ABSTRACT
Ras proteins are small GTPases localized to the plasma membrane (PM), which regulate cellular proliferation, apoptosis and differentiation. After a series of post-translational modifications, H-Ras and N-Ras traffic to the PM from the Golgi via the classical exocytic pathway, but the exact mechanism of K-Ras trafficking to the PM from the ER is not fully characterized. ATP5G1 (also known as ATP5MC1) is one of the three proteins that comprise subunit c of the F0 complex of the mitochondrial ATP synthase. In this study, we show that overexpression of the mitochondrial targeting sequence of ATP5G1 perturbs glucose metabolism, inhibits oncogenic K-Ras signaling, and redistributes phosphatidylserine (PtdSer) to mitochondria and other endomembranes, resulting in K-Ras translocation to mitochondria. Also, it depletes phosphatidylinositol 4-phosphate (PI4P) at the Golgi. Glucose supplementation restores PtdSer and K-Ras PM localization and PI4P at the Golgi. We further show that inhibition of the Golgi-localized PI4-kinases (PI4Ks) translocates K-Ras, and PtdSer to mitochondria and endomembranes, respectively. We conclude that PI4P at the Golgi regulates the PM localization of PtdSer and K-Ras.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: K-Ras, Phosphatidylserine, Phosphatidylinositol 4-phosphate, Phosphatidylinositol 4-kinase, Golgi, Mitochondria
Summary: Overexpression of the mitochondrial targeting sequence of ATP5G1 perturbs glucose metabolism, and translocates K-Ras to mitochondria and phosphatidylserine to endomembranes, through regulation of PI4P content at the Golgi.
INTRODUCTION
Ras proteins are small GTPases that operate like molecular switches for cell proliferation, migration and apoptosis (Hancock, 2003). The three ubiquitously expressed Ras isoforms in mammalian cells, K-Ras, N-Ras and H-Ras, are highly homologous in sequence except for the C-terminal 20 amino acid residues, called the hypervariable region (HVR). Post-translational modifications occur at the HVR and are important for Ras trafficking to and interacting with the plasma membrane (PM), where the Ras family activates its downstream effectors (Hancock et al., 1989, 1990). Newly synthesized Ras proteins are prenylated by farnesyltransferase (FTase), which allows Ras to attach to the cytosolic leaflet of the ER (Gutierrez et al., 1989; Hancock et al., 1989). RAS converting CAAX endopeptidase 1 (RCE1) then removes the AAX tripeptide, followed by the methylation of the now C-terminal prenylated Cys by isoprenylcysteine carboxyl methyltransferase (ICMT) (Clarke et al., 1988; Gutierrez et al., 1989). N-Ras, H-Ras and K-Ras4A, the alternative K-Ras splicing variant, are further modified with the addition of palmitic acids on one or two other Cys residues located in the HVR (Hancock et al., 1989), allowing Ras to interact with and localize to the PM. K-Ras4B (hereafter, K-Ras) is unique in that it has a single farnesyl chain preceded by a polybasic domain of six Lys residues (Hancock et al., 1990). The strong positive charge of this polybasic domain allows K-Ras to interact with anionic phospholipids in the PM through electrostatic interaction. Depletion of phosphatidylserine (PtdSer) from the inner PM leaflet or acute neutralization of PM electrostatic potential by Ca2+ influx results in rapid dissociation of K-Ras from the PM (Cho et al., 2016, 2012; Yeung et al., 2008). Furthermore, K-Ras–PM interaction can be regulated through K-Ras phosphorylation, with phosphorylation at residue Ser181 by protein kinase C or protein kinase G causing mislocalization of K-Ras from the PM to endomembranes, including mitochondria (Bivona et al., 2006; Cho et al., 2016).
Both H-Ras and N-Ras are palmitoylated at the Golgi by a palmitoylacyltransferase (Swarthout et al., 2005), where they are trafficked to the PM through the classical secretory pathway (Apolloni et al., 2000; Choy et al., 1999). H-Ras and N-Ras proteins undergo a cycle of palmitoylation and depalmitoylation, which allows them to cycle between endomembrane and PM (Lin and Conibear, 2015; Rocks et al., 2010, 2005). While the mechanism for K-Ras trafficking from the ER to the PM is not fully elucidated, previous studies have implicated microtubules and possibly mitochondria as having a role (Chen et al., 2000; Thissen et al., 1997; Wang and Deschenes, 2006). K-Ras PM maintenance requires the chaperone protein phosphodiesterase δ (PDEδ, also known as PDE6D). Cytosolic PDEδ binds K-Ras endocytosed from the PM and releases K-Ras to perinuclear membranes in an Arl2-dependent manner. K-Ras then electrostatically interacts with the recycling endosome (RE) and returns to the PM (Chandra et al., 2012; Schmick et al., 2014).
Mitochondrial ATP synthase is a multimeric protein consisting of two linked complexes, F0 and F1 (Jonckheere et al., 2012). Each ATP synthase molecule is anchored to the inner mitochondrial membrane through the F0 complex, with the catalytic F1 core extending out into the mitochondrial matrix (Jonckheere et al., 2012). Protons from the intermembrane space funnel through the proton channel of the F0 complex, which causes the c-subunit oligomer ring to rotate. This rotation confers conformational changes to the structure of F1 that results in the conversion of ADP+Pi to ATP (Boyer, 2000). In mammals, subunit c is encoded by three different genes (ATP5MC1, ATP5MC2, ATP5MC3) yielding three protein isoforms, which differ only in their mitochondrial targeting peptide sequences (Dyer et al., 1989; Dyer and Walker, 1993; Yan et al., 1994). The mitochondrial targeting sequence of one of these isoforms, ATP5G1 (also known as ATP5MC1), is used as a mitochondrial marker (Guy et al., 2002; Vives-Bauza et al., 2010). In this study, we discovered that overexpression of the mitochondrial targeting sequence of ATP5G1 translocates K-Ras and PtdSer to mitochondria and endomembranes, respectively, by mechanisms regulating phosphatidylinositol 4-phosphate (PI4P) contents at the Golgi.
