Abstract
Eliminations of alkyl halides by sodium diisopropylamide (NaDA) in tetrahydrofuran (THF)/hexane or THF/N,N-dimethylethylamine (DMEA) solutions are facile and complementary to analogous reactions of lithium diisopropylamide in THF. Rate studies show a dominance of monomer-based metalations and prevalent secondary-shell solvation effects overlaid on primary-shell effects. 1-Halooctanes exclusively undergo elimination rather than substitution. Rate and isotopic labeling studies on 1-bromooctane reveal an E2-like elimination pathway via trisolvated NaDA monomer. By contrast, 1-chlorooctane is eliminated via disolvated monomer through a carbenoid mechanism. exo-2-Norbornyl chloride and bromide are also eliminated via disolvated monomer; a syn E2 mechanism is inferred for these substrates. The cis- and trans-4-tert-butylcyclohexyl bromides show a preference for the elimination of the cis isomer (kcis/ax/ktrans/eq = 10). Rate and isotopic labeling studies are consistent with a trans-diaxial E2 elimination via trisolvated monomer for the cis isomer and a carbenoid mechanism via disolvated monomer for the trans isomer. Vicinal haloethers show substrate-dependent reactivities, affording alkynes and enol ethers. trans-1-Bromo-2-methoxycyclohexane provides enol ether 1-methoxycyclohexene, while trans-1-bromo-2-methoxycyclooctane provides dimeric products consistent with fleeting cycloocta-1,2-diene (cyclic allene), which was fully characterized as two conformers.
Introduction
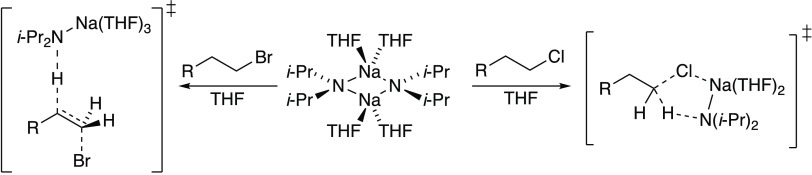
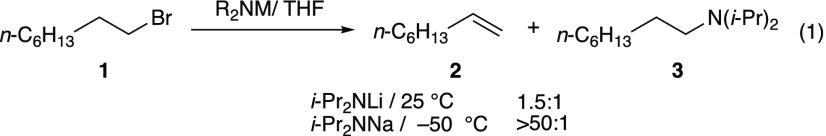
The seemingly subtle factors that dictate whether an anion functions as a base or as a carbon-centered nucleophile can be confounding. Notions of steric effects, nucleophile polarizability, ion pairing, solvent polarity, and counterion effects crucially fail to account for aggregation and primary-shell solvation and are thus too restrictive to fully explain the SN2–E2 dichotomy.1−3 We find it intriguing, for example, that with lithium diisopropylamide (LDA), 1-bromooctane undergoes both elimination (2) and substitution (3), but with sodium diisopropylamide (NaDA), the elimination product (2) is obtained exclusively (eq 1).4
 |
1 |
This paper examines NaDA-mediated dehydrohalogenations as part of an effort to establish the foundational structure–reactivity principles in organosodium chemistry as well as to pique the interest of consumers of strong bases.5−8 What stands out to us is the number of observations that do not follow the script found in standard organic chemistry textbooks.9 Moreover, deconvolution of solvation into primary- and secondary-shell effects brings a noteworthy perspective to the iconic issues of reactivity and selectivity.
Results and Discussion
Methods
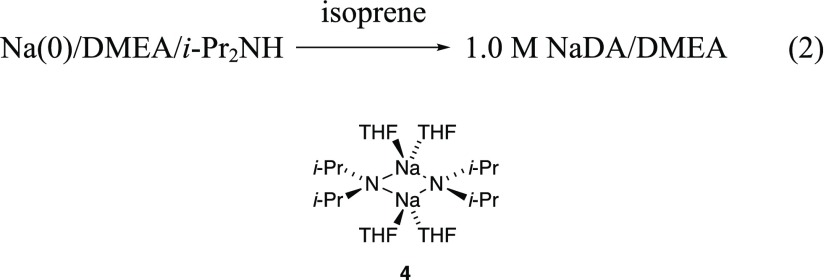
NaDA is prepared from sodium dispersion as a 1.0 M stock solution in N,N-dimethylethylamine (DMEA) as illustrated in eq 2.4 Owing to our emphasis on mechanism, we take the added precaution of recrystallizing NaDA. Although we document secondary shell solvation effects, THF/DMEA and THF/hexane solutions of NaDA would be nearly indistinguishable to the average practitioner owing to the highly favorable substitution of THF for DMEA to form tetrasolvated dimer 4 as the sole observable form10 and relatively minor differences in polarity of hexane and DMEA. Thus, while we use THF/hexane mixtures, others are likely to find the stock solutions of NaDA/DMEA with added THF to be more convenient.
 |
2 |
Because of the volatility of many of the products, yields are often determined using NMR spectroscopy relative to an internal standard (benzene). These calibrated yields are listed in parentheses. Overall, the eliminations are free of significant byproducts unless otherwise noted.
Reaction rates were monitored using 1H NMR spectroscopy, as previously described.11 The often-preferred strategy of monitoring via in situ infrared spectroscopy is not possible in these reactions, as deposition of sodium halides on the detection window (silicon chip) causes spectral distortion. Precipitation is, however, a useful diagnostic for detecting the onset of reaction.
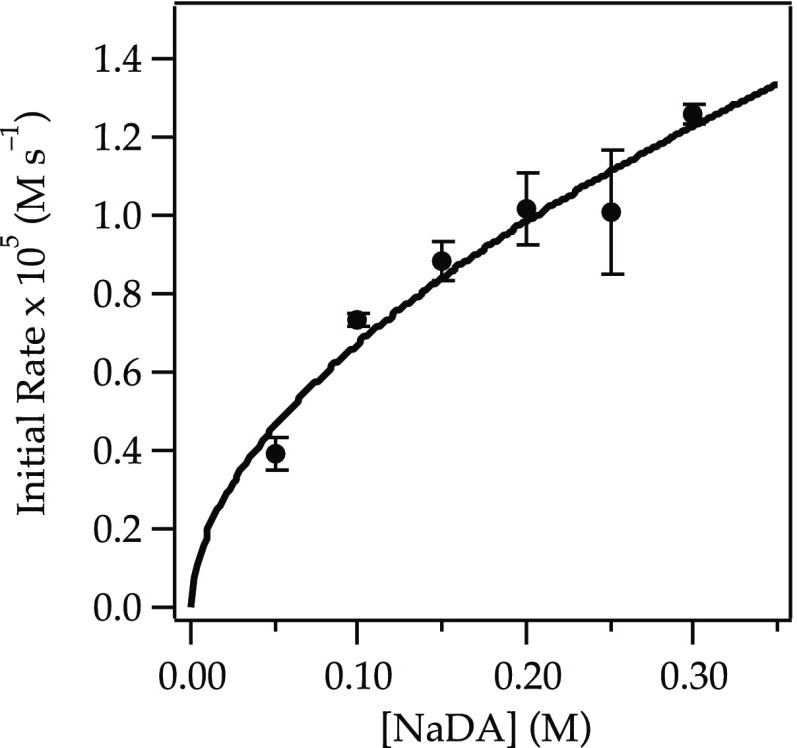
Rate studies of NaDA-mediated dehydrohalogenations show, without exception, half-order NaDA dependencies that implicate monomer-based metalations. Figure 1 is emblematic;12,13 all other measured half orders in NaDA are archived in the Supporting Information.
Figure 1.
Plot of the initial rate19b versus [NaDA] for the dehydrobromination of 1-bromooctane 1 (0.050 M) in 5.1 M THF/hexane at −78 °C. The curve depicts an unweighted least-squares fit to y = k[NaDA]n: k = (24 ± 3) × 10–5; n = 0.55 ± 0.07.
The reaction orders in THF are sensitive to the choice of substrate, eliciting THF concentration dependencies denoted in the generalized and idealized rate law (eq 3; A = i-Pr2NNa).3 Zeroth, first, and second orders in THF appeared to implicate metalations via di-, tri-, and tetrasolvated monomers, respectively (eqs 4–6). However, control experiments, originally developed for the study of lithium amide chemistry, revealed that most THF-concentration-dependent rates stem from a combination of sterically sensitive primary-shell solvation effects overlaid on sterically insensitive secondary-shell solvation effects.12a In these control experiments, the primary and secondary effects may be disentangled through the use of hindered polyalkylated THF ligands (2,2-dimethyltetrahydrofuran, 2,5-dimethyltetrahydrofuran, or 2,2,5,5-tetramethyltetrahydrofuran) as poorly coordinating but still polar cosolvents.14 Secondary-shell (medium) effects on the chemistry of LDA typically account for very low (0–20%) rate changes, spanning <1.0 M THF/hexane to neat THF. Having established that NaDA is stable in 2,5-dimethyltetrahydrofuran (2,5-Me2THF) at ≤ −20 °C,10,15 we found secondary-shell effects to be measurable and consequential to the mechanistic interpretation. Most noticeably, apparent evidence of tetrasolvates (eq 6) proved to be illusory, stemming instead from the influence of the secondary-shell solvation on trisolvated monomer-based pathways (eq 5). Similarly, a number of apparent first-order dependencies are traced to the secondary-shell effects overlaid on zeroth-order dependencies.
