large for-profit chain 1; 482 689 (32.6%) at 2082 facilities of large for-profit chain 2; 225 890 (15.3%) at 997 for-profit small chain facilities; and 98 680 (6.7%) at 434 for-profit independent facilities. During the study period, 121 680 patients (8.2%) were placed on the deceased donor waiting list, 23 762 (1.6%) received a living donor kidney transplant, and 49 290 (3.3%) received a deceased donor kidney transplant. For-profit facilities had lower 5-year cumulative incidence differences for each outcome vs nonprofit facilities (deceased donor waiting list: −13.2% [95% CI, −13.4% to −13.0%]; receipt of a living donor kidney transplant: −2.3% [95% CI, −2.4% to −2.3%]; and receipt of a deceased donor kidney transplant: −4.3% [95% CI, −4.4% to −4.2%]). Adjusted Cox analyses showed lower relative rates for each outcome among patients treated at all for-profit vs all nonprofit dialysis facilities: deceased donor waiting list (hazard ratio [HR], 0.36 [95% CI, 0.35 to 0.36]); receipt of a living donor kidney transplant (HR, 0.52 [95% CI, 0.51 to 0.54]); and receipt of a deceased donor kidney transplant (HR, 0.44 [95% CI, 0.44 to 0.45]).
Conclusions and Relevance
Among US patients with end-stage kidney disease, receiving dialysis at for-profit facilities compared with nonprofit facilities was associated with a lower likelihood of accessing kidney transplantation. Further research is needed to understand the mechanisms behind this association.
This cohort study characterizes the association of for-profit vs nonprofit dialysis facility ownership with placement on the deceased donor kidney transplantation waiting list or receipt of a living donor or a deceased donor kidney transplant.
Introduction
Kidney transplantation is the preferred treatment for most of the 700 000 adults with end-stage kidney disease (ESKD) in the United States as of 2016.1,2,3 However, only 14% of patients with incident ESKD are placed on the deceased donor kidney transplantation waiting list or receive transplants within 1 year of ESKD diagnosis.2 The Centers for Medicare & Medicaid Services (CMS) requires dialysis facilities to provide transplant education and help interested patients pursue kidney transplantation.4 The CMS amended the final rule for the End-Stage Renal Disease Prospective Payment System in 2018 by proposing a new dialysis facility quality metric to the End-Stage Renal Disease Quality Incentive Program to monitor the percentage of prevalent patients with ESKD on the waiting list for transplantation.5 In a July 2019 presidential executive order, the CMS also proposed the End-Stage Renal Disease Treatment Choices Model to improve access to kidney transplantation as part of the administration’s Advancing American Kidney Health initiative.6
Evidence suggests for-profit dialysis facilities have a lower standardized transplantation ratio,7 and their patients are less likely to be waitlisted compared with nonprofit facilities.8,9 Physicians at for-profit dialysis facilities are less likely to have detailed discussions with patients about transplantation or involve families in the discussion.10 This could lead to limited access to living donor kidney transplantation at for-profit dialysis facilities. Encouragement to pursue living donor kidney transplantation is highly advisable due to the limited supply of organs; no published study has assessed this relationship.
It has been suggested that for-profit dialysis facilities strive to reduce operating costs by limiting the provision of low-margin services11,12,13 (such as extended transplant discussions with patients and their families) in the interest of increasing returns to investors,14 and may impede their patients’ access to transplantation. This study aimed to determine the association between dialysis facility ownership and placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, or receipt of a deceased donor kidney transplant. Changes over time in these associations also were examined.
Methods
Data Sources
A waiver of informed consent was granted for this retrospective, deidentified study (Emory University institutional review board decision No. 63645). All patients with incident ESKD from the publicly available US Renal Data System2 (USRDS) database (January 1, 2000-December 31, 2016) were merged with dialysis facility–level data from the Dialysis Facility Compare (2016) and the Dialysis Facility Report (2013-2016) CMS data sets. The USRDS collects information on US patients with ESKD at the start of long-term dialysis or receipt of a kidney transplant from the CMS-2728 form; it is prelinked with the United Network for Organ Sharing data on kidney transplantation waiting list and transplant events.
Dialysis Facility Compare reports the dialysis facility’s profit status and corporate ownership. The Dialysis Facility Report captures information on facility-level patient characteristics (mean age, percentage of males, race), mortality, treatment patterns, and transplantation rates. Patient observations from the USRDS and dialysis facility–level information from the Dialysis Facility Compare and the Dialysis Facility Report were linked using the facility’s CMS certification number. To obtain information on rurality, patient zip codes were linked to 2013 Rural Urban Continuum Codes from the US Department of Agriculture. Addresses from the Dialysis Facility Report were geocoded and used to calculate the distance from each facility to its nearest transplant center, in miles.
Study Population
All incident patients undergoing dialysis registered with the USRDS between January 1, 2000, and December 31, 2016, were considered for inclusion. Patients were excluded if they were younger than 18 years or older than 100 years, had a previous transplant or received multiple organ transplants, resided outside the 50 US states at the time of dialysis start, had unknown race/ethnicity or sex, or were placed on the waiting list or received a transplant prior to starting dialysis. Facilities with records in both the Dialysis Facility Compare and Dialysis Facility Report data sets were eligible for merging with the USRDS patient-level data (Figure 1).
Figure 1. Data Merge and Cohort Selection to Assess the Association Between Dialysis Facility Ownership and Access to Kidney Transplantation.
aThere were 2948 dialysis facilities within the US Renal Data System that could not be linked to the Dialysis Facility Compare and Dialysis Facility Report cohort. There were 13 facilities that were in the Dialysis Facility Compare and Dialysis Facility Report cohort that could not be linked to the US Renal Data System.
bOnly a single observation for when the event (placement on the deceased donor kidney transplantation waiting list or receipt of a kidney transplant) occurred was included. The last observation was included for patients who died or were censored at the end of the study (December 31, 2016).
Study Variables
The primary exposure was dialysis facility profit status and chain ownership obtained from the Dialysis Facility Compare data set. Facility profit status was defined as for-profit or nonprofit. Nonprofit facilities were defined as either small chain facilities or independent facilities based on Dialysis Facility Compare’s use of the term chain organization. Within the for-profit facility ownership categories, chain ownership was collapsed for each chain reported in Dialysis Facility Compare. For-profit chains with more than 1000 facilities were categorized as large chains; DaVita and Fresenius Medical Care (referred to as large chain 1 and large chain 2, respectively) were the only 2 chains with more than 1000 facilities and were categorized separately. For-profit chains with less than 1000 dialysis facilities were categorized collectively as small chains. The remaining facilities were classified as for-profit independent facilities. Patients were assigned to the dialysis facility from which they were receiving treatment when the outcome of interest occurred (ie, placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, receipt of a deceased donor kidney transplant, death, or study end), and contributed all their person-time from dialysis start to event of interest to this facility.
The primary outcome was access to kidney transplantation, which was defined as placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, or receipt of a deceased donor kidney transplant, each analyzed separately. If a patient was waitlisted and received a living or deceased donor kidney transplant, they contributed an event to each model. Patients were censored for either death or the end of the study (December 31, 2016), or the transplant date. The time to an event was calculated from the dialysis start date to the event date or censor date.
Clinical and demographic characteristics of patients were obtained from the USRDS data and collected from the CMS-2728 form by a dialysis facility staff member at dialysis start. Known risk factors for delayed transplantation were considered, including age at the start of dialysis, sex, race/ethnicity (both were classified by dialysis facility staff in fixed categories: sex as either male or female; race/ethnicity as either non-Hispanic white, non-Hispanic black, Hispanic white, or other defined as either Asian, Middle Eastern, Native American, Pacific Islander, or multiracial), rural/urban status, health insurance, primary cause of ESKD, dialysis modality (in-center hemodialysis, peritoneal dialysis, and home hemodialysis), and the presence of comorbidities such as body mass index greater than 35 (calculated as weight in kilograms divided by height in meters squared), cardiovascular disease, diabetes, and hypertension.
Due to the select all that apply format of the comorbidity section on the CMS-2728 form,5 comorbidities were coded as “yes” if a response was recorded or “no” if a response was missing. The primary cause of ESKD was categorized as diabetes, hypertension, glomerulonephritis, or other disease. Cardiovascular disease was defined as the presence of any of the following cardiac or pulmonary conditions: congestive heart failure, atherosclerotic heart disease, pulmonary vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, or other cardiac disease.
Dialysis facility–level characteristics were obtained from either the Dialysis Facility Compare or Dialysis Facility Report data sets. Patient-level variables from the USRDS were aggregated to define the facility-level number of patients and the percentage of patients reported by the facility that were not informed of kidney transplantation due to medical reasons. The Dialysis Facility Report provided the facility-level number of social workers, the ratio of patients to social workers, the ratio of patients to staff, the standardized mortality ratio, and the ESRD Network geographic areas.
The hospitalization rate per 100 person-years was obtained from the Dialysis Facility Compare data set. Transplant-center affiliation was defined through the United Network for Organ Sharing transplant center ID; and the hospital affiliation of a facility was defined by its CMS certification number. The addresses from the Dialysis Facility Compare data set were geocoded and used to calculate the distance from each facility to its nearest transplant center in miles.
Statistical Analysis
A descriptive analysis was performed for the overall population and comparisons between facility ownership were determined using either the χ2 test (for categorical variables), the Kruskal-Wallis test (for nonparametric continuous variables presented as median interquartile range [IQR]), or the t test (for parametric continuous variables presented as mean [SD]). Cumulative incidence differences and 95% CIs were calculated using the Aalen-Johansen estimator at follow-up years 3, 5, and 10 and accounted for the competing risk of death.15 The number needed to treat for each outcome was calculated using the inverse of the 5-year cumulative incidence difference.
To examine whether the cumulative incidence difference between facilities was constant by calendar year, we stratified the cohort into 2-year increments. Follow-up time was truncated for each period on December 31 of each 2-year period, and the unadjusted 2-year incidence rates (per 100 person-years) for placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, and receipt of a deceased donor kidney transplant were calculated by dividing the count of events for incident patients by the number of incident patients in the dialysis facility ownership group for each interval. The incident rate differences (per 100 person-years) were calculated as the 2-year incidence difference between all nonprofit and all for-profit facilities.
