Abstract
Background:
Tissue evaluation for RAS (KRAS or NRAS) gene status in metastatic colorectal cancer (mCRC) patients represent the standard of care to establish the optimal therapeutic strategy. Unfortunately, tissue biopsy is hampered by several critical limitations due to its invasiveness, difficulty to access to disease site, patient’s compliance and, more recently, neoplastic tissue spatial and temporal heterogeneity.
Methods:
The authors performed a systematic literature review to identify available trials with paired matched tissue and ctDNA RAS gene status evaluation. The authors searched EMBASE, MEDLINE, Cochrane, www.ClinicalTrials.gov, and abstracts from international meetings. In total, 19 trials comparing standard tissue RAS mutational status matched paired ctDNA evaluated through polymerase chain reaction (PCR), next generation sequencing (NGS) or beads, emulsions, amplification and magnetics (BEAMing) were identified.
Results:
The pooled sensitivity and specificity of ctDNA were 0.83 (95% CI: 0.80–0.85) and 0.91 (95% CI: 0.89–0.93) respectively. The pooled positive predictive value (PPV) and negative predictive value (NPV) of the ctDNA were 0.87 (95% CI: 0.81–0.92) and 0.87 (95% CI: 0.82–0.92), respectively. Positive likelihood ratio (PLR) was 8.20 (95% CI: 5.16–13.02) and the negative likelihood ratio (NLR) was 0.22 (95% CI: 0.16–0.30). The pooled diagnostic odds ratio (DOR) was 50.86 (95% CI: 26.15–98.76), and the area under the curve (AUC) of the summary receiver operational characteristics (sROC) curve was 0.94.
Conclusion:
The authors’ meta-analysis produced a complete and updated overview of ctDNA diagnostic accuracy to test RAS mutation in mCRC. Results provide a strong rationale to include the RAS ctDNA test into randomized clinical trials to validate it prospectively.
Keywords: circulating tumor DNA, diagnostic accuracy, meta-analysis, metastatic colorectal cancer, liquid biopsy, RAS
Introduction
Colorectal cancer (CRC) represents one-third of neoplasms in the world by incidence, with approximatley1.3 million new cases diagnosed every year.1 Although many screening efforts are being made to diagnose CRC at an early stage, at least 50% of patients will have local or distant disease recurrence, or present with advanced disease.2,3 Retrospective analyses from phase III randomized controlled trials in metastatic colorectal cancer (mCRC) have shown that a tissue-based mutation in one of the RAS family of genes in a more comprehensive evaluation of RAS, commonly called expanded RAS (KRAS exons 2,3,4 and NRAS exons 2,3,4), can more accurately select patients for an anti-EGFR therapy rather than KRAS codons 12 and 13 testing alone.4–7 Consequently, RAS testing is considered a nsuitable methodto negatively predict the efficacy of anti-EGFR monoclonal antibodies (mAbs) in mCRC patients (representing approximately 55% of the total number).8 Therefore, tumor tissue genotyping has become the routine approach in the management of these patients because of its ability to guide through the best first-line options.9,10 Today, more in-depth understanding of the mechanisms linked to tumor heterogeneity enable researchers to determine that a tumor is made up of a multitude of cellular clones with their proper genomic structure (spatial heterogeneity).11–13 Consequently, the monitoring of acquired resistances represents a fundamental step in establishing the best time to stop drug administration because of its ineffectiveness and the number of side effects. In addition, tissue biopsy, although it is able to provide useful genomic information, remains burdened by various limitations related to its invasiveness, difficult reproducibility over time, and poor representation of the entire tumor mass.14 A number of reports have recently shown how liquid biopsy used in the oncology field could be a potential new alternative to traditional tissue biopsy, due to its abundance of genetic material as circulating tumor DNA (ctDNA), circulating-free DNA (cfDNA), plasma exosomes and circulating microRNAs.15 Recently, the results from a large randomized controlled trial (AURA3) demonstrated that the T790M mutation evaluation performed by tissue or plasma through ctDNA detection in EGFR mutated non-small cell lung cancer (NSCLC) patients progressing after a first generation EGFR tyrosine kinase inhibitor achieved similar efficacy outcomes, when compared with a platinum-pemetrexed regimen.16 Outcomes were considered so consistent that the major international scientific societies including the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the National Comprehensive Cancer Network ( NCCN) recommended the evaluation of the T790M mutation status on blood ctDNA as the first option compared with the traditional evaluation of tumor tissue biopsy.17 The authors’ current work systematically evaluated the performance of T790M analysis on blood ctDNA compared with the standard tissue in NSCLC demonstrating high specificity and sensitivity.18 To date, however, the diagnostic accuracy of the RAS test on blood ctDNA in mCRC patients remains controversial. A recent meta-analysis provided an estimate of the prognostic relevance of cfDNA as a source of KRAS mutations by evaluating different conventional polymerase chain reaction (PCR) techniques.19 Therefore, the objective of this article was to carry out a systematic review of trials comparing matched blood and tumor tissue to provide a precise estimate of the diagnostic accuracy of the RAS gene mutation on ctDNA, in addition new generation technologies in mCRC patients will be assessed.20–38
Materials and methods
Search for clinical trials
The authors reviewed all published studies that reported the plasma specificity and sensitivity data of the RAS mutation (KRAS or KRAS/NRAS if available) tested by ctDNA in mCRC patients. The authors selected trials published up to December 2018 using electronic databases including Medline (PubMed), EMBASE, and Cochrane libraries. The search strategy on PubMed used the following terms, ‘colorectal neoplasms’, ‘CRC’, ‘colorectal’, ‘liquid biopsy’, ‘serum’, ‘plasma’, ‘liquid biopsy’, ‘circulating DNA’, ‘circulating cell-free DNA’, ‘cell-free DNA’, ‘ctDNA’, and ‘circulating tumor DNA’, . This search was then modified for searches using the other databases (EMBASE, Controlled Cochrane Trial Register - CCTR).