| 3 |
| 4 |
| 5 |
| 6 |
We attribute the most highly solvated monomers—the trisolvates—to fully open the transition structures and the disolvates to mechanisms accommodating the halogen–sodium contacts in the rate-limiting transition structures.1b Density functional theory (DFT) calculations were carried out at the B3LYP/6-31G(d) level with single-point calculations at the MP2 level of theory.16 All allusions to transition structures are supported by computed transition structures manifesting a single negative frequency archived in the Supporting Information. The computations are used semiquantitatively to show that the proposed mechanism is plausible rather than to rigorously resolve nuanced mechanistic details. Energies are mentioned sparingly, especially when deviations from isodesmicity17 or charge separation would create severe energetic distortions owing to electron correlation problems.18
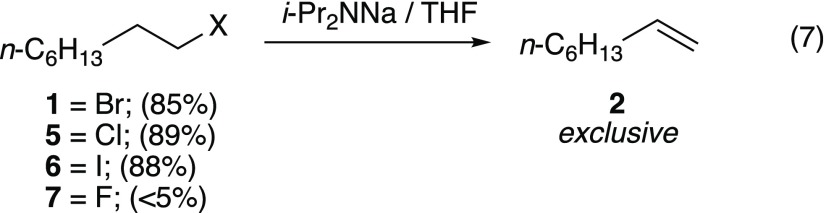
1-Bromooctane (1)
Reaction of 1-bromooctane (0.010 M) with 1.2 equiv of NaDA in THF/hexane at −78 °C affords 1-octene to the exclusion of N-(1-octyl)diisopropylamine in 85% yield by 1H NMR spectroscopy relative to benzene as the internal standard (eq 7). Eliminations under pseudo-first-order conditions, in which NaDA is maintained at synthetically standard concentrations (0.10–0.30 M) in THF/hexane, show clean exponential decays with no evidence of the curvature emblematic of autocatalysis.12b The first-order dependence on 1-bromooctane is confirmed by the concentration-independent pseudo-first-order rate constants (kobsd) versus [1] and by initial rates that linearly correlate with the initial concentrations of 1.12d
 |
7 |
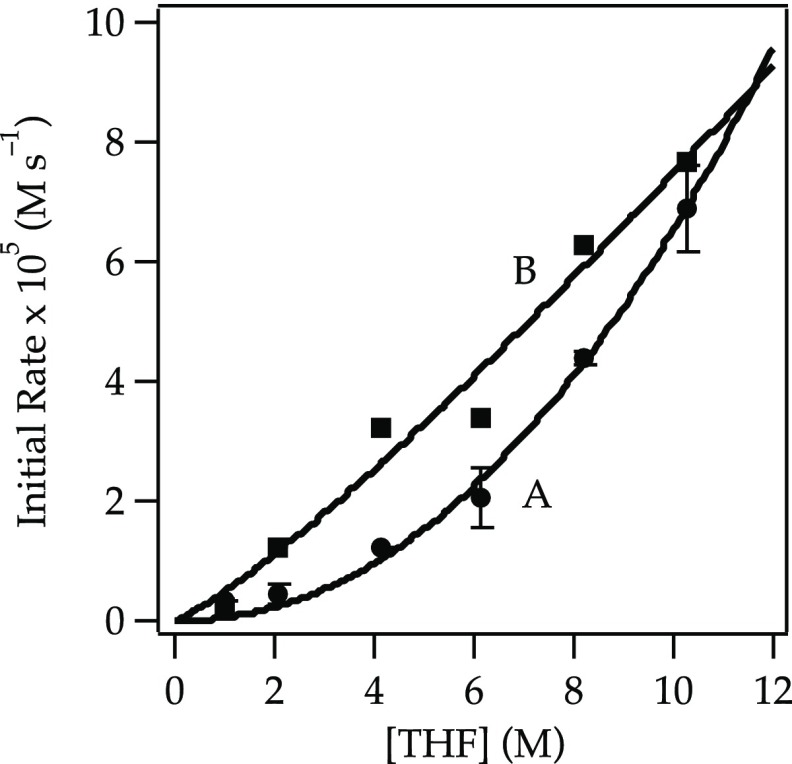
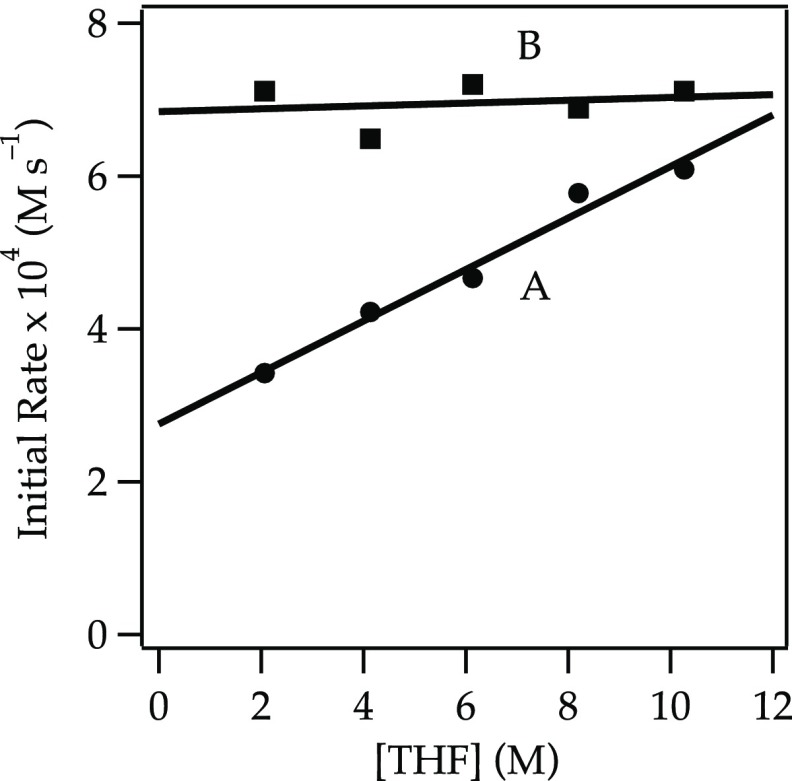
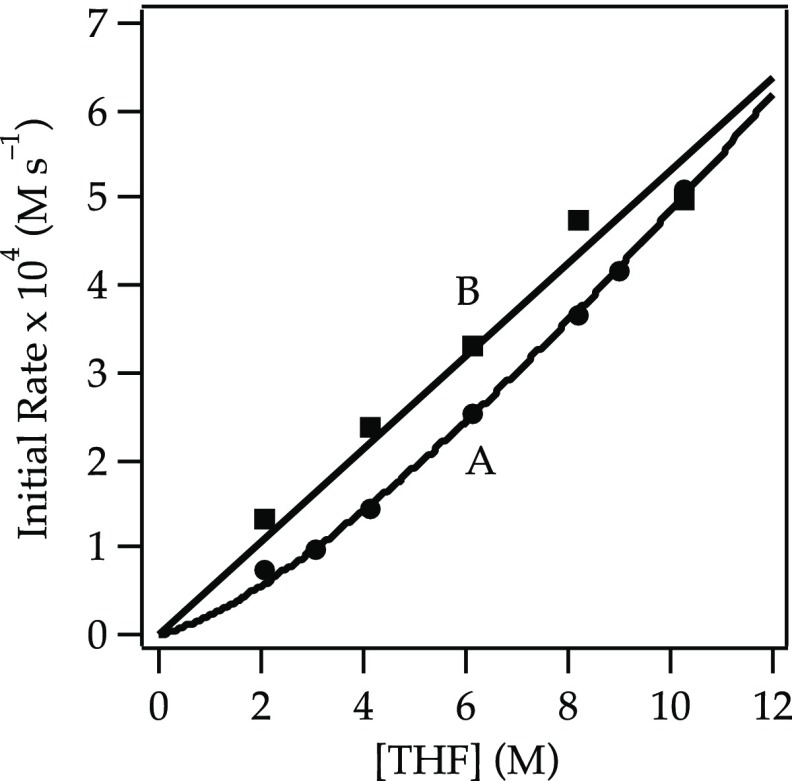
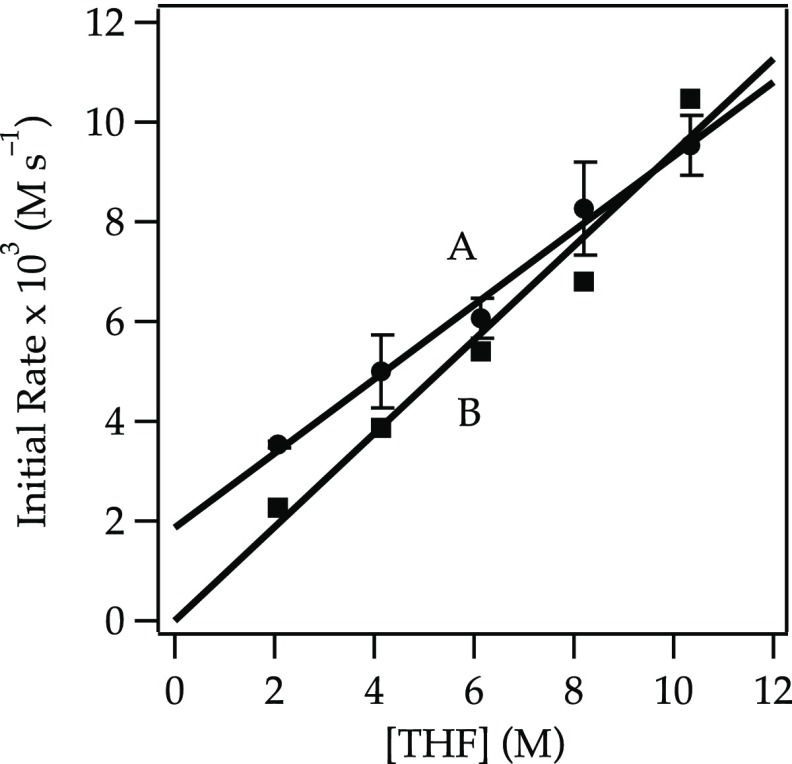
A plot of initial rates for the elimination of 1 versus NaDA concentration shows a half-order dependence (Figure 1), implicating a monomer-based metalation. A plot of the initial rates19 versus THF concentration using hexane as an inert cosolvent reveals an approximate second-order THF dependence (Figure 2, curve A). The data seemed to implicate tetrasolvated monomers (eq 6). However, plotting initial rates versus THF concentration using 2,5-Me2THF as the inert cosolvent reveals an approximate first-order THF dependence (Figure 2, curve B). The rate data are thus consistent with the trisolvated monomer-based mechanism in eq 5.
Figure 2.
Plot of the initial rate19b versus [THF] for the dehydrobromination of alkyl bromide 1 (0.050 M) with NaDA (0.10 M) at −78 °C in hexane (curve A) or 2,5-Me2THF (curve B). The curves depict unweighted least-squares fits. Curve A: y = k[THF]n: k = (5.25 ± 0.02) × 10–5; n = 2.09 ± 0.16. Curve B: y = k[THF]n: k = (0.93 ± 0.03) × 10–4; n = 1.18 ± 0.17.
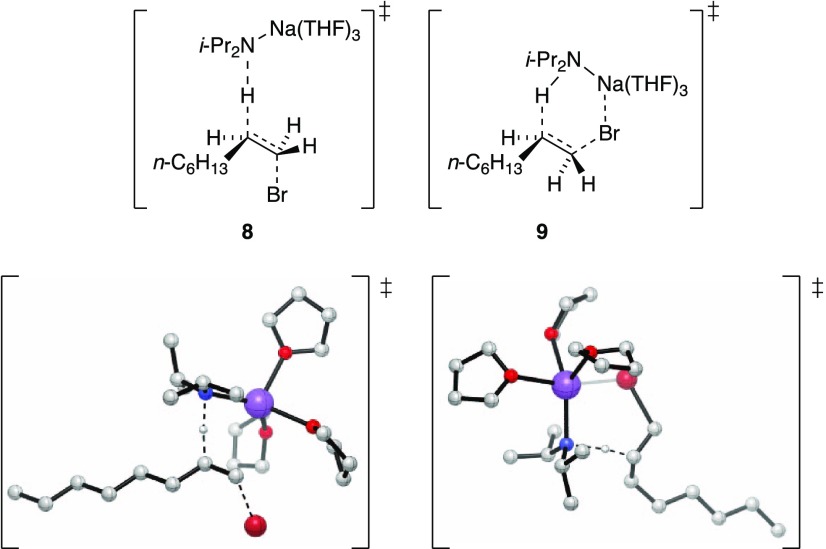
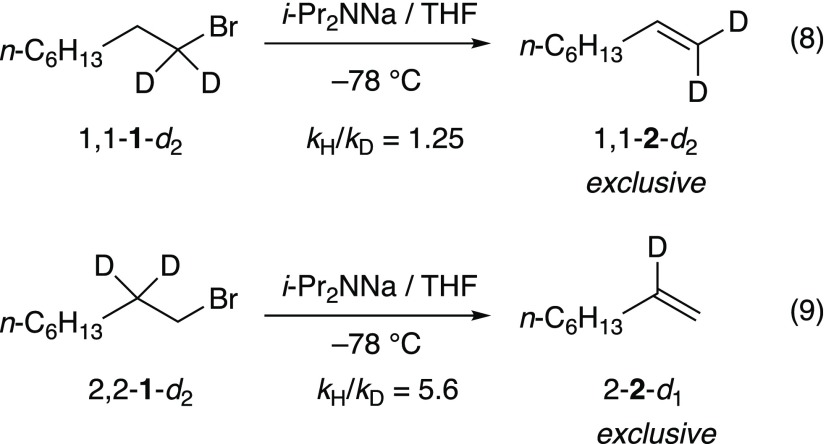
The eliminations of isotopomers 1,1-1-d2 (eq 8)20 and 2,2-1-d2 (eq 9)20 implicate an E2 elimination without an intervening carbenoid. DFT computations show both anti- and syn-periplanar transition structures 8 and 9 to be viable, with 8 being >3 kcal/mol higher than 9. (We routinely found the computed carbene structures to be unusually stable.) The solvent order, when placed in the context of results from other eliminations described below, makes a strong case for 8. Literature precedent supports such a hypothesis.1
 |
8 |
1-Chlorooctane (5)
NaDA-mediated elimination of 5 proceeds smoothly at −20 °C in 89% NMR yield (eq 7). In contrast with 1-bromooctane, monitoring the reaction by 1H NMR spectroscopy reveals a linear dependence of the initial rates on THF concentration with a substantial nonzero intercept (Figure 3, curve A). In conjunction with a half-order NaDA dependence (0.6 ± 0.1), the THF dependence would seem to suggest contributions of both di- and trisolvated monomers (eqs 4 and 5) to the exclusion of tetrasolvated monomer. Yet again, however, the solvent dependence is shown to be illusory: use of a polar, noncoordinating cosolvent 2,5-Me2THF in place of hexane reveals a zeroth-order dependence (Figure 3, curve B).
