Abstract
Background:
Transplantation of autologous minced cartilage is an established procedure to repair chondral lesions. It relies on the migration of chondrocytes out of cartilage particles into a biomaterial. So far, there is no efficient way to finely mince cartilage. No consensus exists on the nature of the biomaterial to be used to promote chondrocyte migration.
Purpose/Hypothesis:
This study aimed to investigate the potential clinical use of a custom-made mincing device as well as a possible alternative biomaterial to fibrin glue. The device was tested for its effect on chondrocyte viability and on subsequent chondrocyte migration into either a fibrin or a collagen gel. We hypothesized that device mincing would allow finer cutting and consequently more cell migration and that the gelation mechanism of the collagen biomaterial, which uses the clotting of platelet-rich plasma, would enhance matrix production by outgrown chondrocytes.
Study Design:
Controlled laboratory study.
Methods:
Cartilage from 12 patients undergoing knee arthroplasty was taken from the femoral condyles and subsequently either hand minced or device minced. The viability and the degree of outgrowth were quantified with live/dead assay on the generated cartilage particles and on the gels in which these particles were embedded, respectively. Matrix deposition in the biomaterials by the outgrown cells was investigated with histology.
Results:
The device allowed rapid mincing of the cartilage and produced significantly smaller pieces than hand mincing. The initial chondrocyte viability in cartilage particles dropped by 25% with device mincing as compared with no mincing. However, the viability in hand-minced, device-minced, and unminced samples was no longer different after 7 and 28 days in culture. Outgrowth scores were similar among the 3 groups. Fibrin and collagen biomaterials equally supported chondrocyte outgrowth and survival, but neither promoted matrix deposition after in vitro culture.
Conclusion:
The outgrowth potential, the viability after 28 days in culture, and the matrix deposition were not different between the mincing techniques and the tested biomaterials, yet device mincing is faster and results in significantly smaller cartilage particles.
Clinical Relevance:
Device mincing could become the standard method to mince cartilage for second-generation cartilage repair techniques.
Keywords: particulated cartilage, knee articular cartilage, biomaterials, collagen, fibrin, outgrowth, platelet-rich plasma
Traumatic and degenerative lesions of knee articular cartilage are a very frequent condition and predispose patients to a higher risk of secondary osteoarthritis. Different surgical techniques are available to treat such lesions, and multiple cell-based methods have been introduced in recent years.18,20,24 Autologous chondrocyte implantation is the most widely used cell-based treatment for full-thickness cartilage defects. However, it is an expensive and invasive 2-step procedure, relying on the in vitro expansion of the patient’s own chondrocytes, isolated from a biopsy. This approximately 3- to 4-week-long in vitro culture of chondrocytes is associated with cellular senescence and dedifferentiation,32 which can impair the quality of the regenerated tissue.
The idea to harvest, mince, and transplant healthy cartilage for a 1-step procedure1 represents an attractive option to avoid the aforementioned drawbacks of autologous chondrocyte implantation. Often, healthy cartilage tissue containing viable cells is discarded during the debridement step of the surgery,5 whereas it could be an alternative to the biopsy. A growing body of evidence7,9 supports the potential of minced cartilage. Studies in animal models have shown that the use of minced cartilage results in better matrix quality as compared with the controls: microfracture, acellular scaffolds, or untreated defects.11,12,16,26 These promising results paved the path to human clinical studies. Phase 1/2 clinical studies have confirmed the safety of the approach as well as an improvement in clinical scores.10,13,31
The use of minced particles for the treatment of cartilage lesions relies on the migration of chondrocytes into a biomaterial and the subsequent cartilaginous extracellular matrix (ECM) deposition by these cells. The degree of fragmentation is an important parameter, as it positively correlates with the surface area exposed to the biomaterial into which the particles are embedded. Particle size has also been shown to be a critical parameter for the amount of ECM production in vitro, hypothetically owing to the activation of chondrocytes upon the mechanical stimulation of the cutting.6 Several surgical techniques describe mincing healthy cartilage, mostly “by hand” with a scalpel29 but also with devices (the single-use CAIS Harvester and Dispenser from DePuy Mitek or Reveille from Exactech).13,15 Mincing by hand is time consuming and does not produce consistent results. Mincing devices could simplify, accelerate, and standardize the procedure of cutting healthy cartilage into small pieces.
Another important parameter for the success of this approach is the nature of the biomaterial used. Protein-based scaffolds, such as collagen and fibrin, provide binding sites for the chondrocytes to adhere to, which make them good candidates to support autologous minced cartilage.3,27 Fibrin glue is currently used to seal the cartilage pieces in the defects, as it supports cell migration21 and is readily available in clinics. However, the source and concentration of its components vary widely, affecting both the mechanical strength and the adhesive properties.4 Some studies have described the potential use of alternative scaffolds19,23,26 for the surgical technique based on the implantation of minced cartilage, but these scaffolds would require extensive characterization before being approved by health authorities for injection in patients.