Bivariable Cox proportional hazard models were used to determine the crude association between covariates and access to transplantation. Cox models were used to determine the crude and adjusted association between dialysis facility ownership and each outcome. Multiple imputation using the fully conditional specification implemented by the chained equations (MICE) algorithm were performed for missing covariate data. For continuous and categorical variables, Ridge Bayesian linear regression, allowing for posterior sampling and random forest, respectively, was conducted.16
We used robust sandwich covariance matrix estimates to account for intracluster dependence17 of patients within dialysis facilities. The adjusted Cox proportional hazard models in this study were developed using a 4-step process: (1) bivariable association between dialysis facility ownership and outcomes of interest, (2) adjustment for patient demographics (age, sex, and race/ethnicity), (3) adjustment for clinical characteristics, and (4) additional adjustment for socioeconomic variables. We tested the proportional hazards assumption by examining the significance of the Schoenfeld residuals with ranked follow-up time, and all proportionality assumptions were met.
Several sensitivity analyses were conducted to ensure the robustness of the results. Supplemental descriptive statistics and multivariable Cox models were generated for this population and were stratified by whether a patient switched dialysis facilities during the study follow-up. Dialysis facility switching was classified by comparing the patients’ first vs last dialysis facility. Sensitivity analyses examined cumulative incidence differences. Cox models were used to assess a cohort of “ideal kidney transplant candidates” that excluded patients (1) aged 66 years or older; (2) diagnosed with peripheral vascular disease, coronary heart failure, or cerebrovascular disease; or (3) not assessed for transplantation due to medical reasons (reported by dialysis facility staff on the CMS-2728 form). The Cox models assessed effect modification of geographic area and the association between facility affiliation with either a transplant center or hospital and each outcome.
Data management and statistical analysis were conducted using SAS version 9.4 (SAS Institute Inc) and Python version 3.6.8 (Python Software Foundation). Two-sided P values were used for all analyses and P < .05 was considered statistically significant.
Results
There were 1 865 462 incident patients with ESKD registered with the USRDS between January 1, 2000, and December 31, 2016. Patients were excluded if (1) younger than 18 years (n = 15 559) or older than 100 years (n = 67), (2) had a prior kidney transplant or index multiple organ transplants (n = 4772), (3) were living outside the United States (n = 27 934), (4) had unknown race/ethnicity (n = 1546) or sex (n = 222), (5) were preemptively waitlisted or received a transplant prior to initiating dialysis (n = 91 722), (6) had duplicate observations that did not occur at the last treatment facility (n = 1045), and (7) did not match to a dialysis facility within the Dialysis Facility Compare or Dialysis Facility Report data sets (n = 243 981), leaving a final cohort of 1 478 564 unique patients included in the primary analysis (Figure 1). The median age was 66 years (IQR, 55-76 years), with 55.3% male, and 28.1% non-Hispanic black patients. Multiple imputation was used in the Cox models to account for the following missing data: health insurance coverage: 115 322 patients (7.8%); type of dialysis: 7805 (0.5%); distance from assigned dialysis facility to nearest transplant center: 319 117 (21.6%); and metropolitan rural-urban classification: 13 322 (0.9%).
The 1 478 564 included patients had a median follow-up time to placement on waiting list or censor date of 2.0 years (IQR, 0.7-4.2 years) and a median follow-up time to transplant or censor date of 2.2 years (IQR, 0.8-4.5 years). The majority of patients with ESKD received care at for-profit large chains with more than 1000 facilities (for-profit large chain 1: n = 483 988 [32.7%] at 2239 facilities; for-profit large chain 2: n = 482 689 [32.6%] at 2082 facilities; P < .001; Table 1). Nonprofit dialysis facilities had a slightly lower percentage of non-Hispanic black patients compared with for-profit facilities (26.8% vs 28.3%, respectively; P < .001), and there was a higher percentage of patients in the South treated at for-profit facilities compared with nonprofit facilities (42.9% vs 29.4%; P < .001) (Table 1 and eFigure in the Supplement).
Table 1. Patient- and Facility-Level Characteristics at Start of Dialysis Within the US Renal Data System Overall and Stratified by Dialysis Facility Ownership, 2000-2016a.
| Characteristicsb | Total | Nonprofit Dialysis Facilitiesc | For-Profit Dialysis Facilitiesc | All Facilities | |||||
|---|---|---|---|---|---|---|---|---|---|
| Small Chains | Independent | Large Chain 1 | Large Chain 2 | Small Chains | Independent | Nonprofit | For-Profit | ||
| Facilities | 6511 (100) | 435 (6.7) | 324 (5.0) | 2239 (34.4) | 2082 (32.0) | 997 (15.3) | 434 (6.7) | 759 (11.7) | 5752 (88.3) |
| Patients | 1 478 564 (100) | 109 030 (7.4) | 78 287 (5.3) | 483 988 (32.7) | 482 689 (32.6) | 225 890 (15.3) | 98 680 (6.7) | 187 317 (12.7) | 1 291 247 (87.3) |
| Patient-Level Characteristics at Start of Dialysisb | |||||||||
| Age, median (IQR), y | 66 (55-76) | 65 (54-75) | 62 (51-73) | 66 (55-76) | 66 (55-76) | 66 (55-76) | 67 (56-76) | 64 (53-74) | 66 (55-76) |
| Age group, y | |||||||||
| 18-29 | 31 745 (2.1) | 2721 (2.5) | 3024 (3.9) | 10 371 (2.1) | 9456 (2.0) | 4379 (1.9) | 1794 (1.8) | 5745 (3.1) | 26 000 (2.0) |
| 30-39 | 67 186 (4.5) | 5372 (4.9) | 5069 (6.5) | 21 785 (4.5) | 21 195 (4.4) | 9936 (4.4) | 3829 (3.9) | 10 441 (5.6) | 56 745 (4.4) |
| 40-49 | 143 339 (9.7) | 11 138 (10.2) | 9988 (12.8) | 46 268 (9.6) | 45 509 (9.4) | 21 812 (9.7) | 8624 (8.7) | 21 126 (11.3) | 122 213 (9.5) |
| 50-59 | 268 858 (18.2) | 20 320 (18.6) | 16 313 (20.8) | 87 683 (18.1) | 86 466 (17.9) | 40 996 (18.1) | 17 080 (17.3) | 36 633 (19.6) | 232 225 (18.0) |
| 60-69 | 364 820 (24.7) | 26 401 (24.2) | 18 569 (23.7) | 119 203 (24.6) | 120 011 (24.9) | 56 176 (24.9) | 24 460 (24.8) | 44 970 (24.0) | 319 850 (24.8) |
| ≥70 | 602 616 (40.8) | 43 078 (39.5) | 25 324 (32.3) | 198 678 (41.1) | 200 052 (41.4) | 92 591 (41.0) | 42 893 (43.5) | 68 402 (36.5) | 534 214 (41.4) |
| Sex | |||||||||
| Male | 818 096 (55.3) | 61 160 (56.1) | 45 077 (57.6) | 268 830 (55.5) | 264 618 (54.8) | 124 064 (54.9) | 54 347 (55.1) | 106 237 (56.7) | 711 859 (55.1) |
| Female | 660 468 (44.7) | 47 870 (43.9) | 33 210 (42.4) | 215 158 (44.5) | 218 071 (45.2) | 101 826 (45.1) | 44 333 (44.9) | 81 080 (43.3) | 579 388 (44.9) |
| Race/ethnicity | |||||||||
| Non-Hispanic white | 805 905 (54.5) | 63 076 (57.9) | 41 721 (53.3) | 258 373 (53.4) | 280 730 (58.2) | 113 594 (50.3) | 48 411 (49.1) | 109 775 (55.7) | 696 130 (54.3) |
| Non-Hispanic black | 415 353 (28.1) | 28 831 (26.4) | 21 050 (26.9) | 134 024 (27.7) | 138 400 (28.7) | 65 881 (29.2) | 27 167 (27.5) | 52 798 (26.8) | 362 555 (28.3) |
| Hispanic white | 178 763 (12.1) | 9222 (8.5) | 9997 (12.8) | 65 480 (13.5) | 48 979 (10.1) | 28 906 (12.8) | 16 179 (16.4) | 20 304 (10.3) | 158 459 (12.4) |
| Otherd | 78 543 (5.3) | 7901 (7.2) | 5519 (7.0) | 26 111 (5.4) | 14 580 (3.0) | 17 509 (7.8) | 6923 (7.0) | 14 197 (7.2) | 6346 (5.0) |
| Insurance coveragee | |||||||||
| Medicare | 542 921 (39.8) | 40 590 (40.4) | 24 339 (33.2) | 172 010 (38.9) | 186 054 (41.5) | 84 647 (40.8) | 35 281 (38.4) | 64 929 (37.3) | 477 992 (40.2) |
