Abstract
Alveolar ridge deficiency hampers placement of dental implants in functionally optimal position. This warrants hard-tissue augmentation using bone grafts. Lately, the use of autogenous tooth bone graft material is gaining a momentum. However, tedious and time-consuming chair-side preparation limits its use. Allograft using human extracted teeth can be a better alternative to tackle these practical issues. Hence, we prepared “Whole Human Tooth Allograft (whole tooth allograft [WTA])” following standard protocols of tissue bank at Tata Memorial Hospital, Mumbai, India. The efficacy of WTA was evaluated in a 43-year-old patient who reported with Seibert Class-III alveolar ridge deficiency in the right maxillary canine region. Three-dimensional changes were evaluated on clinical and radiographic parameters at baseline and at 4-month follow-up. A clinicoradiographic comparative analysis of height and width measurements revealed a successful three-dimensional alveolar ridge augmentation. The finding of the present case report underscores clinical safety and good bone-forming potential of WTA.
Keywords: Autograft, ridge augmentation, sticky bone, tooth graft
INTRODUCTION
Alveolar ridge resorption following tooth extractions, periodontal diseases, trauma, or congenital abnormalities limits clinicians’ ability to rehabilitate dental function.[1] Hard-tissue augmentation procedures using bone grafts are often required.[2] Although autogenous bone graft is still considered as gold standard, it is a less favored option for routine use due to its limited availability, donor-site morbidity, and unpredictable graft resorption rate. Besides, three-dimensional hard-tissue augmentation often requires a large amount of bone graft material that should be osteo-inductive and cost-effective. The quest of finding an “ideal” graft has driven research in the direction of inventing a number of bone graft substitutes.[3] The substitute that is making headlines lately is graft prepared using human extracted teeth. The rationale for its use is structural and biochemical similarities between dentin and bone.[4] Moreover, demineralized dentin matrix (DDM) has shown to possess exogenous bone morphogenetic proteins (BMPs) and other growth factors that promote bone remodeling.[5] Even though autogenous DDM has demonstrated promising results, it comes with its own practical challenges. Especially in routine dental practice, its chair-side preparation can be tedious and time consuming. To overcome these shortcomings, we prepared allografts from human extracted teeth. The present case report evaluates the efficacy of human tooth allograft in combination with autologous fibrin glue (AFG) to treat alveolar ridge defect in maxillary right canine region.
CASE REPORT
A 43-year-old female patient reported to the department of periodontology, with missing right maxillary canine [Figure 1]. The medical history was noncontributory. After two failed attempts of root canal therapies, the canine was ultimately extracted around 2 years ago. After discussing possible options to replace the missing tooth, the patient demanded implant-supported fixed restoration. Clinical and radiographic examination using cone-beam computed tomography (CBCT) was performed (baseline scan) to evaluate the hard-tissue profile of the edentulous area. The scan revealed a Seibert Class III alveolar ridge defect with a remaining ridge width of 2.55 mm and a height of 9.21 mm. We decided to embark on two-stage approach. This article reports the first stage, i.e., ridge augmentation procedure using whole tooth allograft (WTA) in combination with AFG. This was carried out to prepare the edentulous site to receive functionally optimal sized implant.
Figure 1.
