Abstract
Irreversible electroporation has raised great interest in the past decade as a means of destroying cancers in a way that does not involve heat. Irreversible electroporation is a novel ablation technology that uses short high-voltage electrical pulses to enhance the permeability of tumor cell membranes and generate irreversible nano-sized structural defects or pores, thus leading to cell death. Irreversible electroporation has many advantages over thermal therapies due to its nonthermal mechanism: (1) reduced risk of injury to surrounding organs and (2) no “heat-sink” effect due to nearby blood vessels. However, so far, it has been difficult for irreversible electroporation to completely ablate large tumors (eg, >3 cm in diameter). In order to overcome this problem, many preclinical and clinical studies have been performed to improve the efficacy of IRE in the treatment of large size of tumors through a chemical perspective. Due to the distribution of electric field, irreversible electroporation region, reversible electroporation region, and intact region can be found in the treatment of irreversible electroporation. Thus, 2 types of chemical enhancements of irreversible electroporation were discussed in the article, such as the reversible electroporation region enhanced and the irreversible electroporation region enhanced. Specifically, the state-of-the-art results regarding the following approaches that have the potential to be used in the enhancement of irreversible electroporation were systematically reviewed in the article, including (1) combination with cytotoxic drugs, (2) calcium electroporation, (3) modification of cell membrane, and (4) modification of the tumor cell microenvironment. In the end, we concluded with 4 issues that should be addressed in the future for improving irreversible electroporation further in a chemical way.
Keywords: irreversible electroporation, chemical enhancement, calcium electroporation, cytotoxic drugs, surfactants, cell microenvironment
Introduction
For several decades, thermal tumor ablation modalities have been used in the treatment of primary tumors in various organs with favorable clinical outcomes. Of those modalities, cryoablation, radiofrequency ablation, and microwave ablation are the most commonly used ones in the clinical setting.1 These thermal ablation modalities provide minimally invasive treatment options for selected tumors in various organs, including liver, lung, pancreas, kidney, and prostate.2-4
Irreversible electroporation (IRE)5 as a nonthermal tumor ablation modality has also attracted great interest in the field of cancer treatment since the introductory work by Davalos et al. 6 Initially, reversible electroporation (RE) was used to deliver chemotherapeutic drug molecules into tumor cells. The combination of electroporation with chemotherapeutic drugs rapidly attracted great attention in the field of cancer treatment and was used as an independent treatment, known as electrochemotherapy (ECT). In 2005, the concept of electroporation as an independent therapy (ie, not using cytotoxic drugs or combining with thermal effects) was proposed by Davalos et al 6 to destroy tumor tissues. Irreversible electroporation was soon introduced to clinical use as a means of treating cancers using short, high-voltage electric pulses to increase the permeability of tumor cell membranes.7 Although no unified explanation for the mechanism of cell death is available, there is a consensus that when cells are exposed to an external high-voltage pulsed electric field, irreversible nano-sized structural defects or pores are produced in the membrane. As a result of these defects or pores, many events occur in the membrane, such as electroconformational protein denaturation,8 osmotic imbalance, flush in/out of ions, depletion of adenosine triphosphate (ATP),9 and uptake of toxic/foreign molecules,10 which eventually lead to cell death. Once the electric field magnitude exceeds a tissue-specific lethal threshold, cell death occurs.11 Irreversible electroporation has many advantages due to its nonthermal mechanism and applied pulses, including (1) no collateral heat damage to adjacent organs and (2) no “heat-sink” effect due to nearby blood vessels. (For thermal therapies, a blood vessel can dissipate the thermal energy as a heat sink, leading to incomplete tumor ablation in the area adjacent to the vessel. These advantages have raised a great deal of interest in this novel technology, particularly in the treatment of tumors in anatomically sensitive areas where one might be concerned about injury to nearby vessels, nerves, bowel, or ducts.12
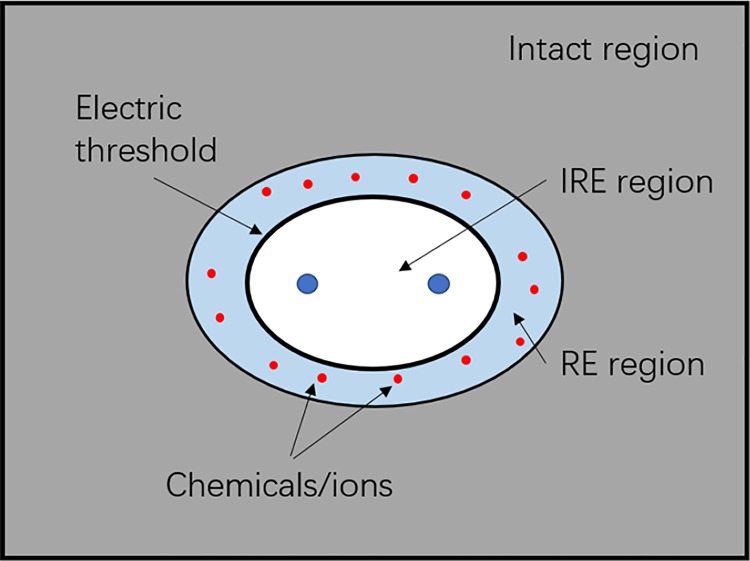
However, as a new tumor ablation modality, one of the critical disadvantages of IRE is the incomplete ablation of large tumors (eg, >3 cm in diameter). To overcome this issue, many preclinical and clinical studies have been performed to improve the efficacy of IRE in the treatment of large size of tumors, using the combination of chemical substances or drugs, which we will refer to as “chemical enhancement of IRE” in this article. It is worth mentioning that usually 3 regions can be discovered in the treatment of IRE, such as IRE region, RE region, and intact region due to the electric field distribution, as shown in Figure 1. (In order to concisely clarify the idea of enhancement of IRE, we ignored the heterogeneity of tumor tissues in the model, as shown in Figure 1. It is noteworthy that the model used is not the real case and any difference in cellular distribution, extracellular matrix, or vascular systems of tumor tissues might cause an uneven distribution of electric field.)6 One possibility to increase the size of tumor ablation in IRE is to introduce chemical drugs or ions to RE region because lots of promising results in the tumor tissue death have been achieved in ECT or calcium electroporation (discussed below). In another word, by introducing chemical drugs or ions, the ablation zone of IRE could be enlarged by ECT or calcium electroporation (IRE region + RE region). Therefore, in this review article, 2 types of chemical enhancements of IRE were discussed, such the RE region enhanced (eg, ECT or calcium electroporation) and the IRE region enhanced (eg, adding surfactants or modification of the chemical microenvironment of tumor cells). Specifically, the following approaches have been tried thus far in literature: (1) combination with cytotoxic drugs,13 such as bleomycin,14 cisplatin,15,16 and carboplatin, (2) calcium electroporation whereby there is an increase in the concentration of free calcium in the cell and decreased viability of the cell as a result,17 (3) modification of cell membrane by surfactants18 (eg, sodium dodecyl sulfate [SDS] and dimethyl sulfoxide [DMSO]), interacting with membrane lipids thereby changing the membrane’s line tension and surface tension, thus impeding the resealing process, and (4) modification of the chemical microenvironment of tumor cells19 (eg, glucose concentration) in the hopes of delivering a greater amount of energy during IRE treatment. This article also mentioned the combination of high-frequency IRE and molecular therapy, which can improve the treatment of diffuse cells outside the tumor margins.20
Figure 1.