| Medicaid | 377 827 (27.7) | 25 996 (25.8) | 20 724 (28.3) | 126 842 (28.7) | 115 911 (25.9) | 57 570 (27.8) | 30 784 (33.5) | 46 720 (26.9) | 331 107 (27.8) |
| Employer | 239 678 (17.6) | 18 328 (18.2) | 14 592 (19.9) | 77 010 (17.4) | 83 322 (18.6) | 34 250 (16.5) | 12 176 (13.3) | 32 920 (18.9) | 206 758 (17.4) |
| None | 103 263 (7.6) | 7957 (7.9) | 6573 (9.0) | 32 806 (7.4) | 33 776 (7.5) | 16 059 (7.7) | 6092 (6.6) | 14 530 (8.4) | 88 733 (7.5) |
| Otherf | 99 553 (7.3) | 7762 (7.7) | 7063 (9.6) | 33 113 (7.6) | 29 064 (6.5) | 14 998 (7.2) | 7553 (8.2) | 14 825 (8.5) | 84 728 (7.1) |
| Attributed cause of ESKD | |||||||||
| Diabetes | 694 455 (47.0) | 51 095 (46.9) | 34 276 (43.8) | 229 209 (47.4) | 226 561 (46.9) | 106 898 (47.3) | 46 416 (47.1) | 85 371 (45.6) | 609 084 (47.2) |
| Hypertension | 439 480 (29.7) | 27 941 (25.6) | 19 561 (25.0) | 143 466 (29.6) | 147 224 (30.5) | 70 466 (31.2) | 30 822 (31.2) | 47 502 (25.4) | 391 978 (30.3) |
| Other disease | 236 794 (16.0) | 20 085 (18.4) | 15 942 (20.3) | 76 880 (15.9) | 75 047 (15.5) | 33 450 (14.8) | 15 390 (15.6) | 36 027 (19.2) | 200 767 (15.5) |
| Glomerulonephritis | 107 835 (7.3) | 9909 (9.1) | 8508 (10.9) | 34 433 (7.1) | 33 857 (7.0) | 15 076 (6.7) | 6052 (6.1) | 18 417 (9.8) | 89 418 (6.9) |
| Dialysis typeg | |||||||||
| In-center hemodialysis | 1 340 123 (91.1) | 98 475 (90.8) | 70 631 (91.4) | 437 359 (90.9) | 444 756 (92.5) | 206 216 (91.6) | 82 686 (84.5) | 169 106 (91.0) | 1 171 017 (91.1) |
| Peritoneal dialysis | 108 819 (7.4) | 9085 (8.4) | 5958 (7.7) | 38 963 (8.1) | 30 590 (6.4) | 17 070 (7.6) | 7153 (7.3) | 15 043 (8.1) | 93 776 (7.3) |
| Home hemodialysis | 21 817 (1.5) | 922 (0.8) | 700 (0.9) | 4632 (1.0) | 5653 (1.2) | 1856 (0.8) | 8054 (8.2) | 1622 (0.9) | 20 195 (1.6) |
|
Patient-Level Comorbidities at Start of Dialysis and Measures of Access to Transplantationb | |||||||||
| Hypertension | 1 247 373 (84.4) | 92 469 (84.8) | 65 469 (83.6) | 405 013 (83.7) | 407 749 (84.5) | 192 857 (85.4) | 83 816 (84.9) | 157 938 (84.3) | 1 089 435 (84.4) |
| Diabetes | 710 345 (48.0) | 52 311 (48.0) | 34 245 (43.7) | 232 745 (48.1) | 230 242 (47.7) | 111 471 (49.3) | 49 331 (50.0) | 86 556 (46.2) | 623 789 (48.3) |
| Congestive heart failure | 490 683 (33.2) | 37 448 (34.3) | 23 981 (30.6) | 157 215 (32.5) | 162 272 (33.6) | 74 983 (33.2) | 34 784 (35.2) | 61 429 (32.8) | 429 254 (33.2) |
| BMI >35h | 275 833 (18.7) | 19 509 (17.9) | 13 701 (17.5) | 88 874 (18.4) | 94 357 (19.5) | 41 854 (18.5) | 17 538 (17.8) | 33 210 (17.7) | 242 623 (18.8) |
| Other cardiac disease | 206 715 (14.0) | 15 800 (14.5) | 9731 (12.4) | 64 309 (13.3) | 68 370 (14.2) | 32 509 (14.4) | 15 996 (16.2) | 25 531 (13.6) | 181 184 (14.0) |
| Atherosclerotic heart disease | 205 331 (13.9) | 17 214 (15.8) | 11 956 (15.3) | 60 848 (12.6) | 68 021 (14.1) | 30 827 (13.6) | 16 465 (16.7) | 29 170 (15.6) | 176 161 (13.6) |
| Peripheral vascular disease | 198 760 (13.4) | 16 117 (14.8) | 10 166 (13.0) | 61 933 (12.8) | 67 045 (13.9) | 29 942 (13.3) | 13 557 (13.7) | 26 283 (14.0) | 172 477 (13.4) |
| Cerebrovascular disease | 143 362 (9.7) | 11 873 (10.9) | 6762 (8.6) | 44 201 (9.1) | 47 882 (9.9) | 22 045 (9.8) | 10 599 (10.7) | 18 635 (9.9) | 124 727 (9.7) |
| COPD | 142 639 (9.6) | 11 763 (10.8) | 6653 (8.5) | 45 302 (9.4) | 47 751 (9.9) | 21 097 (9.3) | 10 073 (10.2) | 18 416 (9.8) | 124 223 (9.6) |
| Cancer | 109 679 (7.4) | 9566 (8.8) | 5686 (7.3) | 34 539 (7.1) | 36 130 (7.5) | 16 387 (7.3) | 7371 (7.5) | 15 252 (8.1) | 94 427 (7.3) |
| Tobacco use | 92 390 (6.2) | 8788 (8.1) | 4514 (5.8) | 29 497 (6.1) | 30 981 (6.4) | 13 295 (5.9) | 5315 (5.4) | 13 302 (7.1) | 79 088 (6.1) |
| Nephrology care before ESKDi | 622 983 (42.1) | 50 017 (45.9) | 33 033 (42.2) | 199 389 (41.2) | 202 491 (42.0) | 97 429 (43.1) | 40 624 (41.2) | 83 050 (69.3) | 539 933 (66.5) |
| Period of dialysis, median (IQR), y | |||||||||
| Before placement on waiting listj | 2.0 (0.7-4.2) | 1.9 (0.7-4.2) | 1.5 (0.6-3.7) | 2.0 (0.7-4.3) | 2.0 (0.7-4.2) | 2.0 (0.7-4.2) | 1.9 (0.7-4.1) | 1.8 (0.6-4.0) | 2.0 (0.7-4.2) |
| Before first transplantk | 2.2 (0.8-4.5) | 2.3 (0.8-4.6) | 2.6 (1.0-5.0) | 2.2 (0.8-4.5) | 2.1 (0.8-4.4) | 2.1 (0.8-4.4) | 2.0 (0.7-4.4) | 2.4 (0.9-4.8) | 2.1 (0.8-4.4) |
| Not informed of transplant options due to medical reasonsl | 80 085 (5.4) | 8040 (7.4) | 5761 (7.4) | 21 567 (4.5) | 25 617 (5.3) | 11 804 (5.2) | 7296 (7.4) | 14 384 (7.3) | 65 701 (5.1) |
| Dialysis Facility-Level Characteristics | |||||||||
| Patients per facility, median (IQR) | 60 (39-87) | 61 (24-103) | 57 (24-103) | 61 (40-87) | 59 (40-84) | 59 (38-88) | 63 (38-92) | 59 (31-102) | 60 (40-86) |
| Patients:staff, median (IQR)m | 3.4 (2.7-4.1) | 3.1 (2.4-3.8) | 2.5 (1.9-3.2) | 3.6 (2.9-4.3) | 3.4 (2.8-4.0) | 3.5 (2.8-4.2) | 3.0 (2.3-3.7) | 2.8 (2.2-3.6) | 3.5 (2.8-4.2) |
| Social workers per facility, median (IQR)m | 1 (0-1) | 1 (0-1) | 1 (1-1) | 1 (0-1) | 1 (0-1) | 1 (1-1) | 1 (1-1) | 1 (0-1) | 1 (0-1) |
| Patients:social workers per facility, median (IQR)m | 64 (47-83) | 66 (50-82.5) | 64 (48-82) | 63 (47-84) | 63 (43-86) | 53 (26-72) | 64.3 (49-85) | 59 (31-102) | 64.5 (48-83) |
| Hospitalization rate/100 person-years, median (IQR)n | 173.8 (142.3-209.4) |
159.7 (128.1-195.7) |
155.4 (122.2-185.5) |
179.4 (147.7-216.8) |
173.8 (142.3-208.6) |
174.2 (143.1-209.2) |
176.6 (139.7-219.7) |
157.1 (125.6-190.1) |
176.1 (144.4-211.7) |
| Standardized mortality ratio, mean (SD)m | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.5) | 1.0 (0.4) | 1.0 (0.3) | 1.0 (0.4) | 1.1 (0.5) | 1.0 (0.4) | 1.0 (0.4) |
| ESRD Network geographic areasm | |||||||||
| Northeasto | 238 682 (16.1) | 12 588 (11.5) | 32 812 (41.9) | 65 160 (13.5) | 76 260 (15.8) | 24 876 (11.0) | 26 986 (27.3) | 46 818 (23.8) | 191 864 (15.0) |
| Southp | 608 050 (41.1) | 38 995 (35.8) | 14 837 (19.0) | 186 182 (38.5) | 215 243 (44.6) | 120 286 (53.2) | 32 507 (32.9) | 58 022 (29.4) | 550 028 (42.9) |
| Midwestq | 333 802 (22.6) | 26 053 (23.9) | 20 166 (25.8) | 114 370 (23.6) | 134 604 (27.9) | 19 280 (8.5) | 19 329 (19.6) | 48 649 (24.7) | 285 153 (22.3) |
| Westr | 298 030 (20.2) | 31 394 (28.8) | 10 472 (13.4) | 118 276 (24.4) | 56 582 (11.7) | 61 448 (27.2) | 19 858 (20.1) | 43 585 (22.1) | 254 445 (19.9) |
| Metropolitan rural-urban classifications | 1 233 267 (84.2) | 88 324 (81.5) | 62 967 (81.6) | 413 497 (86.1) | 395 681 (82.6) | 187 732 (84.0) | 85 066 (88.0) | 34 191 (14.7) | 197 784 (85.3) |
| Distance from assigned facility to nearest transplant center, median (IQR), mt | 16.7 (6.1-53.2) | 14.5 (3.5-55.9) | 5.9 (0.8-46.7) | 15.2 (6.7-45.7) | 21.6 (6.6-60.8) | 16.5 (6.1-57.3) | 14.8 (6.4-53.0) | 10.1 (1.9-50.4) | 17.6 (6.6-54.1) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ESKD, end-stage kidney disease; ESRD, end-stage renal disease; IQR, interquartile range.
Data are expressed as No. (%) unless otherwise indicated. Patients were assigned to the last dialysis facility from which they were receiving treatment when the outcome event of interest occurred.
Baseline patient characteristics were obtained at the time the CMS-2728 form was completed.
P<.001 for all comparisons across nonprofit and for-profit facility ownership categories. The P values were calculated using the χ2 test for categorical variables, the Kruskal-Wallis for nonparametric continuous variables, and the t test for parametric continuous variables.
Defined as Asian, Middle Eastern, Native American, Pacific Islander, or multiracial.
There were missing data for 115 322 patients (7.8%).
This category was collected and defined on the CMS-2728 form and was not further defined.
There are missing data for 7805 patients (0.5%).
Calculated as weight in kilograms divided by height in meters squared.
There were missing data for 546 900 patients (37.0%).