Figure 3.
Plot of the initial rate19b versus [THF] for the dehydrochlorination of 1-chlorooctane 5 (0.050 M) with NaDA (0.10 M) at −20 °C in hexane (curve A) or 2,5-THF (curve B). The curves depict unweighted least-squares fits to y = k[THF] + k′. Curve A: k = (0.37 ± 0.15) × 10–4; k′ = (2.25 ± 0.29) × 10–5. Curve B: k = (0.02 ± 0.05) × 10–4; k′ = (6.84 ± 0.33) × 10–5.
At first glance, the low solvation number appeared
to implicate
a syn-transition structure 10, displaying distinct Na–Cl
contacts as both plausible and computationally viable. Alkoxide-mediated
eliminations in nonpolar (nonhydroxylic) solvents led other investigators
to invoke M–X ion pairing.1b However,
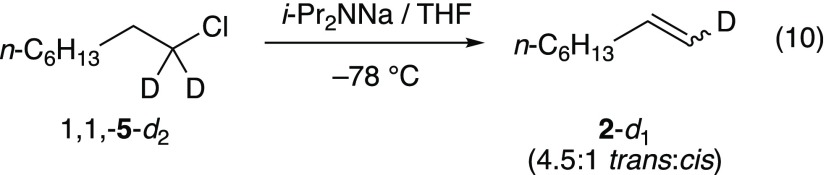
elimination of 1,1-5-d2 afforded
a small but most likely primary isotope effect (kH/kD = 2.5) and an isotopic
distribution (eq 10)
consistent with a carbene-based mechanism via the transition structure 11. Transition structure 11 is calculated to
be >5 kcal/mol more stable than 10.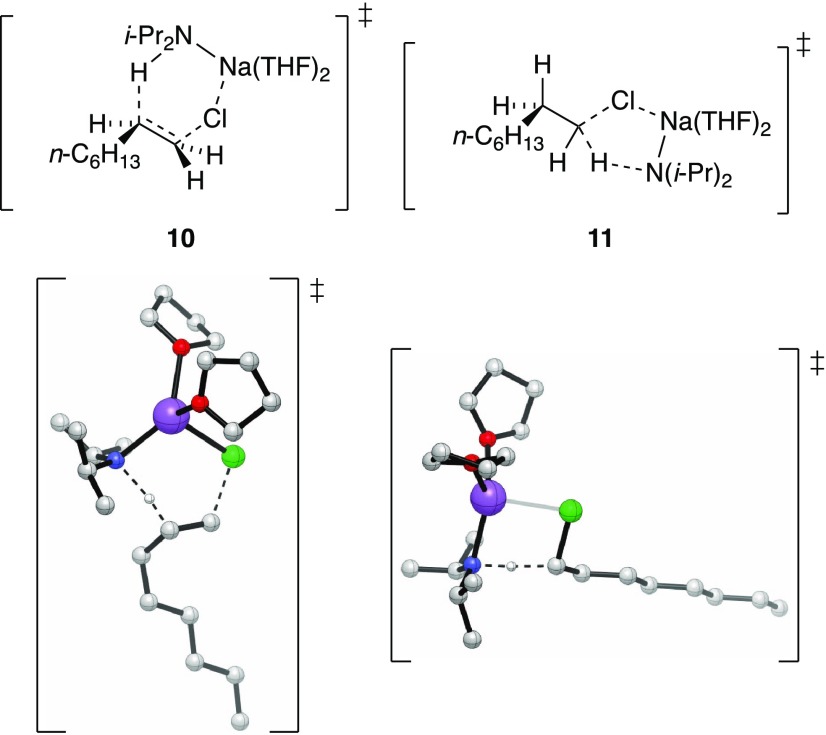
 |
10 |
1-Iodooctane
Reaction of 6 with NaDA in THF/hexane reveals only elimination (eq 7) but is too fast to be monitored conveniently at −78 °C.
1-Fluorooctane
At 25 °C, the elimination of 7 (eq 7) is too slow to compete with THF decomposition.10 We will offer a solution to the problems posed by recalcitrant metalations and competing solvent decomposition in due course.
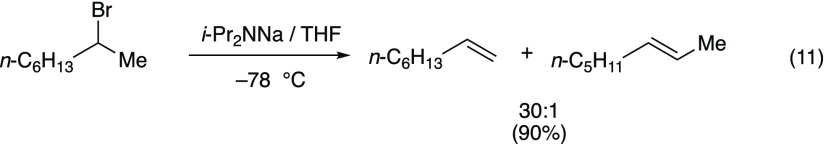
2-Bromooctane
Zaitsev’s rule states that “the alkene formed in greatest amount is the one that corresponds to removal of the hydrogen from the β-carbon having the fewest hydrogen substituents.”21 As a quick check of the veracity of this often-taught rule, we examined the NaDA-mediated elimination of 2-bromooctane. As shown in eq 11, we observed 30:1 selectivity for the terminal alkene; this is the highest reported regioselectivity.22 Removal of Zaitsev’s rule as a centerpiece of our undergraduate curriculum seems overdue.
 |
11 |
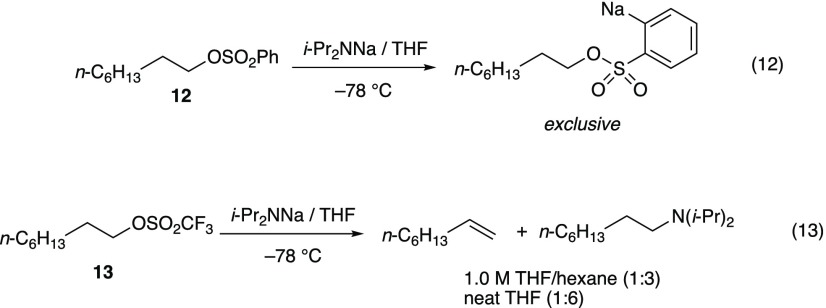
n-Octylsulfonate Esters
A brief excursion to investigate n-octyl sulfonate esters revealed that NaDA/THF-mediated elimination or substitution of benzenesulfonate 12 is precluded by a dominant orthosodiation (eq 12), as confirmed by quenching with D2O. Although this pathway has not been reported for LDA, metalations of benzenesulfonate esters by n-BuLi are well documented.23,24
 |
12 |
Treatment of triflate 13(4) with NaDA/THF at −78 °C provided
a mixture of substitution and elimination products (eq 13).25,26 Although the absolute rates are
too high to conveniently monitor, the relative proportions of substitution
and elimination show THF dependence with a distinct nonzero intercept
(Figure 4). The observed
independence on the NaDA concentration, in conjunction with the dominance
of monomer-based reactions for all NaDA/THF-mediated transformations,
leaves little doubt that monomers are involved. Noting that sulfonates
offer potential Na–X contacts in an SN2 reaction27 less easily attained with alkyl halides,28 we offer computationally viable transition structures 14–16 as compatible with the THF-dependent
selectivities. Computations predict the relative stabilities of 0,
2, and 4 kcal/mol for the transition structures 14, 15, and 16, respectively.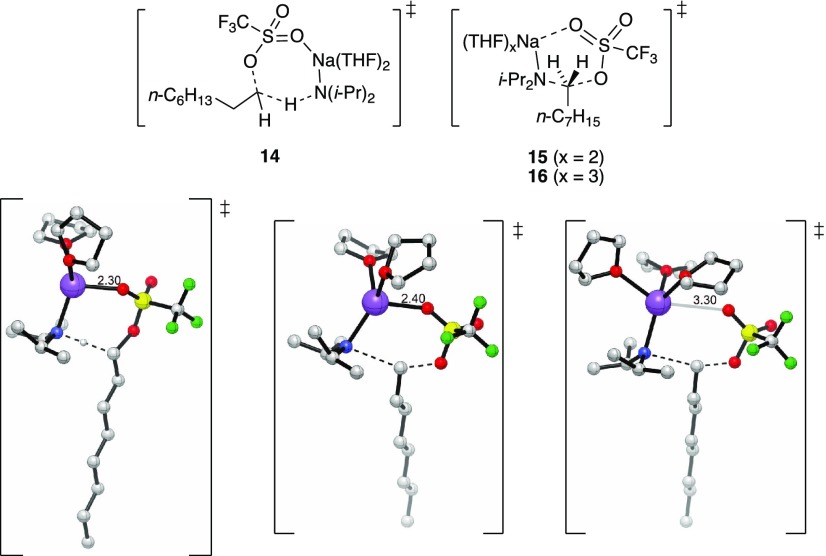
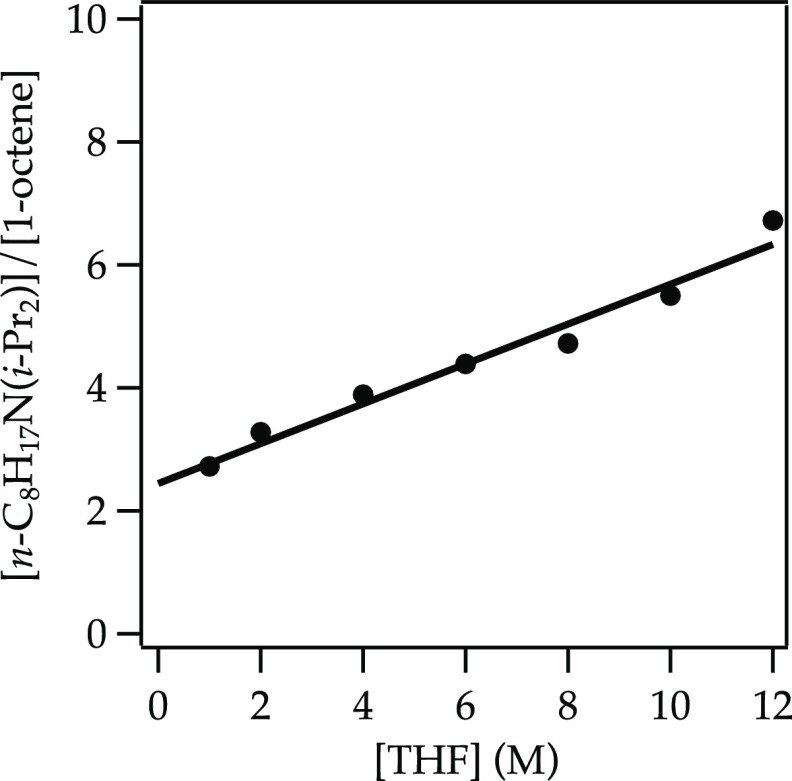
Figure 4.