The aim of this study was to further improve the surgical technique of implanting minced autologous cartilage particles. We compared a custom-made mincing device with the current standard hand-mincing procedure with regard to cellular viability, outgrowth potential of cells into the biomaterial, and ability of the outgrown cells to deposit ECM within the biomaterial. We hypothesized that the device would allow a faster and finer mincing. We also investigated the potential use of a collagen gel (Vergenix; CollPlant Ltd), already clinically approved for chronic wound healing30 and tendinopathies, as an alternative to fibrin glue to support cell migration and cartilaginous ECM deposition. We hypothesized that collagen would equally promote outgrowth but that the presence of platelet-rich plasma (PRP) in CollPlant Virgenix would enhance ECM deposition by the outgrown cells.
Methods
Cartilage Particle Preparation and Characterization
After ethics committee approval and patient informed consent were obtained, we removed cartilage samples from the lateral condyle of 12 patients undergoing total knee replacement at the Schultess Clinic in Zurich.
The lateral condyle samples were transferred in phosphate buffered saline (PBS) supplemented with 50 μg/mL of gentamicin (15710; Gibco) at 4°C on the day of surgery and processed the next day. Samples were washed in PBS supplemented with 50 µg/mL of gentamicin, and macroscopically healthy cartilage was isolated from the lateral condyle with a scalpel. The fibrillated areas were discarded.
Cartilage pieces were randomly divided into 3 groups: control, hand minced, and device minced. The controls (“unminced”) were 3 mm–diameter cylinders punched from a larger cartilage piece with a biopsy punch. Mincing by hand was done with a scalpel into approximately 1-mm3 pieces, as described by Salzmann et al.29 The third group was cut with a mincing device.
The custom-made mincing device consisted of a socle and a blade unit, both gamma sterilized before use. The socle was made of VeroWhitePlus (RGD835), a material used for 3-dimensional printing, and included a rectangle-shaped well in which the cartilage pieces were placed. The blade unit was composed of 10 parallel, round, stainless-steel blades, and it was manually guided horizontally up to 50 times over the cutting chamber (Figure 1A). The time required to obtain cartilage particles was measured from the moment when the relatively big cartilage pieces (450 mg of ∼1 cm–long cartilage piece isolated from a non–load bearing part of a bovine knee joint) were put on the cutting board (hand mincing) or in the cutting chamber (device mincing), until ≤1-mm3 particles were obtained.
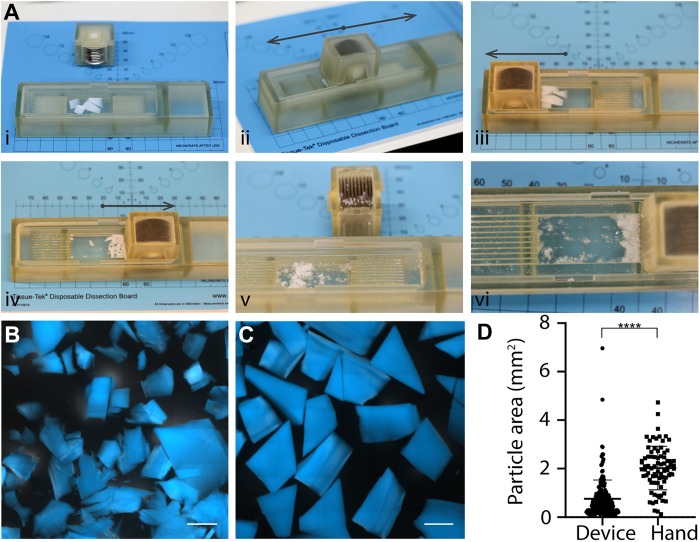
Figure 1.
Outcome of the mincing technique on the cartilage particle size. (A) Sequential steps with the device, from loading in the chamber (i) to collecting the particles (vi). DAPI staining of cartilage particles obtained with (B) the mincing device and (C) hand mincing. Scale bars: 1 mm. (D) Plot of quantified particle area (n = 213 for device mincing and n = 80 for hand minced). ****P < .0001. Bars indicate mean ± SD.
Particle size was measured by staining the cartilage particles with 4′,6-diamidino-2-phenylindole (DAPI; D9542, Sigma-Aldrich) for 30 minutes in the dark at room temperature (RT) and imaging with a ZEISS Axio Observer inverted epifluorescence microscope (ApoTome.2) with a 5× objective. The area of the particles was measured with the software Fiji (which can be downloaded at https://fiji.sc/).
In Vitro Culture of Cartilage Particles: Free-floating or Embedded in a Biomaterial
The culture medium used for all experiments was Dulbecco’s modified Eagle medium (DMEM; 31966; Gibco) supplemented with 10% v/v fetal bovine serum (10270; Gibco), 50 μg/mL of ascorbic acid (A8960; Sigma Aldrich), and 10 μg/mL of gentamicin. All cultures were done in a humidified chamber (37°C, 5% v/v CO2) with media changes 3 times a week.