Calculated as the time receiving dialysis from the first date of dialysis treatment to either the date of first placement on the deceased donor kidney transplantation waiting list, at the end of the study, or time of death.
Calculated as the time receiving dialysis from the first date of dialysis treatment to either the date of first transplant (living or deceased donor kidney transplant), at the end of the study, or at time of death.
Obtained from item 27 on the CMS-2728 form. The attending physician recorded no if the patient had not been informed of their transplant options and entered all the reasons why a kidney transplant was not an option for the patient at that time.
Obtained from the Dialysis Facility Report, 2013-2016.
Obtained from the Dialysis Facility Compare, 2016.
Network 1: Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont; Network 2: New York; Network 3: New Jersey; and Network 4: Delaware and Pennsylvania.
Network 5: District of Columbia, Maryland, Virginia, and West Virginia; Network 6: Georgia, North Carolina, and South Carolina; Network 7: Florida; Network 8: Alabama, Mississippi, and Tennessee; Network 13: Arkansas, Louisiana, and Oklahoma; and Network 14: Texas.
Network 9/10: Illinois, Indiana, Kentucky, and Ohio; Network 11: Michigan, Minnesota, North Dakota, South Dakota, and Wisconsin; and Network 12: Iowa, Kansas, Missouri, and Nebraska.
Network 15: Arizona, Colorado, Nevada, New Mexico, Utah, and Wyoming; Network 16: Alaska, Idaho, Montana, Oregon, and Washington; Network 17: Hawaii and northern California; and Network 18: southern California.
Obtained from the US Department of Agriculture Rural and Urban Continuum Code. There were missing data for 13 322 patients (0.9%).
Calculated using the dialysis facility address from Dialysis Facility Compare (2013-2016) and the nearest transplant center address from the Scientific Registry of Transplant Recipients. There were missing data for 319 177 patients (21.6%).
Patient comorbidities were mostly similar across dialysis facility ownership categories. Treatment location for approximately 84% of patients diagnosed with hypertension ranged from 83.6% at nonprofit independent facilities to 85.4% at for-profit small chain facilities (P < .001). However, for patients diagnosed with diabetes, treatment location ranged from 43.7% at nonprofit independent facilities to 50.0% at for-profit independent facilities (P < .001). A higher percentage of patients treated at nonprofit independent facilities (7.4%), nonprofit small chain facilities (7.4%), and for-profit independent facilities (7.4%) were reported by facility staff as not being informed of kidney transplant options because of medical reasons compared with patients treated at facilities of for-profit large chain 1 (4.5%), facilities of for-profit large chain 2 (5.3%), and for-profit small chain facilities (5.2%) (P < .001). All nonprofit facilities had a median hospitalization rate of 157.1 (IQR, 125.6-190.1) per 100 person-years compared with the higher rate of 176.1 (IQR, 144.4-211.7) per 100 person-years for all for-profit facilities.
Primary Analysis
A total of 121 680 patients (8.2%) with incident ESKD were placed on the deceased donor kidney transplantation waiting list, 23 762 (1.6%) received a living donor kidney transplant, and 49 290 (3.3%) received a deceased donor kidney transplant. The percentage of patients placed on the deceased donor kidney transplantation waiting list during the study period was 11.9% for those treated at nonprofit small chain facilities, 29.8% for those treated at nonprofit independent dialysis facilities, 7.0% for those treated at facilities of for-profit large chain 1, 6.2% for those treated at facilities of for-profit large chain 2, 6.6% for those treated at for-profit small chain facilities, and 6.9% for those treated at for-profit independent facilities (Table 2). There was a lower percentage of patients treated at all for-profit facilities vs all nonprofit facilities who received a living donor kidney transplant (1.3% vs 3.5%, respectively) or a deceased donor kidney transplant (2.7% vs 7.6%).
Table 2. Patient Characteristics and Crude Hierarchical Cox Proportional Hazard Ratios (HRs) Among Patients Who Were Placed on the Deceased Donor Waiting List, Received a Living Donor Kidney Transplant, or Received a Deceased Donor Kidney Transplant During Follow-up, 2000-2016.
| Characteristicsa | Censored Date (n = 1 341 386)b |
Placement on the Deceased Donor Waiting List (n = 121 680) (8.2%) |
Receipt of a Living Donor Kidney Transplant (n = 23 762) (1.6%) |
Receipt of a Deceased Donor Kidney Transplant (n = 49 290) (3.3%) |
|||
|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | HR (95% CI)c | No. (%) | HR (95% CI)d | No. (%) | HR (95% CI)e | |
| Dialysis Facility Ownershipf | |||||||
| Nonprofit | |||||||
| Small chains (n = 109 030) | 94 601 (86.8) | 12 942 (11.9) | 1 [Reference] | 2694 (2.5) | 1 [Reference] | 5337 (4.9) | 1 [Reference] |
| Independent facilities (n = 78 287) | 53 992 (69.0) | 23 324 (29.8) | 2.74 (2.68-2.80) | 3914 (5.0) | 1.90 (1.81-2.00) | 8899 (11.4) | 2.07 (2.00-2.14) |
| For-profit | |||||||
| Large chain 1 (n = 483 988) | 444 858 (91.2) | 34 027 (7.0) | 0.58 (0.57-0.59) | 6719 (1.4) | 0.57 (0.54-0.60) | 14 006 (2.9) | 0.60 (0.58-0.62) |
| Large chain 2 (n = 482 689) | 447 759 (92.8) | 29 812 (6.2) | 0.52 (0.51-0.53) | 6403 (1.3) | 0.55 (0.53-0.58) | 12 652 (2.6) | 0.56 (0.54-0.58) |
| Small chains (n = 225 890) | 209 161 (92.6) | 14 815 (6.6) | 0.55 (0.54-0.57) | 2674 (1.2) | 0.49 (0.47-0.52) | 5926 (2.6) | 0.56 (0.54-0.58) |
| Independent facilities (n = 98 680) | 91 105 (92.2) | 6760 (6.9) | 0.59 (0.57-0.61) | 1358 (1.4) | 0.59 (0.55-0.62) | 2470 (2.5) | 0.54 (0.52-0.57) |
| All facilities | |||||||
| Nonprofit (n = 187 317) | 148 593 (79.3) | 36 266 (19.4) | 1 [Reference] | 6608 (3.5) | 1 [Reference] | 14 236 (7.6) | 1 [Reference] |
| For-profit (n = 1 291 247) | 1 192 793 (92.4) | 85 414 (6.6) | 0.33 (0.32-0.33) | 17 154 (1.3) | 0.40 (0.39-0.41) | 35 054 (2.7) | 0.39 (0.38-0.40) |
|
Patient-Level Characteristics at Start of Dialysisa | |||||||
| Age group, y | |||||||
| 18-29 | 18 152 (57.2) | 11 251 (35.4) | 4.21 (4.12-4.30) | 4206 (13.2) | 10.8 (10.3-11.3) | 5709 (18.0) | 5.48 (5.30-5.68) |
| 30-39 | 46 118 (68.6) | 16 840 (25.1) | 2.94 (2.88-2.99) | 4280 (6.4) | 5.24 (5.01-5.47) | 9699 (14.4) | 4.53 (4.40-4.67) |
| 40-49 | 111 700 (77.9) | 27 361 (19.1) | 2.31 (2.27-2.35) | 5301 (3.7) | 3.09 (2.96-3.22) | 12 296 (8.6) | 2.86 (2.78-2.94) |
| 50-59 | 230 534 (85.7) | 35 491 (13.2) | 1.70 (1.68-1.73) | 5535 (2.1) | 1.83 (1.76-1.91) | 12 694 (4.7) | 1.84 (1.78-1.89) |
| 60-69 | 337 535 (92.5) | 25 839 (7.1) | 1 [Reference] | 3679 (1.0) | 1 [Reference] | 7557 (2.1) | 1 [Reference] |
| ≥70 | 597 347 (99.1) | 4898 (0.8) | 0.14 (0.13-0.14) | 761 (0.1) | 0.15 (0.14-0.17) | 1335 (0.2) | 0.16 (0.15-0.17) |
| Sex | |||||||
| Male | 733 434 (89.7) | 75 081 (9.2) | 1 [Reference] | 14 792 (1.8) | 1 [Reference] | 30 510 (3.7) | 1 [Reference] |
| Female | 607 952 (92.0) | 46 599 (7.1) | 0.75 (0.74-0.76) | 8970 (1.4) | 0.74 (0.72-0.76) | 18 780 (2.8) | 0.73 (0.72-0.74) |
| Race/ethnicity | |||||||
| Non-Hispanic white | 744 464 (92.4) | 51 267 (6.4) | 1 [Reference] | 14 763 (1.8) | 1 [Reference] | 22 036 (2.7) | 1 [Reference] |
| Non-Hispanic black | 373 135 (89.8) | 39 641 (9.5) | 1.53 (1.50-1.55) | 4044 (1.0) | 0.85 (0.82-0.88) | 16 195 (3.9) | 0.96 (0.93-0.98) |
| Hispanic white | 155 183 (86.8) | 21 491 (12.0) | 1.21 (1.19-1.23) | 3572 (2.0) | 0.42 (0.40-0.43) | 7582 (4.2) | 0.88 (0.86-0.90) |
| Otherg | 68 604 (87.3) | 9281 (11.8) | 1.57 (1.54-1.61) | 1383 (1.8) | 0.78 (0.73-0.82) | 3477 (4.4) | 1.08 (1.04-1.12) |
| Insurance coverageh | |||||||
| Medicare | 524 311 (96.6) | 17 125 (3.2) | 0.20 (0.20-0.21) | 2456 (0.5) | 0.11 (0.11-0.12) | 5472 (1.0) | 0.19 (0.18-0.19) |
| Medicaid | 350 489 (92.8) | 24 333 (6.4) | 0.37 (0.37-0.38) | 2998 (0.8) | 0.18 (0.18-0.19) | 9495 (2.5) | 0.35 (0.34-0.36) |
| Employer | 188 766 (78.8) | 43 833 (18.3) | 1 [Reference] | 11 764 (4.9) | 1 [Reference] | 19 349 (8.1) | 1 [Reference] |