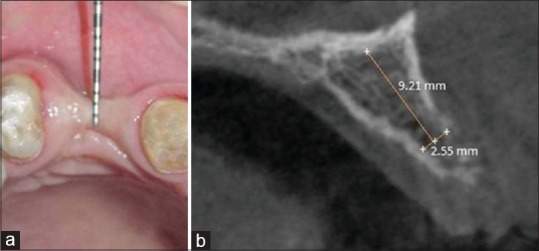
Preoperative (a) clinical, (b) cone-beam computed tomography evaluation
Allograft preparation
The detailed procedure of WTA preparation has already been published.[6] Briefly, full-mouth extraction cases were screened to identify potential donors to collect extracted teeth. Four patients with nonreactive sera for HIV, hepatitis B virus, hepatitis C virus, and syphilis antigens donated their extracted teeth. All these patients signed informed written consent for tooth donation, and their medical histories were noncontributory. Any residual soft-tissue attachments were removed, and all the extracted teeth were scaled using an ultrasonic scaler. The collected extracted teeth were stored at −20°C. Once all the extracted teeth were collected, they were pooled and transported on ice to tissue bank at Tata Memorial Hospital (TMH), Mumbai, India. Upon arrival, the teeth were processed immediately, were sterilized, and were packed as 1 cc graft of particle size 300 μm–500 μm, using the standard protocol of TMH for bone graft preparation [Figure 2].
Figure 2.
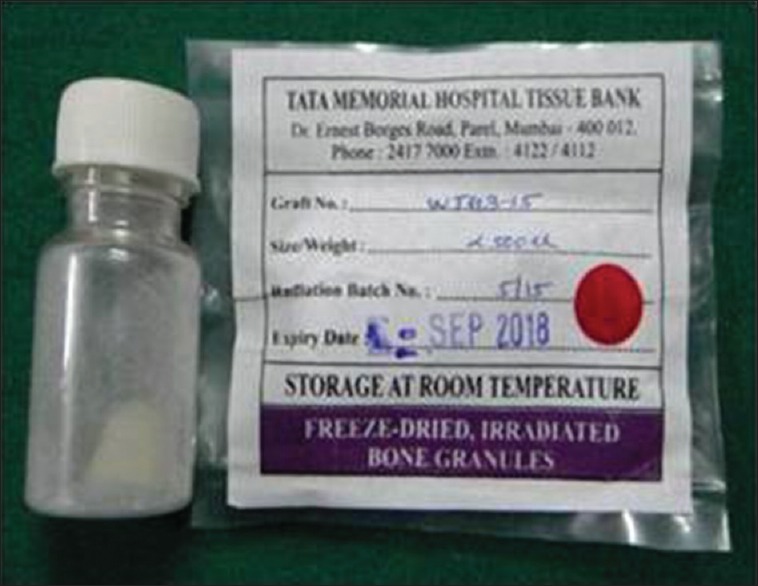
Tooth allograft
Surgical procedure
After obtaining informed consent, a crestal incision was given in the edentulous maxillary right canine area. The crestal incision was continued as sulcular incision on the adjacent teeth. Full-thickness flaps were reflected on the buccal and palatal sides to maximize visualization of the underlying alveolar ridge defect [Figure 3a–f]. Hemostasis was achieved, and the alveolar bone was decorticated using a small round surgical bur (PRIMA CLASSIC carbide bur [HP-8], UK). At the beginning of the surgery, 10 mL intravenous blood was collected through a venipuncture of the median cubital vein in the antecubital fossa in a nonsilica-coated tube. The collected blood was centrifuged at 2700 rpm for 2 min that led to separation of blood into two distinct layers. The lower layer containing red blood cells was discarded, whereas the upper layer containing straw-colored liquid referred to as “AFG” was collected using a syringe. It was then mixed with particulate WTA and left standing for around 5–10 min. This yielded a homogenous mixture of WTA particles interspersed and trapped between the fibrin meshwork. This polymerized pliable mass rich in platelets, leukocytes, and mesenchymal cells was referred to as “sticky bone”[7] [Figure 4]. It was then adapted around the surgical site and covered with a collagen membrane ([Periocol® GTR], Eucare Pharmaceuticals Private Limited, India). The flaps were approximated, and a primary closure was achieved using 4-0 vicryl suture (Polyglactin 910[4-0] suture [VICRYL®, Ethicon], Johnson and Johnson Ltd., India). The patient was given postoperative instructions and prescribed amoxicillin (500 mg), thrice daily for 5 days, and ibuprofen (400 mg), thrice daily for 3 days. The patient was instructed to use 0.12% chlorhexidine mouth rinse twice a day for 14 days without mechanical cleaning of the surgical area. The patient was recalled after 1 week for suture removal. The surgical site healed without any eventful sequelae. Clinical and radiographic analysis was performed at 4-month postoperative follow-up visit. The clinical examination suggested gain in alveolar ridge width and height. A second CBCT scan was taken at this point to ascertain hard-tissue augmentation. Comparison of baseline and 4-month postoperative scans revealed a gain of 5.31 mm and 4.27 mm in ridge width and height, respectively [Figure 5].