An ideal schematic of electric fields distribution around irreversible electroporation (IRE) electrodes.
In this article, a systematic review of the preclinical and clinical results and some future directions for the chemical enhancement of IRE proposed to assist further studies for improving the efficacy of IRE in clinical practice.
Combination With Cytotoxic Drugs
There are no studies in the combination of IRE and cytotoxic drugs available in literature. Thus, this section was focused on the work of ECT due to the reasons mentioned above. (The same reason goes to the section of calcium electroporation.) The first use of electroporation for drug delivery in humans was the delivery of bleomycin to skin tumors.21 The uptake of bleomycin, one of the commonly used cancer chemotherapy drugs, was found to be increased by 300 to 700 times when intravenous administration of the drug was combined with electroporation in a preclinical study of hamster lung fibroblast cells.22 Cisplatin and carboplatin have also been studied in combination with electroporation. A 2.3- to 8-fold increase in the cytotoxicity of cisplatin by combining its administration with electroporation was found in an in vitro study.23 Similarly, Souza et al 24 found that the use of electroporation increased the cytotoxic effect of carboplatin on the equine sarcoid cell line 3-fold.
Electrochemotherapy With Bleomycin
Bleomycin was discovered by Umezawa and colleagues in 1966,25 which was isolated from the fungus berberine (collected from Japanese coal mine soil). Bleomycin causes direct DNA damage, making single- and double-strand DNA breaks in mammalian cells.26 There are 2 mechanisms whereby bleomycin can cause cell death. If only a few thousand bleomycin molecules are present in a cell, the cell arrests in the G2-M phase and becomes enlarged, leading to the leakage of polynuclei and micronuclei.27,28 The cells then die in a slow process lasting about 3 doubling times.28 If, on the other hand, cells contain several million bleomycin molecules, they are destroyed much more rapidly, within a few minutes, due to pseudoapoptosis because bleomycin short-circuits the apoptosis pathway by creating the characteristic DNA fragmentation.29 This is followed by cell shrinkage, membrane blebbing, and chromatin condensation.27,30
Cell toxicity caused by bleomycin can be enhanced by electroporation,22,31 administered 1 to 15 minutes after infusion of bleomycin.32 Domenge et al showed that electroporation applied at 8 to 28 minutes after the intravenous administration of bleomycin is beneficial in the treatment of head and neck squamous cell carcinomas (SCCs).33
Sersa et al 10 demonstrated that in addition to the well-established direct cytotoxic effect on tumor cells, a combination of bleomycin and electroporation also has an indirect effect on tumor necrosis via an effect on the tumor blood supply, something which is not seen with either bleomycin alone or IRE alone. They tested this finding successfully with murine fibrosarcoma SA-1 cells that were obtained from the ascitic form of the tumors in mice with 4 different treatments, including (1) control group (untreated tumors), (2) tumors treated by bleomycin alone, (3) tumors treated by electroporation alone, and (4) tumors treated by ECT with bleomycin. They also observed some important phenomena, which include that (1) ECT with bleomycin had a significantly better inhibition to tumor growth and tumor necrosis, reaching ∼50% at 48 hours after the treatment (compared to other treatment groups), (2) at 8 hours after ECT with bleomycin, in some vessels endothelial cells with apoptotic morphological characteristics and blood vessels were stacked with erythrocytes and extravasation of erythrocytes, and (3) an immediate reduction was found in tumor perfusion (about 10% of the tumor perfusion in control tumors) after application of ECT with bleomycin. The electric pulses and ECT with bleomycin significantly reduced the blood flow of the treated tumor. However, at 48 hours after ECT, a reduced tumor blood flow was still observed after ECT with bleomycin, contrary to electric pulses alone.10 Apoptosis of vascular endothelial cells, as well as vascular accumulation and erythrocyte ex-osmosis, can increase vascular resistance to blood flow, which is why the reduced blood flow in tumors was observed. Moreover, reduced blood flow within the tumor can also induce cell hypoxia, leading to cancer cell death. Therefore, ECT with bleomycin is more effective in tumor ablation than either electroporation alone or bleomycin injection alone. It is worth noting that, in a one-patient case report, Klein et al demonstrated for the first time that IRE combined with ECT (bleomycin) was well tolerated and safe with obviously antitumor activity in the treatment of lymph node metastases from gastric cancer.34 Although there was no information of comparison between IRE alone and IRE combined with ECT, the 8-month of follow-up shows no local recurrence and invasion to surrounding organs and tissues. This pioneered work is a good start for this combining tumor ablation method.
Electrochemotherapy With Cisplatin
Cisplatin is another toxic drug that is commonly used in ECT. Cisplatin is a phase-specific chemotherapeutic drug that forms cross-links between adjacent DNA strands and within the same strand, therefore limiting the tumor cell DNA replication.15 Since bleomycin has been shown to cause alveolar cell damage and subsequent pulmonary inflammation,35 one of the potential advantages of ECT is to limit the dosage of the drug and reduce the side effects. Electrochemotherapy has been trialed clinically in patients with cutaneous and subcutaneous tumors; even for these superficial tumors, there are significant side effects noted and the incidence of toxicity in patients receiving doses of more than 450 mg/m2 rises sharply, and it has been reported that under doses of less than 100 mg, general anesthesia following bleomycin may be associated with postoperative respiratory failure and may be secondary to bleomycin-induced oxygen sensitivity.15 Therefore, for the challenge is to find a drug with a similar mechanism to bleomycin, but with fewer serious side effects.
Michel et al 15 showed that cisplatin was effective in the treatment of pancreatic cancer cells in vitro. They concluded that the lowest viability of cells from the pulmonary metastases of pancreatic cancer can be achieved by using ECT with cisplatin with the electric field of 1000 V/cm and the cisplatin dosage of 10 μM, compared to cisplatin alone, electroporation alone, and nontreated. The mitochondrial activity of tumor cells was also examined for 24, 48, and 72 hours after the experiment, resulting in about 68%, 61%, and 67% of reduction, respectively, compared to the control group. They also noted an increase in the immunoreactivity of human pancreatic ductal adenocarcinoma cells, while the pulse intensity was around 1000 V/cm. The results achieved in this work also suggest that electric pulses around 1000 V/cm can enhance the transportation and anticancer effect of cisplatin, which may be a signal for oxidative stress or apoptosis.15 Most importantly, they identified parameters that affect cell activity, such as drug concentration and pulse parameters. This serves as an important reference for future drug-electroporation (EP) combination therapy for cancer. Since these are largely in vitro studies, much more work is needed before this approach can be tested clinically in patients with pancreatic cancer.
Prevc et al 32 compared the effect of ECT with cisplatin or bleomycin between human papillomavirus (HPV)-negative and HPV-positive human pharyngeal SCC cell lines. They concluded that both HPV-positive cells and tumors were more sensitive to ECT with cisplatin than other treatment groups. In vitro the sensitivity to ECT with cisplatin was found higher in HPV-positive cell line than in HPV-negative cell line and ECT with bleomycin in HPV-positive/negative cell line. There was no difference in the sensitivity to ECT with bleomycin between HPV-positive cell line and HPV-negative cell line. Similarly, a greater proportion of compete responses and survival rate32 was found in ECT with cisplatin compared to ECT with bleomycin (85% vs 33%) in the treatment of HPV-positive tumors.