| None | 84 277 (81.6) | 17 283 (16.7) | 0.74 (0.72-0.75) | 2554 (2.5) | 0.41 (0.40-0.43) | 7800 (7.6) | 0.63 (0.62-0.65) |
| Otheri | 84 717 (85.1) | 13 069 (13.2) | 0.71 (0.69-0.72) | 3070 (3.1) | 0.63 (0.61-0.66) | 5366 (5.4) | 0.66 (0.64-0.68) |
| Attributed cause of ESKD | |||||||
| Diabetes | 636 630 (91.7) | 48 240 (6.9) | 1 [Reference] | 7028 (1.0) | 1 [Reference] | 20 670 (3.0) | 1 [Reference] |
| Hypertension | 407 538 (92.7) | 30 133 (6.9) | 1.02 (1.01-1.04) | 4486 (1.0) | 1.04 (1.00-1.08) | 11 383 (2.6) | 0.85 (0.83-0.87) |
| Other disease | 215 645 (91.1) | 19 283 (8.1) | 1.26 (1.24-1.28) | 4940 (2.1) | 2.22 (2.14-2.30) | 7631 (3.2) | 1.01 (0.99-1.04) |
| Glomerulonephritis | 81 573 (75.6) | 24 024 (22.3) | 3.00 (2.96-3.05) | 7308 (6.8) | 5.95 (5.76-6.15) | 9606 (8.9) | 2.17 (2.12-2.22) |
| Dialysis typej | |||||||
| In-center hemodialysis | 1 235 615 (92.2) | 92 673 (6.9) | 1 [Reference] | 16 947 (1.3) | 1 [Reference] | 37 627 (2.8) | 1 [Reference] |
| Peritoneal dialysis | 80 218 (73.7) | 25 352 (23.3) | 3.86 (3.81-3.92) | 5983 (5.5) | 4.58 (4.45-4.72) | 10 118 (9.3) | 4.53 (4.44-4.64) |
| Home hemodialysis | 17 901 (82.1) | 3612 (16.6) | 2.39 (2.31-2.47) | 772 (3.5) | 2.67 (2.48-2.87) | 1456 (6.7) | 2.25 (2.13-2.37) |
|
Patient-Level Comorbidities at Start of Dialysis and Measures of Access to Transplantationa | |||||||
| Hypertension | 1 130 062 (90.6) | 104 489 (8.4) | 1.07 (1.05-1.09) | 19 403 (1.6) | 0.77 (0.74-0.79) | 42 009 (3.4) | 1.06 (1.03-1.09) |
| Diabetes | 655 158 (92.2) | 46 622 (6.6) | 0.70 (0.70-0.71) | 6413 (0.9) | 0.42 (0.41-0.43) | 18 896 (2.7) | 0.85 (0.84-0.87) |
| Congestive heart failure | 470 977 (96.0) | 18 107 (3.7) | 0.42 (0.41-0.42) | 2451 (0.5) | 0.28 (0.27-0.29) | 5888 (1.2) | 0.39 (0.38-0.40) |
| BMI >35k | 252 850 (91.7) | 21 749 (7.9) | 0.87 (0.86-0.88) | 3177 (1.2) | 0.61 (0.59-0.64) | 7049 (2.6) | 0.63 (0.61-0.65) |
| Other cardiac disease | 199 097 (96.3) | 7126 (3.4) | 0.49 (0.47-0.5) | 945 (0.5) | 0.32 (0.30-0.35) | 1988 (1.0) | 0.44 (0.42-0.46) |
| Atherosclerotic heart disease | 197 145 (96.0) | 7575 (3.7) | 0.47 (0.46-0.49) | 1032 (0.5) | 0.32 (0.31-0.35) | 2075 (1.0) | 0.39 (0.37-0.41) |
| Peripheral vascular disease | 191 483 (96.3) | 6485 (3.3) | 0.41 (0.40-0.42) | 934 (0.5) | 0.30 (0.28-0.32) | 2092 (1.1) | 0.39 (0.37-0.40) |
| Cerebrovascular disease | 138 017 (96.3) | 4878 (3.4) | 0.44 (0.42-0.45) | 642 (0.4) | 0.29 (0.27-0.32) | 1602 (1.1) | 0.41 (0.39-0.43) |
| COPD | 139 765 (98.0) | 2630 (1.8) | 0.26 (0.25-0.27) | 400 (0.3) | 0.21 (0.19-0.23) | 792 (0.6) | 0.25 (0.24-0.27) |
| Cancer | 106 370 (97.0) | 3064 (2.8) | 0.41 (0.40-0.43) | 641 (0.6) | 0.45 (0.42-0.49) | 946 (0.9) | 0.38 (0.35-0.40) |
| Tobacco use | 85 877 (93.0) | 5734 (6.2) | 0.72 (0.70-0.74) | 1008 (1.1) | 0.65 (0.61-0.69) | 2304 (2.5) | 0.73 (0.70-0.76) |
| Nephrology care before ESKDl | 563 625 (90.5) | 53 496 (8.6) | 1.00 (0.99-1.02) | 8777 (1.4) | 1.18 (1.15-1.22) | 19 148 (3.1) | 1.11 (1.08-1.13) |
| Not informed of transplant options due to medical reasonsm | 79 280 (99.0) | 777 (1.0) | 0.16 (0.15-0.17) | 87 (0.1) | 0.09 (0.08-0.12) | 218 (0.3) | 0.15 (0.13-0.17) |
| Dialysis Facility-Level Characteristics | |||||||
| ESRD Network geographic areasn | |||||||
| Northeasto | 214 813 (90.0) | 21 864 (9.1) | 1 [Reference] | 21 684 (9.1) | 1 [Reference] | 8115 (3.4) | 1 [Reference] |
| Southp | 554 157 (91.1) | 48 866 (8.0) | 0.83 (0.82-0.84) | 48 866 (8.0) | 0.67 (0.65-0.70) | 20 171 (3.3) | 0.90 (0.88-0.92) |
| Midwestq | 303 183 (90.8) | 25 581 (7.7) | 0.82 (0.8-0.83) | 25 581 (7.7) | 1.21 (1.16-1.26) | 11 664 (3.5) | 1.04 (1.01-1.07) |
| Westr | 269 233 (90.3) | 25 549 (8.6) | 0.87 (0.86-0.89) | 25 549 (8.6) | 0.84 (0.81-0.88) | 9340 (3.1) | 0.82 (0.79-0.84) |
| Metropolitan rural-urban classifications | |||||||
| Metropolitan area | 1 115 432 (83.9) | 104 943 (8.5) | 1 [Reference] | 20 002 (1.6) | 1 [Reference] | 42 315 (3.4) | 1 [Reference] |
| Rural area | 213 870 (16.1) | 15 626 (6.7) | 0.81 (0.74-0.88) | 3539 (1.5) | 0.97 (0.90-1.05) | 6540 (2.8) | 0.89 (0.82-0.96) |
| Distance from assigned facility to nearest transplant center, median (IQR), mt | 16.7 (6.1-53.2) | 9.0 (2.1-36.1) | 1.00 (1.00-1.00) | 9.9 (2.2-38.3) | 1.00 (1.00-1.00) | 9.0 (2.4-35.3) | 1.00 (1.00-1.00) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ESKD, end-stage kidney disease; ESRD, end-stage renal disease; IQR, interquartile range.
Baseline characteristics were obtained at the time the CMS-2728 form was completed.
Defined as death or end of follow-up (December 31, 2016).
Univariate Cox proportional hazard model determined the association between patient- and facility-level characteristics and placement on the deceased donor waiting list. Time to follow-up was defined as start of dialysis to placement on waiting list or censor date (end of study or death).
Univariate Cox proportional hazard model determined the association between patient- and facility-level characteristics and receipt of a living donor kidney transplant. Time to follow-up was defined as start of dialysis to living donor kidney transplant or censor date (end of study or death).
Univariate Cox proportional hazard model determined the association between patient- and facility-level characteristics receipt of a deceased donor kidney transplant. Time to follow-up was defined as start of dialysis to deceased donor kidney transplant or censor date (end of study or death).
Patients were assigned to the last dialysis facility from which they were receiving treatment when the outcome event of interest occurred.
Defined as Asian, Middle Eastern, Native American, Pacific Islander, or multiracial.
There were missing data for 115 322 patients (7.8%).
This category was collected and defined on the CMS-2728 form and was not further defined.
There were missing data for 7805 patients (0.5%).
Calculated as weight in kilograms divided by height in meters squared.
There were missing data for 546 900 patients (37.0%).
Obtained from item 27 on the CMS-2728 form. The attending physician recorded no if the patient had not been informed of their transplant options and entered all the reasons why a kidney transplant was not an option for the patient at that time.
Obtained from the Dialysis Facility Report, 2013-2016.
Network 1: Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont; Network 2: New York; Network 3: New Jersey; and Network 4: Delaware and Pennsylvania.
Network 5: District of Columbia, Maryland, Virginia, and West Virginia; Network 6: Georgia, North Carolina, and South Carolina; Network 7: Florida; Network 8: Alabama, Mississippi, and Tennessee; Network 13: Arkansas, Louisiana, and Oklahoma; and Network 14: Texas.
Network 9/10: Illinois, Indiana, Kentucky, and Ohio; Network 11: Michigan, Minnesota, North Dakota, South Dakota, and Wisconsin; and Network 12: Iowa, Kansas, Missouri, and Nebraska.
Network 15: Arizona, Colorado, Nevada, New Mexico, Utah, and Wyoming; Network 16: Alaska, Idaho, Montana, Oregon, and Washington; Network 17: Hawaii and northern California; and Network 18: southern California.
Obtained from the US Department of Agriculture Rural and Urban Continuum Code. There were missing data for 13 322 patients (0.9%).
Calculated using the dialysis facility address from Dialysis Facility Compare (2013-2016) and the nearest transplant center address from the Scientific Registry of Transplant Recipients. There were missing data for 319 177 patients (21.6%).
In bivariable Cox proportional hazard models, patients younger than 60 years had an increased probability of being placed on the deceased donor kidney transplantation waiting list, receiving a living donor kidney transplant, or receiving a deceased donor kidney transplant. Compared with patients with ESKD caused by diabetes, those with ESKD attributed to glomerulonephritis were more likely to be placed on the deceased donor kidney transplantation waiting list (hazard ratio [HR], 3.00 [95% CI, 2.96-3.05]), to receive a living donor kidney transplant (HR, 5.95 [95% CI, 5.76-6.15]), or to receive a deceased donor kidney transplant (HR, 2.17 [95% CI, 2.12-2.22]) (Table 2).