Fibrin Glue
Fibrin was prepared with a final concentration of 20 mg/mL of human fibrinogen (Tisseel; Baxter), 1 U/mL of thrombin (Tisseel; Baxter), 7.7 µg/mL of gentamicin, and 2 mmol/L of CaCl2. Cartilage pieces were placed in an empty cylindrical PDMS mold (polydimethylsiloxane; 4-mm diameter) with a spatula, and fibrin glue (25 μL) was added on top and mixed gently with the cartilage particles to fully encapsulate them. The constructs were incubated 25 minutes at 37°C to crosslink before addition of culture medium.
CollPlant Vergenix
CollPlant Vergenix consists of type I recombinant human collagen (Vergenix STR Kit; CollPlant Ltd), clotted with PRP, according to manufacturer’s protocol. PRP was obtained from human blood (Blutspende AG) delivered in citrate-coated vials by using the Arthrex ACP double syringe, according to the manufacturer’s protocol. Briefly, the PRP-containing syringe was connected to the collagen fiber–containing syringe, and the 2 components were mixed by moving the whole content from one syringe into the other until a homogeneous mixture was obtained. The mixture was distributed on cartilage pieces in 6 mm–diameter PDMS molds placed in a 24-well plate. A bigger mold size than that used for fibrin glue was necessary owing to the imprecise delivery system of CollPlant Vergenix and the nature of the gel itself (clot), which makes the tuning of the dispensed volume difficult. Culture medium was added after incubating 25 minutes at 37°C in a humidified chamber to crosslink.
Upon embedding in the biomaterial (fibrin or collagen), constructs were cultured in free-swelling conditions for 4 weeks.
Viability Assay
Gels were incubated for 1 hour in DMEM (31966) containing 2μM calcein AM, 6.6 μg/mL of propidium iodide, and 10 µg/mL of Hoechst 33342. Images of the samples were taken with a ZEISS Axio Observer inverted epifluorescence microscope at 10× magnification. The excitation wavelengths were 405 nm (DAPI), 494 nm (calcein), and 528 nm (propidium iodide). Images from 3 replicates were used for quantification of each condition. A macro was written with Fiji software to systematically quantify the number of living and dead cells (Appendix Figure A1). The viability was calculated as the number of viable cells divided by the number of viable and dead cells.
Outgrowth Assessment
To be able to compare the outgrowth of chondrocytes from hand- and device-minced particles into fibrin glue and CollPlant Vergenix after 28 days of culture (D28), a scoring system was developed (Figure 2A). Gels with no visible cells or containing only the embedded cartilage pieces were given a score of 0. Gels with cells that outgrew from 1 or 2 spots but did not fill the gel were given a score of 1. A score of 2 was given to gels containing outgrown cells invading the whole gel. For each patient's sample, the outgrowth score was calculated by averaging the scores of the technical duplicates or triplicates.
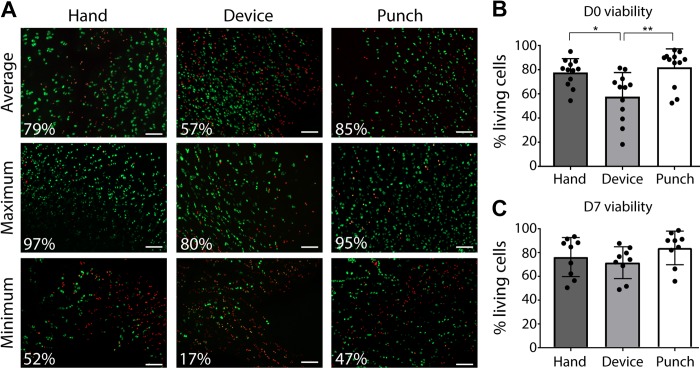
Figure 2.
Effect of mincing on the initial viability of the chondrocytes. (A) Live/dead staining of hand-minced, device-minced, and punched cartilage pieces at D0, showing representative images of the average, maximum, and minimum viability results. Scale bars: 100 μm. Plot of quantified viability at (B) D0 (n = 12) and (C) D7 (n = 9). For panels B and C, triplicates were made for each condition, and the plotted viability data represent the mean ± SD. *P < .05. **P < .01. D0, an hour after mincing; D7, after 7 days in culture.
Some gels were stained with DAPI-phalloidin. Briefly, the gels were fixed and permeabilized for 15 minutes with a solution of 1% bovine serum albumin (BSA; A1391, Applichem) and 0.1% Triton X-100. Subsequently, gels were rinsed and stained with a solution of 0.13 µg/mL of phalloidin-rhodamine at RT in the dark for 1 hour, followed by staining with DAPI for 30 minutes in the dark. Gels were rinsed in PBS and imaged at the Zeiss Axio Observer at 10× magnification.