Other studies comparing bleomycin with IRE or cisplatin with IRE are further summarized in Table 1. However, no clinical trial has yet been done to confirm the safety and effectiveness of ECT with cisplatin in the treatment of head and neck SCCs. In addition, in this study, details on the mechanism of ECT combined with cisplatin were not given, and whether it deepened the tumor necrosis or expanded the ablation area was not described. However, HPV-positive cell lines and tumors can be treated by ECT combined cisplatin because they were more sensitive to cisplatin and had better responses than bleomycin combined with ECT. Therefore, further clinical studies of ECT combined with cisplatin in the treatment of HPV are necessary.
Table 1.
Summary of In Vivo, In Vitro, and Clinical ECT Studies.
| Target | No. of Patients/Mouse | Chemotherapeutic Drug | Number of Electrodes | Pulse Parameters | Dose | Effect | References |
|---|---|---|---|---|---|---|---|
| Human; colorectal liver metastasis | 16 | Bleomycin | 7-needle electrodes | 8 pulses, 100 µs, and 1000 V/cm | 15 000 IU/m2 | 85% CR;15% PR | 36 |
| Human; PVTT from HCC | 6 | Bleomycin | 4- to 6-needle electrodes | 8 pulses, 100 µs | 15 000 IU/m2 | 83% complete necrosis of VP3-VP4, PVTT | 37 |
| Human papillomavirus | In vitro | Cisplatin | NA | 8 pulses, 100 µs, and 1300 V/cm | 4 mg/kg | 85% CR | 32 |
| Mucosal head and neck tumors | 19 | Bleomycin | NA | NA | Ranging from 250 to 1000 IU/cm3 | The tumor-specific 5-year survival was 75% | 38 |
| Murine fibrosarcoma cells | 98 | Cisplatin | Flat parallel electrodes with 8 mm gap | 4 pulses, 100 µs, and 1300 V/cm | 1-8 mg/kg | Longer tumor growth delay and higher tumor curability rate | 39 |
| Ovarian cancers | In vitro | Bleomycin | NA | 8 pulses, 100 µs, and 1000 V/cm | 1; 3; 7.5; 30; 75; 300; and 750 nM | The highest decrease of cell proliferation was observed after EP with bleomycin after 48 hours of incubation for 1000 V/cm | 40 |
| Pancreatic cancers | 11 | Bleomycin | NA | NA | NA | Feasibility and safety | 41 |
Abbreviations: CR, complete response; HCC, hepatocellular carcinoma; NA, not applicable; PR, partial response; PVTT, portal vein tumor thrombus; VP3, Tumor thrombus in the first branch of the portal vein; VP4, tumor thrombus extension to the trunk or the opposite-side branch of the portal vein.
Electrochemotherapy With Carboplatin
Lately, Souza et al 24 investigated the combined effects of ECT with carboplatin using an equine sarcoid cell line. A 3-fold increase in the toxicity of carboplatin to the equine sarcoid cells can be achieved by using electroporation. The electric field used in this study was 1000 V/cm because it was the highest field strength with the minimal impact on cell viability.24 Cells were subjected to multiple drug concentrations ranging from 0 to 2 mM carboplatin. Cell viability was determined at 48 hours after treatment using an 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. When sarcoid cells were exposed to IRE and carboplatin at the highest tested concentration of 2 mM, 11% cell viability of the electroporated cells was noted in comparison with 32% cell viability when the drug alone was used. The dose of carboplatin can be reduced by 2.8 times when it is combined with IRE compared to when carboplatin alone is used. Their findings demonstrated proof of principle for future preclinical studies on different tumor cells to investigate the in vivo effects of electroporation combined carboplatin.
It is worth noting that ECT alone might be able to treat large tumors and can be used for other clinical indications as well, such as the primary local treatment of frail elderly patients who are not suitable for the surgical resection of the primary tumor. Gehl et al 42 found that when ECT treated tumors larger than 3 cm, about 38% of complete response (CR) and 76% of overall response were obtained, respectively. However, the response rate decreased with the increase in tumor size, because of, on the one hand, smaller tumors being responding better, likely due to faster healing (time to clinical response), to larger tumors that have a more aggressive phenotype, and on the other hand, technical limitations when treating very large tumors.43 The side effects of ECT with large dose of cytotoxic drugs while treating large tumors are of severity in clinical practice, such as pulmonary fibrosis, particularly when this treatment is administered to patients who have previously received radiation therapy.44 In addition, in the safety evaluation of ECT, although most patients had no serious consequences, there were also 4 cases of severe adverse events: (1) one nearly lethal bleeding, (2) 2 cases of osteoradionecrosis and fistula, and (3) one patient died of distant metastasis.45 Median functional outcomes of all parameters were worse 1 year after treatment.45 Therefore, the ablation of large tumors with minimal side effect to patients is still a critical issue hitherto. Irreversible electroporation combined with ECT might be able to give us a solution to this issue.
Calcium Electroporation
Enhancing Mechanism
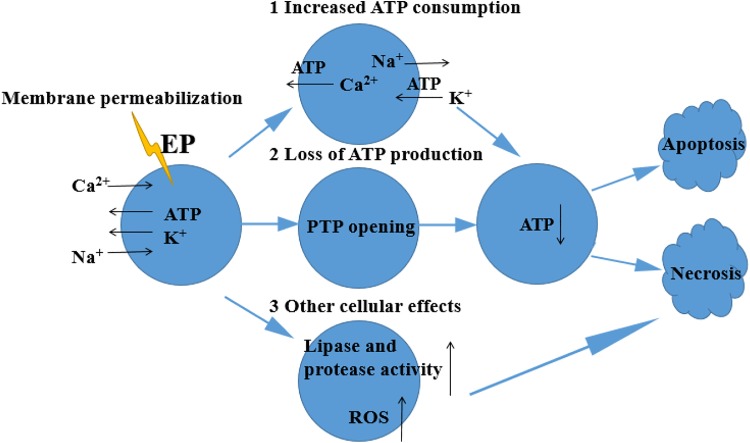
As shown in Figure 2, when cells undergo electroporation by external electric fields, the permeability of cell membrane increases, and Ca2+ and Na+ move into cells, resulting in an increase in both the intracellular calcium concentration and the activity of Ca2+-ATPase, thereby consuming a large amount of ATP.46 This phenomenon usually leads to (1) direct sodium inflow or sodium–calcium exchange and an increase in the activity of Na+/K+-ATPase, resulting in increased ATP consumption,47 (2) an increase in the concentration of calcium ions in cells that may open the permeability transition pores in the mitochondrial membrane, resulting in the loss of electrochemical gradient, which can drive the mitochondria to produce new ATP,48 and (3) other cellular effects, including the activation of lipases and proteases, as well as the production of reactive oxygen species, which may cause cell death.49 Severe calcium overload can lead to cell death through either apoptosis or necrosis, depending on the ATP levels. If most mitochondria are still able to synthesize ATP, ATP loss may only lead to cell apoptosis. On the contrary, if ATP consumption is so severe that the cells cannot synthesize new ATP, cells would undergo necrosis.
Figure 2.