Only human studies were selected and an English language restriction was used. The authors have also explored sources of gray literature including the abstracts presented at the ASCO, ESMO, and Gastrointestinal Cancer Symposium meetings. The web-site www.clinicaltrials.gove was also investigated as a source of unpublished data.
Selection Criteria
To select clinical trials the following criteria were used: (1) patients with histologically confirmed diagnosis of advanced CRC, (2) studies that contain RAS mutational evaluation (KRAS or KRAS / NRAS) in tumor tissue and matched plasma, (3) studies that included the sensitivity and specificity value of the RAS mutation tested by ctDNA using traditional (PCR) or new generation techniques (ddPCR, BEAMing, NGS), and (4) studies in which basal blood sampling was carried out before drug therapy. Any ongoing trials were excluded. In the case of trials with several follow-ups, the most recent trial was selected. The trials that did not include both the specificity and sensitivity values of the detection of blood RAS mutation by ctDNA were excluded from this review.
Data extraction
Data extraction and assessment were performed by two authors separately (A.G and S.T.). Disagreements were solved following discussions with a third author (A.R.). The following data were extracted from the selected studies: name of the first author, author nation, number of patients, year of publication, type of blood sample (plasma or serum) name of the journal, method of detection (PCR, BEAMing or NGS), evaluated gene (KRAS or KRAS/NRAS), true positive (TP), true negative (TN), false positive (FP), and false negative (FN) rates. The meta-analysis was drawn up according to the PRISMA guidelines for reporting of systematic reviews.39
Quality assessment
The analysis of the overall quality of the studies included in the meta-analysis was conducted by two different investigators (A.G. and S.T) according to the quality assessment of diagnostic accuracy studies 2 ( QUASAD-2) tool which designed for diagnostic accuracy analysis.40 The quality assessment included four areas: patient selection, index test, reference standard, and flow and timing. For each study, the authors declared ‘yes’ as a low risk of bias and ‘no’ as a high risk of bias. The authors also declared ‘unclear’ if the data was not sufficient for a precise judgment. In addition, two authors (A.G. and S.T.) evaluated the risk of selective outcome reporting bias and any disagreements were resolved by consensus.
Statistical analysis
The data was extracted as TP, TN, FP, FN and the sample size in each study included the status of RAS mutation on tumor tissue, which is considered as a gold standard, and ctDNA considered as an experimental group (mutations in KRAS or KRAS/NRAS found in both tissue and liquid biopsy). The authors considered: TP as the number of patients with RAS mutation found in both tissue and liquid biopsy, TN as the number of patients with RAS mutation not found either in the tissue or in liquid biopsy, FP as the number of patients with RAS mutation not found in the tissue but found in liquid biopsy, and FN as the number of patients with positive tissue biopsy and negative liquid biopsy. The following values were calculated: specificity, sensitivity, concordance, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR,) and the respective 95% confidence intervals (95% CI). The sensitivity was calculated as the ratio between TP and the sum of TP and FN × 100 (TP/[TP + FN] × 100). The specificity was calculated as the ratio between TN and the sum of TN and FP × 100 (TN/[TN + FP] × 100). The concordance was calculated as ([TP + TN]/[TP + FN + TN + FN]) × 100. PLR was calculated as sensitivity/(1-specificity) and represents the likelihood that a positive liquid biopsy for a RAS mutation can be found in a patient with a positive tissue biopsy for RAS mutation. NLR was calculated as (1-sensitivity)/specificity and represents the likelihood that a negative liquid biopsy for a RAS mutation can be found in a patient with a negative tissue biopsy for RAS mutation. The DOR was calculated as the ratio between PLR and NLR (PLR/NLR). This measure expresses the magnitude of the