Plot of [n-C8H17N(i-Pr)2]/[1-octene] versus [THF] in hexane for the reaction of triflate 13 with 0.10 M NaDA at −78 °C (eq 13). The curve depicts an unweighted least-squares fit to the function f(x) = k[THF] + k′ (k = 0.33 ± 0.03; k′= 2.4 ± 0.2).
4-tert-Butylcyclohexyl bromides
Conformationally constrained cyclohexyl halides offer a pedagogically convenient means to underscore the importance of antiperiplanar (trans-diaxial) alignments in dehydrohalogenations.29 Ironically, we know of only one example of the elimination of a 4-tert-butylcyclohexane derivative with adequate isotopic labeling to demonstrate trans-diaxial elimination,30 and it was not a trivial analysis.31 Unexpectedly, NaDA/THF-mediated eliminations of both cis-17 and trans-17 afford 4-tert-butylcyclohexene (18, eq 14) in excellent yields with similar facilities (kax/cis/keq/trans = 10 in neat THF at −35 °C).32
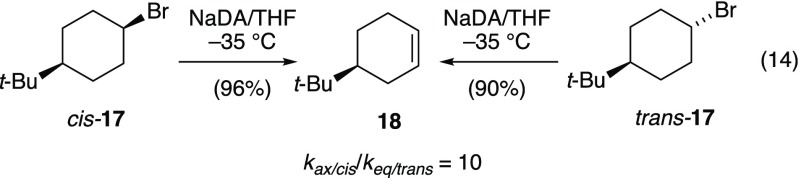 |
14 |
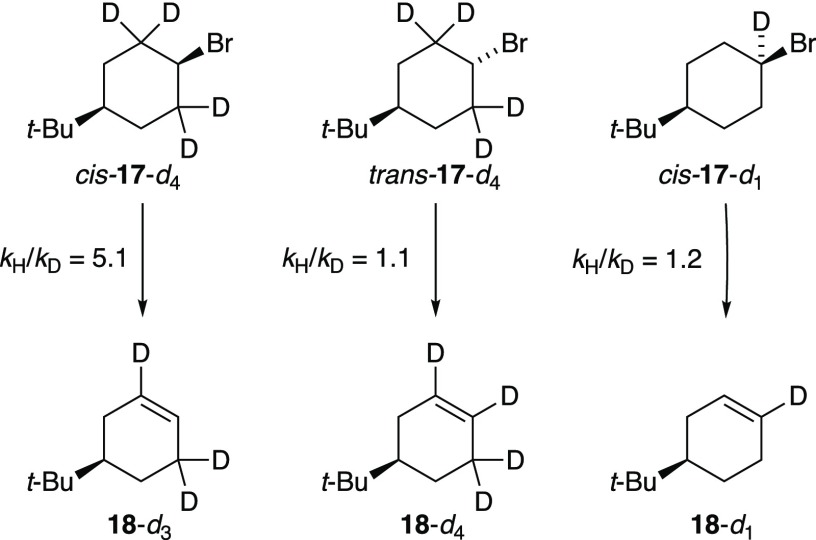
Mono- and tetradeuterated cyclohexyl bromides cis-17-d4, trans-17-d4, and cis-17-d1 were used to interrogate the kinetic isotope effects and isotopic distributions (Scheme 1).31 Elimination of the trans-17 isotopomers reveals geminal metalations to generate carbenoid intermediates,33,34 which are inserted into the proximate C–H(D) bonds. The cis-17 substrates follow a more traditional script for E2 elimination.
Scheme 1. Isotope Effects and Isotope Distributions for NaDA/THF-Mediated Elimination of the Isomers of 17 at −50 °C.
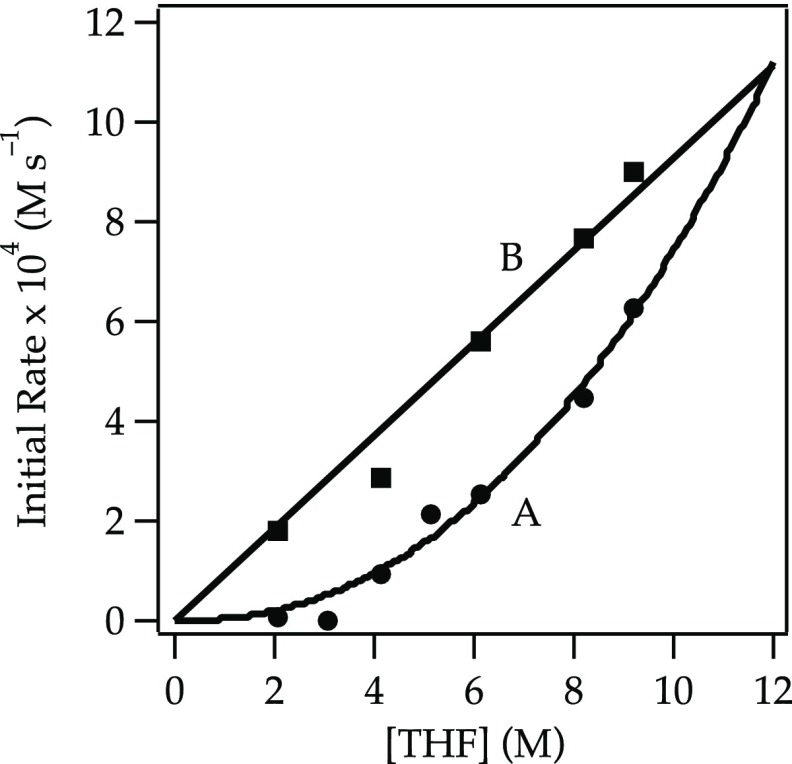
Axial isomer cis-17 follows a half-order
NaDA dependence (0.58 ± 0.08) and a second-order THF dependence
in hexane (Figure 5, curve A), which becomes a first-order dependence when 2,5-Me2THF is used as the cosolvent (curve B).35 Thus, the preferred path is via a trisolvated monomer-based
transition structure 19.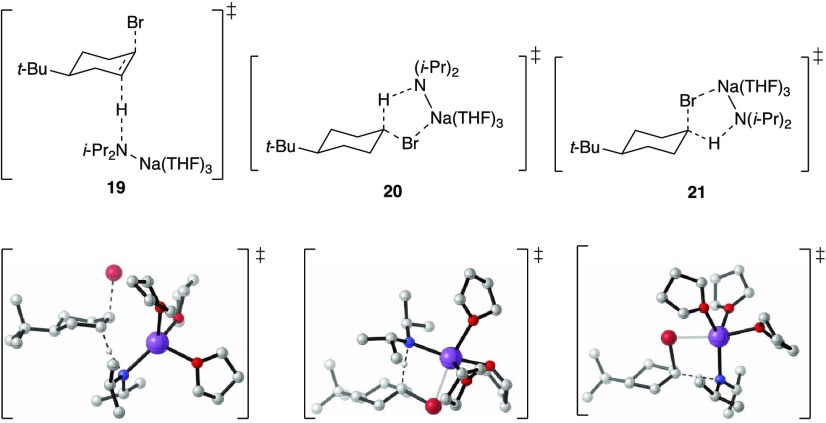
Figure 5.
Plot of the initial rate19b versus [THF] for the dehydrobromination of cis-17 (0.050 M) with NaDA (0.10 M) at −50 °C in hexane (curve A) or 2,5-Me2THF (curve B). The curves depict unweighted least-squares fits. Curve A: y = k[THF]n: k = (3.23 ± 0.01) × 10–6; n = 2.39 ± 0.24. Curve B: y = k[THF]: k = (0.93 ± 0.03) × 10–4.
trans-17, bearing an equatorially disposed bromide, shows a first-order THF dependence (Figure 6), which, in conjunction with a half-order NaDA dependence, is consistent with the trisolvated monomer-based elimination (eq 5). Transition structure 20 is computationally viable and consistent with the rate data and isotopic studies. One might wonder why the axial isomer cis-17 does not also show a carbenoid behavior, especially because the calculated activation barriers for 19 and 20 are 7 and 4 kcal/mol higher (respectively) than that of 21.
Figure 6.
Plot of the initial rate19b versus [THF] for the dehydrobromination of trans-17 (0.050 M) with NaDA (0.13 M) at −25 °C in hexane (curve A) or 2,5-dimethyltetrahydrofuran (curve B). The curves depict unweighted least-squares fits. Curve A: y = k[THF]n: k = (2.22 ± 0.2) × 10–5; n = 1.34 ± 0.04. Curve B: y = k[THF]: k = (0.53 ± 0.02) × 10–4.
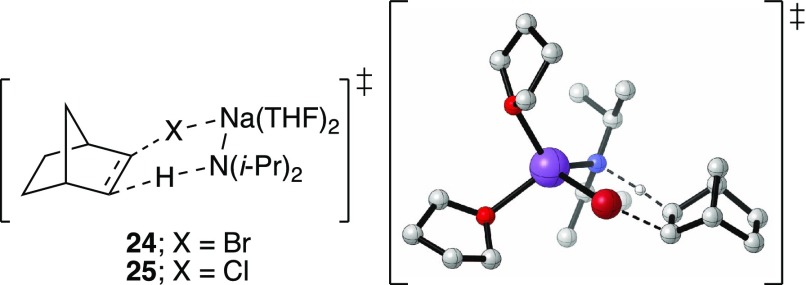
exo-2-Halonorbornanes
Elimination of 2-bromo- and 2-chloronorbornanes (22 and 23, eq 15) at −40 and −20 °C (respectively) shows nearly zeroth-order dependencies on THF even in hexane (Figure 7). This is consistent with syn elimination via the computationally viable transition structures 24 and 25.31,36,37
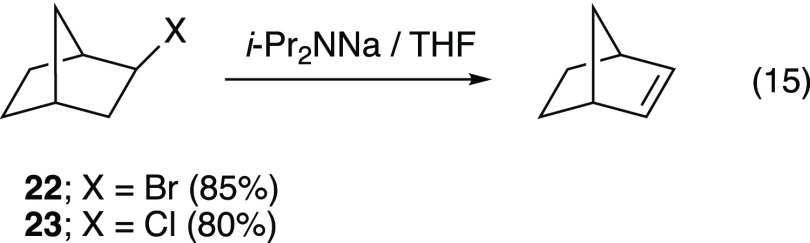 |
15 |
Figure 7.