The enhancing mechanism of calcium electroporation.9
First Clinical Trial With Calcium Electroporation
Recently, Falk et al 50 conducted the first clinical trial of calcium electroporation, whereby calcium chloride is injected directly into the tumor prior to IRE. They compared calcium electroporation with ECT with bleomycin. A total of 47 metastatic skin cancer lesions from breast and malignant melanoma in 7 patients were followed over a period of 6 months as part of this phase I trial. They mainly studied (1) difference in the therapeutic outcomes between calcium electroporation and ECT with bleomycin, (2) toxicity of calcium electroporation, and (3) difference in the electroporation current between calcium electroporation and ECT with bleomycin. Patients were followed up for 6 months after calcium electroporation and ECT with bleomycin. Few adverse events occurred in both treatments during the follow-up time. Five out of the 7 patients with a total of 25 metastases had a long-term follow-up for 12 months after calcium electroporation, and it was found that 3 metastases had relapsed within a year.50 Biopsies collected 7 days after treatment showed a significant decrease in the number of cancer cells and a higher level of cell death in metastatic tumors treated with calcium electroporation compared to ECT with bleomycin. The objective response to calcium electroporation and ECT was 72% (CR = 66% and partial response [PR] = 5%) and 84% (CR = 68% and PR = 15%), respectively, after 6 months without a significant difference. After ECT, 26% of patients had hyperpigmentation, while no similar cases were found in patients treated with calcium electroporation. The incidence of ulcers, itching, and exudation was slightly higher in metastatic tumors treated with ECT with bleomycin compared to calcium electroporation, and pigmentation only appeared in metastatic tumors treated by ECT with bleomycin. Compared with metastatic tumors treated by ECT with bleomycin, tumors treated by calcium electroporation had a higher conductivity, but the difference was not significant. This clinical follow-up study suggested that calcium electroporation has similar therapeutic results as ECT with bleomycin but milder side effects in the treatment of metastatic skin tumors. Therefore, calcium electroporation should be studied further and someday might be used in the treatment of large and internal tumors.50,51
This first clinical trial has shown preliminarily that calcium electroporation can be used in clinical practice with favorable outcomes, but the intrinsic mechanism of calcium injection combined with electroporation remains to be proven by specific experiments. It is noted that plenty of studies on calcium electroporation were focused on its safety and side effects. We believe that some exploratory studies need to be performed to quantitatively determine the efficacy of tumor ablation enhancement by combining calcium injection and IRE in the treatment of various tumors.
Modification of Cell Membrane
Enhancing Mechanism
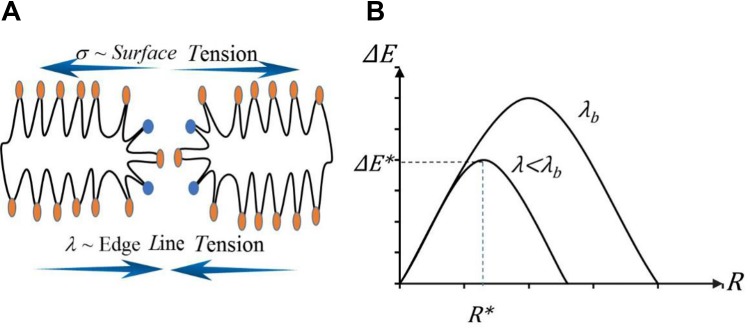
Modifying the cell membrane properties (ie, line tension and surface tension) by surfactants is another feasible way to enhance IRE. As shown in Figure 3A, both line and surface tensions can increase the permeability of membrane and impede the resealing process. It is worth mentioning that this enhancement is relevant to membrane property modification instead of cytotoxicity of surfactants. A key factor in the effectiveness of IRE is the permeability of the cell membrane during electrical pulses.52 Previous studies have shown that membrane rupture may be related to the energy dynamics of an external electric field during electroporation.53 Pore formation on cell membranes is associated with free energy (ΔE), which can be modeled using a modified nucleated model,54 as described in Equation 1:
Figure 3.
The ablation mechanism of IRE: (A) surface tension and edge line tension during pore formation, (B) nucleation based energy model and critical pore radius and energy.52
| 1 |
As shown in Figure 3B, when the pore radius (R) exceeds the standard radius (R*), ΔE will decrease with the increase in R, causing the rupture of the cell membrane and leading to cell death.52
As can be seen from Equation 1, in order to reduce ΔE, in addition to increasing the transmembrane voltage (U), the edge line tension (λ) can also be reduced. This not only reduces ΔE but also affects the formation of pores.
According to the membrane elastic model proposed by Karatekin et al,55 λ is affected by its bending modulus (κ), the membrane thickness (h), the inclusion fraction (θ) of foreign molecules, and the spontaneous curvature (C 0) of the included molecules,39 as shown in Equation 2
| 2 |
When the surfactant group is absorbed by the head region of the lipid bilayer, they reduce λ by decreasing κ and positive curvature (θC0) group.52 Molecular dynamics simulation in Moldovan et al has demonstrated that 11.3 mol.% DMSO leads to over 90% decrease in line tension on modeled lipid bilayer membrane, which may cause a significant reduction in IRE threshold (Figure 3B).56
The nucleation model of Toner and Cravalho53 is determined by Equation 3:
| 3 |
The rate of critical pore formation proposed by Weaver and Chizmadzhev,54 and ΔE* can be given by Equation 4:
| 4 |
Jiang et al 52 assumed that the occurrence of irreversible membrane rupture can be considered as a random fluctuation process, when a large number of cells are under electroporation, and the probability is calculated by Equation 5:
| 5 |
Based on the effect of λ on transmembrane voltage, the threshold voltage (Uth) can be calculated by using above equations. Finally, Jiang et al demonstrated that the reduction in membrane line tension of prostate cancer cells has a dramatic impact on IRE threshold.52 Over one order of magnitude of drop in Uth can be achieved by reducing λ from 5 × 10−11 to 1 × 10−11 J/m.52 Furthermore, they found that 5% vol/vol DMSO decreased membrane line tension by at least 20%. All the parameters used in Equations 1 to 5 were tabulated in Table 2.
Table 2.
Summary of Parameters Used in the Present Study.
| Parameter | Symbol | Unit |
|---|---|---|
| Free energy with pore formation | ΔE | J |
| Pore radius | R | m |
| Transmembrane voltage | U | Volt |
| Membrane line tension | λ | J/m |
| Membrane surface tension | σ | J/m2 |
| Membrane bending modulus | κ | J |
| Membrane thickness | h | m |
| Inclusion fraction of foreign molecules | θ | - |
| Spontaneous curvature of the included molecules | C 0 | 1/m |
| Tissue thermal conductivity | K | W/(m·K) |
| Absolute temperature | Ta | K |
| Avogadro’s number | NA | - |
| Radius of water molecule | rw | m |
| Viscosity of the suspending solution | η | P |
| Molar volume of water | v | m3/mol |
| Membrane surface area | Am | m2 |
Dimethy1 Sulfoxide
Jiang et al 52 found that IRE can be improved by combining DMSO in the treatment of prostate cancers. In an in vitro study, they added 1% to 15% vol/vol DMSO to a precultured prostate cancer cell suspension and then waited for 1 minute to allow the DMSO molecules to fully diffuse into the cell membrane. In order to reduce the effect of DMSO on the extracellular electrical conductivity and reduce the toxicity of DMSO in the IRE process, the cell suspension was diluted below 1% vol/vol before IRE.52 The cell suspension was then exposed to electric pulses (50 pulses, 1000 V/cm, 50 μs per pulse). The results showed that the cell viability was about 50% after IRE alone. When DMSO was added alone with a concentration of <5%, the change of cancer cell viability was almost negligible. However, when DMSO was combined with IRE, the survival rate of cancer cells decreased significantly with the increase in DMSO concentration, and the highest cell killing was increased by 75% with 5% vol/vol DMSO.