odds that a patient is a carrier of a tissue mutation of RAS in the case of positive liquid biopsy or negative liquid biopsy for a RAS mutation. The sample sizes of the included studies were used as weight to pool specificity, sensitivity, PPV, and NPV. PLR, NLR, and DOR were pooled using the DerSimonian Laird method (random effects model) to consider variance between studies.41 Furthermore, the authors have elaborated a summary receiver operating characteristics (sROC) curve and calculated the area under the curve (AUC). A subgroup analysis was also performed with regard to the new generation detection techniques included in this meta-analysis (BEAMing, PCR, and NGS). To assess the presence of a possible bias linked to the threshold effect, Spearman’s rank correlation coefficient was used between the sensitivity logit and the 1-specificity logit. The threshold effect was considered significant if the p value <0.05. The other sources of heterogeneity not dependent on the threshold effect were calculated using t Cochran’s Q test (as the weighted sum of squared differences between individuals) or the index of inconsistency (I2) that indicates the percentage of variance between the studies that is explained by heterogeneity and not by chance. Heterogeneity was considered significant if the p value of Cochran’s Q test was <0.05 or high if I2 value >75%. A meta-regression was also performed to identify other sources of heterogeneity and the publication bias test to evaluate any other bias among the studies was included in our analysis. The related funnel plot (for visual inspection) and Egger’s test were produced and considered significant if p value <0.05. All analyses were conducted using the MetaDisc statistical software (version 1.4).42 The publication bias calculation was performed using the MetaEssential software.43
Results
Characteristics of eligible studies
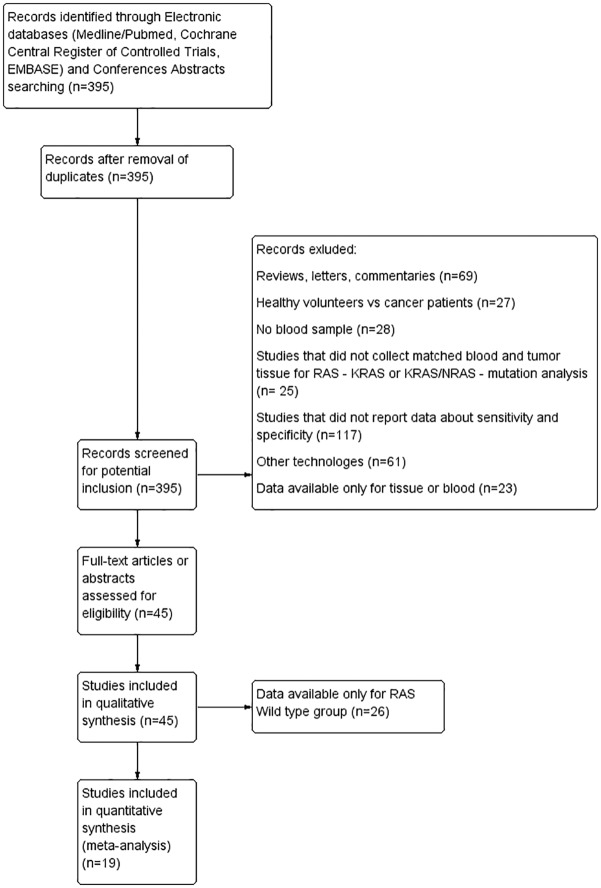
The search of literature trials found a total of 395 articles published up to December 2018. A small number of patients lacked information on treatments (140/1810 = 7.7%)
Of these patients, nineteen met the inclusion criteria and were therefore included in the pooled analysis with a total of 1810 patients (Figure 1). The study by Sefrioui35 evaluated the RAS mutation status by both digital chip PCR and cast PCR and the data was reported in two independent studies, with a total of 20 items included in the pooled analysis. All of the studies included patients with histologically confirmed mCRC with the collection of tissue matched to blood (plasma or serum) for the ctDNA RAS mutation detection. The most frequently used technology was NGS (8/20 studies). Six studies used the PCR technique (6/20) and six studies used BEAMing (6/20). With regard to positivity thresholds, although 2/20 studies did not report a precise threshold, 18/20 studies expressed it as mutant allele concentration (copies/ml) or mutant allele fraction (%). All of the studies which underwent pooled analysis included the values of specificity and sensitivity of ctDNA for the detection of RAS mutation (KRAS or KRAS/NRAS) compared with the tumor tissue considered as the gold standard. The authors included studies in which the basal blood sample was carried out before drug therapy. Only three studies, for which this information was not available and for a small number of patients,21,27,31 were included in the results to guarantee the systematic nature of the meta-analysis. Sensitivity ranged from 50.0 to 100%, while specificity ranged from 66.7 to 100% among the different studies. The main characteristics of the included trials are summarized in Table 1.
Figure 1.
Flow chart of the trials included in the meta-analysis.
Table 1.
Characteristics of the trials included in the meta-analysis.
| Study (reference) | Country | Number of patients n |
Sample | Detection method | Gene | Sensitivity n. (%) | Specificity n. (%) | Concordance n. (%) | NPV n. (%) | PPV n. (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bachet et al.20 | France | 412 | Plasma | NGS | KRAS, NRAS | 184/242 (76) | 167/170 (98.2) | 351/412 (85.2) | 167/225 (74.2) | 184/187 (98.4) |