Histology and Immunohistochemistry
Gels were fixed in 4% formaldehyde for 2 hours, washed twice in PBS, and incubated in PBS supplemented with 10% (w/v) sucrose for 1 hour at RT and subsequently in PBS supplemented with 30% sucrose (w/v) overnight at 4°C. They were then transferred into cryomolds, embedded in optimum cutting temperature (OCT) (4583; Sakura), incubated in a humidified chamber at RT for 2 hours, frozen on dry ice, and stored at –80°C. Slices 5 μm thick were cut with a Cryostat (NX70; Thermo Scientific) and stored at –20°C until staining.
For staining, sections were immersed in PBS for 5 minutes and washed with deionized water to remove OCT. After antigen retrieval was performed by digesting the sections with 1200 U/mL of hyaluronidase (H3506; Sigma-Aldrich) for 30 minutes at 37°C and blocking with 5% BSA in PBS at RT for 1 hour, the sections were incubated with mouse anti–collagen I (Abcam, ab6308, 1:1500 dilution) and rabbit anti–collagen II (1:200 dilution; 600-401-104, Rockland) at 4°C overnight. Primary antibodies were diluted in 1% BSA in PBS. After being washed in PBS twice, slides were treated with 0.3% H2O2 in deionized water for 20 minutes, and goat anti-rabbit IgG-HPR (1:1500 dilution in 1% BSA; ab6721; Abcam) was applied to the slides for 1 hour at RT. After being rinsed 3 times with PBS, a chromogen solution was added to the slides for 5 minutes at RT with the DAB substrate kit (ab64238; Abcam). The slides were counterstained with hematoxylin (MHS1; Sigma-Aldrich) for 3 minutes, washed, dehydrated, and mounted with Eukitt Mounting Media (03989; Sigma-Aldrich).
For the safranin O staining, sections were treated 5 minutes with Weigert iron hematoxylin, washed in distilled water, differentiated in 1% acid-alcohol for 2 seconds, and rinsed in distilled water. The slides were then sequentially incubated in 0.02% fast green for 1 minute, 1% acetic acid for 30 seconds, and 1% safranin O (S8884; Sigma-Aldrich) for 30 minutes. After being rinsed in 95% ethanol, the slides were dehydrated and mounted with Eukitt Mounting Media.
Statistical Analysis
Data are reported as the mean ± SD of technical duplicates or triplicates of at least 10 biological replicates (patients’ samples). Different conditions were compared with 1- and 2-way analyses of variance with Tukey post hoc testing for multiple comparisons, after confirming the normality of the data with the D’Agostino-Pearson test. The analyses of correlations (Appendix Figure A2 ) used the 2-tailed Pearson correlation test. The size of particles obtained with hand and device mincing was compared with an unpaired t test. Statistical significance was considered when P < .05. All statistical tests were done with Prism (v 7.0.3; GraphPad Software Inc).
Results
Mincing Time and Particle Size
Pieces of harvested tissue were deposited into the cutting chamber of the mincing device, and the tissue was minced by sliding the blades. It took 1.3 ± 0.1 minutes to finely mince the cartilage with the device, whereas cutting by hand required 3.4 ± 0.1 minutes to cut in a less fine manner. Indeed, the particles obtained with the device were significantly smaller than the ones obtained with a scalpel (0.76 ± 0.77 vs 2.02 ± 0.90 mm2), although the range of sizes was broader (6.9 vs 4.6 mm2) (Figure 1, B-D). In fact, some pieces remained in the corners of the chamber and could therefore not be completely cut, resulting in the presence of some larger particles.
Effect of Mincing Strategy on Chondrocyte Viability
The ages of the 12 patients from whom cartilage samples were obtained varied between 59 and 77 years (68 ± 5 years; 8 women and 4 men).
Another endpoint to characterize the efficacy of the device was its effect on chondrocyte viability in the cartilage particles within 1 hour after mincing (defined as D0). The initial viability of chondrocytes in the unminced harvested cartilage tissue (punched for the staining) was variable among patients, but there was no correlation between age and viability (P = .58) (Appendix Figure A2, part A). The mean viability of the chondrocytes in the unminced controls was 81.2% ± 15.25%, supporting the fact that the harvested tissue contained a majority of living cells, despite the age and health condition of the patients. We observed a significant decrease in mean chondrocyte viability for device-minced cartilage particles (percentage of living cells, 57.7% ± 19.9%) as compared with chondrocytes in hand-minced samples (77.7% ± 11.37%, P = .011) and unminced controls (P = .002) (Figure 2) at D0. However, after a recovery phase of 7 days in culture medium, the viability was not significantly different among any of the groups (91.1 ± 8.8%, 84.7 ± 11%, 74.4 ± 13.8% in unminced controls, hand-minced and device-minced particles respectively).
Chondrocyte Outgrowth After Hand or Device Mincing
To investigate whether this initial viability has an impact on the ability of the chondrocytes to outgrow, we encapsulated the cartilage particles in a biomaterial and cultured for 28 days using the scoring system described in Figure 3A.
Figure 3.