The same enhancing method was also investigated in an in vivo study using a prostate cancer model. An electrode needle (diameter = 1 mm) is inserted into the center of the tumor and a ring electrode (thickness = 1 mm, height = 2.5 mm, radius = 10 mm) was used to cover the edge of tumor. Two groups of trials were performed to study the enhancement of DMSO, which was designed as follows: (1) the tumor was injected with 5% vol/vol DMSO with the full diffusion of DMSO by waiting for 6 minutes followed by IRE and (2) the tumor was treated by IRE alone. The same amount of pulse energy (50 pulses, 500 V, and 50 μs per pulse) was administrated to each tumor in the 2 groups. Experiment results showed that the tumor ablation volume can be increased from 10 mm3 by IRE alone to 24.3 mm3 by combining DMSO (5% vol/vol) and IRE, indicating an increase in ablation volume by 136%.
Their work in a sense, a proof of concept, that a surfactant can enhance the efficacy of IRE and increase the ablation zone of IRE. They postulated that the enhancement of DMSO is due to the reduction in membrane line tension caused by DMSO molecules in the cancer cells, which lowers the transmembrane voltage threshold accelerating the cell membrane rupture after IRE. However, the enhancing effect of DMSO must be tested in the treatment of various types of tumors during IRE before the clinical application of the proposed method. There was no discussion around regarding adverse effects or phenomena in their work, and further studies on animal models are required to confirm the safety of this combining method.
Modification of the Chemical Microenvironment of Tumor Cells
Culture Medium
A study conducted by Shao et al 57 recently showed that the culture medium can also significantly affect the efficacy of IRE. Pancreatic cancer cells were cultured in phosphate-buffered saline (PBS), Dulbecco's Modified Eagle Medium (DMEM) and Roswell Park Memorial Institue (RPMI)-1640 Medium, respectively, and they showed that the viability of pancreatic cancer cells treated by the same IRE protocols changes with the type of culture medium. Under all 3 tested IRE electric fields (750, 1000, and 1250 V/cm), cancer cells had the lowest viability (50%, 18%, and 10%, respectively) when they were treated in PBS and the highest viability when they were treated in RPMI (97%, 86%, and 74%, respectively), which shows for the first time that the local culture microenvironment of cancer cells can affect the efficacy of IRE significantly. They further concluded that inorganic compounds and ordinary basic molecules play a key role in the enhancement of IRE. For example, the lack of amino acids, vitamins, and glucose in PBS compared with DMEM and RPMI may eventually lead to significant enhancement in IRE.57 In contrast to DMEM, some vitamins such as biotin, para-aminobenzoic acid, and vitamin B12 are added to the RPMI medium, which may inhibit the performance of IRE. Although the exact mechanism is not explicit enough, the theory of medium conductivity58 (the conductivity of the extracellular medium) or electroporation-induced cell sensitization59 has been proposed. A previous study has demonstrated that permeabilization can be achieved with a relatively lower electric field when the cells are cultured in medium with a higher conductivity.60 The cell sensitization is that when the cell membrane integrity decreases, the cell membrane mass transport increases leading to a decrease in cell viability. It has been shown that exposing cells to electric field increases their sensitivity to subsequent millisecond square pulses.59
Glucose Level
In addition, Shao et al found that increasing glucose concentration in tumor environment might reduce the therapeutic effect of IRE.57 For the same pancreatic cancer cells, they found that the cell survival rate increases from 16.5% to 30.4%, with the increase in the glucose concentration from 80 to 380 mg/dL in the culture medium showing that IRE is less effective in high glucose conditions.57 Although the underlying mechanism by which glucose concentration can affect the efficacy of IRE was not fully explained in their work, treatment strategies that target metabolic pathways during hyperthermia in cancer are well known.61 For instance, most cancer cells depend predominantly on glycolysis for energy production, therefore cellular stress due to IRE or heat is likely to be augmented at low glucose concentrations whereas the metabolic function of the cell is compromised.62 In the case of IRE, this stress is probably exacerbated by the loss of intracellular enzymes needed for glycolysis. Therefore, to improve the efficacy of IRE, targeting a low glucose concentration environment for tumors might be reasonable to consider. It is worth noting that, however, the glucose level used in the study is quite higher than the case in vivo (80-380 vs 1.8-7.2 mg/dL63) in tumors. Therefore, the conclusions achieved by Shao et al might not be able to be translated to clinical practice without further studies. But still, the work provides us a hint to study the effect of glucose level in tumor on tumor ablation with IRE.
However, it is interesting to note that the glucose level might be able to be manipulated by ketogenic diet (KD). Ketogenic diet is made up of high fat, medium to low protein, and very low carbohydrates, which forces the body to burn fat instead of glucose to make ATP. Increased fat metabolism and restricted carbohydrate metabolism of KD lead to increased ketone body production in the blood,64 decreased glucose and insulin,65 and maintained blood pH value (about 7.4),66 leading to a physiological ketosis state. Over the past 60 years, numerous animal studies have demonstrated not only the increased glucose consumption in cancer cells but also the importance of glucose for tumor survival and metastasis.67 It is also been well recognized that most human cancer cells require more glucose than surrounding normal tissues. Bhatia et al 64 demonstrated that KD can assist cancer treatment. Its lipid metabolism limits the formation of pyruvate and glucose-6 phosphate which can reduce oxidative stress. As a result, KD has been used to treat cancer because it can reduce glucose levels in cancer cells.68 Therefore, it might be possible to manipulate the glucose level in tumors in favor of IRE using KD.