The crude and adjusted time to event associations between dialysis facility ownership and placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, and receipt of a deceased donor kidney transplant appear in Table 3. Nonprofit small chain dialysis facilities were used as the reference group. Patients receiving dialysis at all for-profit facilities vs all nonprofit facilities had lower 5-year cumulative incidence of placement on the deceased donor kidney transplantation waiting list (cumulative incidence difference, −13.2% [95% CI, −13.4% to −13.0%]), receipt of a living donor kidney transplant (−2.3% [95% CI, −2.4% to −2.3%]), and receipt of a deceased donor kidney transplant (−4.3% [95% CI, −4.4% to −4.2%]).
Table 3. Crude and Adjusted Hierarchical Cox Proportional Hazard Ratios Between Dialysis Facility Ownership and Placement on the Deceased Donor Kidney Transplantation Waiting List, Receipt of a Living Donor Kidney Transplant, or Deceased Donor Kidney Transplant by Patient-Level and Facility-Level Characteristics, 2000-2016a.
| Cumulative Incidence Difference, % (95% CI)b | Hazard Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| At 3 y | At 5 y | At 10 y | Crude Model (n = 1 478 564) |
Adjusted for Demographics (n = 1 478 564)c |
+ Clinical Characteristics (n = 1 478 506)d |
+ Socioeconomic and Geographic Characteristics (n = 1 478 506)e |
|
|
Placement on Deceased Donor Kidney Transplantation Waiting List (n = 121 680) | |||||||
| Nonprofit dialysis facilities | |||||||
| Small chains | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Independent | 17.1 (16.8 to 17.5) | 18.4 (18.1 to 18.8) | 19.1 (18.7 to 19.5) | 2.74 (2.68 to 2.80) | 2.46 (2.41 to 2.51) | 2.48 (2.42 to 2.53) | 2.36 (2.31 to 2.42) |
| For-profit dialysis facilities | |||||||
| Large chain 1 | −4.9 (−5.1 to −4.7) | −5.0 (−5.2 to −4.8) | −4.9 (−5.1 to −4.7) | 0.58 (0.57 to 0.59) | 0.58 (0.57 to 0.59) | 0.57 (0.56 to 0.58) | 0.57 (0.56 to 0.58) |
| Large chain 2 | −5.7 (−5.9 to −5.5) | −5.9 (−6.1 to −5.7) | −5.9 (−6.1 to −5.7) | 0.52 (0.50 to 0.53) | 0.53 (0.52 to 0.54) | 0.55 (0.54 to 0.56) | 0.54 (0.53 to 0.55) |
| Small chains | −5.2 (−5.4 to −5.0) | −5.4 (−5.7 to −5.2) | −5.4 (−5.6 to −5.2) | 0.55 (0.54 to 0.56) | 0.56 (0.55 to 0.57) | 0.56 (0.55 to 0.57) | 0.56 (0.54 to 0.57) |
| Independent | −5.0 (−5.2 to −4.8) | −5.1 (−5.3 to −4.8) | −5.0 (−5.3 to −4.8) | 0.59 (0.57 to 0.61) | 0.61 (0.60 to 0.63) | 0.59 (0.57 to 0.61) | 0.60 (0.58 to 0.61) |
| All facilities | |||||||
| Nonprofit | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| For-profit | −12.5 (−12.7 to −12.3) | −13.2 (−13.4 to −13.0) | −13.4 (−13.6 to −13.2) | 0.33 (0.32 to 0.33) | 0.35 (0.34 to 0.35) | 0.34 (0.34 to 0.35) | 0.36 (0.35 to 0.36) |
|
Receipt of a Living Donor Kidney Transplant (n = 23 762) | |||||||
| Nonprofit dialysis facilities | |||||||
| Small chains | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Independent | 2.3 (2.1 to 2.5) | 2.7 (2.5 to 2.9) | 3.0 (2.8 to 3.2) | 1.90 (1.81 to 2.00) | 1.59 (1.51 to 1.67) | 1.52 (1.44 to 1.59) | 1.33 (1.26 to 1.40) |
| For-profit dialysis facilities | |||||||
| Large chain 1 | −1.0 (−1.1 to −1.0) | −1.1 (−1.2 to −1.0) | −1.2 (−1.3 to −1.1) | 0.57 (0.54 to 0.60) | 0.61 (0.58 to 0.63) | 0.61 (0.58 to 0.63) | 0.61 (0.58 to 0.63) |
| Large chain 2 | −1.1 (−1.2 to −1.0) | −1.2 (−1.3 to −1.1) | −1.3 (−1.4 to −1.2) | 0.55 (0.53 to 0.58) | 0.59 (0.57 to 0.62) | 0.62 (0.60 to 0.65) | 0.60 (0.57 to 0.63) |
| Small chains | −1.2 (−1.3 to −1.1) | −1.4 (−1.5 to −1.2) | −1.4 (−1.5 to −1.3) | 0.49 (0.47 to 0.52) | 0.55 (0.52 to 0.58) | 0.56 (0.53 to 0.59) | 0.61 (0.58 to 0.64) |
| Independent | −1.0 (−1.1 to −0.9) | −1.2 (−1.3 to −1.0) | −1.2 (−1.3 to −1.1) | 0.59 (0.55 to 0.62) | 0.68 (0.64 to 0.72) | 0.69 (0.64 to 0.73) | 0.70 (0.65 to 0.74) |
| All facilities | |||||||
| Nonprofit | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| For-profit | −2.1 (−2.1 to −2.0) | −2.3 (−2.4 to −2.3) | −2.5 (−2.6 to −2.4) | 0.40 (0.39 to 0.41) | 0.47 (0.45 to 0.48) | 0.49 (0.47 to 0.50) | 0.52 (0.51 to 0.54) |
|
Receipt of a Deceased Donor Kidney Transplant (n = 49 290) | |||||||
| Nonprofit dialysis facilities | |||||||
| Small chains | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Independent | 2.5 (2.4 to 2.7) | 5.3 (5.1 to 5.6) | 9.1 (8.8 to 9.4) | 2.07 (2.00 to 2.14) | 1.82 (1.76 to 1.88) | 1.82 (1.76 to 1.88) | 1.71 (1.66 to 1.77) |
| For-profit dialysis facilities | |||||||
| Large chain 1 | −1.2 (−1.3 to −1.1) | −2.0 (−2.1 to −1.8) | −2.5 (−2.6 to −2.3) | 0.60 (0.58 to 0.62) | 0.62 (0.60 to 0.64) | 0.60 (0.58 to 0.62) | 0.60 (0.58 to 0.62) |
| Large chain 2 | −1.2 (−1.3 to −1.1) | −2.1 (−2.3 to −2.0) | −2.9 (−3.1 to −2.8) | 0.56 (0.54 to 0.58) | 0.58 (0.57 to 0.60) | 0.61 (0.59 to 0.63) | 0.59 (0.57 to 0.61) |
| Small chains | −1.2 (−1.3 to −1.1) | −2.1 (−2.3 to −2.0) | −2.8 (−3.0 to −2.7) | 0.56 (0.54 to 0.58) | 0.59 (0.57 to 0.61) | 0.58 (0.56 to 0.61) | 0.60 (0.58 to 0.62) |
| Independent | −1.4 (−1.5 to −1.2) | −2.4 (−2.5 to −2.2) | −2.9 (−3.1 to −2.7) | 0.54 (0.52 to 0.57) | 0.59 (0.57 to 0.62) | 0.58 (0.56 to 0.61) | 0.59 (0.56 to 0.62) |
| All facilities | |||||||
| Nonprofit | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| For-profit | −2.3 (−2.4 to −2.2) | −4.3 (−4.4 to −4.2) | −6.6 (−6.7 to −6.4) | 0.39 (0.38 to 0.40) | 0.43 (0.42 to 0.44) | 0.43 (0.42 to 0.44) | 0.44 (0.44 to 0.45) |
Patients were assigned to the last dialysis facility from which they were receiving treatment when the outcome event of interest occurred.
Calculated using the Aalen-Johansen estimator and was adjusted for competing risk of death.
Age, sex, and race reported at dialysis start.
Body mass index greater than 35, attributed cause of end-stage kidney disease, congestive heart failure, atherosclerotic heart disease, other cardiac disease, cerebrovascular disease, peripheral vascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, tobacco use, cancer reported at dialysis start, and type of dialysis. Multiple imputation was used because of missing data for type of dialysis (7805 patients; 0.5%).
End-Stage Renal Disease Network geographic area of assigned facility; insurance coverage reported at dialysis start; facility located in metropolitan, rural-urban classification; distance from assigned dialysis facility to the nearest transplant center; and not informed of transplant options due to medical reasons. Multiple imputation was used because of missing data for insurance coverage (115 322 patients; 7.8%); distance from assigned dialysis facility to nearest transplant center (319 117 patients; 21.6%); and metropolitan, rural-urban classification (13 322 patients; 0.9%).
Compared with patients treated at nonprofit small chain dialysis facilities, patients treated at nonprofit independent facilities were more likely to be placed on the deceased donor kidney transplantation waiting list (HR, 2.36 [95% CI, 2.31-2.42]) and patients were less likely to be placed on the deceased donor kidney transplantation waiting list at facilities of for-profit large chain 1 (HR, 0.57 [95% CI, 0.56-0.58]), at facilities of for-profit large chain 2 (HR, 0.54 [95% CI, 0.53-0.55]), for-profit small chain dialysis facilities (HR, 0.56 [95% CI, 0.54-0.57]), and for-profit independent chain facilities (HR, 0.60 [95% CI, 0.58-0.61]) (Table 3). Patients treated at all for-profit facilities were less likely to receive a living donor kidney transplant compared with patients treated at all nonprofit facilities (HR, 0.52 [95% CI, 0.51-0.54]).