| Osumi et al.21 | Japan | 101 | Plasma | NGS | KRAS, NRAS | 31/41 (75.6) | 47/60 (78.3) | 78/101 (77.2) | 47/57 (82.4) | 31/44 (70.4) |
| Garcia-Foncillas et al.22 | Spain | 236 | Plasma | Beaming | KRAS, NRAS | 113/131 (86.2) | 97/105 (92.4) | 210/236 (89) | 97/115 (84.3) | 113/121 (93.3) |
| Hsu et al.23 | Taiwan | 32 | Plasma | NGS | KRAS | 11/12 (91.6) | 19/20 (95) | 30/32 (93.7) | 19/20 (95) | 11/12 (91.6) |
| Yao et al.24 | China | 76 | Plasma | NGS | KRAS | 16/24 (66.7) | 36/40 (90) | 52/64 (81.2) | 36/44 (81.8) | 16/20 (80) |
| Normanno et al.25 | Italy | 92 | Plasma | NGS | KRAS, NRAS | 23/33 (70) | 49/59 (83) | 72/92 (78.3) | 49/59 (83) | 23/33 (70) |
| Garcia-Foncillas et al.26 | Spain | 238 | Plasma | Beaming | KRAS, NRAS | 112/121 (92.5) | 110/117 (94) | 222/238 (92.6) | 110/119 (92.4) | 112/119 (94.1) |
| Galbiati et al.27 | Italy | 30 | Plasma | ddPCR | KRAS | 14/21 (66.7) | 6/9 (66.7) | 20/30 (66.7) | 6/13 (46.1) | 14/17 (82.3) |
| Vidal et al.28 | Spain | 115 | Plasma | Beaming | KRAS, NRAS | 53/55 (96.3) | 54/60 (90) | 107/115 (93) | 54/56 (96.4) | 53/59 (89.8) |
| Grasselli et al.29 | Spain | 92 | Plasma | Beaming | KRAS, NRAS | 48/54 (88.8) | 83/92 (90.2) | 131/14 (89.7) | 83/83 (93.2) | 48/57 (84.2) |
| Schmiegel et al.30 | Germany | 98 | Plasma | Beaming | KRAS, NRAS | 47/52 (90.3) | 43/46 (93.5) | 90/98 (91.8) | 43/48 (89.5) | 47/50 (94) |
| Kidess-Sigal et al.31 | USA | 9 | Plasma | NGS | KRAS | 1/2 (50) | 5/7 (71.4) | 6/9 (66.7) | 5/6 (83.3) | 1/3 (33.3) |
| Rachiglio et al.32 | Italy | 35 | Plasma | NGS | KRAS, NRAS | 14/19 (73.6) | 16/16 (100) | 30/35 (85.7) | 16/21 (76.2) | 14/14 (100) |
| Kim et al.33 | Korea | 29 | Plasma | NGS | KRAS | 5/6 (83.3) | 20/23 (87) | 25/29 (86.2) | 20/21 (95.2) | 5/8 (62) |
| Diaz jr et al.34 | USA | 28 | Serum | Beaming | KRAS | 3/4 (75) | 24/24 (100) | 27/28 (96.4) | 24/25 (96) | 3/3 (100) |
| Sefrioui et al.35 | France | 34 | Plasma | dPCR (chip) | KRAS | 11/16 (69) | 18/18 (100) | 29/34 (85.2) | 18/23 (78.2) | 11/11 (100) |
| castPCR | 9/16 (56) | 18/18 (100) | 27/34 (79.4) | 18/25 (72) | 9/9 (100) | |||||
| Thierry et al.36 | France | 95 | Plasma | Intplex PCR | KRAS | 37/40 (92.5) | 56/57 (98.2) | 93/97 (96) | 56/59 (95) | 37/38 (97.3) |
| Kuo et al.37 | Taiwan | 52 | Plasma | PNA-PCR | KRAS | 15/15 (100) | 26/37 (70.2) | 41/52 (78.8) | 26/26 (100) | 15/26 (57.6) |
| Jin et al.38 | Korea | 6 | Plasma | DTBP + PCR | KRAS | 1/2 (50) | 3/4 (75) | 4/6 (66.6) | 3/4 (75) | ½ (50) |
BEAMing, beads, emulsions, amplification and magnetics; CAST, competitive allele-specific TaqMan; CI, confidence intervals; dPCR, digital polymerase chain reaction; DTBP, Dimethyl 3,3′- dithiopropionimidate dihydrochloride; NGS, next generation sequencing; NPV, negative predictive value; PCR, polymerase chain reaction; PNA, peptide nucleic acid; PPV, positive predictive value.
Diagnostic accuracy analysis
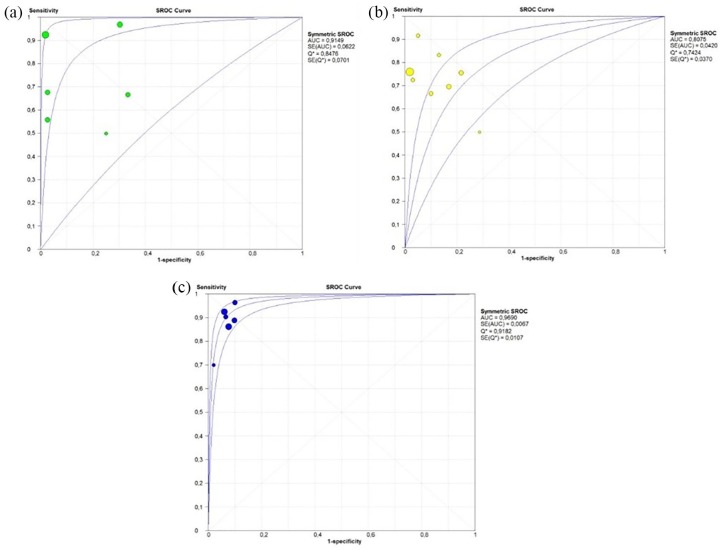
The pooled sensitivity and specificity of ctDNA in the authors’ analysis were 0.83 (95% CI: 0.80–0.85) and 0.91 (95% CI: 0.89–0.93) respectively (Figure 2). The pooled PPV and NPV of the ctDNA were 0.87 (95% CI: 0.81–0.92) and 0.87 (95% CI: 0.82–0.92), respectively. PLR was 8.20 (95% CI: 5.16–13.02) and NLR was 0.22 (95% CI: 0.16–0.30). The pooled DOR was 50.86 (95% CI: 26.15–98.76) and the AUC of the sROC curve was 0.94 (Figure 3). A subgroup analysis was carried out to evaluate if different methods could influence the diagnostic accuracy of the ctDNA and their results are shown in Figures 4and 5 and summarized in Table 2.
Figure 2.
Forest plots of sensitivity and specificity of ctDNA for the detection of RAS (KRAS or KRAS/NRAS) mutation. Figure 2(a) pooled sensitivity, (b) pooled specificity.
Forest plots of overall sensitivity and specificity of ctDNA for the detection of RAS (KRAS or KRAS/NRAS) mutation. Figure 2(a) pooled sensitivity, (b) pooled specificity. The different circle size indicates the different weight of the study.
Figure 3.
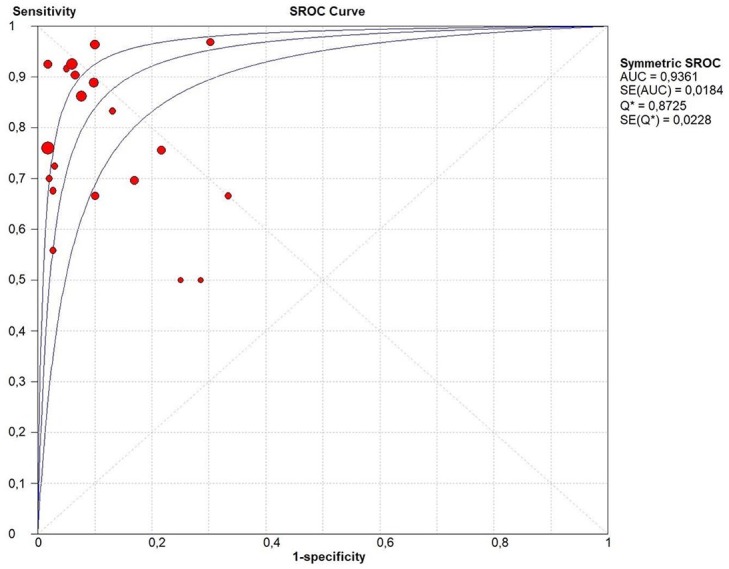
sROC curve of ctDNA for the detection of RAS (KRAS or KRAS/NRAS) mutation.
sROC curve of overall ctDNA for the detection of RAS (KRAS or KRAS/NRAS) mutation. The different circle size indicates the different weight of the study.