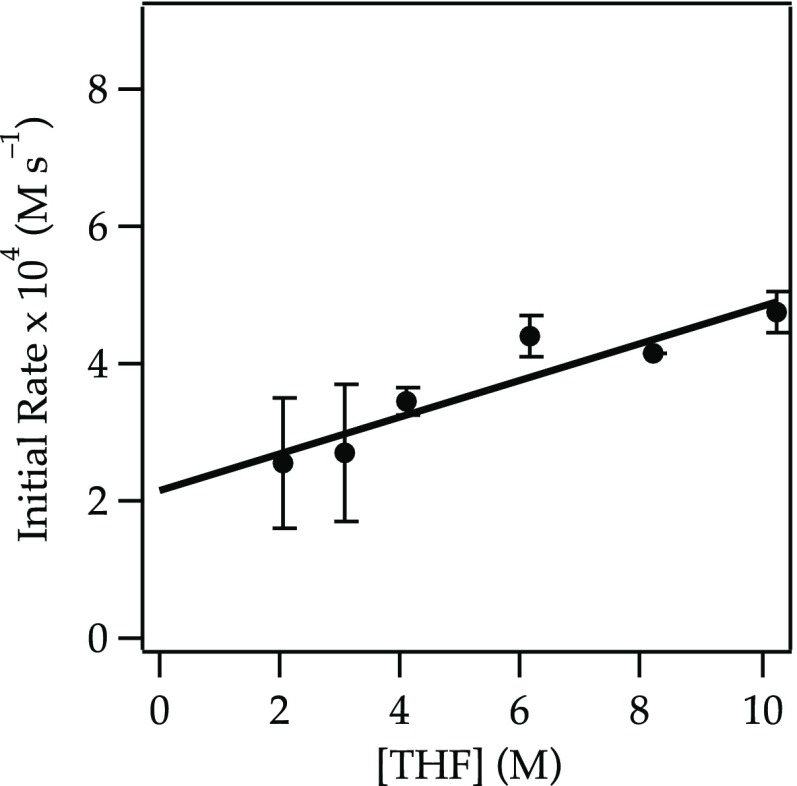
Plot of the initial rate19b versus [THF] in hexane for the dehydrobromination of exo-2-bromonorbornane 22 (0.040 M) with NaDA (0.10 M) at −40 °C. The curve depicts an unweighted least-squares fit to y = k[THF] + k′: k = (2.6 ± 0.5) × 10–4; k′ = (2.2 ± 0.3) × 10–4.
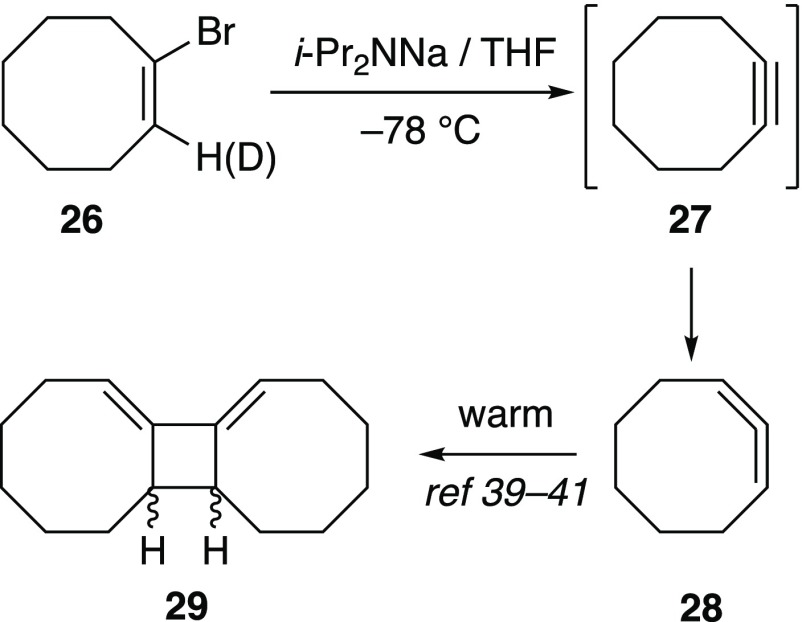
1-Bromocyclooctene
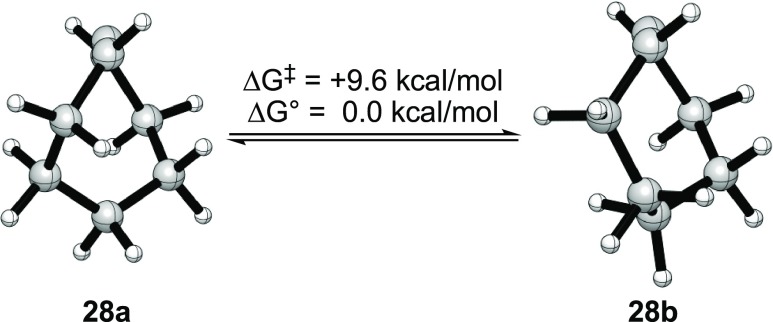
Elimination of 26 to give cyclooctyne (27) necessarily proceeds syn (Scheme 2). Cyclooctyne is not stable under the reaction conditions, cleanly isomerizing to cycloocta-1,2-diene (allene) 28 as a 2:1 mixture of symmetric and unsymmetric conformers, 28a and 28b, respectively (Figure 8). These conformers were decoalesced at −110 °C and characterized by a bevy of two-dimensional (2D-NMR spectroscopic methods (Supporting Information).38 As noted previously,39−4128 self-condenses when warmed to ambient temperatures to give a complex mixture that includes dimer 29 and a trimer (likely the Diels–Alder adduct of 29 and cyclooctyne). An independently prepared sample of 27(31) reacts with NaDA analogously.
Scheme 2. Formation of Cycloocta-1,2-diene.
Figure 8.
Symmetric and unsymmetric conformers of cycloocta-1,2-diene (28a and 28b, respectively).
Rate studies showed a rate-limiting
dehydrohalogenation that necessarily proceeds via 27 rather
than directly to allene 28. Specifically, comparing 26 with 26-d131 affords kH/kD = 10. A linear THF dependence (Figure 9) in conjunction with a half-order
dependence on NaDA (0.50 ± 0.07) are consistent with the generic
mechanisms depicted in eqs 4 and 5. (The nonzero intercept is relatively
minor.) The trisolvated-monomer-based transition structure 30 is computationally viable.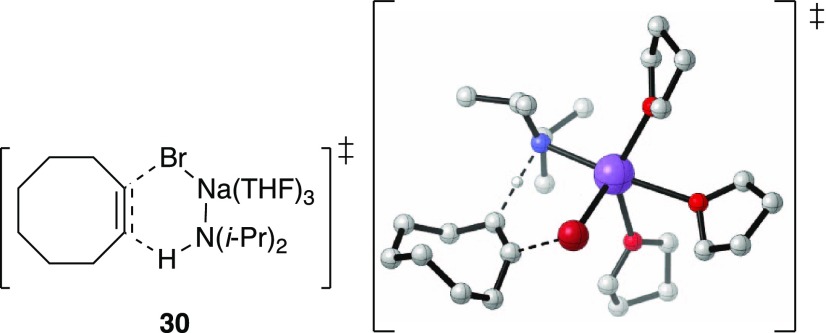
Figure 9.
Plot of the initial rate versus [THF] for the dehydrobromination of 1-bromocyclooctene 26 (0.050 M) with NaDA (0.10 M) at −50 °C in (A) hexane and (B) 2,5-Me2THF. The curve depicts an unweighted least-squares fits. Curve A: y = k[THF] + k′: k = (0.74 ± 0.05) × 10–3; k′ = (1.90 ± 0.05) × 10–3. Curve B: y = k[THF]: k = (0.89 ± 0.12) × 10–3; k′ = (2.61 ± 0.0.03) × 10–5.
trans-1-Bromo-2-methoxycyclohexane (31)42,43
Reaction of 31 (eq 16) cleanly affords the enol ether 32 to the exclusion of allylic ether 33 (>20:1).44 This result contrasts with a reported t-BuOK-mediated elimination to give 33.45 Although it may be tempting to invoke a syn elimination, carbene 34 seems more plausible and has been shown to insert into the C–H bond geminal to the alkoxy moiety with high fidelity.46
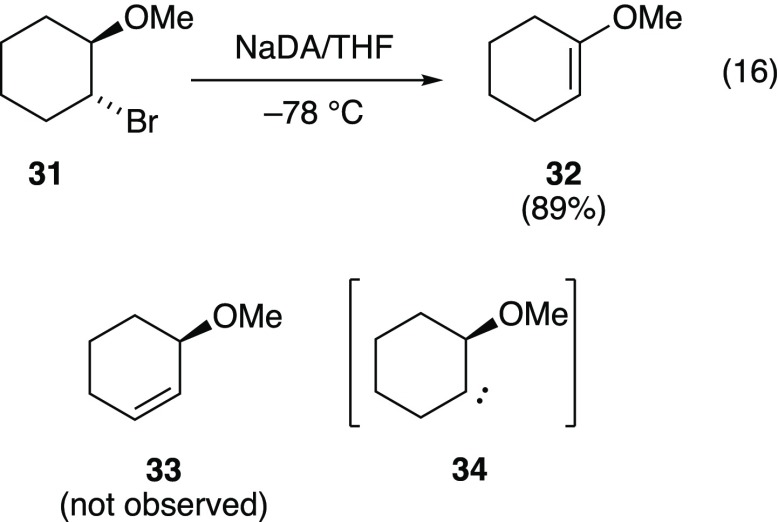 |
16 |
trans-1-Bromo-2-methoxycyclooctane (35)
Dehydrohalogenation of 35 by t-BuOK/18-crown-6 is reported to give allylic ether 36,43 while NaNH2-mediated dehydrohalogenation yields 29 via the transiently stable cycloocta-1,2-diene (allene 28).41 These reports prompted us to examine the reactivity of NaDA/THF with 35. Treatment of 35 with excess NaDA/THF at −78 °C afforded, upon workup, dimer 29 along with other forms (see Scheme 2). Using only 0.80 equiv of NaDA to minimize allene 28 formation, we obtained 1-bromocyclooctene (26) in 62% isolated yield (eq 17). This unusual preferential elimination of the methoxy moiety has meager literature support.47−49
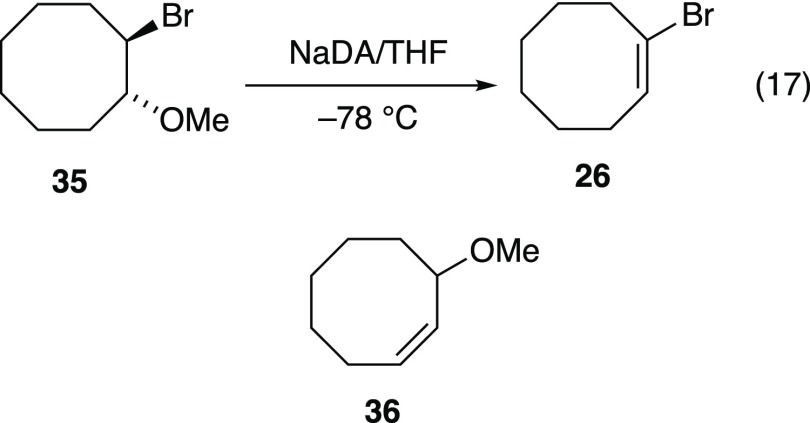 |
17 |
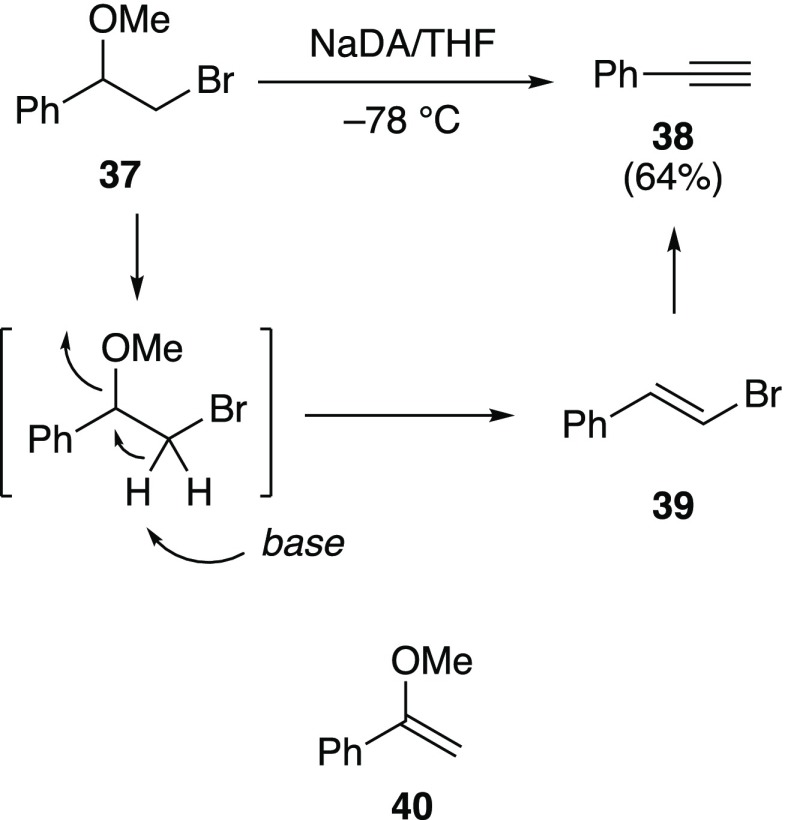
2-Phenyl-2-methoxy-1-bromoethane (37)
Reaction of 37 with NaDA/THF was anticipated to provide enol ether 40 given the considerable acidity of the benzylic proton; however, phenylacetylene was formed instead (Scheme 3).50 Observation of vinyl bromide 39 at partial conversion, along with the failure of an independently prepared sample of enol ether 40 to give phenylacetylene under the reaction conditions, supports the mechanism as drawn.