Other Feasible Enhancing Methods
Sodium Dodecyl Sulfate
Previous studies have shown that SDS enhances the transdermal transport of molecules through electroporation, which is able to promote the destruction of the barrier during the pulse process and also extend the life of electric holes generated by the pulse.69 Sodium dodecyl sulfate has been reported to have a higher distribution coefficient (the ability to penetrate and interact with) on pig epidermis than other tissues, indicating that SDS has a preferential affinity for skin lipids and proteins. Therefore, the use of SDS to modulate cellular impedance and the required electrical dose during electroporation could be considered to enhance the effect of IRE. Kalia and Guy70 found that the impedance of pig epidermis decreased by 40% at 24 hours after the administration of SDS. However, under the same conditions, there is no significant change in impedance for the administration of PBS. They found that a dramatic reduction in skin impedance by using 0.25% wt/wt SDS before electroporation. An increase in propranolol hydrochloride flux in human stratum corneum was also observed by combining SDS and electroporation.71 They believed that the combination of SDS and electroporation could reduce the adhesion between molecules in the skin’s lipid bilayer, thus increasing skin permeability.69 In general, at most given voltages, the presence of surfactants causes greater reduction in impedance than the cases without surfactants. However, they also found that the effect of SDS on impedance was smaller when a high impulse voltage applied. For instance, the impedance can be decreased by 60% using pulses of >100 V, while the reduction in impedance is negligible using pulses of >250 V.69 Murthy et al 69 found that in the absence of SDS, the translocation of glucose through the pulse at 500 V/cm was about 30 μg/cm2. With the administration of SDS, the same transmission can be achieved at 300 V/cm, which means that the presence of SDS was able to reduce the required electric dose for electroporation by about 40%. Therefore, the combination of electroporation and SDS can achieve a higher membrane permeability at a lower voltage. The presence of SDS during electroporation increased glucose transport efficiency by about 5 times at 100 V. The increase in passive transport of the epidermis treated by SDS was approximately twice that treated by PBS.69
Thus, the use of SDS can reduce the resealing rate of membrane pores, enhance the potential of electroporation, and improve the efficiency of transdermal transport of molecules. It is worth mentioning that low threshold voltages can reduce the risk of thermal damage and other side effects in association with high voltage pulses. These results indicate that SDS can increase RE by lowering the electrical threshold or increasing the ablation zone. Due to these positive effects of SDS, it can be studied whether the combination of SDS and IRE can enhance the homogeneity and range of tumor ablation in the future.
Combination With Biologicals
Currently, a relatively new means to enhance tumor ablation through a molecular manipulation of cellular morphology before high-frequency irreversible electroporation (H-FIRE) proposed by Ivey et al.20 Trains of bipolar pulses with less than 2 μs pulse width were used in the study. By reducing the duration of electric pulse and making it less than the charging time of cell membrane, the electric pulse can penetrate into the cell and reduce the dependence of electroporation on cell size.72,73 They combined H-FIRE with eA1 molecular intervention to change nuclear/cytoplasm ratio (NCR) to investigate the effect of NCR on H-FIRE.72 Their earlier work have shown that exogenous soluble eA1 is a functional ligand for EphA2 and progress has been made in creating ephrin-based therapeutic agents through conjugation of a bacterial toxic protein to soluble eA1 that selectively targets GBM cells.74,75
The results showed (1) eA1 can active EphA2 in malignant cell lines, leading to a significant increase in the NCR of the cells.20 However, no significant changes in NCR were observed in normal human astrocyte (NHA) cells with eA1 addition; (2) the increase of NCR in malignant cells corresponds to a smaller lethal threshold for H-FIRE, while the lethal threshold of nonmalignant cells remained unchanged.20 However, when using IRE with pulse width of 100 μs, the lesions of eA1-treated malignant cells was significantly smaller than malignant cells cultured in normal medium, and the lethal threshold was also higher than that of cells not treated with eA1. Therefore, the combination of IRE and this molecular therapy could not enhance tumor ablation; and (3) the combination of H-FIRE and eA1 treatment can improve the selectivity of malignant cells. Meanwhile, malignant killing area was significantly enlarged, while the NHA lesion remained unchanged.
Conclusions and Future Directions
Since the advent of IRE in 2005, a plethora of research efforts in various areas ranging from biomedical engineering, to pharmacology, to clinical studies have looked at ways of improving the results seen with this modality, especially in the treatment of large tumors. This article summarized the chemical enhancement methods that are currently being studied in combination with electroporation and further provided suggestions in improving the chemical enhancement of IRE in future as well.
The combination of IRE with bleomycin, cisplatin, or carboplatin has been used in the treatment of some tumors with clinically promising results. The toxicity and precise delivery of the chemical drugs can be further improved by optimizing the electrical pulses to tumor cells. The advantages of ECT include: (1) ECT with bleomycin had a significant antitumor effect on tumor growth and increased the extent in tumor necrosis than either electroporation alone or bleomycin injection alone and (2) ECT with cisplatin can achieve the lowest viability of cells from the pulmonary metastases of pancreatic cancer compared to either cisplatin or electroporation alone. Electrochemotherapy has been proved to have some side effects when combined with bleomycin, such as postoperative respiratory failure, oxygen sensitivity, alveolar cell damage, and subsequent pulmonary inflammation. This further argues that identifying settings for the delivery of current in IRE that will allow for a reduced dose of bleomycin are worthwhile.
Calcium electroporation appears promising as another modality that can improve the performance of IRE. By increasing the calcium concentration in the treatment zone, it is reasonable to predict that a larger ablation zone can be achieved compared to IRE alone. However, the efficacy of calcium-enhanced IRE in the treatment of large and heterogeneous tumors requires further in vivo study. Due to the easily accessible of calcium chloride, the enhancing method would be significantly beneficial to patients in areas with the paucity of resources.
However, the model (Figure 1) used in the study clarifying the RE region–enhanced IRE is an ideal one. The heterogeneity of tumor tissues needs to be considered while introducing chemical drugs or ions to tumor tissues during IRE. Before being translated to clinical practice, this combining approach must be replenished by meticulous studies on the distribution of electric field and chemicals in a heterogenous tissue environment. The information on side effects and treatment efficacy in the treatment of large tumors must also be achieved in advance by using big patient cohort studies.
Modification of cell membrane is a relatively novel and perhaps less toxic way to enhance IRE compared with ECT with chemical drugs. The mechanism of enhancement may be that the surfactants are able to cause changes to cell membrane properties leading to an increase in cell membrane permeability. One group is working on this enhancing method and achieved some promising results using in vitro prostate cancer cell and in vivo animal prostate cancer models. They found that adding surfactants lead to an increase the ablation zone and reduced the electric threshold value of IRE. Before this approach can be used clinically, much further study is needed, including safety, optimal treatment planning and surfactant, or tumor type dependent using extensive in vivo validation.
Modifying the microenvironment of tumor cells before or after treatment is another chemical means proposed lately to improve the performance of IRE. Although the underlying mechanism is still not clear enough, it is largely due to the different concentrations of glucose and (perhaps) vitamins in the culture medium, leading to different chemical reactions during and after the IRE procedure. Therefore, further carefully designed in vitro and/or in vivo validation with various microenvironments of tumor cells must be performed to characterize quantitatively the enhancement. A brief overview of all the enhancing methods is given in Table 3.
Table 3.
Overview of all the Methods of Chemical Enhancement of IRE With Their Advantages and Drawbacks.
| Methods | Compounds | Advantages | Drawbacks | References |
|---|---|---|---|---|
| ECT | Bleomycin, cisplatin, and carboplatin | Cell toxicity can be enhanced by electroporation Significant antitumor effect on tumor growth and increased tumor ablation zone Significant increase in cell death for the pulmonary metastases of pancreatic cancer |
Postoperative respiratory failure, oxygen sensitivity, alveolar cell damage, and subsequent pulmonary inflammation Strict control of the dosage Clinically difficult injection due to the risk of dissemination of tumor cells |
21,24,32,41,42,43,44,45 |
| Calcium electroporation | Calcium | Larger ablation zone Similar therapeutic results as ECT but milder side effects in the treatment of metastatic skin tumors Higher sensitivity to cancer cells than normal cells Low cost of treatment |
Mechanism not fully understood Efficacy in the treatment of large and heterogeneous tumors unknown |
46,48,50,51,76 |
| Modification of cell membrane | DMSO and SDS | Less toxic and less harmful to normal tissue Increase in cell membrane permeability Increase in the ablation zone and decrease in the electric threshold value of IRE |
Clinical safety unknown Lack of clinical data Few surfactants available |
52,55,56,69 |
| Modifying the microenvironment | Culture medium and glucose | No chemical agents involved Suitable to tumors in a low glucose concentration |
Few modifying methods available Mechanism not fully understood |
57,59,61,62 |
Abbreviations: DMSO, dimethyl sulfoxide; ECT, electrochemotherapy; IRE, irreversible electroporation; SDS, sodium dodecyl sulfate.
