ABSTRACT
WNT/β-catenin signaling is crucial for neural crest (NC) formation, yet the effects of the magnitude of the WNT signal remain ill-defined. Using a robust model of human NC formation based on human pluripotent stem cells (hPSCs), we expose that the WNT signal modulates the axial identity of NCs in a dose-dependent manner, with low WNT leading to anterior OTX+ HOX− NC and high WNT leading to posterior OTX− HOX+ NC. Differentiation tests of posterior NC confirm expected derivatives, including posterior-specific adrenal derivatives, and display partial capacity to generate anterior ectomesenchymal derivatives. Furthermore, unlike anterior NC, posterior NC exhibits a transient TBXT+/SOX2+ neuromesodermal precursor-like intermediate. Finally, we analyze the contributions of other signaling pathways in posterior NC formation, which suggest a crucial role for FGF in survival/proliferation, and a requirement of BMP for NC maturation. As expected retinoic acid (RA) and FGF are able to modulate HOX expression in the posterior NC. Surprisingly, early RA supplementation prohibits NC formation. This work reveals for the first time that the amplitude of WNT signaling can modulate the axial identity of NC cells in humans.
KEY WORDS: Neural crest, WNT dose/magnitude, Anterior-posterior axis, HOX genes, Human embryonic stem cells
Summary: Human neural crest cells generated from pluripotent stem cells acquire cranial (anterior) or trunk (posterior) identity based on the magnitude of the WNT signal used to induce them.
INTRODUCTION
Neural crest cells (NCCs) are multipotent embryonic cells that are exclusive to vertebrates and are endowed with a bewildering differentiation potential, enabling them to form peripheral neurons and glia, craniofacial bone and cartilage, and sympathoadrenal secretory cells, among other derivatives. Defects in NCC development and homeostasis lead to significant human pathologies known as neurocristopathies, which include craniofacial birth defects (i.e. cleft lip/palate) and severe cancers (i.e. melanoma), yet our understanding of how these cells are formed in humans remains ill defined.
Efforts to study NC development in human embryos remain limited (Betters et al., 2010; O'Rahilly and Müller, 2007) owing to ethical and technical obstacles. However, a powerful alternative has emerged through modeling human NC development using human embryonic stem cells (hESCs) (Pomp et al., 2005). Initial efforts relied on co-cultures, the use of serum or serum-replacement cocktails, and either embryoid bodies or neural rosettes (Bajpai et al., 2010; Brokhman et al., 2008; Curchoe et al., 2010; Jiang et al., 2009; Lee et al., 2007; Liu et al., 2012; Rada-Iglesias et al., 2012; Sparks et al., 2018). Simpler models, where cell density was controlled along with defined media components, have enabled a better analysis of contributing factors during human NC development from hESCs (Fukuta et al., 2014; Hackland et al., 2017; Huang et al., 2016; Leung et al., 2016; Menendez et al., 2011). Common to these and to animal models of NC development is the activation of the WNT/β-catenin signaling pathway (Chambers et al., 2012; Fukuta et al., 2014; Hackland et al., 2017; Leung et al., 2016; Menendez et al., 2011; Mica et al., 2013), which recapitulates the known role of WNT in NC induction in model organisms (García-Castro et al., 2002; LaBonne and Bronner-Fraser, 1998; Lewis et al., 2004).
The WNT/β-catenin pathway ultimately results in the accumulation of β-catenin in the nucleus where it modulates the expression of targets of the WNT/β-catenin pathway (reviewed by de Jaime-Soguero et al., 2018; Nelson and Nusse, 2004). This pathway plays different roles throughout development, eliciting a wide range of responses that rely on context-dependent modulation of the signal. Prominent among these modulators are specific LEF/TCF co-factors and other transcription factors, their splice isoforms, chromatin modifiers, non-coding RNAs and physical parameters (such as the initial timing, length of exposure and concentration of the WNT signal) (Goentoro and Kirschner, 2009; Lee et al., 2003; Masuda and Ishitani, 2017; Rogers and Schier, 2011; Tan et al., 2012).
The magnitude of the signal has been recognized as an important parameter affecting the context of the signal (Goentoro and Kirschner, 2009; Lee et al., 2003; Rogers and Schier, 2011; Tan et al., 2012). Although a role for WNT/β-catenin signaling in NC development is well established, our understanding of the effects of time and dose of the signal in NC development remain very limited.
In this study, we have investigated the effects caused by the magnitude of the WNT signal during human NC formation. We unveil a double bell curve of NC formation mediated by the magnitude of the WNT/β-catenin signal, with low and high concentrations leading to anterior and posterior NC formation, respectively. Increasing signal imposes a posterior character in NCCs, as revealed by the induction of posterior identity HOX genes. Interestingly, these posterior NCCs display a mixed differentiation potential, generating peripheral neurons, glia and melanoblasts, in addition to osteoblasts; but unlike anterior NCCs, are not able to differentiate into chondrocytes or adipocytes. Through further interrogation of the formation of posterior NCCs, we evaluated how modulation of other signaling pathways involved in posteriorization affects NC formation by high WNT signaling. We found that BMP is required for the generation of posterior NCCs, whereas FGF signaling appears to regulate proliferation/survival, but not the formation, of posterior NCCs. Finally, FGF and RA signaling modulates axial characteristics and the expression of HOX genes within posterior NCCs. Together, this work demonstrates that modulation of the WNT/β-catenin signal promotes the formation of NCCs endowed with different anterior-posterior characteristics.
RESULTS
A bimodal wave of NC formation is produced by increasing the magnitude of WNT signaling
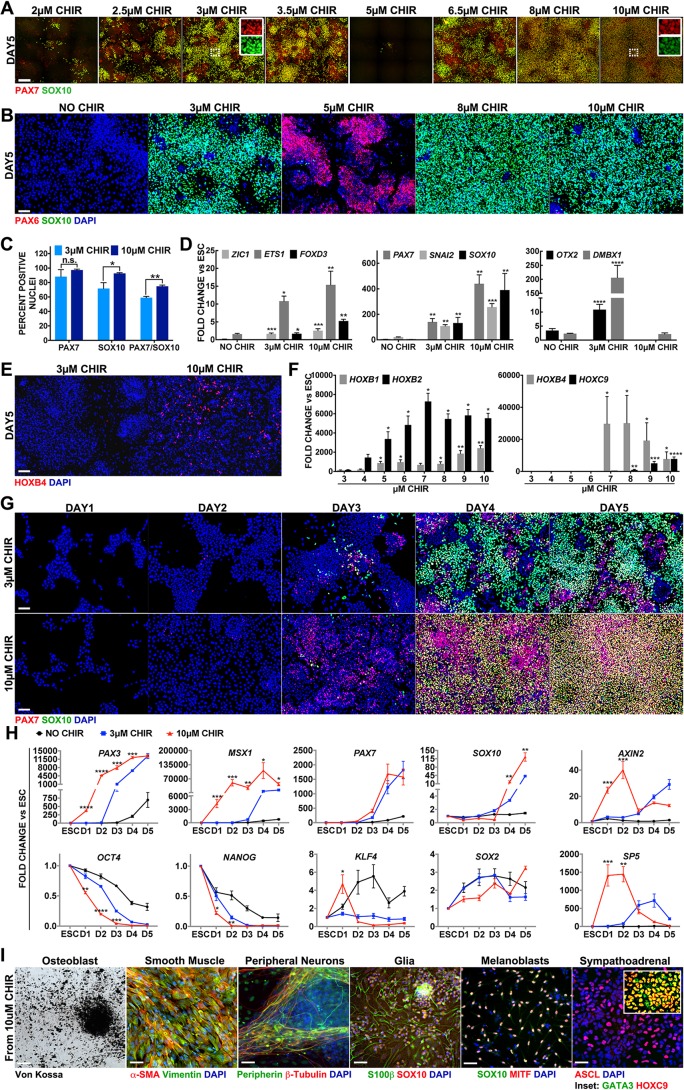
We have previously reported an effective model of human NC formation that generates anterior NCCs from pluripotent stem cells through activation of the WNT/β-catenin pathway either for 5 days (Leung et al., 2016) or just the first 2 days of the 5-day culture (Gomez et al., 2019). To further explore the effect of the magnitude of the WNT signal, we first assessed the effect of different concentrations of CHIR99021 (CHIR), a potent and well-characterized WNT inducer (Ring et al., 2003). To this end, H1 hESCs (expressing pluripotency markers OCT4, SOX2 and NANOG prior to differentiation; Fig. S1A) were initially exposed to 24 concentrations in 0.5 µM increments ranging from 0 to 12 µM CHIR, delivered for the first 2 days of the 5-day NC protocol. Cultures were then fixed and analyzed for the expression of NC markers. We found high PAX7 and SOX10 expression in cultures receiving 2.5-3.5 µM CHIR, with reduced levels from 4 to 6 µM CHIR and increasing levels for both markers from 6.5 to 9 µM CHIR, followed by sustained high levels of NC markers from 9.5 to 12 µM CHIR (Fig. 1A; data not shown). Tests with 15 µM CHIR displayed no PAX7 or SOX10, while 20 and 30 µM CHIR compromised cell survival (data not shown). These results suggest that, under our culture conditions, WNT signaling can trigger efficient NC formation from hESCs at a low dose, ∼3 µM (2.5-3.5 µM), and at a high dose, ∼10 µM (7-12 µM), whereas intermediate doses between these ranges, or at and above 15 µM, do not produce NC. Given these results, we refer to CHIR treatments as low and high WNT for 3 µM and 10 µM, respectively, throughout the rest of the article. Interestingly, unlike low- and high-WNT treatments, cells exposed to the 4-6 µM CHIR range express the earliest neuroectodermal determinant, PAX6 (Zhang et al., 2010) (Fig. 1B; data not shown), suggesting a switch from NCCs (PAX7+, SOX10+, PAX6−) to neural ectoderm progenitors (PAX7−, SOX10–, PAX6+). Immunofluorescence for additional markers SNAI2, AP2A and HNK1 further support the NC characteristic of cells generated under both low- and high-WNT conditions (Fig. S1C). The percentage of nuclei positively stained for PAX7 and SOX10 at low WNT is close to 60% (Gomez et al., 2019; Leung et al., 2016); instead, high-WNT cultures display >75% double-positive cells (Fig. 1C). In line with this evidence, transcripts for PAX7, SNAI2, ZIC1, ETS1, FOXD3 and SOX10 were more strongly expressed in high WNT by reverse transcription-quantitative PCR; RT-qPCR (Fig. 1D).
Fig. 1.