RESULTS
Overexpression of ATP5G1(1–67) translocates K-Ras to mitochondria
Recently, we identified several classes of compounds that mislocalize K-Ras from the PM to endomembranes (Cho et al., 2016; Cho et al., 2012; Salim et al., 2014a,b, 2015; Tan et al., 2018; van der Hoeven et al., 2013, 2017). In yeast, deletion of class C VPS genes results in mitochondrial defects and an accumulation of Ras2 on mitochondrial membranes (Wang and Deschenes, 2006). In mammalian cells, K-Ras translocates to mitochondria through PKC-mediated phosphorylation at Ser181, inducing Bcl-XL (also known as BCL2L1)-dependent apoptosis (Bivona et al., 2006). These studies suggest that mitochondria are involved in K-Ras trafficking and signaling once K-Ras is mislocalized from the PM. To characterize whether the identified compounds translocate K-Ras to mitochondria, MDCK cells stably expressing a monomeric GFP (mGFP)-tagged K-RasG12V were infected with lentivirus expressing a mitochondrial marker, RFP-tagged ATP5G1 (amino acid residues 1–67). ATP5G1 is mitochondrial F0 complex subunit C1 in ATP synthase, and its mitochondrial targeting sequence (amino acid residues 1–61) followed by the first six amino acids of the catalytic domain (amino acid residues 62–67) is used as a mitochondrial marker (Guy et al., 2002; Higuti et al., 1993; Oca-Cossio et al., 2003; Vives-Bauza et al., 2010).
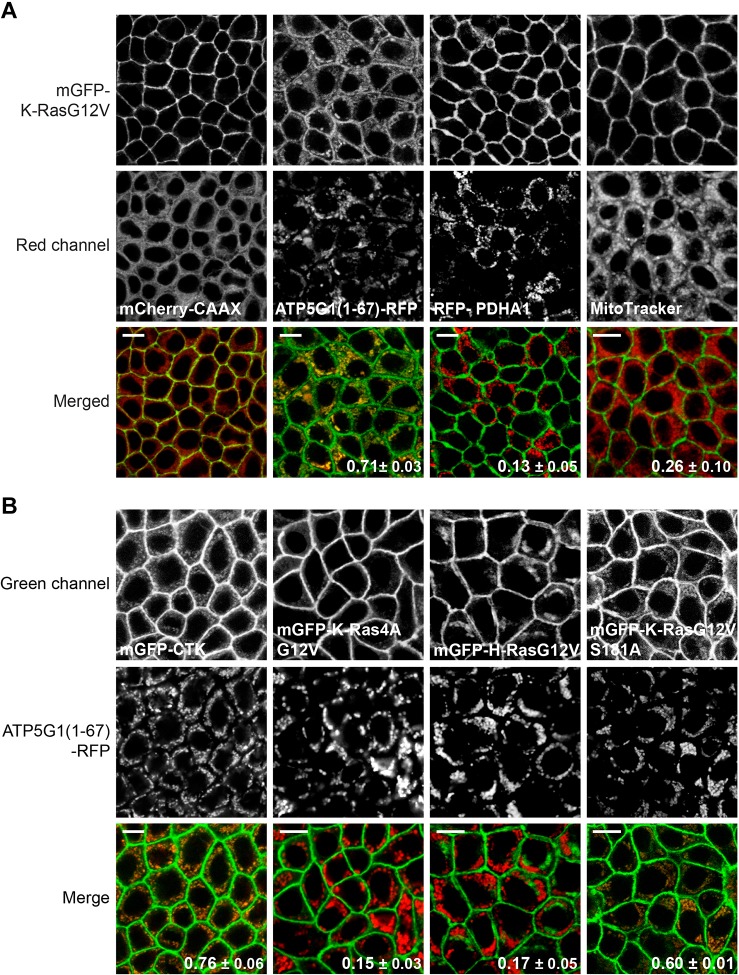
When ATP5G1(1–67)–RFP was overexpressed, mGFP–K-RasG12V unexpectedly colocalized with ATP5G1(1–67)–RFP, suggesting that K-RasG12V is translocated from the PM to mitochondria (Fig. 1A). The same observation was made in baby hamster kidney cells, suggesting it is not a cell type-specific effect (Fig. S1). In control MDCK cells co-expressing mGFP–K-RasG12V and mCherry–CAAX, an endomembrane marker (Cho et al., 2012; Choy et al., 1999), K-RasG12V is localized to the PM (Fig. 1A). Furthermore, incubating MDCK cells stably expressing mGFP–K-RasG12V with MitoTracker or baculovirus expressing the RFP-fused leader sequence of mitochondrial protein pyruvate dehydrogenase E1 α1 subunit (PDHA1) (Sutendra et al., 2014) did not disrupt K-RasG12V PM localization (Fig. 1A). To quantitate the extent of colocalization of mGFP–K-RasG12V with mitochondrial markers, we used Manders’ coefficient, which provides an estimate of the fraction of mitochondrial markers colocalized with mGFP–K-RasG12V. Manders’ coefficient for ATP5G1(1–67) showed a higher value than other mitochondrial markers (Fig. 1A, bottom right of merge images). Taken together with the confocal imaging, our data suggest that overexpression of ATP5G1(1–67) translocates K-RasG12V to mitochondria.