To further improve this chemical-related enhancing method and overcome the limitation of IRE clinically, some suggestions for future studies are provided, such as:
The mechanism of each chemical enhancement method must be reinforced further using preclinical studies, which is necessary to understand and improve those methods.
The optimal treatment planning of IRE with appropriate chemical drugs must be investigated for different tumor cells to help identify specific treatments that are beneficial to certain tumors; drug delivery methods used in ECT for enhancing IRE also require further investigation in vivo.
Future work on the effect of various surfactants (eg, SDS) on IRE needs to be done using different tumor cells in vitro and in vivo.
A hybrid chemical enhancement of IRE with different chemical drugs or enhancing methods might be worth studying for better clinical outcomes.
Abbreviations
- ATP
adenosine triphosphate
- CR
complete response
- DMSO
dimethyl sulfoxide
- ECT
electrochemotherapy
- H-FIRE
high frequency irreversible electroporation
- IRE
irreversible electroporation
- KD
ketogenic diet
- NCR
nuclear/cytoplasm ratio
- NHA
normal human astrocyte
- PBS
phosphate-buffered saline
- PR
partial response
- SCC
squamous cell carcinoma
- SDS
sodium dodecyl sulfate
- RE
reversible electroporation
- EP
electroporation
- HPV
human papillomavirus
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Footnotes
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was supported in part by 111 Project (D18003) and National Natural Science Foundation of China (Grant No. 81801795).
ORCID iD: Bing Zhang  https://orcid.org/0000-0001-5650-2518
https://orcid.org/0000-0001-5650-2518
References
- 1. Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of irreversible electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg. 2015;32(2):90–97. [DOI] [PubMed] [Google Scholar]
- 2. Lencioni R, Cioni D, Della Pina C, Crocetti L. Hepatocellular carcinoma: new options for image-guided ablation. J Hepatobiliary Pancreat Sci. 2010;17(4):399–403. [DOI] [PubMed] [Google Scholar]
- 3. Wagstaff PG, Buijs M, van den Bos W, et al. Irreversible electroporation: state of the art. Onco Targets Ther. 2016;9:2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang B, Moser MA, Zhang EM, Luo Y, Liu C, Zhang W. A review of radiofrequency ablation: large target tissue necrosis and mathematical modelling. Phys Med. 2016;32:961–971. [DOI] [PubMed] [Google Scholar]
- 5. Vroomen LGPH, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: what are the differences? Diagn Interv Imaging. 2017;98(9):609–617. [DOI] [PubMed] [Google Scholar]
- 6. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. [DOI] [PubMed] [Google Scholar]
- 7. Qin Q, Xiong ZA, Liu Y, et al. Effects of irreversible electroporation on cervical cancer cell lines invitro. Mol Med Rep. 2016;14(3):2187–2193. [DOI] [PubMed] [Google Scholar]
- 8. Lee RC, Zhang D, Hannig J. Biophysical injury mechanisms in electrical shock trauma. Ann Rev of Biomed Eng. 2000;2(1):477–509. [DOI] [PubMed] [Google Scholar]
- 9. Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. [DOI] [PubMed] [Google Scholar]
- 10. Sersa G, Jarm T, Kotnik T, et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer. 2008;98(2):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lorenzo MF, Arena CB, Davalos RV. Maximizing Local access to therapeutic deliveries in glioblastoma. Part iii: irreversible electroporation and high-frequency irreversible electroporation for the eradication of glioblastomaIn: De Vleeschouwer S, ed. Glioblastoma [Internet]. Brisbane, Queensland, Australia: Codon Publications; 2017. [PubMed] [Google Scholar]
- 12. Rubinsky J, Onik G, Mikus P, Rubinsky B. Optimal parameters for the destruction of prostate cancer using irreversible electroporation. J Urol. 2008;180(6):2668–2674. [DOI] [PubMed] [Google Scholar]
- 13. Kranjc S, Cemazar M, Sersa G, Scancar J, Grabner S. Invitro and invivoevaluation of electrochemotherapy withtrans-platinum analogue trans-[PtCl2(3-Hmpy)2]. Radiol Oncol. 2017;51(3):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dolinsek T, Prosen L, Cemazar M, Potocnik T, Sersa G. Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol Oncol. 2016;50(3):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michel O, Kulbacka J, Saczko J, et al. Electroporation with cisplatin against metastatic pancreatic cancer: in vitro study on human primary cell culture. Biomed Res Int. 2018;2018:7364539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dermol-Černe J, Vidmar J, Ščančar J, Uršič K, Serša G, Miklavčič D. Connecting the in vitro and in vivo experiments in electrochemotherapy—a feasibility study modeling cisplatin transport in mouse melanoma using the dual-porosity model. J Control Release. 2018;286:33–45. [DOI] [PubMed] [Google Scholar]
- 17. Hansen EL, Sozer EB, Romeo S, Frandsen SK, Vernier PT, Gehl J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. Plos One. 2015;10(5):e0128034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashton P, Walters KA, Brain KR, Hadgraft J. Surfactant effects in percutaneous absorption I. Effects on the transdermal flux of methyl nicotinate. Int J Pharm. 1992;87(1-3):261–264. [Google Scholar]
- 19. Manuchehrabadi N, Gao Z, Zhang J, et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci Transl Med. 2017;9(379):Pii: eaah4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ivey JW, Latouche EL, Richards ML, et al. Enhancing irreversible electroporation by manipulating cellular biophysics with a molecular adjuvant. Biophys J. 2017;113(2):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mir LM, Belehradek M, Domenge C, et al. Electrochemotherapy, a new antitumor treatment: first clinical trial [in French].C R Acad Sci III. 1991;313(13):613–618. [PubMed] [Google Scholar]
- 22. Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9(4):319–325. [DOI] [PubMed] [Google Scholar]
- 23. Sersa G, Cemazar M, Miklavcic D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum(II) in mice. Cancer Res. 1995;55(15):3450–3455. [PubMed] [Google Scholar]
- 24. Souza C, Villarino NF, Farnsworth K, Black ME. Enhanced cytotoxicity of bleomycin, cisplatin, and carboplatin on equine sarcoid cells following electroporation-mediated delivery invitro. J Vet Pharmacol Ther. 2017;40(1):97–100. [DOI] [PubMed] [Google Scholar]
- 25. Umezawa H, Maeda K, Takeuchi T, Okami Y. New antibiotics, bleomycin A and B. J Antibiot (Tokyo). 1966;19(5):200–209. [PubMed] [Google Scholar]
- 26. Poddevin B, Orlowski S, Belehradek J, Jr, Mir LM. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem Pharmacol. 1991;42:S67–S75. [DOI] [PubMed] [Google Scholar]
- 27. Burney I. Cancer Chemotherapy and Biotherapy: Principles and Practice. Philadelphia, PA:Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 28. Tounekti O, Pron G, Belehradek J, Jr, Mir LM. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993;53(22):5462–5469. [PubMed] [Google Scholar]
- 29. Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol. 1996;27(5):745–748. [DOI] [PubMed] [Google Scholar]
- 30. Pron G, Mahrour N, Orlowski S. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol. 1999;57(1):45–56. [DOI] [PubMed] [Google Scholar]