Scheme 3. Elimination of Vicinal Haloether 37 to Form Acetylene 38.
Conclusions
Eliminations of a range of alkyl halides by NaDA in THF/hexane are facile and efficient. The dominance of monomer-based eliminations is a theme that continues from previous studies of NaDA-mediated metalations.8,11 The absence of competing SN2 substitution contrasts with the analogous reaction of 1-bromooctane with LDA/THF in which substitution and elimination pathways compete. The facile elimination of an equatorially disposed cyclohexyl bromide via a carbenoid-based pathway is also notable.33,34
Previous discussions of solvent effects on base-mediated eliminations are couched using the language of ion pairing.1b There is only limited attention to the role of aggregation,3 and solvation is described as bulk properties rather than as molecular phenomena. We trace rate changes to differential solvation numbers that affiliate with the absence or presence of Na–X contacts in the rate-limiting transition structures. The computed disolvated transition structures show Na–X contacts, whereas the trisolvates are more variable. Overlaid on the primary-shell solvation reside sterically insensitive, secondary-shell effects that can be teased out using 2,5-Me2THF as a polar surrogate for hexane. Although the influences of these secondary-shell polarity effects on reaction rates seem small, the influence on the interpretation of the rate laws and mechanisms is far more consequential. Two important points are that (1) macroscopic solvation effects can be described in terms of separate contributions of primary and secondary shells, and (2) secondary-shell effects seem moderately more pronounced for NaDA than for LDA.
The ease of synthesis, physical properties, and reactivities of NaDA continue to support our assertion that NaDA is a long-overlooked base of considerable potential utility. We hope that our studies of structure–reactivity relationships will prompt practitioners to consider NaDA as more than just hyperreactive LDA. The one notable limitation described herein—the failure to eliminate n-alkyl fluorides on the timescales of THF decomposition—reminds us that efforts to exploit the high reactivity of NaDA will be capped by the lability of ethereal solvents. An altogether different class of ligand will be needed to maximize reactivity without eliciting decomposition. Stated more broadly, organosodium chemistry may require more than just the ligands that have served organolithium chemistry well.
Experimental Section
Reagents and Solvents
THF and hexane were distilled from blue or purple solutions containing sodium benzophenone ketyl. NaDA was prepared from sodium dispersion in N,N-dimethylethylamine (DMEA) using a modified4 procedure first reported by Wakefield and recrystallized from DMEA/hexane.51 Solutions of NaDA can be titrated using a literature method.52
NMR Spectroscopic Analyses
An NMR tube under vacuum was flame-dried on a Schlenk line, allowed to cool to room temperature, backfilled with argon, placed in a −78 °C dry ice/acetone bath, and charged with NaDA and solvents using stock solutions. Substrates were added in THF-d8 or THF-d8/cosolvent mixtures via a syringe. The tube was vortexed two times on a vortex mixer for 5 s with cooling between vortexing. Standard 1H NMR spectra were recorded on a 500 MHz spectrometer. The resonances are referenced to residual THF resonances at 1.73 and 3.58 ppm for 1H and 67.57 and 25.37 ppm for 13C. The concentrations of the starting material were referenced to an internal benzene standard (7.27 ppm). Spectra were recorded with a scan rate that varies depending on the rate of reaction.
Elimination of cis-17
To a stirred solution of NaDA (80.0 mg, 0.65 mmol) in dry THF (10 mL) under argon atmosphere at −78 °C was added cis-1-bromo-4-(tert-butyl)cyclohexane (cis-17, 110 mg, 0.50 mmol) in THF (1.0 mL). The reaction mixture was stirred for 1.5 h, quenched by the addition of saturated NH4Cl solution (1.0 mL), and extracted with Et2O (2 × 10 mL). The organic layer was washed with brine (10 mL) and dried over Na2SO4. Evaporation of the solvent, followed by flash chromatography of the resulting crude residue using neat hexane as an eluent, afforded 18 (66.4 mg, 96% yield).
Cycloocta-1,2-diene (28a)
Allene 28a, contaminated by 20% of a minor conformer 28b, was prepared most cleanly from cyclooctyne using the general procedure for NMR spectroscopic analysis using 0.25 equiv of NaDA in neat THF-d8. The NMR spectroscopic data recorded at −110 °C for the major isomer, 28a, is as follows: 1H NMR (500 MHz, THF-d8): δ 5.3 (m, 1H), 5.11 (t, J = 6.1 Hz, 1H), 2.29 (dd, J = 20.2 Hz, 14.4 Hz, 1H); 2.01 (dd, J = 10.0, 8.0 Hz, 1H), 1.91–1.95 (m, 2H), 1.78–1.84 (m, 3H), 1.33 (q, J = 14.5 Hz, 1H), 1.29 (t, J = 16.2 Hz, 1H), 0.84 (t, J =16.2 Hz, 1H). 13C NMR (73.57 MHz, THF-d8): δ: 208.6, 95.1, 90.5, 33.4, 31.6, 30.1, 29.1, and 27.4. Note: the multiplicities were derived from 2D 1H/13C HSQC experiments and represent approximate multiplet structure. J values smaller than 6 Hz could not be resolved.
Acknowledgments
We thank the National Institutes of Health (GM131713) for support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b01428.
NMR spectroscopic studies; 1H NMR spectrum of 1-bromooctane; partial 1H NMR spectrum of 1-octene (2) in THF-d; spectroscopic rate; and computational data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Smith M.; March J.. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; 6th ed.; Wiley-Interscience: New York, 2007, Chapters 10 and 17. [Google Scholar]; b Bartsch R. A.; Zavada J. Stereochemical and Base Species: Dichotomies in Olefin-Forming E2 Eliminations. Chem. Rev. 1980, 80, 453. 10.1021/cr60328a001. [DOI] [Google Scholar]; c Anslyn E. V.; Dougherty D. A.. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, 2006; pp 590–591. [Google Scholar]
- Csizmar C. M.; Daniels J. P.; Davis L. E.; Hoovis T. P.; Hammond K. A.; McDougal O. M.; Warner D. L. Modeling SN2 and E2 Reaction Pathways and Other Computational Exercises in the Undergraduate Organic Chemistry Laboratory. J. Chem. Educ. 2013, 90, 1235. 10.1021/ed2008735. [DOI] [Google Scholar]
- “Associated” forms (aggregates) in alkoxide-mediated metalations have been discussed in the context of reported fractional reaction orders. See ref (1b).
- Ma Y.; Algera R. F.; Collum D. B. Sodium Diisopropylamide in N,N-Dimethylethylamine: Reactivity, Selectivity, and Synthetic Utility. J. Org. Chem. 2016, 81, 11312. 10.1021/acs.joc.6b02287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser M.Organometallics in Synthesis: A Manual, 2nd ed.; Schlosser M., Ed.; John Wiley & Sons: Chichester, 2002, Chapter 1. [Google Scholar]
- a For a relatively short but comprehensive bibliography of NaDA, see ref (10); b For an extensive review of alkali metal amides, see:Mulvey R. E.; Robertson S. D. Synthetically Important Alkali-Metal Utility Amides: Lithium, Sodium, and Potassium Hexamethyldisilazides, Diisopropylamides, and Tetramethylpiperidides. Angew. Chem., Int. Ed. 2013, 52, 11470. 10.1002/anie.201301837. [DOI] [PubMed] [Google Scholar]; c For an interesting historical perspective on organoalkali metal chemistry, see:Seyferth D. Alkyl and Aryl Derivatives of the Alkali Metals: Useful Synthetic Reagents as Strong Bases and Potent Nucleophiles. 1. Conversion of Organic Halides to Organoalkali-Metal Compounds. Organometallics 2009, 28, 2. 10.1021/om801047n. [DOI] [Google Scholar]
- Transition metal catalyzed eliminations of n-alkylhalides and n-alkyltosylates have received attention recently:Bissember A. C.; Levina A.; Fu G. C. A Mild, Palladium-Catalyzed Method for the Dehydrohalogenation of Alkyl Bromides: Synthetic and Mechanistic Studies. J. Am. Chem. Soc. 2012, 134, 14232. 10.1021/ja306323x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel and coworkers exploited NaDA in DMEA solutions to achieve challenging arene orthometalation-functionalization sequences in a flow system:Wiedmann N.; Ketels M.; Knochel P. Sodiation of Arenes and Heteroarenes in Continuous Flow. Angew. Chem., Int. Ed. 2018, 57, 10748. 10.1002/anie.201803961. [DOI] [PubMed] [Google Scholar]
- Buonora P. T.; Lim Y. J. The Substitution–Elimination Mechanistic Disc Method. J. Chem. Educ. 2004, 81, 368 10.1021/ed081p368. [DOI] [Google Scholar]
- Algera R. F.; Ma Y.; Collum D. B. Sodium Diisopropylamide: Aggregation, Solvation, and Stability. J. Am. Chem. Soc. 2017, 139, 7921. 10.1021/jacs.7b03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Algera R. F.; Ma Y.; Collum D. B. Sodium Diisopropylamide in Tetrahydrofuran: Selectivities, Rates, and Mechanisms of Alkene Isomerizations and Diene Metalations. J. Am. Chem. Soc. 2017, 139, 11544. 10.1021/jacs.7b05218. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Algera R. F.; Ma Y.; Collum D. B. Sodium Diisopropylamide in Tetrahydrofuran: Selectivities, Rates, and Mechanisms of Arene Metalations. J. Am. Chem. Soc. 2017, 139, 15197. 10.1021/jacs.7b08734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Collum D. B.; McNeil A. J.; Ramírez A. Lithium Diisopropylamide: Solution Kinetics and Implications for Organic Synthesis. Angew. Chem., Int. Ed. 2007, 46, 3002. 10.1002/anie.200603038. [DOI] [PubMed] [Google Scholar]; b Algera R. F.; Gupta L.; Hoepker A. C.; Liang J.; Ma Y.; Singh K. J.; Collum D. B. Lithium Diisopropylamide: Non-Equilibrium Kinetics and Lessons Learned about Rate Limitation. J. Org. Chem. 2017, 82, 4513. 10.1021/acs.joc.6b03083. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hsieh H. L.; Quirk R. P.. Anionic Polymerization: Principles and Practical Applications; Marcel Dekker: New York, 1996. [Google Scholar]; d Espenson J. H.Chemical Kinetics and Reaction Mechanisms, 2nd ed.; McGraw-Hill: New York, 1995. [Google Scholar]
- We define the idealized rate law as that obtained by rounding the observed reaction orders to the nearest rational order.