Axial identity of NCCs formed from hESCs is dependent on WNT magnitude, and posterior NCCs retain NC differentiation potential. All data reflect cultures treated with CHIR for 0-2 days. (A) Immunofluorescent expression of PAX7 (red) and SOX10 (green) on day 5. Insets show the co-labeling of PAX7 and SOX10 in the areas outlined with white boxes. (B) Immunofluorescent expression of PAX6 (red) and SOX10 (green). (C) Average percentage of nuclei expressing PAX7 and SOX10 on day 5 by immunofluorescence. (D) RT-qPCR expression levels of NC genes ZIC1, ETS1 and FOXD3 (left); PAX7, SNAI2 and SOX10 (center); and anterior NCCs OTX2 and DMBX1 (right). Fold change is relative to hESCs and is normalized by housekeeping genes. Unpaired Student's t-test; each condition is compared with NO CHIR. (E) Immunofluorescent expression of HOXB4 (red). (F) RT-qPCR on day 5 from 3 to 10 µM CHIR, assessing HOXB1, HOXB2, HOXB4 and HOXC9 fold change is relative to ESCs and normalized by housekeeping genes. Each condition is compared with 3 μM CHIR. (G) Immunofluorescence of PAX7 (red) and SOX10 (green) after treatment with 3 µM CHIR (top row) or 10 µM CHIR (bottom row). Each column represents a different day of culture. (H) RT-qPCR expression levels of NC markers PAX3, MSX1, PAX7 and SOX10 (top row, columns 1-4), pluripotency markers OCT4, NANOG, KLF4 and SOX2 (bottom row, columns 1-4) or WNT response markers AXIN2 and SP5 (column 5). NO CHIR (black), 3 µM CHIR (blue) and 10 µM CHIR (red). Fold change is relative to hESCs and is normalized by housekeeping genes. Statistics are 10 µM CHIR versus 3 µM CHIR per given day. (I) Terminal derivatives from day 5 NCCs induced with 10 µM CHIR. Osteoblasts stained with Von Kossa stain. Immunofluorescence stains for: smooth muscle, α-SMA (red) and vimentin (green); peripheral neurons, peripherin (green) and β-tubulin (red); glia, S100β (green) and SOX10 (red), and MITF negative (data not shown); melanoblasts, SOX10 (green) and MITF (red); sympathoadrenal, ASCL (red), GATA3 (green, inset) and HOXC9 (red, inset). Nuclei are stained with DAPI (blue) where indicated. Data are mean±s.e.m. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005. Scale bars: 225 µm in A; 100 µm in B,E,G; 50 µm in I.
To confirm that WNT signaling is responsible for this unusual double curve of NC formation, we tested the effect of the WNT3A ligand on hESCs at a wide range of concentrations. In agreement with our CHIR experiments, WNT3A titration also revealed a double curve with NC appearing at low and high concentrations, with a NC-negative range in between (Fig. S1D). Furthermore, knockdown of β-catenin via siRNA in high-WNT cultures revealed that NC formation requires canonical WNT/β-catenin function (Fig. S1E).
The magnitude of WNT signal dictates axial identity of NCCs
Increasing levels of WNT/β-catenin signaling have been shown to impart posterior characteristics to multiple cell types (Greco et al., 1996; Kiecker and Niehrs, 2001; Kim et al., 2000; Nordström et al., 2002). Therefore, we examined the axial identity of the NCCs induced by low or high WNT. First, we evaluated expression of OTX2 and DMBX1, genes associated with anterior NC territories. Although both are induced by low-WNT treatment, they are considerably reduced in cells exposed to high WNT (Fig. 1D). In agreement with our results, elevated WNT signaling has been shown to result in the downregulation of OTX2 in neural ectoderm (Kudoh et al., 2002; McGrew et al., 1997). To determine whether higher WNT triggers a posteriorization of the NC, we assessed the expression of posterior HOX genes. Immunofluorescence revealed expression of HOXB4 and HOXC9 in high-, but not low-, WNT conditions (Fig. 1E and Fig. S1F). Further transcript analysis revealed a gradual progressive posteriorization profile of HOX gene expression in relation to increasing CHIR concentrations. Cervical HOX genes, HOXB1 and HOXB2, were minimally detected at a concentration of 3 µM, in agreement with our previous findings under low-WNT treatment, suggesting an anterior NC character (Leung et al., 2016). At 5 µM CHIR, HOXB1 and HOXB2 are robustly expressed with amplitude levels increasing as CHIR doses are increased (Fig. 1F). HOXB4, which is normally expresses at vagal levels, first appeared at 7 µM CHIR and gradually diminishes at higher CHIR concentrations. Thoracic HOXC9 appears at 8 µM CHIR and increases thereafter (Fig. 1F). More-posterior HOX genes, including lumbar (HOXA10 and HOXA11) and sacral (HOXD13) levels reveal no significant induction in 10 µM CHIR conditions (Fig. S1G).
To confirm our results in other lines, we tested 3, 5 and 10 µM CHIR treatments in Shef6 (hESCs) and Yale6 (iPSCs). In both cases, we replicated NC generation at low- and high-, but not under intermediate-, WNT conditions, and in both cases, high WNT led to HOX expression confirming posterior character (Fig. S2). The downregulation of anterior genes and upregulation of posterior HOX genes in NCCs generated with high WNT shows that an increased dose of CHIR can posteriorize the NC population. Here, the NCs produced by 10 µM CHIR apparently adopt a thoracic/trunk character, where HOX paralog 9 groups are expressed. Importantly, the degree of NC posteriorization/HOX regulation seems to respond to the magnitude of the WNT stimulus in a very sensitive fashion, suggesting a rheostat-type of response.
The transition from hESC to NC is accelerated in posterior NCCs
To further characterize the NC generated with a high-WNT dose, we explored their temporal kinetics. Cultures exposed to low- and high-WNT conditions were analyzed on a daily basis. Few cells displaying robust PAX7 and SOX10 appear at day 3 in both conditions, and gradually increase at 4 and 5 days of culture (Fig. 1G). Transcripts of these two genes show a similar trend. Instead, transcripts of neural plate border-specifier genes, PAX3 and MSX1 (Pla and Monsoro-Burq, 2018; Stuhlmiller and García-Castro, 2012a), are expressed above basal levels on day 2 in high-WNT, but not in low-WNT cultures (Fig. 1H, top row). Thus, increasing the levels of WNT activation to 10 µM CHIR appears to have slightly accelerated acquisition of the NC program, but similar to anterior NCCs, the full NC profile arises after 5 days of differentiation.
Transcriptional time-course analysis of core pluripotency markers showed that OCT4 and NANOG follow the same downward trend, but their expression is reduced sooner in high-WNT conditions (Fig. 1H, bottom row). By contrast KLF4 remains unchanged by low WNT through the 5 days; instead, under high WNT a surge of KLF4 appears on day 1 that is diminished below hESC levels from day 2 onwards. Conversely, SOX2 expression is increased over twofold during the first 2 days in low WNT, whereas it rises about 1.5-fold in high-WNT conditions. Overall, higher WNT stimulus appears to alter the pluripotency status sooner than the lower WNT treatment.
To assess the status of canonical WNT activity we monitored transcripts of direct targets of β-catenin, SP5 and AXIN2 (Jho et al., 2002; Park et al., 2013). Both were clearly induced to higher levels during the first 2 days in high-WNT compared with low-WNT treatment. However, on day 3, once CHIR was removed from both conditions, a surprising reversal occurred and stronger levels of AXIN2 and SP5 are seen in low WNT (Fig. 1H, last column). Interestingly, this higher level of WNT-responsive targets from day 3 onwards is associated with a lower level of expression of NC markers in low-WNT conditions. Instead, the intermediate dose of 5 µM CHIR promotes a mid-range response in both AXIN2 and SP5 during the first 2 days that is abruptly reduced to lower levels than both low- and high-WNT conditions on days 3-5, while NC markers PAX7 and SOX10 are not induced (Fig. S3). These results suggest that a transient and early high-WNT dose (0-2 days) leads to a transiently high expression of WNT targets, which correlate with faster departure from the stemness state and robust posterior NC formation.
High-WNT NCCs display posterior differentiation potential and restricted ectomesenchymal capacity
We have previously reported that low-WNT anterior NCCs generate melanoblasts, peripheral neurons, glia and ectomesenchymal derivatives according to their axial identity (Gomez et al., 2019; Leung et al., 2016). We therefore evaluated the differentiation potential of posterior NCCs induced with high WNT. Interestingly, NC generated with high WNT are able to differentiate into peripheral neurons (99.74% peripherin+/nuclei), glia (93.74% S100B+/nuclei, 92.08% SOX10+/nuclei), melanoblasts (85.61% MITF+/nuclei, 83.52% SOX10+/nuclei) and sympathoadrenal derivatives (49.73% ASCL+/nuclei, 100% GATA3+/nuclei, 99.5% HOXC9+/nuclei), but failed to generate chondrocytes and adipocytes according to their suspected posterior NC character. However, posterior NCCs also generated smooth muscle (99.74% SMA+/Nuclei) and osteoblasts (Fig. 1I). These results demonstrate that, similar to the anterior NCCs generated with low WNT, posterior NCCs are also multipotent. However, unlike anterior NCCs, high-WNT-induced posterior NCCs produce sympathoadrenal derivatives and, somewhat surprisingly, these posterior NCCs are also able to efficiently generate osteoblasts and smooth muscle, which are thought to be exclusive to anterior NCCs.
Transient versus continued high WNT promotes posterior NC versus mesodermal fate
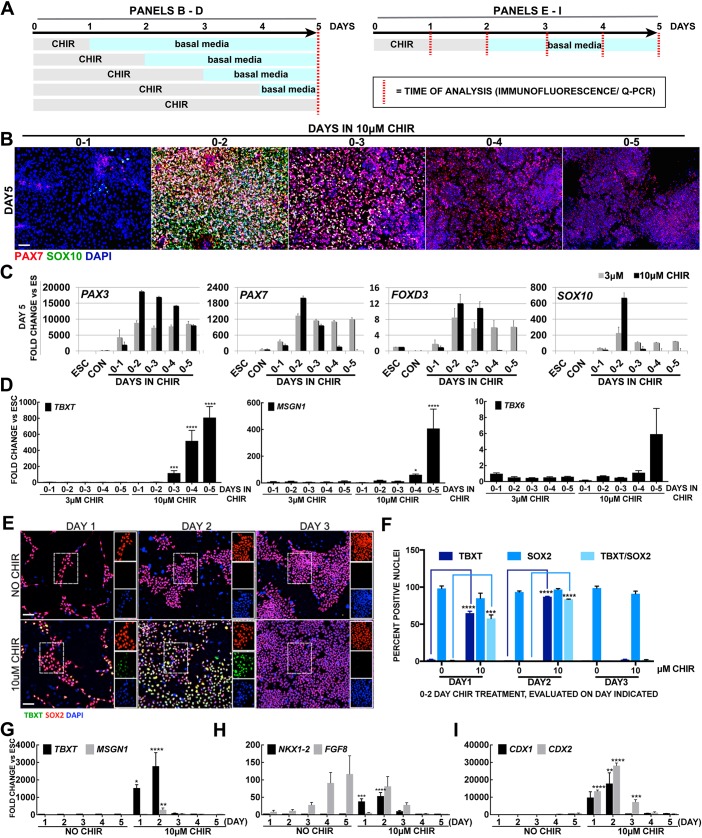
To test the effect of varying the duration of the high-WNT stimulus on NC formation, we exposed hESCs to WNT for 1 to 5 days of the 5 day culture and assessed multiple NC markers. Immunofluorescence of PAX7 and SOX10 suggest that 1-, 4- or 5-day treatments do not lead to NC formation, while few SOX10 cells are produced by 3 days of treatment. Instead, when CHIR is added for the first 2 days, robust signals are detected (Fig. 2B). This is further supported by transcriptional output of PAX3, PAX7, FOXD3 and SOX10, which also exhibit the highest expression levels with 2 days of high-WNT treatment (Fig. 2C).