Patients treated at nonprofit independent facilities were more likely to receive a deceased donor kidney transplant (HR, 1.71 [95% CI, 1.66-1.77]), whereas patients treated at facilities of for-profit large chain 1 (HR, 0.60 [95% CI, 0.58-0.62]), facilities of for-profit large chain 2 (HR, 0.59 [95% CI, 0.57-0.61]), for-profit small chain dialysis facilities (HR, 0.60 [95% CI, 0.58-0.62]), and for-profit independent chain facilities (HR, 0.59 [95% CI, 0.56-0.62) were less likely to receive a deceased donor kidney transplant compared with their counterparts in nonprofit small chain dialysis facilities. Based on 5-year cumulative incidence differences between for-profit and nonprofit facilities, the number needed to treat for placement on the deceased donor kidney transplantation waiting list is 7.6 (95% CI, 7.5-7.7); for receipt of a living donor kidney transplant, 43.5 (95% CI, 41.7-45.4); and for receipt of a deceased donor kidney transplant, 23.2 (95% CI, 22.7-23.8).
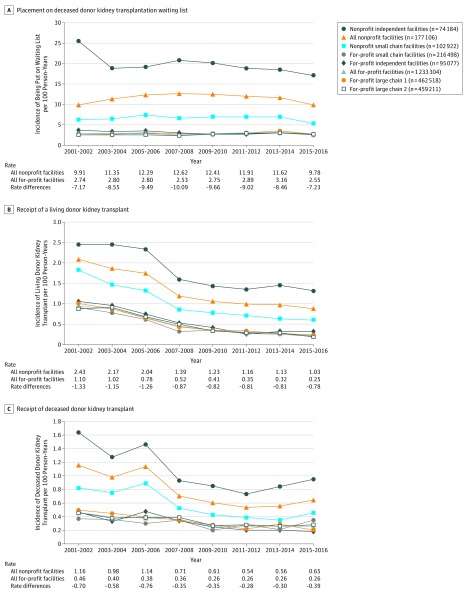
Two-year event incidence rates during the study period showed a general decrease during the last 17 years across dialysis facility ownership categories in patient placement on the deceased donor kidney transplantation waiting list (Figure 2A), receipt of a living donor kidney transplant (Figure 2B), and receipt of a deceased donor kidney transplant (Figure 2C). From 2001-2016, for-profit dialysis facilities had a lower 2-year incidence rate per 100 person-years for each event compared with other dialysis facility ownership categories. The for-profit vs nonprofit facility rate differences ranged from −7.17 to −10.09 per 100 person-years for placement on the deceased donor kidney transplantation waiting list; −0.78 to −1.33 per 100 person-years for receipt of a living donor kidney transplant; and −0.28 to −0.76 per 100 person-years for receipt of a deceased donor kidney transplant (Figure 2).
Figure 2. Two-Year Incidence Rates and Rate Differences for Kidney Transplant, 2001-2016.
Follow-up time was truncated on December 31 of each 2-year period. The unadjusted 2-year incidence rates (per 100 person-years) for placement on the deceased donor waiting list, receipt of a living donor kidney transplant, and receipt of a deceased donor kidney transplant were calculated by dividing the count of events for incident patients by the number of incident patients in the dialysis facility ownership group for each interval. The 2-year incident rate differences (per 100 person-years) were calculated between all nonprofit facilities and all for-profit facilities.
Overall, 76.8% of patients did not switch dialysis facilities during the study period, 19.1% switched facilities within the same profit status, 1.1% switched from for-profit facilities to nonprofit facilities, and 3.0% switched from nonprofit facilities to for-profit facilities (eTable 1 in the Supplement). Among the patients who switched facilities, the median time from first to last facility was 1.2 years (IQR, 0.3-3.0 years).
Sensitivity Analyses
The sensitivity analysis for the association between switching dialysis facilities and the outcomes of interest, stratified by the last dialysis facility where the patients received treatment appears in eTable 2 in the Supplement. Patients who switched from a nonprofit facility to a for-profit facility were more likely to be placed on the deceased donor kidney transplantation waiting list or receive a living or deceased donor kidney transplant compared with patients who started and continued treatment at for-profit facilities. For example, patients who switched from a nonprofit facility to a for-profit large chain 2 facility had a greater hazard of being placed on the deceased donor kidney transplantation waiting list (HR, 2.84 [95% CI, 2.76-2.93]) than patients who started and continued dialysis at the same for-profit large chain 2 facility.
The sensitivity analysis among the ideal kidney transplant candidate cohort reported similar relative risks to the primary analysis (eTable 3 in the Supplement). The 5-year cumulative incidence differences between all nonprofit facilities and all for-profit facilities were higher in the ideal cohort analysis for placement on the deceased donor kidney transplantation waiting list (−23.9% [95% CI, −24.3% to −23.5%]), receipt of a living donor kidney transplant (−4.8% [95% CI, −5.0% to −4.6%]), and receipt of a deceased donor kidney transplant (−8.5% [95% CI, −8.8% to −8.3%]). The sensitivity analyses found consistent results of lower rates for each outcome among nonprofit facilities vs for-profit facilities regardless of geographic area (eTable 4 in the Supplement). A reclassification based on either transplant center–affiliated dialysis facilities (eTable 5 in the Supplement) or hospital-affiliated dialysis facilities (eTable 6 in the Supplement) by profit status showed similar associations between nonprofit facilities and placement on the deceased donor kidney transplantation waiting list, receipt of a living donor kidney transplant, and receipt of a deceased donor kidney transplant.
Discussion
Among US patients with incident ESKD, nonprofit dialysis facilities had a higher percentage of patients who were placed on the deceased donor kidney transplantation waiting list, who received a living donor kidney transplant, and who received a deceased donor kidney transplant compared with the other dialysis facility ownership categories.
To our knowledge, no studies have examined the relationship between dialysis facility profit status and both a living donor or a deceased donor kidney transplantation, and only 2 studies have examined the relationship between facility profit status and placement on the deceased donor kidney transplantation waiting list, reporting that patients were less likely to be waitlisted if initially treated at for-profit dialysis facilities vs nonprofit facilities.8,9 This study extends the work of Zhang et al8 by using hierarchical survival analysis to examine how dialysis facility ownership is associated with living donor and deceased donor kidney transplant and placement on the deceased donor kidney transplantation waiting list in a large national cohort of patients with ESKD from 2000 to 2016.
A strength of this analysis vs prior research was a novel approach of assigning dialysis facilities to patients. Nearly 23.2% of patients switched dialysis facilities during follow-up; however, only 4.1% of patients switched dialysis facilities by profit status. Recognizing this fact, the typically short follow-up these patients have with their initial dialysis facilities (median, 1.2 years), and the intensity of clinical follow-up leading up to placement on the deceased donor kidney transplantation waiting list or receipt of a kidney transplant (median, 2.2 years to transplantation), indicate that the last facility where a patient was treated may be more representative of the preparatory transplant care he or she receives.
Patients undergoing home dialysis also were included in this study (8.9% of cohort). Patients who receive home dialysis are more likely to undergo transplantation,18,19 but previous studies have excluded these patients.8,9
The sensitivity analysis that stratified patients by dialysis facility switch status showed that patients switching from nonprofit facilities to for-profit facilities were more likely to access transplantation vs patients who started and remained at the same for-profit facility. The 2-year incidence rates for receipt of a living donor and a deceased donor kidney transplant decreased each year, and the incidence rate for placement on the deceased donor kidney transplantation waiting list increased from 2001-2014 and then decreased in 2015, congruent with declines observed in national reports.2,20 The decrease in placement on the deceased donor kidney transplantation waiting list since the new kidney allocation system took place in December 2014 is similar to the decline reported in the study by Zhang et al21 of 3.45 waitlisted events per month per 10 000 patients with ESKD. The profit-status disparity in the 2-year incidence rates for each outcome of interest persisted every year within this 17-year study cohort despite research identifying this problem nearly 2 decades ago.9
In 2014, the CMS began administering the In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems (ICH CAHPS) survey to collect patient-reported satisfaction.22 The Dialysis Facility Compare website allows users to review patient satisfaction scores for local facilities and state- and national-level metrics under the “survey of patients experiences” tab. Based on the ICH CAHPS survey responses, only an estimated 67% of patients reported that their nephrologists “always communicated well and cared for them as a person.”23 These national data are similar to previous reports that more than 30% of incident patients with ESKD were uninformed of transplant options24 and nephrologists working at for-profit dialysis facilities were significantly less likely to spend longer than 20 minutes with their patients, counsel their patients about transplantation, or involve family members in the discussion.10
Clinician-level barriers, including clinician perception of the appropriateness of the possible transplantation,25,26,27 poor medical follow-up, time spent with patients,10 and format of transplant education,10 may lead to delays in access to transplantation, and could explain some of these findings, but are unmeasured in national data. Additional barriers, such as resource allocation of staffing to enable transplant education, could also play a role because prior research found that increased staff may improve access to kidney transplantation.7 Although this study reported more staff and social workers per patient at for-profit facilities vs nonprofit facilities, these findings did not explain the observed associations.
Limitations
This study has several limitations. First, given the availability of the data, it was not possible to determine the differences between profit status and chain affiliations regarding staffing resources,10,24,25,28,29 education policies,30,31,32,33 and transplant referral practices,34,35 all of which may be associated with increased access to kidney transplantation.
Second, although this analysis clustered dialysis facility ownership categories, we were unable to account for the nonrandom geographical location of for-profit and nonprofit chains, which may lead to unmeasured differences in patient characteristics across profit status categories.
Third, it is difficult to capture steps in the transplantation process that precede placement on the deceased donor kidney transplantation waiting list or receipt of a living donor or a deceased donor kidney transplant, such as transplant evaluation or referral for a transplant evaluation, and the effects may not be consistent by transplant step. A study by Patzer et al34 found that, among patients with ESKD in Georgia, the patients treated at for-profit facilities were more likely to be referred for a kidney transplant evaluation compared with patients treated at nonprofit facilities (odds ratio, 1.51 [95% CI, 1.20-1.91]), and there was no difference in placement on the deceased donor kidney transplantation waiting list among those referred within 1 year (odds ratio, 1.09 [95% CI, 0.83-1.44]). The sensitivity analyses found the association between profit vs nonprofit facilities and access to transplantation was consistent across all geographic areas; however, further research on transplant referral would permit analyses examining geographic differences in steps more closely aligned with dialysis facility behavior.36
Fourth, because of the wide variations in placement on the deceased donor kidney transplantation waiting list and in the practices of transplant centers across the United States,37,38 and the limitation of patient-level data collected at the start of dialysis, this study was unable to identify the patients truly eligible for transplantation. The sensitivity analysis of an ideal kidney transplantation cohort showed higher cumulative incidence differences and relative risks reporting the profit status disparity as presented in our primary analysis.