Figure 3.
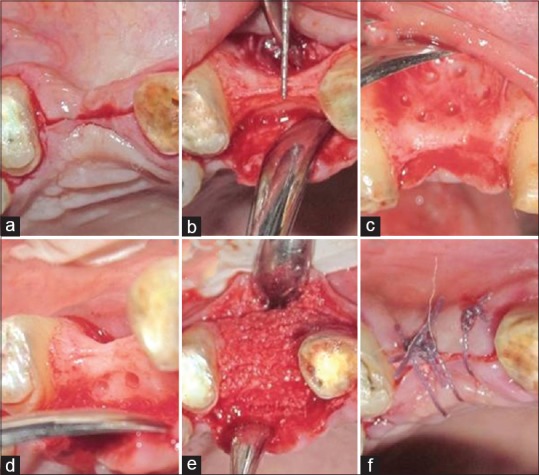
Surgical procedure (a) incision, (b) full-thickness flap raised, (c) decortication done on the buccal aspect, (d) decortication done on the palatal aspect, (e) sticky bone placed, (f) suturing
Figure 4.
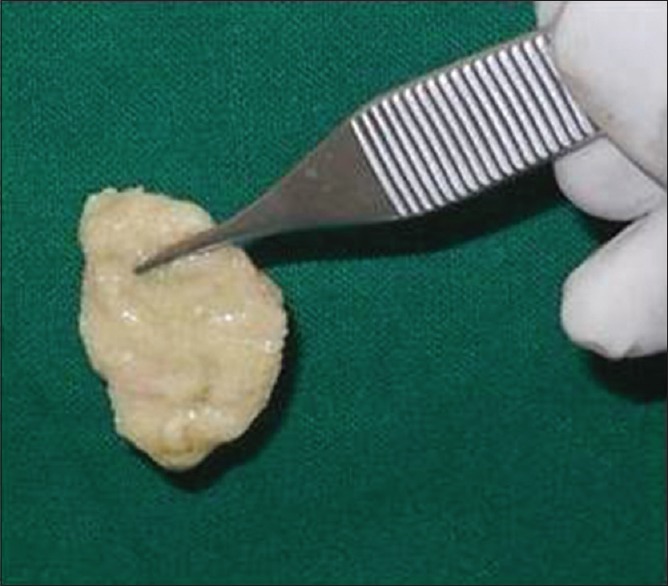
Sticky bone
Figure 5.