Ability of chondrocytes to outgrow in a biomaterial. (A) The scoring system. Top view of fibrin glue or CollPlant Vergenix gels at D28, stained by calcein/Hoechst. Blue, nuclei; green, live cells. Cartilage pieces are outlined in white and gels in yellow. White arrows indicate the presence of outgrown cells. Scale bars: 500 μm. Close-ups are shown underneath each image, outlined with dotted lines (scale bars: 100 µm). (B) Quantification of outgrowth. The mean ± SD score of the triplicates was used to obtain the final score for each condition for each patient. CollPlant, n = 11; fibrin glue, n = 12. (C) Sample images of 1 gel per condition. The related scores are indicated in the white frame, and arrows point at the presence of cells. Scale bars: 500 μm. Insets are shown underneath each image, outlined with dotted lines (scale bars: 100 µm). D28, after 28 days in culture.
Chondrocytes were able to outgrow from hand-minced as well as device-minced cartilage pieces into both fibrin glue and CollPlant Vergenix. Neither the mincing technique (hand vs device, P = .67) nor the type of biomaterial (fibrin glue or CollPlant Vergenix, P = .69) had a statistically significant role in the final outgrowth score (Figure 3, B and C). The cells that outgrew from embedded cartilage particles displayed an elongated shape (Appendix Figure A2, part B), supporting their migratory behavior. Both biomaterials were stable over the course of the culture, and we noticed that the round shape of the gel was better retained with fibrin glue, owing to the difficult distribution of the CollPlant Vergenix clot in the molds and its initial softer consistency.
Chondrocyte Viability After 1-Month Culture of Hand- or Device-Minced Cartilage Pieces in Fibrin and Collagen Hydrogels
Live/dead stainings after D28 did not reveal significant differences between gels containing hand- or device-minced cartilage embedded in fibrin glue and in CollPlant Vergenix (P = .38) (Figure 4). The mean viability of chondrocytes within and outgrown from device-minced particles (D28) was higher than the viability of chondrocytes at D0, owing to the migration and proliferation of viable cells to D28. There was no difference between the viability at D0 and D28 for both mincing techniques (Appendix Figure A3).
Figure 4.
Effect of the mincing technique and biomaterial on D28 viability in the scaffolds, which includes the viability of chondrocytes residing in cartilage particles and outgrowing in the biomaterial. (A) Live/dead stainings of hand- and device-minced cartilage pieces embedded in fibrin glue and CollPlant Vergenix gels at D28. Representative images of cartilage particles outlined in white (i) and outgrown cells (ii). Scale bars: 100 μm. (B) Quantified viability of aforementioned conditions. CollPlant: hand minced, n = 10; device minced, n = 10. Fibrin glue: hand minced, n = 12; device minced, n = 10. Values are presented as mean ± SD. D28, after 28 days in culture.
Matrix Deposition by Outgrown Cells
ECM deposition by outgrown chondrocytes was assessed by histological stainings for glycosaminoglycans (detected by safranin O staining) and collagen II, 2 markers of hyaline cartilage, as well as collagen I, which is a marker of fibrocartilage28 (Figure 5). All cartilage pieces embedded in the gels stained positive for collagen II. Some of them were stained positively at the periphery for collagen I, which may be explained by the fact that the tissue was taken from around lesions and may include some parts of degenerated tissue. Safranin O staining was positive for most pieces, but some displayed a much fainter staining, suggesting some matrix diffusion, as described by Zheng et al35 upon freezing. As for the outgrown chondrocytes within the gels, no proteoglycan deposition was observed in any of the groups. The area between the cartilage pieces was positive for fast green, indicating the presence of noncollagenous matrix, which supports the stability of the constructs to D28. In line with the glycosaminoglycan stainings, no collagen II or collagen I deposition could be detected in any of the groups.
Figure 5.
Extracellular matrix deposition by chondrocytes derived from hand- and device-minced cartilage particles embedded in fibrin glue and CollPlant Vergenix after 28 days of culture. Representative images of safranin O staining (n = 8) and collagen I and II immunostainings (n = 4 for both). *Cartilage pieces. Scale bars: 200 μm for close-up and 2 mm for images of whole gels.
Discussion
The aim of this study was to provide in vitro evidence on the viability of the minced cartilage used in the treatment of articular cartilage lesions. In particular, it is of great importance to optimize these techniques by providing an efficient and fast way to mince cartilage and to investigate alternative biomaterials that could support the regeneration of the injured tissue. This study introduced the use of a tailor-made device that allows an easy mincing of cartilage tissue, finer and faster than the currently used hand mincing. Additionally, we could show that >50% of the cells in the generated particles were alive, which was enough to observe outgrowth from the tissue to a similar extent as from hand-minced particles. The results also support the use of fibrin glue as an encapsulating biomaterial, as it promotes cell migration. Finally, our work did not show an improvement in terms of outgrowth and ECM deposition when the cartilage particles were encapsulated in the collagen gel, CollPlant Vergenix, despite the presence of integrin binding sites on the collagen fibrils and the addition of PRP.