Fig. 2.
Prolonged treatment with CHIR re-directs the fate of NCCs toward paraxial mesoderm progenitors. (A) Scheme of data presented in B-D and E-I. (B) Immunofluorescence expression on day 5 of PAX7 (red) and SOX10 (green) after 10 µM CHIR for the durations indicated; nuclei were counterstained with DAPI. (C) RT-qPCR evaluation of NC markers PAX3, PAX7, FOXD3 and SOX10 on day 5 in NO CHIR (CON), 3 µM or 10 µM CHIR for durations indicated on the x-axis. Fold change is relative to hESCs and normalized to housekeeping genes. (D) RT-qPCR for mesoderm genes TBXT, MSGN1 and TBX6 on day 5 after treatment with 3 µM or 10 µM CHIR for the durations indicated on the x-axis. Comparisons are between matching days of treatment between two doses. (E) Immunofluorescent expression of SOX2 (red) and TBXT (green) during the first 3 days in NO CHIR and after 0-2 days treatment with 10 µM CHIR. (F) Average percentage of nuclei expressing TBXT, SOX2 or TBXT/SOX2 on the day indicated on the x-axis. (G-I) Daily RT-qPCR for genes associated with NMPs: TBXT, MSGN1, NKX1-2, FGF8, CDX1 and CDX2. Comparisons are between treated and untreated groups on each corresponding day. Data are mean±s.e.m. *P<0.05, **P <0.005, ***P<0.0005, ****P<0.00005. Nuclei are stained with DAPI (blue). Scale bars: 100 µm.
PAX3 is important for NC and mesoderm development (Epstein et al., 1995; Plouhinec et al., 2014), and WNT signaling has also been associated with mesodermal precursors (Funa et al., 2015), thus prompting us to inquire whether other mesodermal markers appear in our cultures. Low-WNT cultures, regardless of the length of exposure, produced no significant signal for TBXT, MSGN1 and TBX6, similar to high-WNT cultures treated for 1, 2 or 3 days. Instead, treatment with high WNT for 4 or 5 days display robust expression of TBXT, MSGN1 and TBX6 (Fig. 2D). Therefore, prolonged high-WNT treatment appears to inhibit the formation of posterior NC and instead leads to a mesodermal expression profile.
Posterior NCCs transiently express an NMP-like precursor profile
We have previously reported that anterior NCCs produced with low WNT arise through an intermediate that transiently expresses low levels of TBXT transcript, but not the protein (Leung et al., 2016). TBXT is a well-known target of WNT/β-catenin signaling (Yamaguchi et al., 1999) and a prominent mesodermal precursor. The co-expression of TBXT along with SOX2 defines neuromesodermal progenitors (NMPs) that contribute to posterior axial tissues, including spinal cord and mesoderm (Henrique et al., 2015). Importantly, NMPs have also been suggested to contribute to NCC formation (Cambray and Wilson, 2007; McGrew et al., 2008; Wymeersch et al., 2016), and recently NCs have been generated from NMPs (Frith et al., 2018). Therefore, we evaluated the expression of SOX2 and TBXT in high-WNT cultures. At day 1, most cells express SOX2 and a few express TBXT. The proportion of cell expressing TBXT and SOX2 at day 2 increases considerably, but at day 3 or thereafter, no TBXT protein was found. Similarly, we detected significant transcript levels for TBXT, MSGN1, NKX1-2, FGF8, CDX1 and CDX2 on day 2 of culture, whereas only CDX2 displays significantly high expression on day 3 (Fig. 2E-I). These results suggest that posterior NCCs generated by high magnitude of WNT transiently express several markers associated with an NMP-like precursor profile.
BMP signaling is required for induction of posterior NCCs
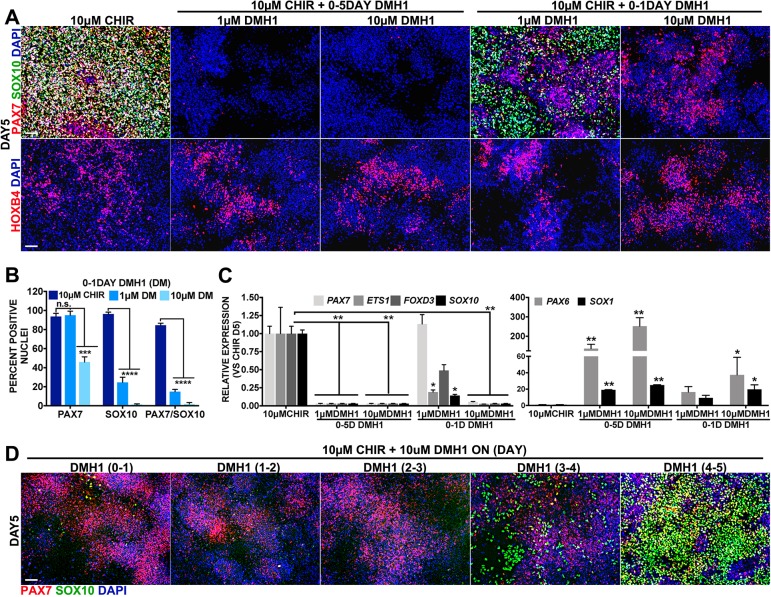
Once we established that high WNT can lead to posterior NC formation, we assessed how other signaling pathways known to participate in NC development function in this context. BMP signaling is considered to be a key player in the induction of NC in multiple model organisms (Pla and Monsoro-Burq, 2018; Stuhlmiller and García-Castro, 2012a). Human NC formation models have reported mixed and even contradictory information regarding the contribution of BMP signaling. Whereas some groups required BMP inhibition (Chambers et al., 2012; Mica et al., 2013), others found BMP inhibition dispensable for NC formation from hPSCs (Fukuta et al., 2014; Huang et al., 2016; Menendez et al., 2011). By contrast, our model of anterior NC formation requires BMP signaling (Leung et al., 2016). We therefore tested whether BMP signaling is also required for posterior NC induction. To this end, we applied the BMP receptor inhibitor DMH1 (Hao et al., 2010) throughout the 5-day posterior NC regimen (2-day high WNT). Unlike control posterior NC cultures, cells treated with 1 µM or 10 µM DMH1 do not display PAX7 or SOX10, or transcripts for PAX7, ETS1, FOXD3 and SOX10 (Fig. 3A-C).
Fig. 3.
Induction of posterior NCCs requires BMP signaling. All data were analyzed on day 5. Controls are 0-2 days treatment with 10 µM CHIR. (A) Immunofluorescent expression of SOX10 (green) and PAX7 (red) (top) or HOXB4 (red) (bottom). DMH1 was added on days 0-5 (columns 2 and 3) or day 0-1 (columns 4 and 5) at the doses indicated. (B) Average percentage of nuclei expressing PAX7, SOX10 or PAX/SOX10 on day 5 in 10 µM CHIR without or with 0-1 day 1 µM or 10 µM DMH1. (C) RT-qPCR analysis for experimental conditions defined in A. NC genes PAX7, ETS1, FOXD3 and SOX10 (left), and neural precursor genes PAX6 and SOX1 (right). Data are evaluated using one-way ANOVA and changes noted are relative to 10 µM CHIR. (D) Immunofluorescent expression of PAX7 (red) and SOX10 (green). 10 µM DMH1 was added on the days indicated. Nuclei are stained with DAPI (blue). Data are mean±s.e.m. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005. Scale bars: 100 µm.
To assess an early requirement for BMP signaling, we deployed DMH1 exclusively on the first day. DMH1 at a low dose of 1 µM does not result in a change in PAX7 protein expression (Fig. 3A,B), whereas SOX10+ and PAX7+/SOX10+ cells are considerably reduced. Similarly, PAX7 transcripts are not altered under this condition, even though ETS1, FOXD3 and SOX10 transcript levels are diminished (Fig. 3C). Instead, treatment with 10 µM DMH1 during the first 24 h results in reduced PAX7+, SOX10+ and co-expressing PAX7+/SOX10+ cells (Fig. 3A, column 1 versus column 5; Fig. 3B), as well as reduced PAX7, ETS1, FOXD3 and SOX10 transcripts (Fig. 3C). Interestingly, HOXB4 protein was unaltered under all these conditions, suggesting the retention of a posterior character (Fig. 3A).
We then assessed whether BMP inhibition had redirected the cells in the high-WNT cultures from a posterior NC status towards neural precursors, as occurs in low-WNT cultures (Leung et al., 2016). To this end, we measured the expression of PAX6 and SOX1, two prominent neuronal precursor genes. Compared with high-WNT conditions, cultures treated for 5 days with DMH1 at 1 µM or 10 µM concentrations show significant increase for both markers (Fig. 3C, right). Strong PAX6 and SOX1 expression is also triggered by a 1-day treatment with 10 µM, but not with 1 µM DMH1. Moreover, addition of 10 µM DMH1 on days 2-5 also promotes conversion to a neural fate at the expense of NCCs (Fig. S4), suggesting that the cells with an NMP-like profile induced on day 2 have the potential to produce neural cells. These results suggest that BMP signaling is crucial during posterior NC formation and its inhibition shifts the fate of hESCs towards a neuronal precursor identity.
To test the temporal contribution of BMP signaling to posterior NC formation, we delivered the DMH1 inhibitor at 24 h intervals. Although PAX7 was modestly changed, SOX10 expression was amiss in DMH1 treatments at 0, 1 and 2 days, weak in cultures treated at day 3, and apparently normal when deployed at day 4 (Fig. 3D). These results indicate that BMP signaling is required for posterior NC formation during the first 3 days.