- The dielectric constants of substituted tetrahydrofurans are only slightly lower than THF.; a Harada Y.; Salomon M.; Petrucci S. Molecular Dynamics and Ionic Associations of Lithium Hexafluoroarsenate (LiAsF6) in 4-Butyrolactone Mixtures with 2-Methyltetrahydrofuran. J. Phys. Chem. A 1985, 89, 2006. 10.1021/j100256a041. [DOI] [Google Scholar]; b Carvajal C.; Tolle K. J.; Smid J.; Szwarc M. Studies of Solvation Phenomena of Ions and Ion Pairs in Dimethoxyethane and Tetrahydrofuran. J. Am. Chem. Soc. 1965, 87, 5548. 10.1021/ja00952a005. [DOI] [Google Scholar]
- NaDA has a half-life of 1.0 h at 25 °C in THF.10 By contrast, NaDA in 5.0 M 2,5-dimethyltetrahydrofuran/THF decomposes with a half-life of 2–3 h at 0 °C.
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Zakrzewski V. G.; Montgomery J. A. Jr.; Stratmann R. E.; Burant J. C.; Dapprich S.; Millam J. M.; Daniels A. D.; Kudin K. N.; Strain M. C.; Farkas O.; Tomasi J.; Barone V.; Cossi M.; Cammi R.; Mennucci B.; Pomelli C.; Adamo C.; Clifford S.; Ochterski J.; Petersson G. A.; Ayala P. Y.; Cui Q.; Morokuma K.; Malick D. K.; Rabuck A. D.; Raghavachari K.; Foresman J. B.; Cioslowski J.; Ortiz J. V.; Baboul A. G.; Stefanov B. B.; Liu G.; Liashenko A.; Piskorz P.; Komaromi I.; Gomperts R.; Martin R. L.; Fox D. J.; Keith T.; Al-Laham M. A.; Peng C. Y.; Gill A.; Nanayakkara C.; Gonzalez M.; Challacombe P. M. W.; Johnson B.; Chen W.; Wong M. W.; Andres J. L.; Gonzalez C.; Head-Gordon M.; Replogle E. S.; Pople J. A.. Gaussian 09, revision A.02, Gaussian, Inc.: Wallingford, CT, 2009.
- From Wikipedia, an isodesmic reaction is a chemical reaction in which the type of chemical bonds broken in the reactant are the same as the type of bonds formed in the reaction product.
- Cohen A. J.; Mori-Sánchez P.; Yang W. Insights into Current Limitations of Density Functional Theory. Science 2008, 321, 792. 10.1126/science.1158722. [DOI] [PubMed] [Google Scholar]
- a Casado J.; Lopez-Quintela M. A.; Lorenzo-Barral F. M. The Initial Rate Method in Chemical Kinetics: Evaluation and Experimental Illustration. J. Chem. Educ. 1986, 63, 450. 10.1021/ed063p450. [DOI] [Google Scholar]; b Initial rates (slopes) were determined by fitting 10% of the decay to a third-order polynomial (at2 + bt+c). The parameter b represents the rate at time zero. Initial rate = f′(0) = b). The experimental observable (NMR intensity, IR absorbance etc.) is converted to concentration to ensure a valid comparison between initial rates of varying concentrations.
- Preparations of 1,1-1-d2 and 2,2-1-d2:Boden N.; Bushby R. J.; Clark L. D. The Synthesis of Specifically and Selectively Deuteriated 4,4′- Bis-alkoxyazoxybenzene Derivatives. J. Chem. Soc., Perkin Trans. 1 1983, 543. 10.1039/P19830000543. [DOI] [Google Scholar]
- Braida B.; Prana V.; Hiberty P. C. The Physical Origin of Saytzeff’s Rule. Angew. Chem., Int. Ed. 2009, 48, 5724. 10.1002/anie.200901923. [DOI] [PubMed] [Google Scholar]
- a Barry J.; Bram G.; Decodts G.; Loupy A.; Pigeon P.; Sansoulet J. Solid–Liquid Phase-Transfer Catalysis Reactions without Solvent; Very Mild Conditions for β-eliminations. J. Org. Chem. 1984, 49, 1138. 10.1021/jo00180a040. [DOI] [Google Scholar]; b Wolkoff P. Dehydrobromination of Secondary and Tertiary Alkyl and Cycloalkyl Bromides with 1,8-Diazabicyclo[5.4.0]undec-7-ene. Synthetic Applications. J. Org. Chem. 1982, 47, 1944. 10.1021/jo00349a023. [DOI] [Google Scholar]
- a Jakubec P.; Muratore M. E.; Aillaud I.; Thompson A. L.; Dixon D. J. Design, Synthesis and Applications of New families of Chiral Sulfonic Acids. Tetrahedron: Asymmetry 2015, 26, 251. 10.1016/j.tetasy.2015.02.002. [DOI] [Google Scholar]; b Kawamorita S.; Ohmiya H.; Hara K.; Fukuoka A.; Sawamura M. Directed Ortho Borylation of Functionalized Arenes Catalyzed by a Silica-Supported Compact Phosphine–Iridium System. J. Am. Chem. Soc. 2009, 131, 5058. 10.1021/ja9008419. [DOI] [PubMed] [Google Scholar]
- Sodiation of sulfonate 12-d5 can be monitored by 2H NMR spectroscopy at −110 °C.
- Bashore C. G.; Vetelino M. G.; Wirtz M. C.; Brooks P. R.; Frost H. N.; McDermott R. E.; Whritenour D. C.; Ragan J. A.; Rutherford J. L.; Makowski T. W.; Brenek S. J.; Coe J. W. Enantioselective Synthesis of Nicotinic Receptor Probe 7,8-Difluoro-1,2,3,4,5,6-hexahydro-1,5-methano-3-benzazocine. Org. Lett. 2006, 8, 5947. 10.1021/ol0623062. [DOI] [PubMed] [Google Scholar]
- Substitution of an alkyl triflate by a sodium amide:Cruciani G.; Valeri A.; Goracci L.; Pellegrino R. M.; Buonerba F.; Baroni M. Flavin Monooxygenase Metabolism: Why Medicinal Chemists Should Matter. J. Med. Chem. 2014, 57, 6183. 10.1021/jm5007098. [DOI] [PubMed] [Google Scholar]
- For DFT computations of a lithium amide-based SN2 on a sulfonate ester showing a Li-SO2 contact at the transition state, see:Gupta L.; Ramírez A.; Collum D. B. Reaction of Lithium Diethylamide with an Alkyl Bromide and Alkyl Benzenesulfonate: Origins of Alkylation, Elimination, and Sulfonation. J. Org. Chem. 2010, 75, 8392. 10.1021/jo101505x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitwieser A.; Jayasree E. G.; Hasanayn F.; Leung S. S.-H. A Theoretical Study of SN2′ Reactions of Allylic Halides: Role of Ion Pairs. J. Org. Chem. 2008, 73, 9426. 10.1021/jo8020743. [DOI] [PubMed] [Google Scholar]
- Kirk D. N.; Shaw P. M.. Elimination Reactions and Configurations of 1-Methylcyclohexyl Derivatives, including Steroid Analogues. J. Chem. Soc. C 1970, 182.and references cited therein. 10.1039/j39700000182 [DOI] [Google Scholar]
- a Cross B.; Whitham G. H. Conformationally Fixed Olefins. Part I. The Epimeric 1-Methyl-4-t-butylcyclohexanols and 1-Methylene-4-t-butylcyclohexane. J. Chem. Soc. 1960, 3892. 10.1039/jr9600003892. [DOI] [Google Scholar]; b Kwart H.; Takeshita T. Evaluation of the Relative Importance of Charge-Dipole Interactions and Steric Strain Acceleration in Conformationally Mobile Systems. J. Am. Chem. Soc. 1964, 86, 1161. 10.1021/ja01060a042. [DOI] [Google Scholar]; c Baker R.; Hudec J.; Rabone K. L. An Unusual Entropy Effect in the Solvolysis of Some Steroidal Toluene-p-sulphonates. J. Chem. Soc. D 1969, 197. 10.1039/c29690000197. [DOI] [Google Scholar]; d Allinger N. L.; Liang C. D. Conformational Analysis. LVI. Chlorocyclohexane and 1-Chloro-1-methylcyclohexane. J. Org. Chem. 1967, 32, 2391. 10.1021/jo01283a007. [DOI] [Google Scholar]
- Ma Y.; Ramírez A.; Singh K. J.; Keresztes I.; Collum D. B. Lithium Diisopropylamide Solvated by Hexamethylphosphoramide: Substrate-Dependent Mechanisms for Dehydrobrominations. J. Am. Chem. Soc. 2006, 128, 15399. 10.1021/ja060964b. [DOI] [PubMed] [Google Scholar]
- For examples of eliminations of equatorial halides see ref (1b) and (21).
- a Ashby E. C.; Park B. Competing Radical, Carbanion, and Carbene Intermediates in the Reaction of a Primary Alkyl Halide with Lithium Diisopropylamide. Acta Chem. Scand. 1990, 44, 291. 10.3891/acta.chem.scand.44-0291. [DOI] [Google Scholar]; b Ashby E. C.; Park B.; Patil G. S.; Gadru K.; Gurumurthy R. Competing Radical, Carbanion, and Carbene Pathways in the Reactions of Hindered Primary Alkyl Halides with Lithium Dialkylamides. J. Org. Chem. 1993, 58, 424. 10.1021/jo00054a028. [DOI] [Google Scholar]; c Ashby E. C.; Deshpande A. K.; Patil G. S. A Mechanistic Study of the Reactions of Geminal Dihalides with LDA. Evidence for a Single Electron Transfer Pathway in the Reaction of Geminal Diiodides. J. Org. Chem. 1995, 60, 663. 10.1021/jo00108a032. [DOI] [Google Scholar]; d Taber D. F.; Christos T. E.; Neubert T. D.; Batra D. Cyclization of 1,1-Disubstituted Alkenes to Cyclopentenes. J. Org. Chem. 1999, 64, 9673. 10.1021/jo991311z. [DOI] [Google Scholar]; e Friedman L.; Berger J. G. Dehydrohalogenation of Simple Alkyl Halides by Strong Base; Evidence of Carbene Intermediates, Extent of α-Elimination. J. Am. Chem. Soc. 1961, 83, 492. 10.1021/ja01463a061. [DOI] [Google Scholar]; f Ashby E. C.; Mehdizadeh A.; Deshpande A. K. Mechanistic Study of the Reactions of 1,1-Dihalo-2-methyl-2-phenylpropanes with LDA. Evidence for Radical and Carbene Pathways. J. Org. Chem. 1996, 61, 1322. 10.1021/jo951299k. [DOI] [Google Scholar]
- a Boche G.; Lohrenz J. C. W. The Electrophilic Nature of Carbenoids, Nitrenoids, and Oxenoids. Chem. Rev. 2001, 101, 697. 10.1021/cr940260x. [DOI] [PubMed] [Google Scholar]; b Shao Y.; Huang X.; Zhao C.; Ke Z. Making More Efficient Lithium Carbenoid Reagents for Cyclopropanation by Hetero-aggregation: A DFT Prediction on a New Factor to Control the SN2-Type Organometallic Reaction. J. Organomet. Chem. 2018, 864, 110. 10.1016/j.jorganchem.2018.02.021. [DOI] [Google Scholar]
- Before fully appreciating the importance of secondary shell solvation effects, with considerable effort we located a computationally viable transition structure bearing four coordinated THF ligands. We originally assumed the difficulties underscored the limitations of DFT in sterically congested cases.