Fifth, the inclusion of dialysis facility staff–reported race/ethnicity could create a misclassification bias. However, Roach et al39 found high agreement between staff-reported race on the CMS-2728 form and patient-reported race in the Medicare enrollment database.
Conclusions
Among US patients with end-stage kidney disease, receiving dialysis at for-profit facilities compared with nonprofit facilities was associated with a lower likelihood of accessing kidney transplantation. Further research is needed to understand the mechanisms behind this association.
eFigure. Geographic distribution of US dialysis facilities by dialysis facility ownership (n=6,511) obtained from the Dialysis Facility Compare, 2016
eTable 1. Patient and facility demographic characteristics of patients with ESRD at the time of dialysis start within the United States Renal Data System, 2000-2016, stratified by whether or not the patient changed dialysis facilities based on their first and last facility
eTable 2. Fully adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant and whether or not a patient changed dialysis facilities and facility profit status
eTable 3. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant and dialysis facility ownership by patient-level and facility-level characteristics, among ideal kidney transplantation candidates, 2000-2016 (n=454,726)
eTable 4. Fully adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics, stratified by end stage renal disease network region, 2000-2016
eTable 5. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics for dialysis facilities that are (vs are not) affiliated with transplant centers, 2000-2016
eTable 6. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics for dialysis facilities that are (vs are not) affiliated with hospitals, 2000-2016
References
- 1.Tonelli M, Wiebe N, Knoll G, et al. . Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093-2109. doi: 10.1111/j.1600-6143.2011.03686.x [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System US Renal Data System 2018 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 3.Vanholder R, Annemans L, Brown E, et al. ; European Kidney Health Alliance . Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13(7):393-409. doi: 10.1038/nrneph.2017.63 [DOI] [PubMed] [Google Scholar]
- 4.Office of the Federal Register; National Archives and Records Administration Conditions for coverage for ESRD facilities; 2008. https://www.govinfo.gov/app/details/FR-2008-04-15/08-1102. Accessed August 8, 2019.
- 5.Department of Health and Human Services; Centers for Medicare & Medicaid Services End-stage renal disease medical evidence report: Medicare entitlement and/or patient registration; 2005. https://secure.ssa.gov/apps10/poms/images/Other/G-CMS-2728-U3-1.pdf. Accessed August 8, 2019.
- 6.Centers for Medicare & Medicaid Services ESRD Treatment Choices Model; 2019. https://innovation.cms.gov/initiatives/esrd-treatment-choices-model/. Accessed July 24, 2019.
- 7.Patzer RE, Plantinga L, Krisher J, Pastan SO. Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant. 2014;14(7):1562-1572. doi: 10.1111/ajt.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Thamer M, Kshirsagar O, Cotter DJ, Schlesinger MJ. Dialysis chains and placement on the waiting list for a cadaveric kidney transplant. Transplantation. 2014;98(5):543-551. doi: 10.1097/TP.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 9.Garg PP, Frick KD, Diener-West M, Powe NR. Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med. 1999;341(22):1653-1660. doi: 10.1056/NEJM199911253412205 [DOI] [PubMed] [Google Scholar]
- 10.Balhara KS, Kucirka LM, Jaar BG, Segev DL. Disparities in provision of transplant education by profit status of the dialysis center. Am J Transplant. 2012;12(11):3104-3110. doi: 10.1111/j.1600-6143.2012.04207.x [DOI] [PubMed] [Google Scholar]
- 11.Hirth RA, Chernew ME, Orzol SM. Ownership, competition, and the adoption of new technologies and cost-saving practices in a fixed-price environment. Inquiry. 2000;37(3):282-294. [PubMed] [Google Scholar]
- 12.Farley DO. Effects of Competition of Dialysis Facility Service Levels and Patient Selection. Santa Monica, CA: RAND Corp; 1993. [Google Scholar]
- 13.Held PJ, García JR, Pauly MV, Cahn MA. Price of dialysis, unit staffing, and length of dialysis treatments. Am J Kidney Dis. 1990;15(5):441-450. doi: 10.1016/S0272-6386(12)70362-1 [DOI] [PubMed] [Google Scholar]
- 14.Friedman BS, Hattis PA, Bogue RJ. Tax exemption and community benefits of not-for-profit hospitals. Adv Health Econ Health Serv Res. 1990;11:131-158. [PubMed] [Google Scholar]
- 15.Edwards JK, Hester LL, Gokhale M, Lesko CR. Methodologic issues when estimating risks in pharmacoepidemiology. Curr Epidemiol Rep. 2016;3(4):285-296. doi: 10.1007/s40471-016-0089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 17.Lee EW, Wei L, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Survival Analysis: State of the Art. New York, NY: Springer; 1992:237-247. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 18.Issa N, Lankireddy S, Kukla A. Should peritoneal dialysis be the preferred therapy pre-kidney transplantation? Adv Perit Dial. 2012;28:89-93. [PubMed] [Google Scholar]
- 19.van de Luijtgaarden MW, Jager KJ, Segelmark M, et al. . Trends in dialysis modality choice and related patient survival in the ERA-EDTA registry over a 20-year period. Nephrol Dial Transplant. 2016;31(1):120-128. doi: 10.1093/ndt/gfv295 [DOI] [PubMed] [Google Scholar]
- 20.United Network of Organ Sharing Transplant trends: 2018 report. https://www.unos.org/data/transplant-trends/2018/. Accessed August 8, 2019.
- 21.Zhang X, Melanson TA, Plantinga LC, et al. . Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant. 2018;18(8):1936-1946. doi: 10.1111/ajt.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services In-center hemodialysis CAHPS; 2016. https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/CAHPS/ichcahps.html. Accessed July 25, 2019.
- 23.Centers for Medicare & Medicaid Services Dialysis facility compare; 2018. https://www.medicare.gov/dialysisfacilitycompare/. Accessed February 22, 2019.
- 24.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12(2):351-357. doi: 10.1111/j.1600-6143.2011.03865.x [DOI] [PubMed] [Google Scholar]
- 25.Purnell TS, Hall YN, Boulware LE. Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Adv Chronic Kidney Dis. 2012;19(4):244-251. doi: 10.1053/j.ackd.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanson CS, Chadban SJ, Chapman JR, Craig JC, Wong G, Tong A. Nephrologists’ perspectives on recipient eligibility and access to living kidney donor transplantation. Transplantation. 2016;100(4):943-953. doi: 10.1097/TP.0000000000000921 [DOI] [PubMed] [Google Scholar]
- 27.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM. Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis. 2004;43(2):350-357. doi: 10.1053/j.ajkd.2003.10.022 [DOI] [PubMed] [Google Scholar]
- 28.Kucirka LM, Segev DL. The other half of informed consent: Transplant education practices in dialysis centers. Clin J Am Soc Nephrol. 2015;10(9):1507-1509. doi: 10.2215/CJN.08280815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gander J, Browne T, Plantinga L, et al. . Dialysis facility transplant philosophy and access to kidney transplantation in the Southeast. Am J Nephrol. 2015;41(6):504-511. doi: 10.1159/000438463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salter ML, Orandi B, McAdams-DeMarco MA, et al. . Patient- and provider-reported information about transplantation and subsequent waitlisting. J Am Soc Nephrol. 2014;25(12):2871-2877. doi: 10.1681/ASN.2013121298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon EJ. “They don’t have to suffer for me”: why dialysis patients refuse offers of living donor kidneys. Med Anthropol Q. 2001;15(2):245-267. doi: 10.1525/maq.2001.15.2.245 [DOI] [PubMed] [Google Scholar]
- 32.Browne T, Amamoo A, Patzer RE, et al. . Everybody needs a cheerleader to get a kidney transplant: a qualitative study of the patient barriers and facilitators to kidney transplantation in the Southeastern United States. BMC Nephrol. 2016;17(1):108. doi: 10.1186/s12882-016-0326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gander JC, Gordon EJ, Patzer RE. Decision aids to increase living donor kidney transplantation. Curr Transplant Rep. 2017;4(1):1-12. doi: 10.1007/s40472-017-0133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patzer RE, Plantinga LC, Paul S, et al. . Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314(6):582-594. doi: 10.1001/jama.2015.8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis. 2005;46(4):734-745. doi: 10.1053/j.ajkd.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Fowler KJ. Accountability of dialysis facilities in transplant referral: CMS needs to collect national data on dialysis facility kidney transplant referrals. Clin J Am Soc Nephrol. 2018;13(2):193-194. doi: 10.2215/CJN.13741217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 pt 2):1412-1423. doi: 10.1111/j.1600-6143.2007.01785.x [DOI] [PubMed] [Google Scholar]
- 38.Reese PP, Feldman HI, Bloom RD, et al. . Assessment of variation in live donor kidney transplantation across transplant centers in the United States. Transplantation. 2011;91(12):1357-1363. doi: 10.1097/TP.0b013e31821bf138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roach JL, Turenne MN, Hirth RA, Wheeler JR, Sleeman KS, Messana JM. Using race as a case-mix adjustment factor in a renal dialysis payment system: potential and pitfalls. Am J Kidney Dis. 2010;56(5):928-936. doi: 10.1053/j.ajkd.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Geographic distribution of US dialysis facilities by dialysis facility ownership (n=6,511) obtained from the Dialysis Facility Compare, 2016
eTable 1. Patient and facility demographic characteristics of patients with ESRD at the time of dialysis start within the United States Renal Data System, 2000-2016, stratified by whether or not the patient changed dialysis facilities based on their first and last facility
eTable 2. Fully adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant and whether or not a patient changed dialysis facilities and facility profit status
eTable 3. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant and dialysis facility ownership by patient-level and facility-level characteristics, among ideal kidney transplantation candidates, 2000-2016 (n=454,726)
eTable 4. Fully adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics, stratified by end stage renal disease network region, 2000-2016
eTable 5. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics for dialysis facilities that are (vs are not) affiliated with transplant centers, 2000-2016
eTable 6. Crude and adjusted hierarchical Cox proportional hazard ratios between placement on the deceased donor kidney transplant waitlist, receipt of a living donor kidney transplant, or deceased donor kidney transplant by patient-level and facility-level characteristics for dialysis facilities that are (vs are not) affiliated with hospitals, 2000-2016