Figure 4.
Forest plots of sensitivity and specificity of ctDNA for the detection of RAS (KRAS or KRAS/NRAS) according to the different diagnostic methods: Figure 4(a) PCR, (b) NGS, (c) BEAMing.
Forest plots of sensitivity and specificity of ctDNA for the detection of RAS (KRAS or KRAS/NRAS) according to the different diagnostic methods: Figure 4(a) PCR, (b) NGS, (c) BEAMing. The different circle size indicates the different weight of study.
Figure 5.
sROC curve of ctDNA for detection of RAS (KRAS or KRAS/NRAS) mutation according to the different diagnostic methods: Figure 5(a) PCR, (b) NGS, (c) BEAMing.
sROC curve of ctDNA for detection of RAS (KRAS or KRAS/NRAS) mutation according to the different diagnostic methods: Figure 5(a) PCR, (b) NGS, (c) BEAMing. The different circle size indicates the different weight of the study.
Table 2.
Meta-analysis results.
| Number of patients n |
Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) | NLR (95% CI) | PLR (95% CI) | AUC | |
|---|---|---|---|---|---|---|---|
| All Studies | 1810 | 0.83 (0.80 – 0.85) | 0.91 (0.89 – 0.93) | 51.35 (26.85 – 98.18) | 0.22 (0.16 – 0.30) | 8.27 (5.25 – 13.03) | 0.94 |
| NGS | 786 | 0.75 (0.71 – 0.80) | 0.91 (0.88 – 0.94) | 28.28 (10 – 79.94) | 0.27 (0.23 – 0.32) | 7.39 (3.2 – 17.13) | 0.81 |
| BEAMing | 807 | 0.90 (0.87 – 0.93) | 0.93 (0.90 – 0.95) | 113.26 (68.72 – 186.66) | 0.11 (0.08 – 0.17) | 11.41 (8.23 – 15.82) | 0.97 |
| PCR | 217 | 0.79 (0.70 – 0.86) | 0.89 (0.82 – 0.93) | 36.6 (5.6 – 239.55) | 0.29 (0.14 – 0.63) | 6.72 (1.84 – 24.54) | 0.91 |
AUC, area under the curve; BEAMing, beads, emulsions, amplification, and magnetics; CI, confidence intervals; DOR, diagnostic odds ratio; NGS, next generation sequencing; NLR, negative likelihood ratio; PCR, polymerase chain reaction; PLR, positive likelihood ratio.
Threshold effect and heterogeneity
To evaluate the presence of bias associated with the threshold effect the authors calculated Spearman’s rank correlation coefficient and the relative p value. Spearman’s rank correlation coefficient was –0.186 (p value = 0.433) and therefore not significantly related to bias. However, a source of high heterogeneity that was not dependent on the threshold effect was found and, therefore, a meta-regression was performed. The results demonstrated how the different methods of ctDNA analysis were not associated with heterogeneity.
Quality analysis and publication bias
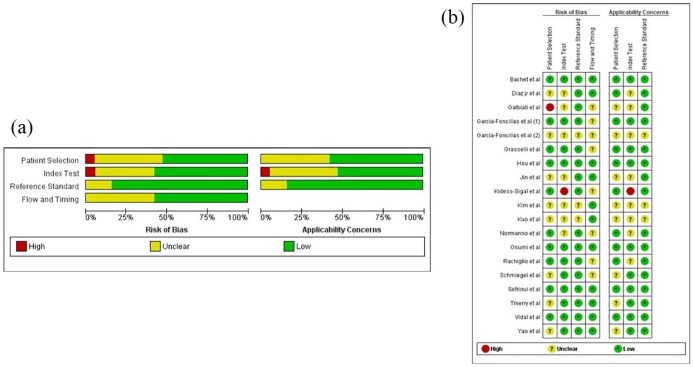
The presence of publication bias was investigated using the MetaEssential software. The plot did not reflect particular asymmetries and in addition, the Egger test was not significant (p = 0.89) (Figure 6). The overall quality of the trials included in the meta-analysis was assessed as good by using the QUADAS-2 tool (Figure 7).
Figure 6.
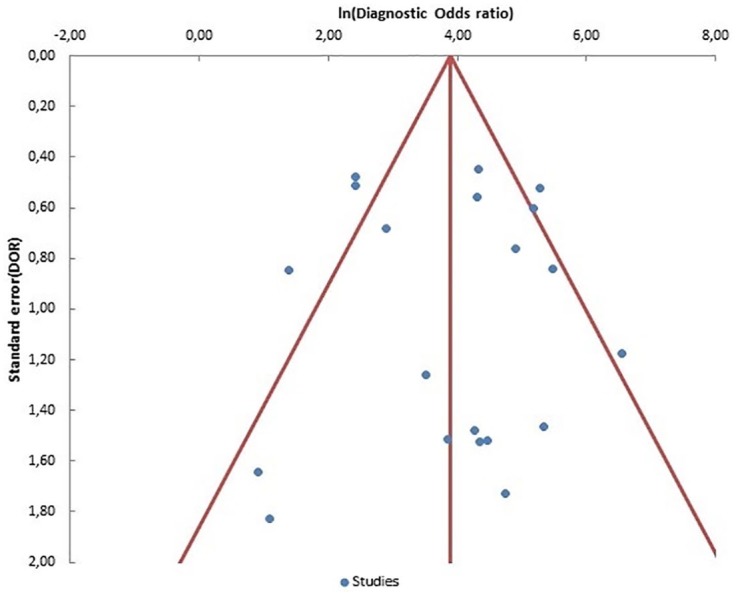
Funnel plot of DOR for ctDNA detection of RAS (KRAS or KRAS/NRAS) mutation. Each circle represents a study and the vertical line represents the pooled effect estimate.