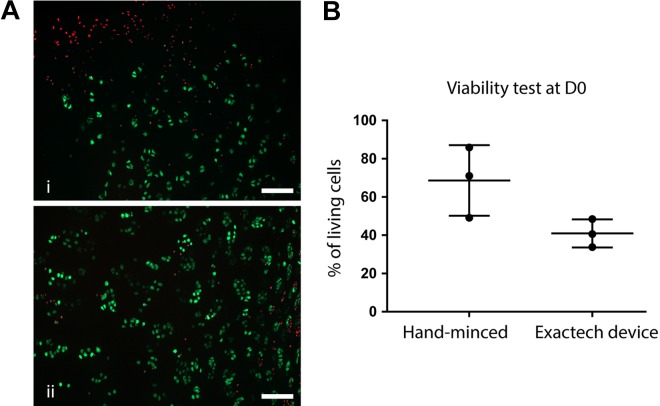
We observed a significant decrease in chondrocyte viability at D0 in device-minced particles as compared with hand-minced ones. This is most probably inherent to the design of the device, which consists of a blade unit being slid from one side to the other of a cutting chamber (see Figure 1). The viability can be affected when the particles are compressed against the walls of the chamber, which would represent a supraphysiological compression stimulus,14,22 more so than because of the sharp cut of the blades, since the hand mincing with a scalpel did not affect the viability. To put this drop in viability in context, we tested the Reveille Cartilage Processor from Exactech, already used clinically. We observed a similar decrease in viability of about 40% as compared with hand-minced samples (Appendix Figure A3). This drop in viability was higher than the 25% decrease that we observed with our mincing device. Although this result should be considered carefully, since it was performed with only 1 patient’s sample, it shows that the initial viability drop was not a limitation to the development and approval of a commercially available mincing device.
After a recovery time of 7 days, there was no longer a statistical difference in viability within the 3 groups. Lu et al23 showed increased proliferation of chondrocytes at the surface of scalpel-cut cartilage pieces over 6 days postmincing, which would explain the absence of correlation between the initial (D0), intermediate (7 days of culture), and final (D28) viability results (Appendix Figure A2) and the fact that outgrowth was still observed despite the initial significant decrease in viability.
We found that chondrocytes could outgrow from the cartilage pieces without any enzymatic digestion, despite previous findings from Andjelkov et al,2 who did not observe outgrowth from “healthy” particulated cartilage unless the cartilage fragments were digested with collagenase. This could have been due to the health status of the patients undergoing total knee arthroplasty, as the joint was probably inflamed, therefore leading to some ECM disruption despite the macroscopically healthy appearance. Moreover, in our experience, the use of collagenase can be detrimental, as the enzyme remains active and overdigests the cartilage even after washing and inhibition with ethylenediaminetetraacetic acid (data not shown). The need for enzymatic digestion is still debated, as many of the in vitro studies cited in this article6,23,25,29 report good results without any digestion step, solely because of the increased surface area following mincing.
We did not notice any significant effect of the mincing technique (ie, by hand or with the device) or the type of biomaterial on the outgrowth behavior of the cells. The presence of trophic and chemotactic factors contained in the PRP in the CollPlant Vergenix gels did not seem to play a role in the amount of outgrowth. This confirms the observations of Zingler et al,36 who reported no increase in the level of outgrowth following exposure to cell lysate, HMGB-1 (high mobility group box 1), TFF3 (trefoil factor 3), BMP-2 (bone morphogenetic protein 2), or TGF-β1 (transforming growth factor β1). However, the use of growth factors to stimulate chondrocyte migration into a scaffold is another variable parameter in the literature, since Marmotti et al,25 for instance, found a positive effect of G-CSF (granulocyte–colony stimulating factor) and, to a lesser extent, TGF-β1. These variable results can be explained by the different administration routes of these factors (loaded in a scaffold or soluble in the culture medium), the quantification methods of outgrowth, or the biomaterial used for the study.
Because the cells were able to outgrow, we characterized them for their ability to deposit matrix. We aimed to reproduce the surgical technique by not supplementing the culture medium with growth factors. We observed diffusion of glycosaminoglycans out of some cartilage particles, which has already been described.35 The absence of deposited hyaline cartilage matrix in the gels was most probably due to the in vitro culture conditions, which are not physiological and cannot reproduce the clinical situation, as suggested by the presence of ECM deposition for in vivo studies.11,12,16,26 Tsuyuguchi et al33 also investigated a clinically available collagen gel (Atelocollagen) and showed little matrix production. However, they could demonstrate a clear advantage of minced cartilage pieces as compared with isolated and embedded chondrocytes, in terms of proliferation potential and growth factor secretion. We believe that the constant supplementation with growth factors in the culture medium would lead to higher amounts of deposited ECM in the 2 biomaterials investigated in our study.6,17,25
The addition of PRP did not lead to increased ECM deposition by outgrown cells, which may be due to the fast diffusion of trophic and chemotactic factors in the medium and their subsequent removal with medium changes. The use of PRP is still debated in the field, and various compositions have been reported depending on the patient’s status and the production method, which can explain the variable efficiency reported in the literature.8 Additionally, the setup could be implemented with mechanical loading to further mimic in vivo conditions. Indeed, Wang et al34 showed that chondrocytes from bovine articular cartilage particles proliferate and produce de novo cartilaginous ECM when subjected to compression and shear within a 3-dimensional matrix with a knee joint–specific bioreactor. A longer time point could also give interesting information, as the onset of cartilaginous ECM was seen after 2 months by Marmotti et al.25 It is noteworthy that most of the studies demonstrating the capacity of outgrown chondrocytes to deposit cartilaginous matrix have been done in vivo.11,12,16,26 An in vitro study is necessary for the preliminary investigation of the mincing efficiency and characterization of cell behavior; however, the in vitro conditions cannot mimic an in vivo environment, notably with regard to the glucose concentration, the viscosity of the culture medium, and the regular changes of medium. In vivo trials will bring more insight into the potential beneficial effects of PRP addition to the Vergenix system in terms of stimulation of collagenous ECM deposition.