- 31. Orlowski S, Belehradek J, Jr, Paoletti C, Mir LM. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37(24):4727–4733. [DOI] [PubMed] [Google Scholar]
- 32. Prevc A, Niksic Zakelj M, Kranjc S, et al. Electrochemotherapy with cisplatin or bleomycin in head and neck squamous cell carcinoma: improved effectiveness of cisplatin in HPV-positive tumors. Bioelectrochemistry. 2018;123:248–254. [DOI] [PubMed] [Google Scholar]
- 33. Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29(5):371–387. [DOI] [PubMed] [Google Scholar]
- 34. Klein N, Zapf S, Gunther E, Stehling M. Treatment of lymph node metastases from gastric cancer with a combination of irreversible electroporation and electrochemotherapy: a case report. Clin Case Rep. 2017;5(8):1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81–94. [DOI] [PubMed] [Google Scholar]
- 36. Edhemovic I, Brecelj E, Gasljevic G, et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110(3):320–327. [DOI] [PubMed] [Google Scholar]
- 37. Tarantino L, Busto G, Nasto A, et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: a feasibility study. World J Gastroenterol. 2017;23(5):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landström FJ, Reizenstein J, Adamsson GB, Beckerath Mv, Möller C. Long-term follow-up in patients treated with curative electrochemotherapy for cancer in the oral cavity and oropharynx. Acta Otolaryngol. 2015;135(10):1070–1078. [DOI] [PubMed] [Google Scholar]
- 39. Cemazar M, Todorovic V, Scancar J, et al. Adjuvant TNF-α therapy to electrochemotherapy with intravenous cisplatin in murine sarcoma exerts synergistic antitumor effectiveness. Radiol Oncol. 2015;49(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saczko J, Pilat J, Choromanska A, et al. The effectiveness of chemotherapy and electrochemotherapy on ovarian cell lines in vitro. Neoplasma. 2016;63(3):450–455. [DOI] [PubMed] [Google Scholar]
- 41. Tafuto S, von Arx C, De Divitiis C, et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int J Surg. 2015;21(Suppl 1):S78–S82. [DOI] [PubMed] [Google Scholar]
- 42. Kunte C, Letulé V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176(6):1475–1485. [DOI] [PubMed] [Google Scholar]
- 43. Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50(5):621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Probst U, Fuhrmann I, Beyer L, Wiggermann P. Electrochemotherapy as a new modality in interventional oncology: a review. Technol Cancer Res Treat. 2018;17:1533033818785329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Landström FJ, Reizenstein JA, Nilsson CO, et al. Electrochemotherapy—possible benefits and limitations to its use in the head and neck region. Acta Otolaryngol. 2015;135(1):90–95. [DOI] [PubMed] [Google Scholar]
- 46. Frandsen SK, Gehl J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. Plos One. 2017;12(7):e0181839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hojman P, Gissel H, Andre FM, et al. Physiological effects of high- and low-voltage pulse combinations for gene electrotransfer in muscle. Hum Gene Ther.2008;19(11):1249–1260. [DOI] [PubMed] [Google Scholar]
- 48. Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol. 2010;2010:546163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8(2):119–130. [DOI] [PubMed] [Google Scholar]
- 50. Falk H, Matthiessen LW, Wooler G, Gehl J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319. [DOI] [PubMed] [Google Scholar]
- 51. Szewczyk A, Gehl J, Daczewska M, Saczko J, Frandsen SK, Kulbacka J. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget. 2018;9(14):11604–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang C, Qin Z, Bischof J. Membrane-targeting approaches for enhanced cancer cell destruction with irreversible electroporation. Ann Biomed Eng. 2014;42(1):193–204. [DOI] [PubMed] [Google Scholar]
- 53. Toner M, Cravalho EG. Kinetics and likelihood of membrane rupture during electroporation. Phys Lett A. 1990;143(8):409–412. [Google Scholar]
- 54. Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41(2):135–160. [Google Scholar]
- 55. Karatekin E, Sandre O, Guitouni H, Borghi N, Puech PH, Brochard-Wyart F. Cascades of transient pores in giant vesicles: line tension and transport. Biophys J. 2003;84:1734–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moldovan D, Pinisetty D, Devireddy RV. Molecular dynamics simulation of pore growth in lipid bilayer membranes in the presence of edge-active agents. Appl Phys Lett. 2007;91(20):42. [Google Scholar]
- 57. Shao Q, Liu F, Chung C, et al. Physical and chemical enhancement of and adaptive resistance to irreversible electroporation of pancreatic cancer. Ann Biomed Eng. 2018;46(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. [DOI] [PubMed] [Google Scholar]
- 59. Dermol J, Pakhomova ON, Pakhomov AG, Miklavčič D. Cell electrosensitization exists only in certain electroporation buffers. Plos One. 2016;11(7):e0159434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ivorra A, Villemejane J, Mir LM. Electrical modeling of the influence of medium conductivity on electroporation. Phys Chem Chem Phys. 2010;12:10055–10064. [DOI] [PubMed] [Google Scholar]
- 61. Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–277. [DOI] [PubMed] [Google Scholar]
- 62. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Y, He B, Liu K. et al. A novel peptide specifically binding to VEGF receptor suppresses angiogenesis in vitro and in vivo. Signal Transduct Target Ther. 2017;2:17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028–1035. [DOI] [PubMed] [Google Scholar]
- 66. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rigo P, Paulus P, Kaschten BJ, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996;23(12):1641–1674. [DOI] [PubMed] [Google Scholar]
- 68. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab. 2010;7:33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murthy SN, Sen A, Hui SW. Surfactant-enhanced transdermal delivery by electroporation. J Control Release. 2004;98(2):307–315. [DOI] [PubMed] [Google Scholar]
- 70. Kalia YN., Guy RH. Interaction between penetration enhancers and iontophoresis: effect on human skin impedance in vivo. J Control Release. 1997;44(1):33–42. [Google Scholar]
- 71. Chesnoy S, Durand D, Doucet J, Couarraze G. Structural parameters involved in the permeation of propranolol HCl by iontophoresis and enhancers. J Control Release. 1999;58(2):163. [DOI] [PubMed] [Google Scholar]
- 72. Ivey JW, Latouche EL, Sano MB, et al. Targeted cellular ablation based on the morphology of malignant cells. Sci Rep. 2015;5:17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arena CB, Sano MB, Rossmeisl JH, Jr, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online. 2011;10(1):102–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wykosky J, Palma E, Dm Ringler S, Turner C, Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27(58):7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6(12 pt 1):3208–3218. [DOI] [PubMed] [Google Scholar]
- 76. Frandsen SK, Gehl J. A review on differences in effects on normal and malignant cells and tissues to electroporation-based therapies: a focus on calcium electroporation. Technol Cancer Res Treat. 2018;17:153303381878807. [DOI] [PMC free article] [PubMed] [Google Scholar]