FGF signaling plays a passive role in the induction of posterior NCCs
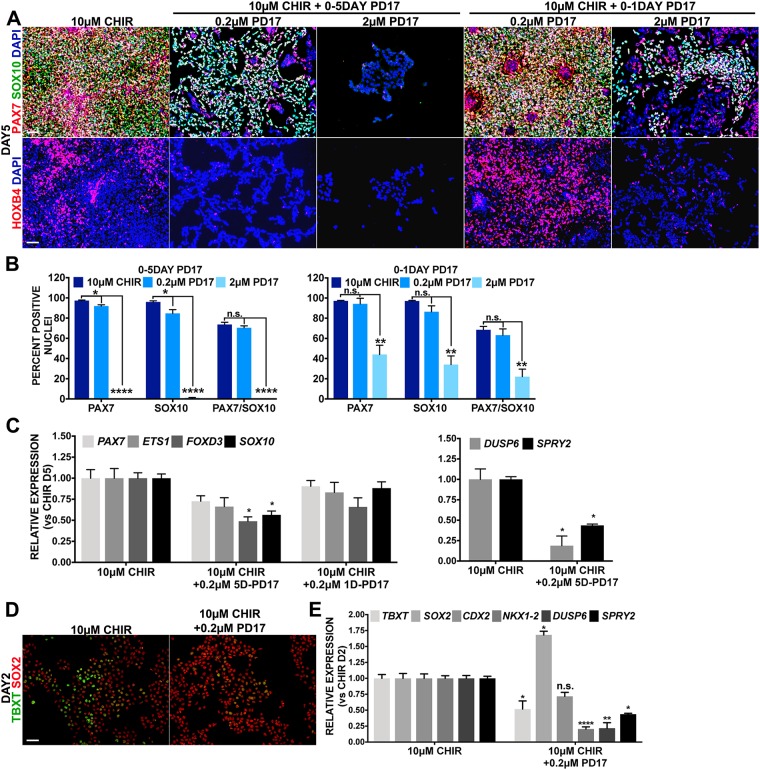
FGF signaling has also been implicated in NC formation (Betters et al., 2018; Hong et al., 2008; Leung et al., 2016; Stuhlmiller and García-Castro, 2012b; Villanueva et al., 2002; Yardley and García-Castro, 2012). To assess whether FGF is required for posterior NC induction, we exposed high-WNT cultures to PD173074 (PD17) (Bansal et al., 2003), a potent FGF receptor inhibitor. High-WNT cultures treated with 0.2 µM PD17 for 5 days display fewer cells than control cultures, appear to maintain a similar proportion of PAX7/SOX10 double-positive cells, but compared with high-WNT controls reveal reduced FOXD3, SOX10, PAX7 and ETS1 transcripts (Fig. 4A-C). Treatment with 2 µM PD17 lead to cultures with no PAX7 or SOX10 signal, and a severe reduction of cell numbers that prevented further analysis (Fig. 4A,B).
Fig. 4.
FGF plays a passive role in the induction of posterior NCCs. A-C were evaluated on day 5; controls are treatment on days 0-2 with 10 µM CHIR. (A) Immunofluorescent expression of SOX10 (green) and PAX7 (red) (top) or HOXB4 (red) (bottom). PD173074 (PD17) was added on days 0-5 (columns 2 and 3) or days 0-1 (columns 4 and 5) at the doses indicated. Nuclei are stained with DAPI (blue). (B) Average percentage of nuclei expressing PAX7, SOX10 or PAX/SOX10 by immunofluorescence in 10 µM CHIR without or with 0-5 day PD17 (left) or 0-1 day PD17 (right). (C) RT-qPCR for NC genes PAX7, FOXD3, ETS1 and SOX10 (left), or FGF response genes DUSP6 and SPRY2 (right) for conditions indicated on the x-axis. Unmarked are not significant. (D) Immunofluorescent expression of SOX2 (red) and TBXT (green) on day 2 after treatment with 10 µM CHIR or 10 µM CHIR plus 0.2 µM PD17 for 2 days. (E) RT-qPCR corresponding to conditions shown in D for NMP-related genes TBXT, SOX2, CDX2 and NKX1-2, and FGF response genes DUSP6 and SPRY2. Data are mean±s.e.m. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005. Scale bars: 100 µm.
To assess whether FGF plays an early role in posterior NC formation, we treated high-WNT cultures with 0.2 µM or 2 µM of PD17 during the first day, and analyzed NC development at day 5. The 0.2 µM PD17 dose had no effect on PAX7 and SOX10 protein or on transcript levels for PAX7, ETS1, FOXD3 and SOX10 (Fig. 4A-C). Instead, the first day-2 µM PD17 treatment produced fewer cells, with a reduced proportion of PAX7/SOX10 double-positive cells (Fig. 4A,B). Reduced expression of known direct targets of FGF, DUSP6 and SPRY2 (Ekerot et al., 2008; Minowada et al., 1999), confirm PD17 efficacy in our system (Fig. 4C). Overall, these data expose an FGF-dependent survival/proliferation role in posterior NCCs, in which remaining cells do acquire NC characteristic.
Given the known role of FGF in axis specification (Villanueva et al., 2002), we also tested whether its inhibition with PD17 altered HOXB4 expression in posterior NC cultures. FGF inhibition for 5 days with 0.2 µM and 2 µM PD17 led to a reduction and elimination of HOXB4 expression, respectively (Fig. 4A). Instead, FGF inhibition with 0.2 µM PD17 for the first day lead to an apparent increase in HOXB4 levels, while the 2 µM PD17 treatment lead to a strong reduction of HOXB4. Thus, FGF seems to modulate the degree of posteriorization elicited by a high-WNT input.
As FGF signaling is known to contribute to NMP formation (Gouti et al., 2014; Henrique et al., 2015; Turner et al., 2014), we also monitored whether PD17 affects NMP-like markers. FGF inhibition (0.2 µM PD17) during the first 2 days lead to reduced TBXT, and increased SOX2 protein and transcripts (Fig. 4D,E). Whereas levels of transcripts for CDX2 were mildly reduced, those for NKX1-2 were significantly downregulated. Reiterating the inhibitory effect of PD17, we detected downregulation of DUSP6 (∼80%) and SPRY2 (∼60%). These results demonstrate that cells exposed to high WNT can still adopt a NC fate, even when NMP markers are compromised.
Exogenous RA inhibits early facets of NC formation
Retinoic acid (RA) signaling has been linked with posteriorization of embryonic tissues and neural crest (Fattahi et al., 2016; Fukuta et al., 2014; Huang et al., 2016; Mica et al., 2013), promoting vagal identity in NC. Additionally, RA is proposed to antagonize FGF to modulate the anterior-posterior characteristics of the neural tube and NCCs (Cunningham et al., 2015; Diez del Corral et al., 2003; Olivera-Martinez and Storey, 2007). We therefore examined the role of RA in our human NCC model. First, we tested whether addition of RA (1 µM or 10 µM RA on days 1-5) affected the posterior character of NCCs. RA induced robust HOXB4 expression in no CHIR control and low WNT, but in high-WNT cultures a mild reduction of HOXB4 was noted (Fig. 5A, left). RA treatment induced HOXB4 but not HOXC9 transcripts in low-WNT cultures. However, in high-WNT cultures, HOXB4 levels did not change, but HOXC9 levels were reduced (Fig. 5B). RA activity in low- and high-WNT conditions lead to graded upregulation of CYP26A1 (Loudig et al., 2000) but not of CRABP2, another putative target of RA (Aström et al., 1992) (Fig. 5C). Importantly, addition of RA to low- and high-WNT cultures lead to no and very few SOX10+ cells, respectively (Fig. 5A, right), along with severe reductions in SNAI, SOX10, ETS1 and FOXD3 transcripts (Fig. 5D). Interestingly, the expression of PAX7 protein and transcripts in high-WNT cultures treated with RA was accompanied by strong and significant upregulation of PAX6 (Fig. S5A). Similarly, prospective posterior NC cells (2 day high WNT) treated with10 µM RA (from days 2-5) suppress SOX10 and reveal robust PAX6 expression (Fig. S5B). Altogether, this suggests that activation of RA signaling under these conditions modulates axial characteristics, but antagonizes NC and apparently promotes a neural fate.
Fig. 5.
RA is necessary for neither NC induction nor posteriorization in high-WNT conditions. All data were analyzed on day 5 following 0-2 days treatment with 3 µM CHIR or 10 µM CHIR. (A) Immunofluorescent expression of HOXB4 (red) in the left three columns, and PAX7 (red) and SOX10 (green) in right two columns. DMSO (0 RA), 1 µM RA or 10 µM RA was added on days 1-5 (rows 1-3). (B-D) RT-qPCR for (B) posterior identity genes HOXB4 and HOXC9, (C) RA response genes CRABP2 and CYP26A1, and (D) NC genes SNAI2 and SOX10 (left) and ETS1 and FOXD3 (right). Fold changes are relative to ESCs and normalized to housekeeping genes. Comparisons are between RA-treated or untreated controls. (E) Immunofluorescent expression of SOX10 (green) and PAX7 (red, top row) or HOXB4 (red, bottom row) for the conditions indicated. AGN193109 (AGN) was added on days 0-5. (F) RT-qPCR corresponding to conditions shown in H. Expression changes are relative to 10 µM CHIR for HOXB1, HOXB2, HOXB4 and HOXC9. Nuclei are stained with DAPI (blue). Data are mean±s.e.m. *P<0.05, **P<0.005, ***P<0.0005, ****P<0.00005. Scale bars: 100 µm.
Late RA does not significantly modulate posterior characteristics imposed by high WNT
Because previous reports show posteriorization of anterior NC through RA treatment (Fattahi et al., 2016; Fukuta et al., 2014; Huang et al., 2016; Mica et al., 2013), we tested whether different treatment times could replicate posteriorization of our low-WNT-induced anterior NC. Indeed, adding RA on the last day to low-WNT cultures led to robust NC formation (SOX10 and PAX7 protein, and SOX10, FOXD3, ETS1 and SNAI2 transcripts) with a significant upregulation of cervical HOX gene expression (Fig. S6A-C). Furthermore, this RA effect could be reversed with the high-affinity pan-retinoic acid receptor inverse agonist AGN193109 (AGN) (Engberg et al., 2010) (Fig. S6D).
To extend our analysis on the effects of RA in our model, we tested whether RA is required for posterior NC formation during our high-WNT conditions. Surprisingly, NC formation (PAX7, SOX10) appears unaffected in high-WNT cultures exposed to AGN (at 0.1 µM, 1 µM or 10 µM; from 0-5 days; Fig. 5E). Importantly, neither HOXB4 protein nor HOXB2 or HOXB4 transcripts were altered. Instead HOXB1 appears reduced, while HOXC9 increased but not significantly (Fig. 5F). Moreover, lumbar/sacral level HOX genes in paralog groups 10-13 were not induced (data not shown). These results suggest that RA does not alter the axial identity of the NCs produced by high WNT.
DISCUSSION
WNT/β-catenin promotes NC formation and its magnitude sets their axial identity
Our experiments suggest that hESCs can adopt distinct axial NC characteristics guided by temporal and dose parameters of the WNT signal exposure. Low-WNT signal generating head/anterior NCs bearing midbrain markers and, instead, a transient high-WNT signal generates posterior NCCs. The immediate induction of CDX factors by high WNT further supports the notion that WNTs set up the axial identity of posterior NCCs, as WNT/β-catenin signaling induces CDX expression (Ikeya and Takada, 2001; Sanchez-Ferras et al., 2012; Shimizu et al., 2005) and CDX2 mediates NCC induction via PAX3, MSX1 and FOXD3 (Sanchez-Ferras et al., 2016, 2012).