Fig. 1.
Overexpression of ATP5G1(1–67) translocates K-Ras to mitochondria. (A) To evaluate expression of mitochondrial markers, MDCK cells stably expressing mGFP–K-RasG12V were infected with lentivirus expressing ATP5G1(1–67)–RFP, incubated with modified baculovirus encoding RFP-tagged PDHA1 for 16 h, or stained with 100 nM MitoTracker Deep Red for 1 h. Cells were fixed with 4% PFA and imaged using a confocal microscope. As a control, MDCK cells stably co-expressing mGFP–K-RasG12V with endomembrane marker mCherry–CAAX were used. Values bottom right of merge panels represent mean±s.e.m. of the fraction of mitochondrial markers colocalized with mGFP–K-RasG12V calculated by Manders' coefficient from three independent experiments. Scale bars: 10 µm. (B) MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP-tagged CTK, K-Ras4AG12V, H-RasG12V or K-RasG12V S181A were fixed with 4% PFA and imaged using a confocal microscope. Inserted values represent mean±s.e.m. of the fraction of ATP5G1(1–67)–RFP colocalized with mGFP-tagged Ras isoforms calculated by Manders' coefficient from three independent experiments. Scale bars: 10 µm. All cells were maintained in complete growth medium (DMEM+10% FBS+2 mM L-glutamine).
To further characterize the mechanism of ATP5G1(1–67)-induced K-Ras mislocalization, we examined the localization of other Ras isoforms and the C-terminal hypervariable region of K-Ras (CTK). ATP5G1(1–67)–RFP was overexpressed in MDCK cells stably expressing mGFP–CTK, mGFP–K-Ras4AG12V, the alternate K-Ras splicing variant (McGrath et al., 1983), or mGFP–H-RasG12V, and images were taken using a confocal microscope. Our data show that CTK was colocalized with ATP5G1(1–67), whereas the PM localization of K-Ras4AG12V (Cho et al., 2015; Tsai et al., 2015), and the PM and Golgi localization of mGFP–H-RasG12V (Roy et al., 2005) were not perturbed (Fig. 1B). K-Ras translocates to mitochondria through PKC-mediated K-Ras phosphorylation at Ser181 (Bivona et al., 2006). To test whether K-Ras translocation to mitochondria in cells overexpressing ATP5G1(1–67) is through phosphorylation of Ser181, we generated MDCK cells stably co-expressing ATP5G1(1–67)–RFP and a mGFP–K-RasG12V S181A mutant (Bivona et al., 2006; Cho et al., 2016). Confocal microscopy shows that K-RasG12V S181A is co-localized with ATP5G1(1–67)–RFP, suggesting ATP5G1(1–67)-mediated K-RasG12V translocation to mitochondria is independent of K-Ras phosphorylation. Taken together, Fig. 1 shows that ATP5G1(1–67) overexpression translocates K-Ras from the PM to mitochondria through its HVR in an isoform-specific manner and it is independent of K-Ras phosphorylation.
ATP5G1(1–67) overexpression perturbs cellular phosphatidylserine distribution
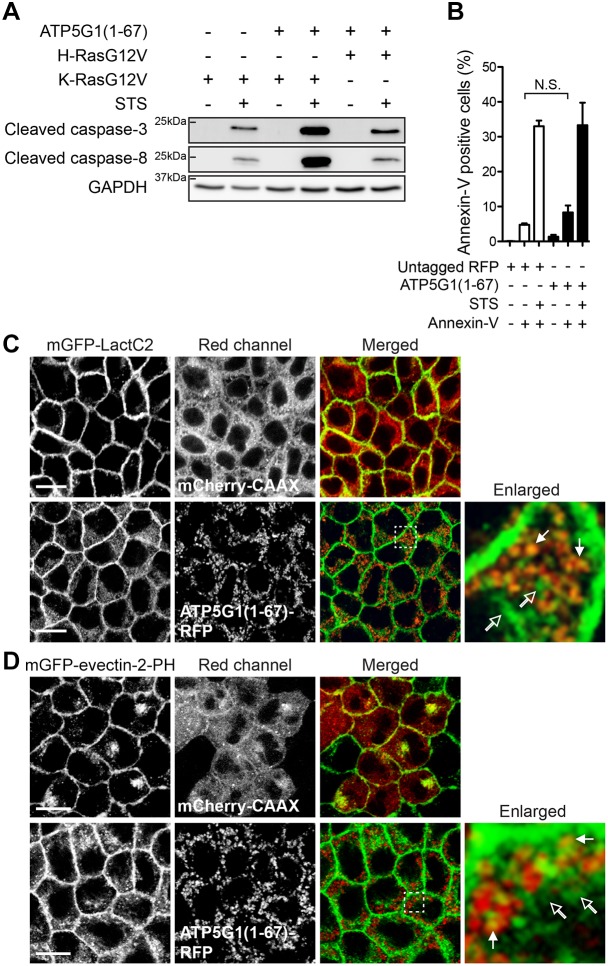
During apoptosis, cardiolipin translocates to the outer mitochondrial membrane, which changes mitochondrial surface charge and recruits proteins with positively charged amino acid residues, including K-Ras (Heit et al., 2011). To test whether ATP5G1(1–67)-mediated K-Ras translocation to mitochondria is due to apoptosis, cell lysates of MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–K-RasG12V or mGFP–H-RasG12V were immunoblotted to measure cleaved caspase-3 and caspase-8 levels. Caspase cleavage is an early apoptosis event, before phosphatidylserine (PtdSer) externalization (Mariño and Kroemer, 2013). Our data show that there were no detectable levels of cleaved caspase-3 or caspase-8 in these cells (Fig. 2A). Interestingly, when cells co-expressing K-RasG12V and ATP5G1(1–67) were treated with staurosporine, an apoptosis-inducing agent, cleaved caspase-3 and caspase-8 levels were much greater than in cells expressing K-RasG12V alone