- For leading references to base-mediated eliminations of norbornyl halides, see ref (31) as well asBartsch R. A.; Lee J. G. Stereochemistry of Base-Promoted 1,2-Elimination from exo-2-Bicyclo[2.2.1]heptyl Tosylate and Chloride. J. Org. Chem. 1991, 56, 212. 10.1021/jo00001a041. [DOI] [Google Scholar]
- Ramírez A.; Lobkovsky E.; Collum D. B. Hemilabile Ligands in Organolithium Chemistry: Substituent Effects on Lithium Ion Chelation. J. Am. Chem. Soc. 2003, 125, 15376. 10.1021/ja030322d. [DOI] [PubMed] [Google Scholar]
- Yavari carried out essentially the same force field calculations on 28 almost four decades ago. The final line of his paper suggests that the substantial calculated barrier (8.8 kcal/mol) “makes a dynamic nuclear magnetic resonance spectroscopic study...quite attractive.” We inadvertently took his cue.Yavari I. Conformational Energy Surface of 1,2-Cyclooctadiene. J. Mol. Struct. 1980, 65, 169. 10.1016/0022-2860(80)85189-1. [DOI] [Google Scholar]
- a Ball W. J.; Landor S. R. The Synthesis of Cyclic Allenes. J. Chem. Soc. 1962, 2298–2304. 10.1039/JR9620002298. [DOI] [Google Scholar]; b Pietruszka J.; König W. A.; Maelger H.; Kopf J. Dimerisierungsprodukte von 1,2-Cyclooctadien?. Chem. Ber. 1993, 126, 159.and references cited therein 10.1002/cber.19931260124. [DOI] [Google Scholar]
- Kawase T.Product Class 3. Science of Synthesis; 2007; 44, 395. [Google Scholar]
- Base-mediated eliminations of 1-halocyclooctenes are reported to give the allene dimer:; a Ball W. J.; Landor S. R. Allenes. Part III. The Synthesis of Cyclic Allenes. J. Chem. Soc. 1962, 2298. 10.1039/jr9620002298. [DOI] [Google Scholar]; b Caubere P.; Coudert G. Les Bases Complexes—VII: Eliminations syn en Serie Cyclohexanique. Examen Rapide de Derives Cyclopenta-et-heptaniques. Bull. Assoc. Chim. 1973, 3067. [Google Scholar]; c Christl M.; Groetsch S.; Gunther K. The Dimerization of Chiral Allenes: Pairs of Enantiomers and Pairs of Homomers Furnish Different Diastereomers. Angew. Chem., Int. Ed. 2000, 39, 3261.. [DOI] [PubMed] [Google Scholar]
- a Dewkar G. K.; Narina S. V.; Sudalai A. NaIO4-Mediated Selective Oxidative Halogenation of Alkenes and Aromatics Using Alkali Metal Halides. Org. Lett. 2003, 5, 4501. 10.1021/ol0358206. [DOI] [PubMed] [Google Scholar]; b Fresnet P.; Dubois J.-E. Evidence d’un Intermediaire Carbonium dans la Methoxybromation du Cyclooctene cis. Tetrahedron Lett. 1979, 20, 2137. 10.1016/S0040-4039(01)86283-3. [DOI] [Google Scholar]
- Rodriguez J.; Dulcere J. P.; Bertrand M. Cohalogenation des Olefines: Application a la Synthese d’Alcools Allyliques. Tetrahedron Lett. 1984, 25, 527. 10.1016/S0040-4039(00)99928-3. [DOI] [Google Scholar]
- Elimination of trans vicinal iodo/alkyl ethers derived from cyclohexane using KOH/EtOH affords enol ethers analogous to 31:; a Mekhtiyeva V. Z.; Karayev S. F.; Talybov G. M. Some Properties of 1-Iodo-2-propargyloxycyclohexane. Izv. Vyssh. Uchebn. Zaved., Khim. Khim. T. 2003, 46, 83. [Google Scholar]; b Kuznetsov N. Y.; Tikhov R. M.; Godovikov I. A.; Medvedev M. G.; Lyssenko K. A.; Burtseva E. I.; Kirillova E. S.; Bubnov Y. N. Stereoselective Synthesis of Novel Adamantane Derivatives with High Potency against Rimantadine-Resistant Influenza A Virus Strains. Org. Biomol. Chem. 2017, 15, 3152. 10.1039/C7OB00331E. [DOI] [PubMed] [Google Scholar]; c Dhanjee H.; Minehan T. G. Indium-Mediated Allylation of Aldehydes, Ketones and Sulfonimines with 2-(Alkoxy)allyl Bromides. Tetrahedron Lett. 2010, 51, 5609. 10.1016/j.tetlet.2010.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ley S. V.; Diez E.; Dixon D. J.; Guy R. T.; Michel P.; Nattrass G. L.; Sheppard T. D. Preparation of Enantiopure Butane-2,3-Diacetals of Glycolic Acid and Alkylation Reactions Leading to α-Hydroxyacid and Amide Derivatives. Org. Biomol. Chem. 2004, 2, 3608. 10.1039/B412788A. [DOI] [PubMed] [Google Scholar]
- For a t-BuOK-mediated elimination of vicinal alkoxy halides analogous to 29 to give allylic ethers, see:Dulcère J.-P.; Rodriguez J. Cohalogenation of Alkenes in Ethylene Oxide: Efficient Methodology for the Preparation of Allyl Vinyl Ether Precursors of γ,δ-Unsaturated Aldehydes. Synthesis 1993, 1993, 399. 10.1055/s-1993-25872. [DOI] [Google Scholar]
- a Mohamadi F.; Collum D. B. Conversion of Ketones to Trisubstituted Olefins Under Neutral Conditions. Tetrahedron Lett. 1984, 25, 271. 10.1016/S0040-4039(00)99859-9. [DOI] [Google Scholar]; b Xiao F.; Zhang Z.; Zhang J.; Wang J. Reaction of β-Trimethylsiloxy α-Diazocarbonyl Compounds with Trimethylsilyl Halides: A Novel Diazo Decomposition Process. Tetrahedron Lett. 2005, 46, 8873. 10.1016/j.tetlet.2005.10.070. [DOI] [Google Scholar]; c Aggarwal V. K.; Sheldon C. G.; Macdonald G. J.; Martin W. P. A New Method for the Preparation of Silyl Enol Ethers from Carbonyl Compounds and (Trimethylsilyl)diazomethane in a Regiospecific and Highly Stereoselective Manner. J. Am. Chem. Soc. 2002, 124, 10300. 10.1021/ja027061c. [DOI] [PubMed] [Google Scholar]; d Yoon C. H.; Zaworotko M. J.; Moulton B.; Jung K. W. Regio- and Stereocontrol Elements in Rh(II)-Catalyzed Intramolecular C–H Insertion of α-Diazo-α-(phenylsulfonyl)acetamides. Org. Lett. 2001, 3, 3539. 10.1021/ol016647l. [DOI] [PubMed] [Google Scholar]
- Examples of base-mediated elimination of enol ethers to form allenes:; a Lyapkalo I. M.; Webel M.; Reißig H. U. Synthesis and Heck Reactions of Ethenyl- and (Z)- Butadien-1-yl Nonaflate Obtained by the Fragmentation of Furan Derivatives. Eur. J. Org. Chem. 2001, 4189.. [DOI] [Google Scholar]; b Harmata M.; Gamlath C. B. Intramolecular 4 + 3 Cycloadditions of 2-Alkoxyallylic Cations Derived from 2-Alkoxyallylic Sulfones. J. Org. Chem. 1988, 53, 6154. 10.1021/jo00261a043. [DOI] [Google Scholar]
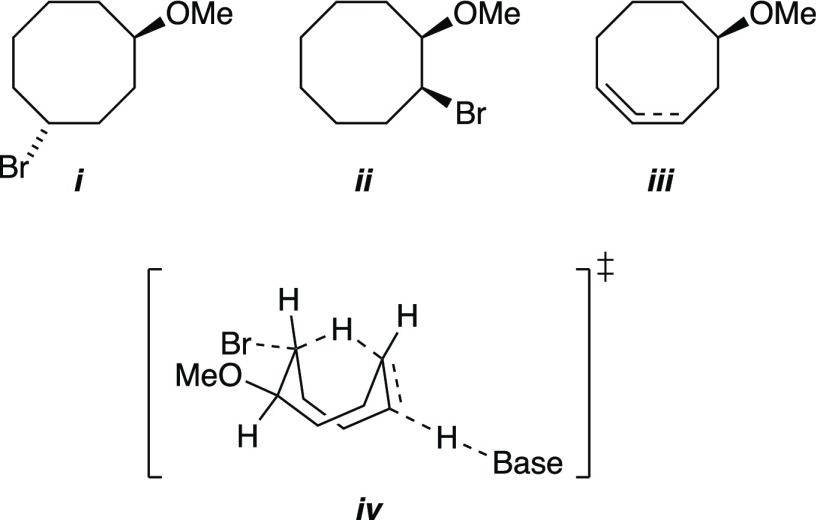
- As a humorous aside, 35 was
contaminated with significant concentrations of trans-4-bromo-1-methoxycyclooctane (i); see ref (38). Thinking we had isolated
the cis-1-bromo-2-methoxycyclooctane (ii)—these things happen—we proceeded
to dehydrohalogenate it to give cyclooctenes iii. Inspired by numerous transannular effects of cyclooctanes
(see ref (49)), with
help from a colleague we concocted a homodehydrobromination via transition
structure iv. Although fictional, iv is also computationally viable.
- Cope A. C.; Lee H. H.; Petree H. E. Proximity Effects. XII. Reaction of cis- and trans-Cyclooctene Oxide with Bases. J. Am. Chem. Soc. 1958, 80, 2849. 10.1021/ja01544a064. [DOI] [Google Scholar]
- Elimination of halo ethers to acetylenes is facile with NaNH2:; a Matovic N.; Matthias A.; Gertsch J.; Raduner S.; Bone K. M.; Lehmann R. P.; DeVoss J. J. Stereoselective Synthesis, Natural Occurrence and CB2 Receptor Binding Affinities of Alkylamides from Herbal Medicines Such as Echinacea sp. Org. Biomol. Chem. 2007, 5, 169. 10.1039/B615487E. [DOI] [PubMed] [Google Scholar]; b Kikuchi K.; Tatewaki Y.; Okada S. Synthesis and Solid-State Polymerization of a Macrocyclic Compound with Two Butadiyne Units. Bull. Chem. Soc. Jpn. 2017, 90, 387. 10.1246/bcsj.20160418. [DOI] [Google Scholar]
- Barr D.; Dawson A. J.; Wakefield B. J. A Simple, High-Yielding Preparation of Sodium Diisopropylamide and Other Sodium Dialkylamides. J. Chem. Soc., Chem. Commun. 1992, 204. 10.1039/c39920000204. [DOI] [Google Scholar]
- Kofron W. G.; Baclawski L. M. A Convenient Method for Estimation of Alkyllithium Concentrations. J. Org. Chem. 1976, 41, 1879. 10.1021/jo00872a047. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.