We suggest that the low-WNT response might be related to the known early role of WNT/β-catenin during axial specification and head formation (Fossat et al., 2011; Heasman et al., 1994; Lemaire et al., 1995; McMahon and Moon, 1989; Smith and Harland, 1991; Sokol et al., 1991). Whereas a high WNT graded signal could be related to gastrulation (Loh et al., 2016; Morkel et al., 2003; Steinhart and Angers, 2018). We propose that, posteriorly, transient WNT levels would be experienced by young pNC progenitors, as they distance themselves from the WNT source.
It is interesting that SOX10+/PAX7+ NCCs can be formed from pluripotent cells in an apparent bimodal wave, with optimal doses of low ∼3 µM and high ∼10 µM CHIR generating anterior (OTX2+/DMBX1+, HOX−) and posterior (OTX2−/DMBX1−, HOX+) NCCs, respectively. At intervening (4-6 µM) and higher (>15 µM) doses, NCCs are not produced. Interestingly, cultures at ∼5 µM CHIR display abundant PAX6+ cells negative for PAX7/SOX10 expression, suggestive of a neural precursor fate. We have previously reported that anterior NCCs arise independently from PAX6 (Leung et al., 2016). The WNT/β-catenin targets AXIN2 and SP5 are induced to intermediate levels during the first 2 days of exposure to 5 µM CHIR (compared with 3 and 10 µM). However, unlike low- and high-WNT conditions, on days 3-5 they are reduced to basal levels. One possible mechanism behind such response could be that, at ∼5 µM CHIR, but not at low or high WNT, WNT/β-catenin signaling activates a negative-feedback mechanism that represses the continued WNT signaling. Alternatively, an intermediate dose might activate other signaling pathways that repress both WNT signaling and NC induction. Our finding that low WNT forms NCCs devoid of HOX expression, whereas high WNT generates HOX+ posterior NC, unveils a switch between anterior and posterior NC mediated by WNT, which can gradually regulate the expression of HOX genes in the posterior NC in a concentration-dependent manner. WNT signaling is known to play a role in early NC formation (García-Castro et al., 2002; Saint-Jeannet et al., 1997) and to modulate the axial identity of neural tissues (McGrew et al., 1995; Michaelidis and Lie, 2008); however, no study has characterized the additional role that WNT signaling plays in setting the axial identity of NCCs.
NC formation and WNT signaling during embryonic development
The bimodal response of hESCs to WNT/β-catenin magnitude is intriguing. In the embryo, anterior and posterior NCCs arise at different time points and in distinct locations, providing a unique context for each NCC fate. Expression profiles for some WNT/β-catenin targets, such as AXIN2 and SP5, as well as β-catenin reporter models, suggest a weaker early WNT signal anteriorly and a stronger signal posteriorly slightly later, with a gap in WNT activity seen along the anterior-posterior axis for several of these (Harrison et al., 2000; Jho et al., 2002; Maretto et al., 2003; Moro et al., 2012). NCCs are considered to arise throughout the entire axis of the neural tube, except from the anterior forebrain. However, multiple expression patterns for NC markers in diverse studies offer a distinct gap between the midbrain and the hindbrain (Khudyakov and Bronner-Fraser, 2009; Sakai and Wakamatsu, 2005). Given that migratory NCCs can move antero-posteriorly at their most dorsal locations (Kulesa and Fraser, 1998), the suggested gap in NC formation could be masked. Further studies are needed to assess this possibility.
Putative additional players triggered by CHIR99021
We have previously shown that both WNT3A or low CHIR could trigger anterior NC development from hPSCs in a β-catenin-dependent fashion (Leung et al., 2016). Here, we also corroborate our CHIR dose results using WNT3A and a requirement for β-catenin through si-RNA-mediated knockdown. Although CHIR99021 has been used extensively as a WNT agonist because of its high specificity and affinity for GSK3 kinases (Ring et al., 2003), at ∼10 µM, CHIR might also inhibit other kinases (Wagner et al., 2016). Furthermore, WNT/FZD/LRP6 inhibition of GSK3 is known to trigger the WNT/STOP and WNT/TOR pathways that modify broad protein stability and translation, respectively. In addition, GSK3 kinases modify various signaling pathways beyond WNT/β-catenin (Acebron et al., 2014; Taelman et al., 2010). Evaluating the possible role of these alternative players in NC development seems worthy of future studies.
Effects of other signaling pathways on posterior NC generated via high WNT (CHIR)
We have previously reported that, in the context of the low-WNT stimulus, BMP and FGF inhibition has a deleterious effect on anterior human NC induction (Leung et al., 2016). Here, we report that BMP signaling is required under low- and high-WNT conditions for anterior and posterior NC formation. This perspective is in alignment with experiments performed with avian embryos (García-Castro et al., 2002; Liem et al., 1997; Patthey et al., 2009; Patthey and Gunhaga, 2014; Selleck et al., 1998; Villanueva et al., 2002).
Under a high- but not low-WNT regimen, FGF inhibition triggered severe survival/proliferation effects but did not prevent NC formation. Interestingly, although FGF inhibition prevents NC-mediated formation of anterior NC (Betters et al., 2018; Leung et al., 2016; Stuhlmiller and García-Castro, 2012b), FGF inhibition in posterior regions of chicken embryos does not prevent NC formation (Martínez-Morales et al., 2011), supporting a different role for FGF in anterior and posterior NC in humans. In agreement, our group has developed an independent xeno-free approach to generate posterior NCCs in which FGF inhibition also fails to prevent NC formation (Hackland et al., 2019).
Avian studies have reported that RA participates in NC migration, and possibly formation (Martínez-Morales et al., 2011), but murine knockout models affecting RA signaling still form NC (Dupé and Pellerin, 2009; Niederreither et al., 2000). Here, we find that, similar to other reports (Fattahi et al., 2016; Frith et al., 2018; Fukuta et al., 2014; Huang et al., 2016; Mica et al., 2013), exogenous RA can posteriorize NCs during maturation stages. However, early addition of RA prevents NC specification. Given that RA can reduce or contribute to β-catenin signaling (Vijayasurian Easwaran and Byers, 1999; Liu et al., 2002; Tice et al., 2002) and can also modulate FGF (Stavridis et al., 2010), studies are needed to decipher its mechanism of action. Finally, as inhibition of RARs with AGN in high-WNT cultures did not prevent HOXC9 expression, or NC formation, we postulate that an RA-independent mechanism posteriorizes NC under high-WNT conditions, which provides a thoracic identity.
Neuromesodermal progenitors and posterior NC
Interestingly, unlike anterior NCCs, high-WNT posterior NCCs emerge from a progenitor that transiently co-expresses TBXT and SOX2 (and transcripts for NKX1-2, MSGN1, FGF8, CDX1 and CDX2), resembling axial neuro-mesodermal precursors or NMPs (Henrique et al., 2015). Once the WNT agonist is removed, these cells continue to acquire the NC fate and express negligible neural or mesodermal markers. However, they can be re-directed to either a neural fate by repression of BMP or addition of exogenous RA; or to a mesodermal profile through continued high WNT.
NMPs produced from hPSCs can generate NCCs (Frith et al., 2018), and we have found that posterior NC generated in a xeno-free platform (BSA free, N2 instead of B27 supplement and under BMP modulation; Hackland et al., 2019) also transiently express an NMP-like profile. Lineage-tracing experiments in chick and mice support the contribution of NMPs to NCCs (Cambray and Wilson, 2007; McGrew et al., 2008; Wymeersch et al., 2016). Here, we have found that destabilization of the NMP-like profile by FGF inhibition in the presence of high-WNT stimulus still allows NC formation, suggesting that posterior NC can arise independently of this progenitor state. Importantly, the expression profile of the early NC specifier PAX7 in chick embryos labels the whole posterior open neural plate border, surrounding the NMPs; thus, if these cells contribute to the neural folds laterally, they would join neural folds already containing prospective NCCs (PAX7+ cells). Alternatively, the NMPs could contribute to the most posterior region of the neural fold, below the existing PAX7+cells.
Differentiation potential of posterior NC
Low- and high-WNT-induced NCCs display distinct differentiation potential. While posterior NCCs can be differentiated to sympathoadrenal derivatives, anterior NCCs die out under these conditions (not shown). Conversely, posterior NCCs die out in conditions that promote chondrocytes and adipocytes in anterior NC (not shown). Nonetheless, posterior NCCs produce osteoblasts and smooth muscle, derivatives normally ascribed to anterior NCCs (Gomez et al., 2019; Leung et al., 2016). Here, in agreement with Frith et al. (2018), we found that posterior NCCs express ETS1, previously thought to be restricted to anterior NCCs, and promote chondrocyte differentiation (Sugiura and Ito, 2010). Moreover, ETS1 has been linked to trunk melanocytes (Gao et al., 2010; Saldana-Caboverde et al., 2015; Ye et al., 2010). NC culture conditions for differentiation reveal a wider potential for NCCs in vitro than in in vivo, and thus inductive signals in vitro appear stronger than those present in vivo (Abzhanov et al., 2003; Dupin et al., 2018; McGonnell and Graham, 2002). It is therefore crucial to address minimal signal conditions in culture that effectively differentiate NCCs into their derivatives according to their axial identity.
Conclusions
Here, we present evidence implicating the WNT/β-catenin pathway as a prime instructor of the NC fate and their axial identity. In this context, BMP operates secondary to WNT signaling, FGF has a role in cell survival and/or proliferation, but not in NC fate acquisition. RA is not required either for NCC formation or for setting their thoracic axial identity, while early addition of exogenous RA derails NC formation. This study offers insight into the parameters that are relevant for human NC formation and sheds light on the inputs that produce NCCs with distinct anterior-posterior identity.