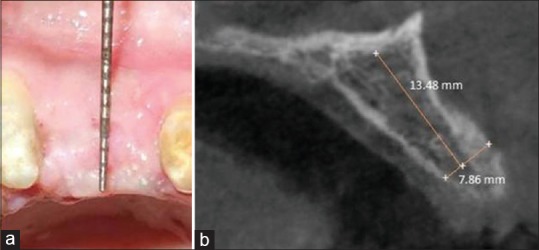
Postoperative follow-up after 4 months (a) clinical, (b) cone-beam computed tomography evaluation
DISCUSSION
Alveolar bone deficiency is a common roadblock in the successful prosthetic rehabilitation of missing teeth, especially using dental implants. For dental implants to be placed in its functionally ideal position, three-dimensional hard-tissue reconstruction of recipient site by guided bone regeneration (GBR) is often necessary.[8] A vast variety of bone grafts and resorbable barrier membranes are available for the purpose of GBR technique. However, for successful hard-tissue regeneration, a bone graft should be biomechanically stable, able to degrade and replaced by new bone within an appropriate period. At the same time it should exhibit osteoconductive, osteogenic and osteoinductive properties. It should also provide a favorable environment for invading blood vessels and bone-forming cells.[9] Human extracted teeth are reported to possess most of these properties and thus can serve as a bone graft substitute.[10] The osteoinductive potential of dentin was first discovered in 1967.[11] Since then, a number of studies have shown promising results using dentin in comparison to various commercially available bone graft substitutes.[12,13,14,15] This is possibly due to the fact that bone and dentin possess similar biochemical properties. Dentin has shown to contain BMPs, insulin-like growth factor (IGF)-II, vascular endothelial growth factor, and transforming growth factor (TGF)-β. Cementum, a bone-like material surrounding tooth roots, also contains TGF-β, IGF-I, and type I and type III collagen, possibly contributing to its osteoinductive potential.[16,17] In our previous randomized controlled study, we demonstrated that allogenic WTA and dentin graft (DA) have superior results than the conventional freeze-dried bone allograft in maintaining alveolar crest height and width, postextraction (P < 0.05). Between WTA and DA sites, there was no statistically significant difference, suggesting that there is no need to separate dentin from tooth when the entire tooth can be utilized as a bone graft material with similar results. Histological analysis confirmed more new bone formation at WTA as well as at DA sites.[6] Findings of this case report reiterate the efficacy of using whole tooth as bone graft. In this case, we decided to mix WTA with platelet concentrates as they have shown to act as the source of growth factors promoting angiogenesis around bone graft and newly formed bone.[18,19] This has been supported by findings of Gomes et al. who evaluated the effects of homogenous DDM (HDDM) slices and platelet-rich plasma (PRP) in the treatment of surgical defects created in diabetic rabbits using GBR technique. Their histological analysis revealed that HDDM promoted not only increased bone formation but also superior bony microstructure owing to graft's osteoinductive and osteoconductive activities, while PRP stimulated angiogenesis and red bone marrow formation.[20] In our experience, the use of “sticky bone” preparation was particularly useful for alveolar ridge augmentation as bone graft trapped within cross-linked fibrin meshwork prevents any undesired movement of graft particles during healing phase. This allows stabilization of the bone graft onto the defect without having to use any bone tacks or titanium mesh, thereby accelerating tissue healing.[7] In the present case, we used a bio-resorbable collagen membrane that aided in compartmentalizing grafted space by blocking any undesirable migration of fibroblasts that originated from the underside of flaps. Moreover, it has been shown that the osteoblasts and fibroblasts can attach to the inner aspect of the collagen membrane irrespective of its origin, thereby promoting wound healing.[21] As collagen membranes lack rigidity, use of particulate graft is advocated to maintain space and to prevent membrane from collapsing.[22]
CONCLUSION
The clinical and radiographic outcomes confirmed substantial gain in alveolar bone width as well as height. The results strengthened the benefits of using human tooth allograft mixed with autologous platelet concentrates in improving alveolar bone profile. Optimal integration of graft particle compounded with uneventful clinical healing demonstrates the potential of WTA to be used as an alternative to conventional bone grafts. It is a cost-effective alternative to most of the conventional graft materials. This becomes even more important as extracted teeth, which otherwise considered as biomedical waste, can be utilized for graft preparation. Its potential as a graft material should further be explored and validated using systematically conducted randomized controlled trials with long-term follow-ups.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Manjunath N, Arjun M. An ounce of prevention is worth a pound of cure: A review on ridge augmentation. J Interdiscip Dent. 2015;5:97–104. [Google Scholar]