Conclusion
According to the endpoints of this in vitro study, device-minced human cartilage behaves similarly to hand-minced tissue. In this study, we have presented a mincing device that allows an easy, fast, and efficient way to finely mince cartilage in the operation room and that can be combined with the use of a standard fibrin sealant. Based on our clinical experience, such a device could be translated into clinical practice to ease handling of the cartilage-mincing procedure. Indeed, it could improve the convenience and speed of the operative procedure and provide a first level of standardization of cartilage particle dimensions. The CollPlant Vergenix showed similar results to fibrin glue in terms of outgrowth and viability but was technically more challenging to administer in a defined volume. Moreover, Vergenix requires the production of PRP—in other terms, some medical personnel to perform a peripheral blood draw on the patient, as well as some added expenses for the autologous conditioned plasma machine. Consequently, Vergenix offers no apparent benefit as compared with fibrin glue. Additionally, Vergenix presents some practical downsides that make it inappropriate for clinical use as a biomaterial to encapsulate cartilage particles in a lesion site, based on this pilot study.
Appendix
Figure A1.
Macro written for the viability analysis with Fiji software (https://fiji.sc/).
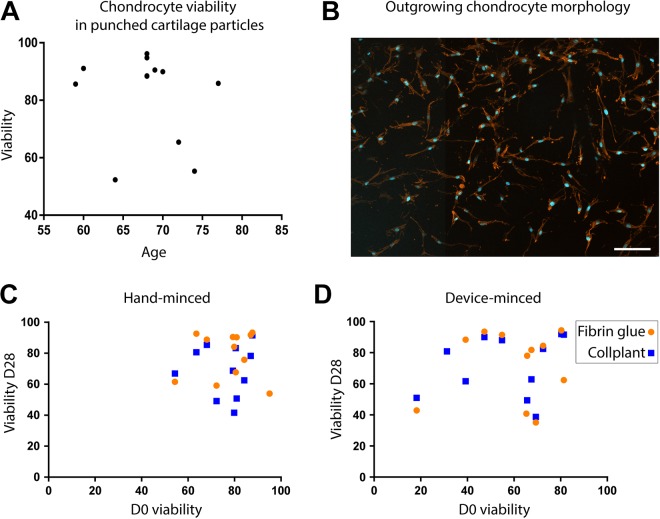
Figure A2.
Correlation between initial viability and age of the patients and between final and initial viability. (A) Plotting of D0 viability in punched cartilage (nonminced) as a function of the patients’ age (n = 12). (B) DAPI-phalloidin staining of outgrown cells. Scale bar = 100 µm. Plotting of final viability after 28 days of culture in (C) hand-minced or (D) device-minced particles, in fibrin glue (round dots) or CollPlant Vergenix (squares), as a function of D0 viability. Pearson 2-tailed correlation test: r values are 0.91 (fibrin glue) and 0.96 (CollPlant) for panel C and 0.73 (fibrin glue) and 0.48 (CollPlant) for panel D. D0, an hour after mincing; D28, after 28 days in culture.
Figure A3.
Assessment of chondrocyte viability in cartilage minced by hand or by the Reveille Cartilage Processor (Exactech) in 1 patient. (A) Representative image of a chip obtained by mincing with the Exactech device (i) and a scalpel (ii). Scale bar = 100 µm. (B) Plot of the technical replicates for each mincing technique. Values are presented as mean ± SD. D0, an hour after mincing.
Footnotes
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Ethikkommission des Kantons Zürich (No. 2014-0390).