or H-RasG12V with ATP5G1(1–67) (Fig. 2A), suggesting that ATP5G1(1–67) overexpression in the presence of oncogenic K-Ras, but not H-Ras, enhances sensitivity to staurosporine-induced apoptosis. PKC-induced K-Ras translocation to mitochondria has previously been shown to stimulate apoptosis in a Bcl-XL-dependent manner (Bivona et al., 2006). Combining this with our data, it suggests that mitochondrial K-Ras in cells overexpressing ATP5G1(1–67) confers high sensitivity to staurosporine-induced apoptosis through interaction with Bcl-XL. To further test cellular apoptosis, we performed an annexin V binding assay. Annexin V is a non-permeable PtdSer-binding protein that only binds to cells when PtdSer is exposed in the outer PM leaflet during early apoptosis (Balasubramanian et al., 2007; Segawa et al., 2014). MDCK cells stably expressing RFP alone or ATP5G1(1–67)–RFP were incubated with annexin V, and annexin V-positive cells were counted using a cytometer. Our data show that ATP5G1(1–67) overexpression did not have any effect on annexin V binding (Fig. 2B), suggesting it does not induce apoptosis. Taken together, our data suggest that ATP5G1(1–67) overexpression does not induce apoptosis, and that ATP5G1(1–67)-mediated K-Ras mislocalization to mitochondria is independent of apoptosis.
Fig. 2.
ATP5G1(1–67) overexpression redistributes PtdSer from the PM independent of apoptosis. (A) MDCK cells stably expressing mGFP–K-RasG12V or mGFP–H-RasG12V with or without ATP5G1(1–67)–RFP were treated with 1 µM staurosporine (STS) to induce apoptosis or vehicle (DMSO) for 6 h. Cell lysates were blotted with anti-cleaved caspase-3 or caspase-8 antibodies as markers of early apoptosis. A GAPDH blot was used for a loading control. Representative blots are shown from three independent experiments. (B) MDCK cells stably expressing RFP alone (open bars) or ATP5G1(1–67)–RFP (closed bars) were incubated with FITC-conjugated annexin V, and annexin V-positive cells were counted in a cytometer. Cells treated with 1 µM STS for 6 h were used as positive controls to induce apoptosis. A difference between annexin V-treated cells with or without ATP5G1(1–67) were assessed using a Student's t-test (N.S., not significant). (C,D) MDCK cells co-expressing mGFP–LactC2 (C) or mGFP–evectin-2-PH (D) with mCherry–CAAX or ATP5G1(1–67)–RFP were fixed with 4% PFA and imaged using a confocal microscope. A selected region indicated by the white square is shown at a higher magnification. mGFP–LactC2 or mGFP–evectin-2-PH colocalized and not colocalized with ATP5G1(1–67)–RFP are indicated by closed and open arrows, respectively. Scale bars: 10 µm. All cells were maintained in complete growth medium (DMEM+10% FBS+2 mM L-glutamine).
PtdSer is a phospholipid concentrated in the inner PM leaflet, where its anionic head group interacts with the polybasic domain and farnesyl anchor of K-Ras, allowing K-Ras PM binding (Yeung et al., 2008; Zhou et al., 2017). To further validate the effect of ATP5G1(1–67) overexpression on cellular PtdSer distribution, ATP5G1(1–67)–RFP was overexpressed in MDCK cells stably expressing well-characterized mGFP-tagged PtdSer markers, the C2 domain of lactadherin (LactC2) (Cho et al., 2012; Yeung et al., 2008) or the PH domain of evectin-2 (also known as PLEKHB2) (evectin-2-PH) (Uchida et al., 2011). While LactC2 and evectin-2-PH were predominantly localized to the PM when mCherry-CAAX was co-expressed, they were redistributed to mitochondria (indicated by closed arrows) and other endomembranes (indicated by open arrows) in cells overexpressing ATP5G1(1–67) (Fig. 2C,D). Taken together, our data suggest that ATP5G1(1–67) overexpression redistributes PtdSer to mitochondria and other endomembranes.