- 2.Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J Clin Periodontol. 2008;35:203–15. doi: 10.1111/j.1600-051X.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 3.Sollazzo V, Carinci F, Lauritano D. The biophysical stimulation of osteogenesis: A review. Eur J Inflamm. 2012;10:65–70. [Google Scholar]
- 4.Huang YC, Lew WZ, Feng SW, Lai WY, Abiko Y, Huang HM. Histomorphometric and transcriptome evaluation of early healing bone treated with a novel human particulate dentin powder. Biomed Mater. 2016;12:015004. doi: 10.1088/1748-605X/12/1/015004. [DOI] [PubMed] [Google Scholar]
- 5.Murata M, Sato D, Hino J, Akazawa T, Tazaki J, Ito K, et al. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J Biomed Mater Res A. 2012;100:571–7. doi: 10.1002/jbm.a.33236. [DOI] [PubMed] [Google Scholar]
- 6.Joshi CP, D’Lima CB, Samat UC, Karde PA, Patil AG, Dani NH. Comparative alveolar ridge preservation using allogenous tooth graft versus free-dried bone allograft: A randomized, controlled, prospective, clinical pilot study. Contemp Clin Dent. 2017;8:211–7. doi: 10.4103/ccd.ccd_147_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn DS, Huang B, Kim J, Park WE, Park CC. Utilization of autologous Concentrated Growth Factors (CGF) enriched bone graft matrix (Sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J Implant Adv Clin Dent. 2015;7:11–29. [Google Scholar]
- 8.Wessing B, Lettner S, Zechner W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33:87–100. doi: 10.11607/jomi.5461. [DOI] [PubMed] [Google Scholar]
- 9.Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury. 2011;42(Suppl 2):S77–81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999–1008. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, et al. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e39–45. doi: 10.1016/j.oooo.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res. 2017;28:809–15. doi: 10.1111/clr.12885. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt CM, Doering H, Schmidt T, Lutz R, Neukam FW, Schlegel KA. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin Oral Implants Res. 2013;24:576–85. doi: 10.1111/j.1600-0501.2012.02431.x. [DOI] [PubMed] [Google Scholar]
- 15.Joshi CP, Dani NH, Khedkar SU. Alveolar ridge preservation using autogenous tooth graft versus beta-tricalcium phosphate alloplast: A randomized, controlled, prospective, clinical pilot study. J Indian Soc Periodontol. 2016;20:429–34. doi: 10.4103/0972-124X.188335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YK, Lee J, Um IW, Kim KW, Murata M, Akazawa T, et al. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surg. 2013;39:103–11. doi: 10.5125/jkaoms.2013.39.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis-Filho CR, Silva ER, Martins AB, Pessoa FF, Gomes PV, de Araújo MS, et al. Demineralised human dentine matrix stimulates the expression of VEGF and accelerates the bone repair in tooth sockets of rats. Arch Oral Biol. 2012;57:469–76. doi: 10.1016/j.archoralbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Qiao J, An N, Ouyang X. Quantification of growth factors in different platelet concentrates. Platelets. 2017;28:774–8. doi: 10.1080/09537104.2016.1267338. [DOI] [PubMed] [Google Scholar]
- 19.Eskan MA, Greenwell H, Hill M, Morton D, Vidal R, Shumway B, et al. Platelet-rich plasma-assisted guided bone regeneration for ridge augmentation: A randomized, controlled clinical trial. J Periodontol. 2014;85:661–8. doi: 10.1902/jop.2013.130260. [DOI] [PubMed] [Google Scholar]
- 20.Gomes MF, Valva VN, Vieira EM, Giannasi LC, Salgado MA, Vilela-Goulart MG. Homogenous demineralized dentin matrix and platelet-rich plasma for bone tissue engineering in cranioplasty of diabetic rabbits: Biochemical, radiographic, and histological analysis. Int J Oral Maxillofac Surg. 2016;45:255–66. doi: 10.1016/j.ijom.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Dimitriou R, Mataliotakis GI, Calori GM, Giannoudis PV. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012;10:81. doi: 10.1186/1741-7015-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–96. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]