MATERIALS AND METHODS
Neural crest differentiation
Human pluripotent stem cell line H1 (WA01), Shef6 (hESC) and Yale6 (iPSC) were maintained in mTeSR1 (Stem Cell Technologies) on matrigel-coated dishes at 37°C in 5% CO2/5% O2 and passaged regularly with Dispase (Stem Cell Technologies) or Versene (Thermo Fisher Scientific). For NCC differentiation, hESCs were collected 4-5 days after last passage at 80% to 90% confluency. Cells were first rinsed three times in Ca2+- and Mg2+-free 1× PBS (Thermo Fisher Scientific), then dissociated in Accutase (Stem Cell Technologies) for 4 min 30 s at 37°C in 5% CO2/5% O2. Accutase was immediately quenched with warmed 1× DMEM/F12 (Invitrogen4) containing 10 µM Rock Inhibitor (Y-27632; Tocris) then cells were centrifuged at 280 g for 4 min at room temperature. 1× DMEM/F12 was discarded leaving cell pellet intact, then replaced with NC induction media [1× DMEM/F12 (Invitrogen), 1× serum-free B27 supplement (Invitrogen), 1× Glutamax (Thermo Fisher Scientific) and 0.5% BSA (Sigma-Aldrich, A7979)] containing 10 µM Y-27632. hESC clusters were further dissociated to single cells mechanically by trituration through a 5 ml serological pipette a total of 20 times. Cells were counted on a hemocytometer, diluted to an optimal seeding density of 20×103 cells/cm2 per culture vessel, then seeded on culture vessels pre-coated with Matrigel (BD Matrigel hESC-qualified Matrix). CHIR99021 (Tocris) was added to cells in induction media during trituration prior to seeding. Induction efficiency is sensitive to the CHIR99021 concentration CHIR used, which varies depending on the different batches of CHIR99021, resuspension, pipetting, etc. Optimal inductions obtained in low WNT can vary over a range of ∼2.5 µM to 3 µM, but we report all results at 3 µM for consistency. Rock Inhibitor (10 µM Y-27632) was added from days 0-2 in all cases and left out of induction media during the remainder of culture. Induction media was changed daily until the day of collection for further analysis. Cells were cultured at 37°C in 5% CO2/5% O2 throughout the procedure. The following small molecules were used where indicated: PD173074 (Santa Cruz Biotechnology); DMH1 (Tocris); retinoic acid (Sigma-Aldrich); AGN193109 (Santa Cruz Biotechnology); and human recombinant WNT3A (R&D systems).
Terminal differentiation of neural crest derivatives
All protocols were adapted and derived from the Studer lab (Sloan Kettering Institute, NY, USA), except chondrocyte differentiation. (Chambers et al., 2012; Lee et al., 2007; Mica et al., 2013). Multilineage differentiation was tested three times, successfully, with the same efficiency achieved across trials. For osteogenic differentiation, day 5 NCs were plated at 300 K/cm2 in osteogenic differentiation media (10 mM β-glycerol phosphate, 0.1 μM dexamethasone and 200 μM AA in α-MEM medium containing 10% FBS) with media changed every 2-3 days for 21 days when calcification was seen. Cells were stained with Von Kossa stain. For chondrogenic differentiation, Mesencult-Chondrogenic Differentiation media (Stem Cell Technologies) was used as per the supplier's protocol. Chondrocyte pellets at the end of the protocol were paraffin sectioned and stained with Alcian Blue. Adipogenic differentiation: Neural crest cells at day 5 were dissociated with Accutase and plated at 50 K/cm2 density in adipogenic media (1 mM dexamethasone, 10 μg/ml insulin and 0.5 mM IBMX in α-MEM medium containing 10% FBS) with media changes every 2-4 days for 21 days and then stained with Oil Red. For, peripheral neuron differentiation, on day 5 of NC induction, nociceptor induction was initiated with the addition of perineural media (3 μM CHIR99021, 10 μM SU5402 and 10 μM DAPT, in N2/DMEM/F12). Cells were harvested after 5 days to test for peripherin and β-tubulin. Glial cell differentiation: NC cells on day 5 were plated on to PO/laminin/fibronectin-coated wells in DMEM/F12/N2 medium supplemented with 10 ng/ml of FGF2 and 10 ng/ml of EGF for 14 days. After FGF2/EGF culture, cells are differentiated towards Glia in N2 medium supplemented with ciliary neurotrophic factor (10 ng/ml), neuregulin (20 ng/ml), FGF2 (10 ng/ml) and cyclic AMP (0.5 mM) for 21 days. Cells were stained with S100β and SOX10. For melanoblast differentiation, on day 5 of NC induction, melanocyte differentiation media was added (25 ng/ml BMP4 and 100 nM EDN3 in DMEM/F12/N2) for 5 days. Cells were further passed on to PO/lamini/fibronectin-coated wells in Mel Maturation media (Neurobasal/Mel Media, as described by Mica et al., 2013) for another three passages then stained for Sox10 and MITF. Sympathoadrenal precursor differentiation: day 5 NCs were dissociated using Accutase and re-plated in media containing DMEM/F12, 1× B27, 1× N2, 1× NEAA, 1× Glutamax, 50 ng/ml recombinant SHH and purmorphamine 1.25 µM according to Frith et al. (2018), and cells were immunostained after 4 days.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde, rinsed in PBS, permeabilized with 0.4% Triton X-100, blocked with 10% fetal bovine serum and incubated with primaries overnight. Alexa Fluor-conjugated secondary antibodies (Invitrogen) were used at 1:2000 dilution. Antibodies used were mouse anti-SOX10 (Santa Cruz Biotechnology, SC271163, 1:200); mouse anti-PAX7 (1:50), mouse anti-PAX6 (1:100) and rat anti-HOXB4 (1:200) (all Developmental Studies Hybridoma Bank); mouse anti-smooth muscle actin (Sigma-Aldrich, A2547, 1:500; mouse anti-peripherin (Santa Cruz Biotechnology, sc-377093, 1:200; mouse anti-β-tubulin (Santa Cruz Biotechnology, sc-365791, 1:300); mouse anti-ASCL1 (Santa Cruz Biotechnology, sc-374104, 1:100); mouse anti-HOXC9 (Santa Cruz Biotechnology, sc-81100, 1:500; mouse anti-GATA3 (Santa Cruz Biotechnology, sc-268, 1:100; Mouse anti-vimentin (Sigma-Millipore, MAB3400, 1:1000; rabbit anti-S100β (Abcam, ab52642, 1:200); goat anti-MITF (R&D systems, AF5769, 1:100); mouse anti-SOX2 (Santa Cruz Biotechnology, sc-365823, 1:200; and goat anti-TBXT (R&D, AF2085, 1:100).
Microscopy
Images were captured on a Nikon eclipse 80i microscope on a Spot SE camera and software, or on an inverted Nikon Eclipse Ti microscope with NIS Elements software. All images were compiled and adjusted in Adobe Photoshop CS5. No primary controls were used to establish baseline signal in immunofluorescence (IF) images.
Cell counts
Immunofluorescence images taken on the NIKON eclipse 80i were converted to nd2 format and counted on NIS-Elements AR Analysis software (version 4.6). Nuclei were counted by clustered method at 5px/spot, at a typical diameter of 10 µM, with variable contrast levels and dark objects removed.
RT-qPCR
Total RNA was collected in TRIzol (Thermo Fisher Scientific), cDNA was prepared with Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and RT-qPCR was performed with SYBR Premix Ex Taq II (Clontech) on an Applied Biosystems Step One Plus system. Fold-changes were evaluated using the ΔΔ-Ct method and plotted on Excel or Graphpad prism. qPCR primers used listed in Table S1.
Statistics
Statistical analysis of cell counts and RT-qPCR was performed on Prism7.0 (GraphPad Software). All experiments were performed with a sample size of n=3 independent cultures, immunofluorescence images are representative of one of these. RT-qPCR was performed from separate wells and three technical replicates were assessed for each of three independent cultures. In graphs where data were normalized to a control reference condition, unpaired Student's t-tests reflect comparisons of fold-change values calculated by the ΔΔ-Ct method relative to ESCs and standardized to housekeeping genes.
siRNA-mediated knockdown
siRNA-mediated knockdown was performed using β-catenin siRNA (Thermo Fisher Scientific) and non-targeting control siRNA (Thermo Fisher Scientific) transfected with Lipofectamine RNAiMAX (Thermo Fisher Scientific). Reverse transfection was performed as per the manufacturer's instructions. Briefly, siRNA/RNAiMAX mixture was pre-plated into the 96-well plate in technical replicates for each biological replicate. Human ES cells were plated in neural crest induction media as described above into each of the wells containing siRNA/RNAiMAX mixture. For each well, 1.2 pmoles of siRNA was used with 0.1 ml of lipofectamine RNAiMAX reagent in total 20 ml of OptiMEM medium. Medium was changed every 24 h and cells were harvested at day 5 for immunofluorescence.
Supplementary Material
Acknowledgements
We thank the University of California Riverside Stem Cell Core for the use of the Nikon eclipse Ti microscope. We also thank the reviewers for their insightful comments and feedback.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: G.A.G., M.I.G.-C.; Methodology: G.A.G., M.S.P., A.W.L.; Validation: M.W., J.C.H.; Formal analysis: G.A.G., M.I.G.-C.; Investigation: G.A.G., M.S.P., M.W., R.M.C., P.B.S., N.S., J.O.S.H., J.C.H., M.I.G.-C.; Resources: M.I.G.-C.; Data curation: G.A.G., M.S.P., M.W., R.M.C., P.B.S., N.S., J.O.S.H., J.C.H., M.I.G.-C.; Writing - original draft: G.A.G.; Writing - review & editing: G.A.G., A.W.L., M.I.G.-C.; Supervision: M.G.; Project administration: M.I.G.-C.; Funding acquisition: M.I.G.-C.