ATP5G1(1–67) overexpression inhibits oncogenic K-Ras signal output
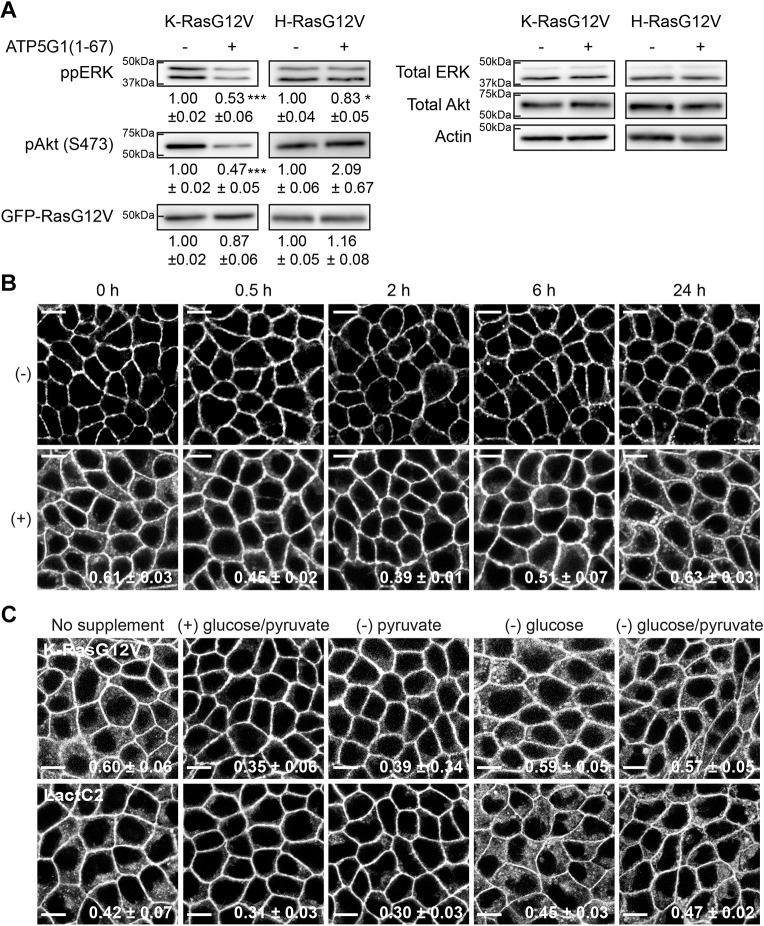
Ras proteins must interact primarily with the PM to stimulate Raf/MEK/ERK and PI3K/Akt signaling pathways (Hancock, 2003; Willumsen et al., 1984). Thus, we studied the effect of ATP5G1(1–67) overexpression in oncogenic Ras signal output. Cell lysates from MDCK cells stably co-expressing mGFP–K-RasG12V or mGFP–H-RasG12V together with RFP only or ATP5G1(1–67)–RFP were immunoblotted for phosphorylated ERK (ppERK) and Akt (pAkt) proteins. Our data show that ATP5G1(1–67)–RFP overexpression significantly reduced ppERK and pAkt levels in K-RasG12V, and to a lesser extent in H-RasG12V cell lines (Fig. 3A). Taken together with confocal microscopy results (Fig. 1), our data demonstrate that ATP5G1(1–67) overexpression inhibits signaling of oncogenic K-Ras, but not H-Ras, through PM mislocalization.
Fig. 3.
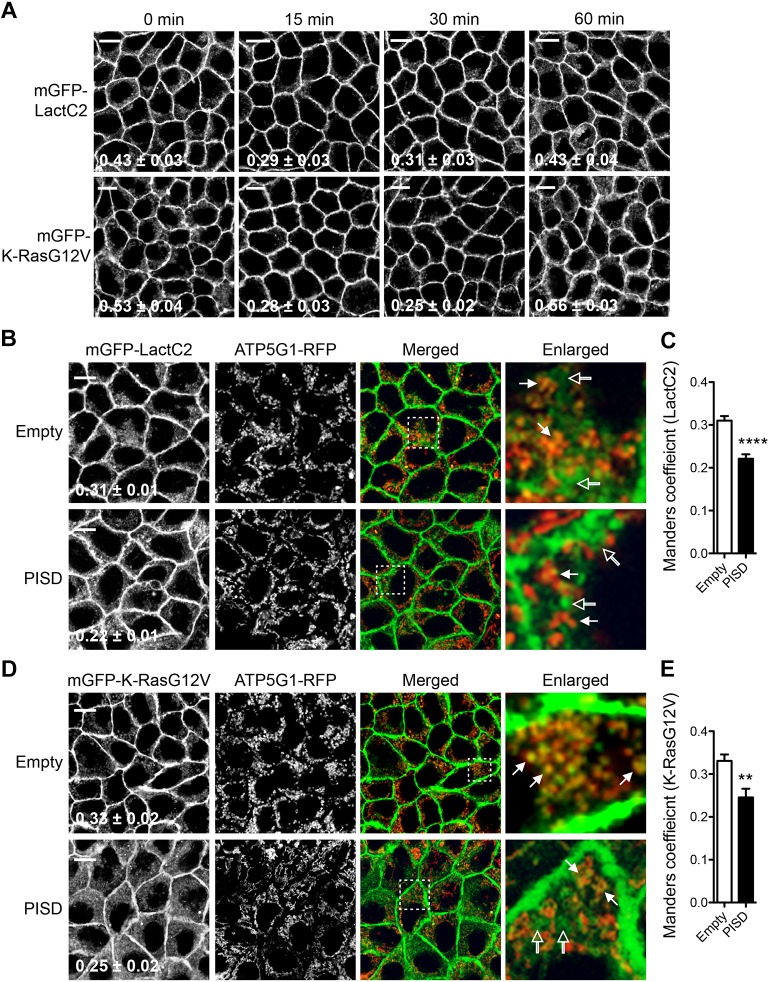
Glucose is required for the PM localization of K-RasG12V and LactC2 in cells overexpressing ATP5G1(1–67). (A) Cell lysates of MDCK cells stably co-expressing mGFP–K-RasG12V or mGFP–H-RasG12V with RFP alone (−) or ATP5G1(1–67)–RFP (+) were immunoblotted for phospho-ERK, phospho-Akt (S473), and mGFP–RasG12V, and quantified (mean±s.e.m.) from three independent experiments. Significant differences between cells not expressing (−) or expressing (+) ATP5G1(1–67) were assessed using Student's t-test (*P<0.05; ***P<0.001). Representative blots of are shown, with total ERK, total Akt and actin used as loading controls. (B) MDCK cells stably expressing mGFP–K-RasG12V in the presence (+) or absence (−) of ATP5G1(1–67)–RFP were maintained in complete growth medium (DMEM+10% FBS+2 mM L-glutamine). When cells reached full confluence, the growth medium was replaced with fresh complete growth medium and cells were further incubated for indicated time points. Cells were fixed with 4% PFA and imaged using a confocal microscope. Inserted values represent mean±s.e.m. of the fraction of ATP5G1(1–67)–RFP colocalized with mGFP–K-RasG12V calculated by Manders' coefficient from three independent experiments. Shown are representative mGFP–K-RasG12V images. Scale bars: 10 µm. (C) MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–K-RasG12V, or mGFP–LactC2 were maintained in complete growth medium (DMEM+10% FBS+2 mM L-glutamine). When cells reached full confluence, the growth medium was left unchanged (no supplement) or replaced with fresh complete growth medium without pyruvate only [(−)pyruvate], without glucose only [(−)glucose], without glucose and pyruvate [(−)glucose/pyruvate], or in complete growth medium [(+)glucose/pyruvate], and incubated for another 2 h. Cells were fixed with 4% PFA and imaged using a confocal microscope. Inserted values represent mean±s.e.m. of the fraction of ATP5G1(1–67)–RFP colocalized with mGFP–K-RasG12V or mGFP–LactC2 calculated by Manders' coefficient from three independent experiments. Shown are representative mGFP–K-RasG12V and mGFP–LactC2 images. Scale bars: 10 µm.