Figure 7.
Quality assessment of included trials by the QUADAS-2 tool. Figure 7(a) methodological quality graph, (b) methodological quality summary.
Discussion
The authors’ meta-analysis included 20 studies with a total of 1810 mCRC patients in which the tissue matched paired with blood were available for the assessment of the mutational status of RAS (KRAS or KRAS/NRAS) due to ctDNA. The results demonstrated acceptable performance of ctDNA in terms of overall specificity (0.91) and sensitivity (0.83) for the RAS mutation if compared with tumor tissue, considered as gold standard. However, this analysis demonstrated that approximately 20% of patients defined as negative by liquid biopsy were actually positive for tissue analysis. This could potentially be due to the different technologies used although the analysis did not appear to indicate this (Figures 4 and 5, and Table 2).
In addition, the novelty of the results resides in the overall finding that liquid biopsy influences by 30% the probability of having an RAS mutated ctDNA in patients with RAS mutated tissue test, as well as the probability of having an RAS wild type ctDNA in patients with wild type RAS tissue tests, therefore indicating a moderate impact according to the PLR and NLR values.44 Finally, sROC curve (AUC 0.94) suggests a high performance of ctDNA in terms of sensitivity and specificity. Overall, according to Youden and colleagues45 it appears that 0.74 should be the pooled threshold value able to minimize the FP rates. BEAMing has the most acceptable performance (Youden Index 0.83).45 However, the authors’ results reinforce the concept that these tools should be used at the most opportune time and at the most appropriate phase of the disease course.
The mutations in ctDNA can be detected with PCR-based or sequencing-based assays. NGS as a high throughput method allows deep sequencing of amplicons, which is useful for the detection of somatic mutations in ctDNA.46 Given the multiplex nature of NGS, it is possible broadly to adopt this method for the identification of a gene’s mutational status with ctDNA as the analyte. While the PCR-based methods can detect preferentially specific hotspot mutations, NGS has the benefit of detecting novel mutations in addition to the hotspots. NGS, in addition, also the advantage of being cost effective in screening for multiple genes and hotspots in a single assay. The NGS assay is a highly sensitive technology that allows the detection of mutations in ctDNA with high accuracy when compared with other next generation technologies including digital real time PCR.
Originally, the potential of liquid biopsies was limited technically by the relatively high error rate of NGS systems, as real ctDNA mutations were masked by intrinsic errors in the DNA library preparation and sequencing. Modern NGS systems produce errors at a per-base rate of 10−2 to 10−3, but clinically relevant mutations have been revealed to be at, or below, that level making many true variants undetectable.47 In recent years error correction strategies have been developed and today NGS and liquid biopsy have a great potential in a gene’s mutational status detection and impact positively on diagnostics and cancer management. Recently, ctDNA liquid biopsies have been adopted for several personalized medicine applications, including guiding treatment selection during monitoring, minimum residual disease detection, and even screening (ClinicalTrials.gov NCT02808884; NCT02889978).
Technologies including quantitative PCR and NGS could potentially be reserved for the first mutational assessment in the metastatic settings, reserving other technologies such as BEAMing for the monitoring of already identified genetic alterations. Despite the fact that the different technologies under investigation have shown an overall good performance, making circulating DNA analysis a promising and unavoidable area of research, the authors’ data suggest that ctDNA has some limitations for its use in routine clinical practice due predominantly to the lack of a real standardization of sensitivity limit that could increase the concordance between tissue and plasma. The early knowledge of the disease’s mutational profile is one of the most relevant clinical needs because it can have a considerable impact at different phases of clinical histories including diagnosis, first-line approach, and dynamic monitoring for the detection of acquired resistance.48 New generation technologies allow the analysis of multigenic panels and several patient samples at the same time, with high performance standards and economic savings. It has also been hypothesized that elevated levels of ctDNA in CRC are correlated with poor outcome, as its primary post-surgery finding may suggest the presence of minimal residual disease and its potential role for the definition of actionable targets. As mentioned previously, although data is fragmented, some evidence seems to suggest a potential role in the monitoring of secondary resistances. Misale and colleagues49 demonstrated for the first time in mCRC patients treated with anti-EGFR (panitumumab or cetuximab) that the discovery of KRAS amplification through liquid biopsy was able to anticipate the radiological progression by 10 months with a sensitivity of 60%. Furthermore, Siravegna and colleagues50 demonstrated that the early discontinuation of treatment with anti-EGFR reduced the number of mutated KRAS clones allowing reacquisition of the sensitivity to anti-EGFR agents. This finding suggested novel rationales potentially useful for the management and continuum of mCRC care. Recently, Goldberg and colleagues51 proposed that ctDNA evaluated by liquid biopsy can become an optimal tool for decision making between the strategy of cetuximab continuum or re-challenge, in order to extend the number of available therapeutic options and optimize the management. Rossini and colleagues52 reported the results of the CRICKET study in which 27 mCRC wild type RAS/BRAF patients with acquired resistance during the first-line anti-EGFR regimen were treated with irinotecan-cetuximab as re-challenge. In this experience, the ctDNA evaluated by ddPCR and NGS at the re-challenge baseline was able to predict a greater efficacy of irinotecan-cetuximab in wild type ctDNA RAS patients compared with RAS mutant ctDNA in terms of progression-free survival (3.9 versus 1.9 months, Hazard Ratio 0.48), identifying RAS mutations in 9/25 among patients evaluated by liquid biopsy (48%). These data show how liquid biopsy can offer a possible solution to the important challenge of inter- and intra-tumor heterogeneity and consequently to drug resistance. Although CRC is highly heterogeneous, their intra-tumor heterogeneity features have been less investigated than in other cancer types. Recent findings indicate that intra-tumor heterogeneity can be tested by single cell analysis, the single cell genome sequencing allows quantitative characterization of both single nucleotide and somatic copy number variations in individual tumor cells.53 Recently, a Nature opinion article suggested that the main operative unit of cancer is the genetically and epigenetically modified single cell. The single cell analysis can allow clarification of the intra-tumor genetic heterogeneity and cancer genome evolution in order to develop new tools able to provide robust interpretation of the mechanisms related to diagnosis, tumor recurrence and the activity of new generation molecular targeted agents, and the development of secondary resistance modifying cancer patient management strategies.54 Despite this potential, the use of liquid biopsy in routine clinical practice has historically been burdened by substantial costs, heterogeneity in methodology, and results from studies or in different threshold values that could be influenced by the noise caused by normal tissue cfDNA. For these reasons, the level of evidence to date is not yet considered as enough to recommend the use of ctDNA RAS testing in routine clinical practice. The authors’ meta-analysis, therefore, provides for the first time clear and comprehensive evidence of the feasibility and potentially acceptable performance of ctDNA RAS testing in mCRC patients offering a strong rationale for prospective clinical trial validation.