Funding
This research was supported by funding from the National Institutes of Health National Institute of Dental and Craniofacial Research (5R01DE017914 and F32DE027862 to R.M.C). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.175604.supplemental
References
- Abzhanov A., Tzahor E., Lassar A. B. and Tabin C. J. (2003). Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 130, 4567-4579. 10.1242/dev.00673 [DOI] [PubMed] [Google Scholar]
- Acebron S. P., Karaulanov E., Berger B. S., Huang Y.-L. and Niehrs C. (2014). Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell 54, 663-674. 10.1016/j.molcel.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Aström A., Pettersson U. and Voorhees J. J. (1992). Structure of the human cellular retinoic acid-binding protein II gene. Early transcriptional regulation by retinoic acid. J. Biol. Chem. 267, 25251-25255. [PubMed] [Google Scholar]
- Bajpai R., Chen D. A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C.-P., Zhao Y., Swigut T. and Wysocka J. (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463, 958-962. 10.1038/nature08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Magge S. and Winkler S. (2003). Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J. Neurosci. Res. 74, 486-493. 10.1002/jnr.10773 [DOI] [PubMed] [Google Scholar]
- Betters E., Liu Y., Kjaeldgaard A., Sundström E. and García-Castro M. I. (2010). Analysis of early human neural crest development. Dev. Biol. 344, 578-592. 10.1016/j.ydbio.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betters E., Charney R. M. and García-Castro M. I. (2018). Early specification and development of rabbit neural crest cells. Dev. Biol. 444 Suppl. 1, S181-S192. 10.1016/j.ydbio.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokhman I., Lina G.-Z., Oz P., Aharonowiz M., Reubbinoff B. E. and Goldstein R. S. (2008). Peripheral sensory neurons differentiate from neural precursors derived from human embryonic stem cells. Differentiation 76, 145-155. 10.1111/j.1432-0436.2007.00196.x [DOI] [PubMed] [Google Scholar]
- Cambray N. and Wilson V. (2007). Two distinct sources for a population of maturing axial progenitors. Development 134, 2829-2840. 10.1242/dev.02877 [DOI] [PubMed] [Google Scholar]
- Chambers S. M., Qi Y., Mica Y., Lee G., Zhang X.-J., Niu L., Bilsland J., Cao L., Stevens E., Whiting P. et al. (2012). Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 30, 715-720. 10.1038/nbt.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Brade T., Sandell L. L., Lewandoski M., Trainor P. A., Colas A., Mercola M. and Duester G. (2015). Retinoic acid activity in undifferentiated neural progenitors is sufficient to fulfill its role in restricting Fgf8 expression for somitogenesis. PLoS ONE 10, e0137894 10.1371/journal.pone.0137894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curchoe C. L., Maurer J., McKeown S. J., Cattarossi G., Cimadamore F., Nilbratt M., Snyder E. Y., Bronner-Fraser M. and Terskikh A. V. (2010). Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE 5, e13890 10.1371/journal.pone.0013890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jaime-Soguero A., de Oliveira W. A. and Lluis F. (2018). The pleiotropic effects of the canonical Wnt pathway in early development and pluripotency. Genes 9, 93 10.3390/genes9020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R., Olivera-Martinez I., Goriely A., Gale E., Maden M. and Storey K. (2003). Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40, 65-79. 10.1016/S0896-6273(03)00565-8 [DOI] [PubMed] [Google Scholar]
- Dupé V. and Pellerin I. (2009). Retinoic acid receptors exhibit cell-autonomous functions in cranial neural crest cells. Dev. Dyn. 238, 2701-2711. 10.1002/dvdy.22087 [DOI] [PubMed] [Google Scholar]
- Dupin E., Calloni G. W., Coelho-Aguiar J. M. and Le Douarin N. M. (2018). The issue of the multipotency of the neural crest cells. Dev. Biol. 444 Suppl. 1, S47-S59. 10.1016/j.ydbio.2018.03.024 [DOI] [PubMed] [Google Scholar]
- Ekerot M., Stavridis M. P., Delavaine L., Mitchell M. P., Staples C., Owens D. M., Keenan I. D., Dickinson R. J., Storey K. G. and Keyse S. M. (2008). Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem. J. 412, 287-298. 10.1042/BJ20071512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg N., Kahn M., Petersen D. R., Hansson M. and Serup P. (2010). Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells 28, 1498-1509. 10.1002/stem.479 [DOI] [PubMed] [Google Scholar]
- Epstein J. A., Lam P., Jepeal L., Maas R. L. and Shapiro D. N. (1995). Pax3 inhibits myogenic differentiation of cultured myoblast cells. J. Biol. Chem. 270, 11719-11722. 10.1074/jbc.270.20.11719 [DOI] [PubMed] [Google Scholar]
- Fattahi F., Steinbeck J. A., Kriks S., Tchieu J., Zimmer B., Kishinevsky S., Zeltner N., Mica Y., El-Nachef W., Zhao H. et al. (2016). Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105-109. 10.1038/nature16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat N., Jones V., Khoo P.-L., Bogani D., Hardy A., Steiner K., Mukhopadhyay M., Westphal H., Nolan P. M., Arkell R. et al. (2011). Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development 138, 667-676. 10.1242/dev.052803 [DOI] [PubMed] [Google Scholar]
- Frith T. J., Granata I., Wind M., Stout E., Thompson O., Neumann K., Stavish D., Heath P. R., Ortmann D., Hackland J. O. et al. (2018). Human axial progenitors generate trunk neural crest cells in vitro. eLife 7, e35786 10.7554/eLife.35786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta M., Nakai Y., Kirino K., Nakagawa M., Sekiguchi K., Nagata S., Matsumoto Y., Yamamoto T., Umeda K., Heike T. et al. (2014). Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 9, e112291 10.1371/journal.pone.0112291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funa N. S., Schachter K. A., Lerdrup M., Ekberg J., Hess K., Dietrich N., Honoré C., Hansen K. and Semb H. (2015). β-Catenin regulates primitive streak induction through collaborative interactions with SMAD2/SMAD3 and OCT4. Cell Stem Cell 16, 639-652. 10.1016/j.stem.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Gao Z., Kim G. H., Mackinnon A. C., Flagg A. E., Bassett B., Earley J. U. and Svensson E. C. (2010). Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development 137, 1543-1551. 10.1242/dev.047696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Castro M. I., Marcelle C. and Bronner-Fraser M. (2002). Ectodermal Wnt function as a neural crest inducer. Science 297, 848-851. [DOI] [PubMed] [Google Scholar]
- Goentoro L. and Kirschner M. W. (2009). Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol. Cell 36, 872-884. 10.1016/j.molcel.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G. A., Prasad M. S., Sandhu N., Shelar P. B., Leung A. W. and García-Castro M. I. (2019). Human neural crest induction by temporal modulation of WNT activation. Dev. Biol. 449, 99-106. 10.1016/j.ydbio.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M., Tsakiridis A., Wymeersch F. J., Huang Y., Kleinjung J., Wilson V. and Briscoe J. (2014). In vitro generation of neuromesodermal progenitors reveals distinct roles for Wnt Signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12, e1001937 10.1371/journal.pbio.1001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T. L., Takada S., Newhouse M. M., McMahon J. A., McMahon A. P. and Camper S. A. (1996). Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 10, 313-324. 10.1101/gad.10.3.313 [DOI] [PubMed] [Google Scholar]
- Hackland J. O. S., Frith T. J. R., Thompson O., Marin Navarro A., García-Castro M. I., Unger C. and Andrews P. W. (2017). Top-down inhibition of BMP signaling enables robust induction of hPSCs into neural crest in fully defined, Xeno-free conditions. Stem Cell Reports 9, 1043-1052. 10.1016/j.stemcr.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackland J. O. S., Shelar P. B., Sandhu N., Prasad M. S., Charney R. M., Gomez G. A., Frith T. J. R. and García-Castro M. I. (2019). FGF modulates the axial identity of trunk hPSC-derived neural crest but not the cranial-trunk decision. Stem Cell Reports 12, 920-933. 10.1016/j.stemcr.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Ho J. N., Lewis J. A., Karim K. A., Daniels R. N., Gentry P. R., Hopkins C. R., Lindsley C. W. and Hong C. C. (2010). In vivostructure−activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem. Biol. 5, 245-253. 10.1021/cb9002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Houzelstein D., Dunwoodie S. L. and Beddington R. S. (2000). Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev. Biol. 227, 358-372. 10.1006/dbio.2000.9878 [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford A., Goldstone K., Garner-Hamrick P., Gumbiner B., McCrea P., Kintner C., Noro C. Y. and Wylie C. (1994). Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79, 791-803. 10.1016/0092-8674(94)90069-8 [DOI] [PubMed] [Google Scholar]
- Henrique D., Abranches E., Verrier L. and Storey K. G. (2015). Neuromesodermal progenitors and the making of the spinal cord. Development 142, 2864-2875. 10.1242/dev.119768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C.-S., Park B.-Y. and Saint-Jeannet J.-P. (2008). Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 135, 3903-3910. 10.1242/dev.026229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Miller M. L., McHenry L. K., Zheng T., Zhen Q., Ilkhanizadeh S., Conklin B. R., Bronner M. E. and Weiss W. A. (2016). Generating trunk neural crest from human pluripotent stem cells. Sci. Rep. 6, 19727 10.1038/srep19727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M. and Takada S. (2001). Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech. Dev. 103, 27-33. 10.1016/S0925-4773(01)00338-0 [DOI] [PubMed] [Google Scholar]
- Jho E.-H., Zhang T., Domon C., Joo C.-K., Freund J.-N. and Costantini F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172-1183. 10.1128/MCB.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Gwye Y., McKeown S. J., Bronner-Fraser M., Lutzko C. and Lawlor E. R. (2009). Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 18, 1059-1071. 10.1089/scd.2008.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudyakov J. and Bronner-Fraser M. (2009). Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev. Dyn. 238, 716-723. 10.1002/dvdy.21881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C. and Niehrs C. (2001). A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189-4201. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Oda T., Itoh M., Jiang D., Artinger K. B., Chandrasekharappa S. C., Driever W. and Chitnis A. B. (2000). Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913-916. 10.1038/35038097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T., Wilson S. W. and Dawid I. B. (2002). Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 129, 4335-4346. 10.3410/f.1008982.116157 [DOI] [PubMed] [Google Scholar]
- Kulesa P. M. and Fraser S. E. (1998). Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev. Biol. 204, 327-344. 10.1006/dbio.1998.9082 [DOI] [PubMed] [Google Scholar]
- LaBonne C. and Bronner-Fraser M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403-2414. [DOI] [PubMed] [Google Scholar]
- Lee E., Salic A., Krüger R., Heinrich R. and Kirschner M. W. (2003). The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 1, E10 10.1371/journal.pbio.0000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V. and Studer L. (2007). Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 25, 1468-1475. 10.1038/nbt1365 [DOI] [PubMed] [Google Scholar]
- Lemaire P., Garrett N. and Gurdon J. B. (1995). Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81, 85-94. 10.1016/0092-8674(95)90373-9 [DOI] [PubMed] [Google Scholar]
- Leung A. W., Murdoch B., Salem A. F., Prasad M. S., Gomez G. A. and García-Castro M. I. (2016). WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 143, 398-410. 10.1242/dev.130849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. L., Bonner J., Modrell M., Ragland J. W., Moon R. T., Dorsky R. I. and Raible D. W. (2004). Reiterated Wnt signaling during zebrafish neural crest development. Development 131, 1299-1308. 10.1242/dev.01007 [DOI] [PubMed] [Google Scholar]
- Liem K. F., Tremml G. and Jessell T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127-138. 10.1016/S0092-8674(01)80015-5 [DOI] [PubMed] [Google Scholar]
- Liu T., Lee Y.-N., Malbon C. C. and Wang H.-Y. (2002). Activation of the beta-catenin/Lef-Tcf pathway is obligate for formation of primitive endoderm by mouse F9 totipotent teratocarcinoma cells in response to retinoic acid. J. Biol. Chem. 277, 30887-30891. 10.1074/jbc.M203852200 [DOI] [PubMed] [Google Scholar]