References
- 1. Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue [in German]. Fortschritte der Medizin. 1983;101:1650–1652. [PubMed] [Google Scholar]
- 2. Andjelkov N, Hamberg H, Bjellerup P. No outgrowth of chondrocytes from non-digested particulated articular cartilage embedded in commercially available fibrin matrix: an in vitro study. J Orthop Surg Res. 2016;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellis SL. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials. 2011;32(18):4205–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhagat V, Becker ML. Degradable adhesives for surgery and tissue engineering. Biomacromolecules. 2017;18(10):3009–3039. [DOI] [PubMed] [Google Scholar]
- 5. Biant LC, Bentley G. Stem cells and debrided waste: two alternative sources of cells for transplantation of cartilage. J Bone Joint Surg Br. 2007;89(8):1110–1114. [DOI] [PubMed] [Google Scholar]
- 6. Bonasia DE, Marmotti A, Mattia S, et al. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: an in vitro study. Arthroscopy. 2015;31(12):2335–2341. [DOI] [PubMed] [Google Scholar]
- 7. Bonasia DE, Marmotti A, Rosso F, et al. Use of chondral fragments for one stage cartilage repair: a systematic review. World J Orthop. 2015;6(11):1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. [DOI] [PubMed] [Google Scholar]
- 9. Christensen BB. Autologous tissue transplantations for osteochondral repair. Danish Med J. 2016;63(4):1–27. [PubMed] [Google Scholar]
- 10. Christensen BB, Foldager CB, Jensen J, et al. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage. 2015;6(3):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen BB, Foldager CB, Olesen ML, et al. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects: a study in Göttingen minipigs. Am J Sports Med. 2016;44(6):1597–1604. [DOI] [PubMed] [Google Scholar]
- 12. Christensen BB, Olesen ML, Lind M, et al. Autologous cartilage chip transplantation improves repair tissue composition compared with marrow stimulation. Am J Sports Med. 2017;45(7):1490–1496. [DOI] [PubMed] [Google Scholar]
- 13. Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170–1179. [DOI] [PubMed] [Google Scholar]
- 14. Dlima DD, Hashimoto S, Chen PC, et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712–719. [DOI] [PubMed] [Google Scholar]
- 15. Farr J, Gomoll AH. Cartilage Restoration—Particulated/Minced Cartilage. New York, NY: Springer Science + Business Media; 2014. [Google Scholar]
- 16. Frisbie DD, Lu Y, Kawcak CE, et al. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37 (suppl 1):71S–80S. [DOI] [PubMed] [Google Scholar]
- 17. Henson FMD, Vincent T. Chondrocyte outgrowth into a gelatin scaffold in a single impact load model of damage/repair—effect of BMP-2. BMC Musculoskelet Disord. 2007;8:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irawan V, Sung TC, Higuchi A, et al. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med. 2018;15(6):673–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalamegam G, Memic A, Budd E, et al. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Adv Exp Med Biol. 2018;1089:23–36. [DOI] [PubMed] [Google Scholar]
- 21. Kirilak Y, Pavlos NJ, Willers CR, et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: possible involvement of thrombin and protease-activated receptors. Int J Mol Med. 2006;17(4):551–558. [PubMed] [Google Scholar]
- 22. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205–212. [DOI] [PubMed] [Google Scholar]
- 23. Lu Y, Dhanaraj S, Wang Z, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261–1270. [DOI] [PubMed] [Google Scholar]
- 24. Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marmotti A, Bonasia DE, Bruzzone M, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg Sports Traumatol Arthrosc. 2012;21(8):1819–1833. [DOI] [PubMed] [Google Scholar]
- 26. Marmotti A, Bruzzone M, Bonasia DE, et al. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur Cell Mater. 2013;26:15–32. [DOI] [PubMed] [Google Scholar]
- 27. Morales TI. Chondrocyte moves: clever strategies? Osteoarthritis Cartilage. 2007;15(8):861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts S, Menage J, Sandell LJ, et al. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salzmann GM, Calek AK, Preiss S. Second-generation autologous minced cartilage repair technique. Arthrosc Tech. 2017;6(1):e127–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shilo S, Roth S, Amzel T, et al. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng Part A. 2013;19(13-14):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stone KR, Pelsis JR, Na K, et al. Articular cartilage paste graft for severe osteochondral lesions of the knee: a 10- to 23-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3824–3833. [DOI] [PubMed] [Google Scholar]
- 32. Thompson CL, Plant JCT, Wann AKT, et al. Chondrocyte expansion is associated with loss of primary cilia and disrupted hedgehog signaling. Eur Cell Mater. 2017;34:128–141. [DOI] [PubMed] [Google Scholar]
- 33. Tsuyuguchi Y, Nakasa T, Ishikawa M, et al. The benefit of minced cartilage over isolated chondrocytes in atelocollagen gel on chondrocyte proliferation and migration [published online October 12, 2018]. Cartilage. doi:10.1177/1947603518805205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang N, Grad S, Stoddart MJ, et al. Particulate cartilage under bioreactor-induced compression and shear. Int Orthop. 2014;38(5):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng S, Xia Y, Bidthanapally A, et al. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging. 2009;27(5):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zingler C, Carl HD, Swoboda B, et al. Limited evidence of chondrocyte outgrowth from adult human articular cartilage. Osteoarthritis Cartilage. 2016;24(1):124–128. [DOI] [PubMed] [Google Scholar]