Conclusion
The results of the authors’ analysis demonstrates that ctDNA is a very promising testing tool. In particular, the technology used (PCR, BEAMing, NGS) and the disease course phase (initial diagnosis, real time monitoring) can influence the diagnostic accuracy of the test. Therefore, there is a need both to standardize ctDNA analysis and that the results are validated prospectively within large randomized trials in order to improve the clinical applicability of ctDNA in mCRC.
Supplemental Material
Supplemental material, Search_strategy_-_Supplemental_Material_1_final for Detection of RAS mutations in circulating tumor DNA: a new weapon in an old war against colorectal cancer. A systematic review of literature and meta-analysis by Antonio Galvano, Simona Taverna, Giuseppe Badalamenti, Lorena Incorvaia, Marta Castiglia, Nadia Barraco, Francesco Passiglia, Fabio Fulfaro, Giordano Beretta, Giovanni Duro, Bruno Vincenzi, Pierosandro Tagliaferri, Viviana Bazan and Antonio Russo in Therapeutic Advances in Medical Oncology
Acknowledgments
All authors read and approved the final manuscript. Conceptualization, G.A, S.T and A.R; Data curation, G.A, S.T, M.C, F.F; formal analysis, G.A, S.T, N.B, methodology G.A, F.P, software G.A, L.I; supervision, V.B, A.R; validation G.A, S.T and F.P; writing - original draft G.A, S.T; writing - review G.A, A.R; and editing G.B, G.D, B.V, P.T.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: G.B receives research support from Roche, Lilly, Thaiho Pharmaceutical. All other authors have no conflicts of interest to declare.
ORCID iD: Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Antonio Galvano, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Simona Taverna, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy; Institute of Biomedicine and Molecular Immunology ‘A. Monroy’, National Research Council, Palermo, Italy.
Giuseppe Badalamenti, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Lorena Incorvaia, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Marta Castiglia, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Nadia Barraco, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Francesco Passiglia, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Fabio Fulfaro, Medical Oncology, Department of Surgical, Oncological and Stomatological Sciences, University of Palermo, Palermo, Italy.
Giordano Beretta, Medical Oncology Unit, Humanitas Gavazzeni, Bergamo, Italy.
Giovanni Duro, Institute of Biomedicine and Molecular Immunology ‘A. Monroy’, National Research Council, Palermo, Italy.
Bruno Vincenzi, Medical Oncology Department, Campus Bio-Medico University of Rome, Rome, Italy.
Pierosandro Tagliaferri, Medical Oncology Unit, AUO ‘Materdomini and Department of Experimental and Clinical Medicine’, Magna Grecia University, Catanzaro, Italy.
Viviana Bazan, Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, University of Palermo, Palermo, Italy.
Antonio Russo, Medical Oncology Director, Department of Oncology, A.O.U.P. P. Giaccone University Hospital, 2013 ESMO Designated Centers of Integrated Oncology and Palliative Care, Via del Vespro 129, Palermo, 90127, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Kievit J. Follow-up of patients with colorectal cancer: numbers needed to test and treat. Eur J Cancer 2002; 38: 986–999. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Sahar L, Portier KM, et al. Cancer death rates in US congressional districts. CA Cancer J Clin 2015; 65: 339–344. [DOI] [PubMed] [Google Scholar]
- 4. Bokemeyer C, Köhne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015; 51: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 6. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 7. Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res 2015; 21: 5469–5479. [DOI] [PubMed] [Google Scholar]
- 8. Peeters M, Kafatos G, Taylor A, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer 2015; 51: 1704–1713. [DOI] [PubMed] [Google Scholar]
- 9. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 10. Bronte G, Silvestris N, Castiglia M, et al. New findings on primary and acquired resistance to anti-EGFR therapy in metastatic colorectal cancer: do all roads lead to RAS? Oncotarget 2015; 6: 24780–24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perakis S, Speicher MR. Emerging concepts in liquid biopsies. BMC Med 2017; 15: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Carmen S, Sayagués JM, Bengoechea O, et al. Spatio-temporal tumor heterogeneity in metastatic CRC tumors: a mutational-based approach. Oncotarget 2018; 9: 34279–34288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venesio T, Siravegna G, Bardelli A, et al. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology 2018; 85: 146–154. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Chang S, Li G, et al. Application of liquid biopsy in precision medicine: opportunities and challenges. Front Med 2017; 11: 522–527. [DOI] [PubMed] [Google Scholar]
- 15. Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018; 10: 1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl. 4): iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 18. Passiglia F, Rizzo S, Di Maio M, et al. Publisher correction: the diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis. Sci Rep 2018; 8: 17270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spindler KG, Boysen AK, Pallisgård N, et al. Cell-free DNA in metastatic colorectal cancer: a systematic review and meta-analysis. Oncologist 2017; 22: 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bachet JB, Bouché O, Taieb J, et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann Oncol 2018; 29: 1211–1219. [DOI] [PubMed] [Google Scholar]