- Liu Q., Spusta S. C., Mi R., Lassiter R. N. T., Stark M. R., Höke A., Rao M. S. and Zeng X. (2012). Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl. Med. 1, 266-278. 10.5966/sctm.2011-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K. M., van Amerongen R. and Nusse R. (2016). Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev. Cell 38, 643-655. 10.1016/j.devcel.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Loudig O., Babichuk C., White J., Abu-Abed S., Mueller C. and Petkovich M. (2000). Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol. Endocrinol. 14, 1483-1497. 10.1210/mend.14.9.0518 [DOI] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M. and Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304. 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Morales P. L., del Corral R. D., Olivera-Martinez I., Quiroga A. C., Das R. M., Barbas J. A., Storey K. G. and Morales A. V. (2011). FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J. Cell Biol. 194, 489-503. 10.1083/jcb.201011077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T. and Ishitani T. (2017). Context-dependent regulation of the β-catenin transcriptional complex supports diverse functions of Wnt/β-catenin signaling. J. Biochem. 161, 9-17. 10.1093/jb/mvw072 [DOI] [PubMed] [Google Scholar]
- McGonnell I. M. and Graham A. (2002). Trunk neural crest has skeletogenic potential. Curr. Biol. 12, 767-771. 10.1016/S0960-9822(02)00818-7 [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Lai C. J. and Moon R. T. (1995). Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev. Biol. 172, 337-342. 10.1006/dbio.1995.0027 [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Hoppler S. and Moon R. T. (1997). Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech. Dev. 69, 105-114. 10.1016/S0925-4773(97)00160-3 [DOI] [PubMed] [Google Scholar]
- McGrew M. J., Sherman A., Lillico S. G., Ellard F. M., Radcliffe P. A., Gilhooley H. J., Mitrophanous K. A., Cambray N., Wilson V. and Sang H. (2008). Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development 135, 2289-2299. 10.1242/dev.022020 [DOI] [PubMed] [Google Scholar]
- McMahon A. P. and Moon R. T. (1989). Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58, 1075-1084. 10.1016/0092-8674(89)90506-0 [DOI] [PubMed] [Google Scholar]
- Menendez L., Yatskievych T. A., Antin P. B. and Dalton S. (2011). Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 108, 19240-19245. 10.1073/pnas.1113746108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mica Y., Lee G., Chambers S. M., Tomishima M. J. and Studer L. (2013). Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. CellReports 3, 1140-1152. 10.1016/j.celrep.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelidis T. M. and Lie D. C. (2008). Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res. 331, 193-210. 10.1007/s00441-007-0476-5 [DOI] [PubMed] [Google Scholar]
- Minowada G., Jarvis L. A., Chi C. L., Neubüser A., Sun X., Hacohen N., Krasnow M. A. and Martin G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Morkel M., Huelsken J., Wakamiya M., Ding J., van de Wetering M., Clevers H., Taketo M. M., Behringer R. R., Shen M. M. and Birchmeier W. (2003). Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development 130, 6283-6294. 10.1242/dev.00859 [DOI] [PubMed] [Google Scholar]
- Moro E., Ozhan-Kizil G., Mongera A., Beis D., Wierzbicki C., Young R. M., Bournele D., Domenichini A., Valdivia L. E., Lum L. et al. (2012). In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 366, 327-340. 10.1016/j.ydbio.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Nelson W. J. and Nusse R. (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483-1487. 10.1126/science.1094291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Schuhbaur B., Chambon P. and Dollé P. (2000). Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 127, 75-85. [DOI] [PubMed] [Google Scholar]
- Nordström U., Jessell T. M. and Edlund T. (2002). Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525-532. 10.1038/nn0602-854 [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I. and Storey K. G. (2007). Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development 134, 2125-2135. 10.1242/dev.000216 [DOI] [PubMed] [Google Scholar]
- O'Rahilly R. and Müller F. (2007). The development of the neural crest in the human. J. Anat. 211, 335-351. 10.1111/j.1469-7580.2007.00773.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.-S., Seo J.-H., Hong M., Bang W., Han J.-K. and Choi S.-C. (2013). Role of Sp5 as an essential early regulator of neural crest specification in xenopus. Dev. Dyn. 242, 1382-1394. 10.1002/dvdy.24034 [DOI] [PubMed] [Google Scholar]
- Patthey C. and Gunhaga L. (2014). Signaling pathways regulating ectodermal cell fate choices. Exp. Cell Res. 321, 11-16. 10.1016/j.yexcr.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Patthey C., Edlund T. and Gunhaga L. (2009). Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 136, 73-83. 10.1242/dev.025890 [DOI] [PubMed] [Google Scholar]
- Pla P. and Monsoro-Burq A. H. (2018). The neural border: induction, specification and maturation of the territory that generates neural crest cells. Dev. Biol. 444 Suppl. 1, S36-S46. 10.1016/j.ydbio.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Plouhinec J.-L., Roche D. D., Pegoraro C., Figueiredo A. L., Maczkowiak F., Brunet L. J., Milet C., Vert J.-P., Pollet N., Harland R. M. et al. (2014). Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Dev. Biol. 386, 461-472. 10.1016/j.ydbio.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp O., Brokhman I., Ben-Dor I., Reubinoff B. and Goldstein R. S. (2005). Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells 23, 923-930. 10.1634/stemcells.2005-0038 [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Prescott S., Brugmann S. A., Swigut T. and Wysocka J. (2012). Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11, 633-648. 10.1016/j.stem.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring D. B., Johnson K. W., Henriksen E. J., Nuss J. M., Goff D., Kinnick T. R., Ma S. T., Reeder J. W., Samuels I., Slabiak T. et al. (2003). Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes 52, 588-595. 10.2337/diabetes.52.3.588 [DOI] [PubMed] [Google Scholar]
- Rogers K. W. and Schier A. F. (2011). Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377-407. 10.1146/annurev-cellbio-092910-154148 [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J.-P., He X., Varmus H. E. and Dawid I. B. (1997). Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. USA 94, 13713-13718. 10.1073/pnas.94.25.13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. and Wakamatsu Y. (2005). Regulatory mechanisms for neural crest formation. Cells, tissues, organs 179, 24-35. 10.1159/000084506 [DOI] [PubMed] [Google Scholar]
- Saldana-Caboverde A., Perera E. M., Watkins-Chow D. E., Hansen N. F., Vemulapalli M., Mullikin J. C., Program N. C. S., Pavan W. J. and Kos L. (2015). The transcription factors Ets1 and Sox10 interact during murine melanocyte development. Dev. Biol. 407, 300-312. 10.1016/j.ydbio.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ferras O., Coutaud B., Djavanbakht Samani T., Tremblay I., Souchkova O. and Pilon N. (2012). Caudal-related homeobox (Cdx) protein-dependent integration of canonical Wnt signaling on paired-box 3 (Pax3) neural crest enhancer. J. Biol. Chem. 287, 16623-16635. 10.1074/jbc.M112.356394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ferras O., Bernas G., Farnos O., Touré A. M., Souchkova O. and Pilon N. (2016). A direct role for murine Cdx proteins in the trunk neural crest gene regulatory network. Development 143, 1363-1374. 10.1242/dev.132159 [DOI] [PubMed] [Google Scholar]
- Selleck M. A., García-Castro M. I., Artinger K. B. and Bronner-Fraser M. (1998). Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development 125, 4919-4930. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Bae Y.-K., Muraoka O. and Hibi M. (2005). Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 279, 125-141. 10.1016/j.ydbio.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Smith W. C. and Harland R. M. (1991). Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67, 753-765. 10.1016/0092-8674(91)90070-F [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T. and Melton D. A. (1991). Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67, 741-752. 10.1016/0092-8674(91)90069-B [DOI] [PubMed] [Google Scholar]
- Sparks N. R. L., Martinez I. K. C., Soto C. H. and Zur Nieden N. I. (2018). Low osteogenic yield in human pluripotent stem cells associates with differential neural crest promoter methylation. Stem Cells 36, 349-362. 10.1002/stem.2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavridis M. P., Collins B. J. and Storey K. G. (2010). Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development 137, 881-890. 10.1242/dev.043117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z. and Angers S. (2018). Wnt signaling in development and tissue homeostasis. Development 145, dev146589 10.1242/dev.146589 [DOI] [PubMed] [Google Scholar]
- Stuhlmiller T. J. and García-Castro M. I. (2012a). Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 69, 3715-3737. 10.1007/s00018-012-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller T. J. and García-Castro M. I. (2012b). FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development 139, 289-300. 10.1242/dev.070276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K. and Ito K. (2010). Roles of Ets-1 and p70S6 kinase in chondrogenic and gliogenic specification of mouse mesencephalic neural crest cells. Mech. Dev. 127, 169-182. 10.1016/j.mod.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Taelman V. F., Dobrowolski R., Plouhinec J.-L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D. and De Robertis E. M. (2010). Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136-1148. 10.1016/j.cell.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. W., Gardiner B. S., Hirokawa Y., Layton M. J., Smith D. W. and Burgess A. W. (2012). Wnt signalling pathway parameters for mammalian cells. PLoS ONE 7, e31882 10.1371/journal.pone.0031882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice D. A., Szeto W., Soloviev I., Rubinfeld B., Fong S. E., Dugger D. L., Winer J., Williams P. M., Wieand D., Smith V. et al. (2002). Synergistic induction of tumor antigens by Wnt-1 signaling and retinoic acid revealed by gene expression profiling. J. Biol. Chem. 277, 14329-14335. 10.1074/jbc.M200334200 [DOI] [PubMed] [Google Scholar]
- Turner D. A., Hayward P. C., Baillie-Johnson P., Rué P., Broome R., Faunes F. and Arias A. M. (2014). Wnt/β-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141, 4243-4253. 10.1242/dev.112979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasurian Easwaran M. P. S. and Byers S. (1999). Cross-regulation of β-catenin–LEF/TCF and retinoid signaling pathways. Curr. Biol. 9, 1415-1419. 10.1016/S0960-9822(00)80088-3 [DOI] [PubMed] [Google Scholar]
- Villanueva S., Glavic A., Ruiz P. and Mayor R. (2002). Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 241, 289-301. 10.1006/dbio.2001.0485 [DOI] [PubMed] [Google Scholar]
- Wagner F. F., Bishop J. A., Gale J. P., Shi X., Walk M., Ketterman J., Patnaik D., Barker D., Walpita D., Campbell A. J. et al. (2016). Inhibitors of glycogen synthase kinase 3 with exquisite kinome-wide selectivity and their functional effects. ACS Chem. Biol. 11, 1952-1963. 10.1021/acschembio.6b00306 [DOI] [PubMed] [Google Scholar]
- Wymeersch F. J., Huang Y., Blin G., Cambray N., Wilkie R., Wong F. C. K. and Wilson V. (2016). Position-dependent plasticity of distinct progenitor types in the primitive streak. eLife 5, e10042 10.7554/eLife.10042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. P., Takada S., Yoshikawa Y., Wu N. and McMahon A. P. (1999). T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 13, 3185-3190. 10.1101/gad.13.24.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley N. and García-Castro M. I. (2012). FGF signaling transforms non-neural ectoderm into neural crest. Dev. Biol. 372, 166-177. 10.1016/j.ydbio.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Coldren C., Liang X., Mattina T., Goldmuntz E., Benson D. W., Ivy D., Perryman M. B., Garrett-Sinha L. A. and Grossfeld P. (2010). Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum. Mol. Genet. 19, 648-656. 10.1093/hmg/ddp532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang C. T., Chen J., Pankratz M. T., Xi J., Li J., Yang Y., Lavaute T. M., Li X.-J., Ayala M. et al. (2010). Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90-100. 10.1016/j.stem.2010.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.