Glucose supplementation returns K-Ras to the PM
Efficient silencing of ATP5G1 induces mitochondrial dysfunction such as reduction of ATP production, the number of fully assembled ATP synthase molecules, mitochondrial fitness, and altered mitochondrial morphology (Vives-Bauza et al., 2010). We therefore hypothesized that cellular ATP level is reduced in cells overexpressing ATP5G1(1–67) by disrupting mitochondrial ATP synthase, which results in disruption of PtdSer and K-Ras4B PM localization. To test this, fully confluent MDCK cells grown in complete growth medium and expressing mGFP–K-RasG12V in the presence or absence of ATP5G1(1–67)–RFP were transferred to fresh complete growth medium, and cell images were taken at different time points. Manders’ coefficients for ATP5G1(1–67) colocalization with K-RasG12V were calculated to quantitate K-RasG12V localization to mitochondria (Fig. 3B, values bottom right of image panels). Our data show that in cells overexpressing ATP5G1(1–67), K-RasG12V returned to and remained at the PM from 0.5 to 6 h after the supplementation, with cells at the 2 h time point showing the most K-Ras PM localization. However, K-RasG12V was again mislocalized to mitochondria 24 h after the supplementation (Fig. 3B). K-RasG12V remained at the PM during these time points in the absence of ATP5G1(1–67)–RFP (Fig. 3B). These data suggest that in cells overexpressing ATP5G1(1–67), nutrients in complete growth medium can rapidly correct K-Ras PM localization, but once these nutrients are depleted, K-Ras translocates back to mitochondria. To further elucidate the nutrients required for K-Ras PM localization in cells overexpressing ATP5G1(1–67), fully confluent MDCK cells stably co-expressing mGFP–K-RasG12V and ATP5G1(1–67)–RFP were incubated with fresh complete growth medium without glucose only [(−)glucose], without pyruvate only [(−)pyruvate], or without glucose and pyruvate [(−)glucose/pyruvate] for 2 h. Cell images were taken and Manders’ coefficients for ATP5G1(1–67) colocalization with K-RasG12 V were calculated to quantitate K-Ras localization to mitochondria. Our data show that in cells incubated with (−)pyruvate medium, K-RasG12V returned to the PM, whereas in cells incubated with (−)glucose medium, it did not (Fig. 3C). The same results were observed for PtdSer localization (Fig. 3C). MDCK cells co-expressing mGFP–LactC2 and ATP5G1(1–67)–RFP were incubated with the same panel of growth media, and cell images were taken. Our data show that in cells incubated with (−)pyruvate and complete growth medium, but not in (−)glucose medium, LactC2 returned to the PM 2 h after the incubation (Fig. 3C). Taken together, these data suggest that sufficient cellular glucose levels are required for K-Ras and PtdSer PM localization in cells overexpressing ATP5G1(1–67).