- 21. Osumi H, Shinozaki E, Takeda Y, et al. Clinical relevance of circulating tumor DNA assessed through deep sequencing in patients with metastatic colorectal cancer. Cancer Med. Epub ahead of print 21 December 2018. DOI: 10.1002/cam4.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García-Foncillas J, Tabernero J, Élez E, et al. Prospective multicenter real-world RAS mutation comparison between OncoBEAM-based liquid biopsy and tissue analysis in metastatic colorectal cancer. Br J Cancer 2018; 119: 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu HC, Lapke N, Wang CW, et al. Targeted sequencing of circulating tumor DNA to monitor genetic variants and therapeutic response in metastatic colorectal cancer. Mol Cancer Ther 2018; 17: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 24. Yao J, Zang W, Ge Y, et al. RAS/BRAF circulating tumor DNA mutations as a predictor of response to first-line chemotherapy in metastatic colorectal cancer patients. Can J Gastroenterol Hepatol 2018; 2018: 4248971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Normanno N, Esposito Abate R, Lambiase M, et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol 2018; 29: 112–118. [DOI] [PubMed] [Google Scholar]
- 26. García-Foncillas J, Alba E, Aranda E, et al. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol 2017; 28: 2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galbiati S, Damin F, Burgio V, et al. Evaluation of three advanced methodologies, COLD-PCR, microarray and ddPCR, for identifying the mutational status by liquid biopsies in metastatic colorectal cancer patients. Clin Chim Acta 2018; 489: 136–143. [DOI] [PubMed] [Google Scholar]
- 28. Vidal J, Muinelo L, Dalmases A, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol 2017; 28: 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grasselli J, Elez E, Caratù G, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol 2017; 28: 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmiegel W, Scott RJ, Dooley S, et al. Blood-based detection of RAS mutations to guide anti-EGFR therapy in colorectal cancer patients: concordance of results from circulating tumor DNA and tissue-based RAS testing. Mol Oncol 2017; 11: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kidess-Sigal E, Liu HE, Triboulet MM, et al. Enumeration and targeted analysis of KRAS, BRAF and PIK3CA mutations in CTCs captured by a label-free platform: comparison to ctDNA and tissue in metastatic colorectal cancer. Oncotarget 2016; 7: 85349–85364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rachiglio AM, Esposito Abate R, Sacco A, et al. Limits and potential of targeted sequencing analysis of liquid biopsy in patients with lung and colon carcinoma. Oncotarget 2016; 7: 66595–66605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim ST, Lee WS, Lanman RB, et al. Prospective blinded study of somatic mutation detection in cell-free DNA utilizing a targeted 54-gene next generation sequencing panel in metastatic solid tumor patients. Oncotarget 2015; 6: 40360–40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diaz LA, Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sefrioui D, Sarafan-Vasseur N, Beaussire L, et al. Clinical value of chip-based digital-PCR platform for the detection of circulating DNA in metastatic colorectal cancer. Dig Liver Dis 2015; 47: 884–890. [DOI] [PubMed] [Google Scholar]
- 36. Thierry AR, Mouliere F, El Messaoudi S, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med 2014; 20: 430–435. [DOI] [PubMed] [Google Scholar]
- 37. Kuo YB, Chen JS, Fan CW, et al. Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer. Clin Chim Acta 2014; 433: 284–289. [DOI] [PubMed] [Google Scholar]
- 38. Jin CE, Koo B, Lee TY, et al. Simple and low-cost sampling of cell-free nucleic acids from blood plasma for rapid and sensitive detection of circulating tumor DNA. Adv Sci (Weinh) 2018; 5: 1800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 41. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. West Sussex: Wiley, 2008. [Google Scholar]
- 42. Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods 2017; 8: 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17: 646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 46. Veldore VH, Choughule A, Routhu T, et al. Validation of liquid biopsy: plasma cell-free DNA testing in clinical management of advanced non-small cell lung cancer. Lung Cancer (Auckl) 2018; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pel J, Choi WWY, Leung A, et al. Duplex proximity sequencing (Pro-Seq): a method to improve DNA sequencing accuracy without the cost of molecular barcoding redundancy. PLoS One 2018; 13: e0204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rolfo C, Castiglia M, Hong D, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta 2014; 1846: 539–546. [DOI] [PubMed] [Google Scholar]
- 49. Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012; 486: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015; 21: 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldberg RM, Montagut C, Wainberg ZA, et al. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open 2018; 3: e000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rossini D, Cremolini C, Conca E, et al. Liquid biopsy to predict benefit from rechallenge with cetuximab (cet) + irinotecan (iri) in RAS/BRAF wild-type metastatic colorectal cancer patients (pts) with acquired resistance to first-line cet+iri: final results and translational analyses of the CRICKET study by GONO. J Clin Oncol 2018; 36: abstract 12007. [Google Scholar]
- 53. Liu M, Liu Y, Di J, et al. Multi-region and single-cell sequencing reveal variable genomic heterogeneity in rectal cancer. BMC Cancer 2017; 17: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baslan T, Hicks J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat Rev Cancer 2017; 17: 557–569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Search_strategy_-_Supplemental_Material_1_final for Detection of RAS mutations in circulating tumor DNA: a new weapon in an old war against colorectal cancer. A systematic review of literature and meta-analysis by Antonio Galvano, Simona Taverna, Giuseppe Badalamenti, Lorena Incorvaia, Marta Castiglia, Nadia Barraco, Francesco Passiglia, Fabio Fulfaro, Giordano Beretta, Giovanni Duro, Bruno Vincenzi, Pierosandro Tagliaferri, Viviana Bazan and Antonio Russo in Therapeutic Advances in Medical Oncology