ATP5G1(1–67) overexpression increases glucose consumption and inhibits glycolysis
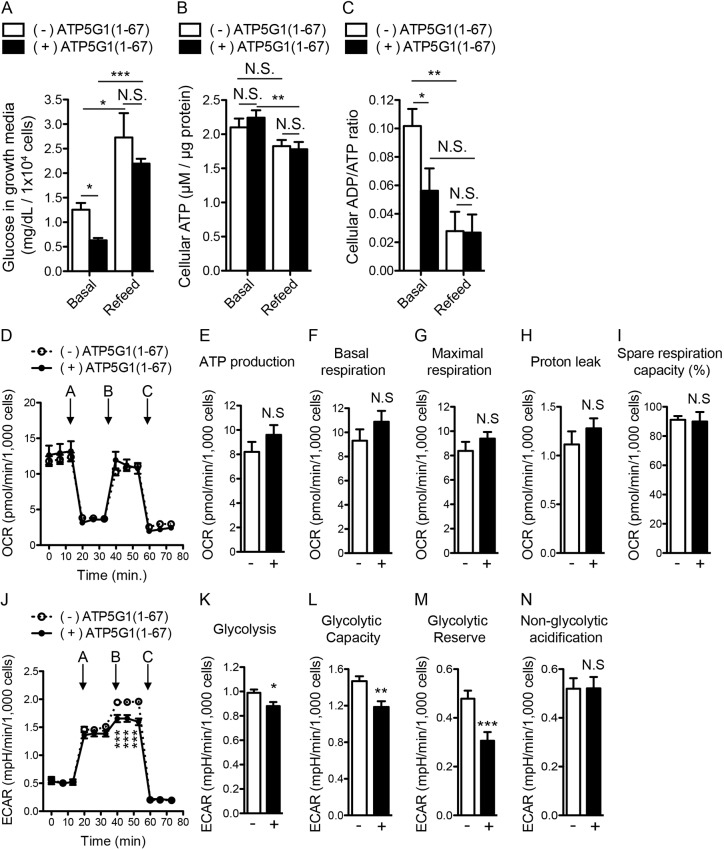
To characterize the effect of ATP5G1(1–67) on glucose metabolism, we measured glucose consumption, cellular ATP levels, and the ADP/ATP ratio. MDCK cells stably co-expressing mGFP–K-RasG12V together with RFP only or ATP5G1(1–67)–RFP were cultured in complete growth medium for 48 h, then incubated with fresh complete growth medium for another 2 h. The growth medium and cell lysates were harvested before (basal) and after (refeed) the medium was changed to measure glucose levels, and cellular ATP levels and the ADP/ATP ratio, respectively. We found that the glucose level was significantly reduced in cells overexpressing ATP5G1(1–67) in the basal condition, suggesting that cells overexpressing ATP5G1(1–67) consumed more glucose (Fig. 4A). After the medium was changed, glucose in the growth medium was significantly increased in both cell lines to a similar level (Fig. 4A). For cellular ATP levels, no difference was observed between the cell lines in the basal condition, whereas the change of medium slightly reduced ATP levels in both cell lines (Fig. 4B). We further found that the ADP/ATP ratio was significantly lower in cells overexpressing ATP5G1(1–67) in the basal condition (Fig. 4C). Since cellular ATP level was unchanged in both cell lines (Fig. 4B), our data suggest a reduction of ADP level in cells overexpressing ATP5G1(1–67) in the basal condition. After medium was changed, the ADP/ATP ratio was reduced in both cell lines to a similar level (Fig. 4C).
Fig. 4.
ATP5G1(1–67) overexpression increases glucose consumption and inhibits glycolysis. (A–C) MDCK cells stably co-expressing K-RasG12V with RFP alone (−) or ATP5G1(1–67)–RFP (+) were grown to full confluence in complete growth medium. The growth medium was left unchanged (basal) or replaced with fresh complete growth medium (refeed), and cells were incubated for another 2 h. The growth medium were collected for glucose consumption assay (A), and cell lysates were harvested for cellular ATP assay (B) and calculation of the ADP/ATP ratio (C). (D–N) MDCK cells stably co-expressing K-RasG12V with RFP alone (−) or ATP5G1(1–67)–RFP (+) were grown to full confluence in complete growth medium. Medium was replaced with fresh XF assay medium, which does not contain glucose and pyruvate, and cells further incubated at 37°C in 0% CO2 for 45–60 min. Using a Seahorse Analyzer, oxygen consumption rate (OCR) was measured to analyze mitochondrial respiration after treating cells with compounds at different time points (A, oligomycin; B, FCCP; C, rotenone and antimycin A) (D–I), or extracellular acidification rate (ECAR) was measured to analyze glycolytic rate after treating cells with compounds at different time points (A, glucose; B, oligomycin; C, 2-deoxy-D-glucose) (J–N). Significant differences between cells not expressing (−) or expressing (+) ATP5G1(1–67) were assessed using Student's t-test (N.S., not significant; *P<0.05; **P<0.01; ***P<0.001).
To further characterize the effect of ATP5G1(1–67) overexpression on glucose consumption and mitochondrial ATP synthesis, we measured the glycolytic rate and mitochondrial respiration using a Seahorse Analyzer. Briefly, MDCK cells stably co-expressing mGFP–K-RasG12V together with RFP only or ATP5G1(1–67)–RFP were grown to full confluence in complete growth medium. Cells were then incubated with XF assay medium, which does not contain glucose, in a non-CO2 37°C incubator for 45–60 min. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), indicators of the mitochondrial respiration and glycolytic rate, respectively, were measured after adding compounds that regulate mitochondrial activity or glycolysis at indicated time points. We found that OCRs in all parameters of mitochondrial respiration that we measured were unchanged in the presence or absence of ATP5G1(1–67) after adding compounds regulating mitochondrial activity (Fig. 4D–I). These data suggest that the mitochondrial respiration, including ATP production, is unaffected by ATP5G1(1–67) overexpression, consistent with the cellular ATP levels (Fig. 4B). However, we observed that ECAR was significantly inhibited in cells overexpressing ATP5G1(1–67) after adding oligomycin (Fig. 4J), which shifts the energy production from mitochondria to glycolysis. Further analyses show that glycolysis, glycolytic capacity and glycolytic reserve were also significantly inhibited in cells overexpressing ATP5G1(1–67) (Fig. 4K–N). Glycolytic capacity measures the maximum rate of glycolysis, whereas glycolytic reserve indicates the capability of glycolytic response to an increased ATP demand (Mookerjee et al., 2016). Taken together, our data indicate that ATP5G1(1–67) overexpression enhances cellular glucose consumption while inhibiting the glycolytic process.
K-Ras translocation to mitochondria is mediated by PtdSer accumulated at mitochondria
We showed that ATP5G1(1–67) overexpression redistributes PtdSer from the PM (Fig. 2C,D). To test whether K-Ras mislocalization is induced by PtdSer redistribution, we performed lipid addback experiments. MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–LactC2 or mGFP–K-RasG12V were incubated with 10 µM PtdSer or phosphatidylcholine (PtdCho), and cells were imaged at different time points. Manders’ coefficients for ATP5G1(1–67) colocalization with K-RasG12V or LactC2 were calculated to quantitate the mitochondria localization. Our data show that LactC2 predominantly decorated the PM after 15 min of PtdSer supplementation, which suggests that the exogenous PtdSer was displayed on the inner leaflet of the PM (Fig. 5A), consistent with the time frame reported previously (Cho et al., 2012, 2015). An hour after PtdSer supplementation, however, LactC2 was redistributed to endomembranes, including mitochondria, as indicated by increased Manders’ coefficients. A similar observation was made with K-RasG12V. PtdSer supplementation restored K-RasG12V PM localization within 15 min (Fig. 5A), but K-RasG12V was translocated back to mitochondria 60 min after the supplementation. Supplementation with PtdCho, which is not required for LactC2 and K-Ras PM binding (Cho et al., 2015), did not correct LactC2 and K-RasG12V mislocalization (Fig. S2A,B). Taken together, our data suggest that K-Ras PM mislocalization in cells overexpressing ATP5G1(1–67) is induced by PtdSer content depletion at the PM.
Fig. 5.
K-Ras translocation to mitochondria is induced by mitochondrial PtdSer. (A) MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–LactC2 or mGFP–K-RasG12V were supplemented with 10 µM exogenous PtdSer or PtdCho (Fig. S2) in complete growth medium without glucose and further incubated for indicated time points. Cells were fixed with 4% PFA and imaged using a confocal microscope. Inserted values represent mean±s.e.m. of the fraction of ATP5G1(1–67)–RFP colocalized with mGFP–LactC2 or mGFP–K-RasG12V calculated by Manders' coefficient from three independent experiments. Shown are representative images of mGFP–LactC2 and mGFP–K-RasG12V. Scale bars: 10 µm. (B–E) MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–LactC2 (B) or mGFP–K-RasG12V (D) were infected with lentivirus expressing PISD or an empty vector. Cells were maintained in complete growth medium. Cells were fixed with 4% PFA and imaged using a confocal microscope. A selected region indicated by the white square is shown at a higher magnification. K-RasG12V and LactC2 colocalized and not colocalized with ATP5G1(1–67) are indicated by closed and open arrows, respectively. Inserted values and the graphs represent mean±s.e.m. of the fraction of mGFP–LactC2 (C) or mGFP–K-RasG12V (E) colocalized with ATP5G1(1–67)–RFP calculated by Manders' coefficient in cells overexpressing an empty vector or PISD from three independent experiments. The difference between control (empty) and cells overexpressing PISD (PISD) cells was assessed using Student's t-test (**P<0.01; ****P<0.0001). Scale bars: 10 µm.
To further examine whether K-Ras translocation to mitochondria is mediated by PtdSer accumulation at mitochondria, we depleted PtdSer content at mitochondria by overexpressing phosphatidylserine decarboxylase (PISD), an enzyme that converts PtdSer to phosphatidylethanolamine in the inner mitochondrial membrane (Percy et al., 1983). Briefly, MDCK cells stably co-expressing ATP5G1(1–67)–RFP and mGFP–K-RasG12V or mGFP–LactC2 were infected with lentivirus expressing PISD or an empty vector, and cell images were taken using a confocal microscope. Manders' coefficients for colocalization of K-RasG12V or LactC2 with ATP5G1(1–67) were calculated to quantitate the fraction of K-RasG12V or LactC2 localized to the mitochondria. Our data show that LactC2 was distributed to endomembranes including mitochondria in control cells and cells overexpressing PISD (Fig. 5B), suggesting that PtdSer distribution was not affected on PISD overexpression. However, Manders' coefficient values were significantly reduced from 0.31 to 0.22 on PISD overexpression, indicating that the fraction of LactC2 localized to mitochondria was decreased from 31% to 22% when PISD was overexpressed (Fig. 5C). These data suggest that PISD overexpression partially reduces PtdSer content at mitochondria. PISD overexpression also redistributed K-RasG12V to endomembranes, including mitochondria, while it was predominantly localized to mitochondria in control cells (Fig. 5D). Manders' coefficients were also significantly reduced from 0.33 to 0.25 (Fig. 5E), indicating that the fraction of K-RasG12V localized to mitochondria was decreased from 33% to 25% when PtdSer content at mitochondria was reduced. PISD overexpression did not disrupt the PM localization of K-RasG12V and LactC2 in cells not expressing ATP5G1(1–67) (Fig. S2C). Taken together with phospholipid supplementation experiments, our data suggest that K-Ras translocation to mitochondria is induced by PtdSer accumulation at mitochondria in cells overexpressing ATP5G1(1–67).
Phosphatidylinositol 4-phosphate distribution is perturbed in cells overexpressing ATP5G1(1–67)
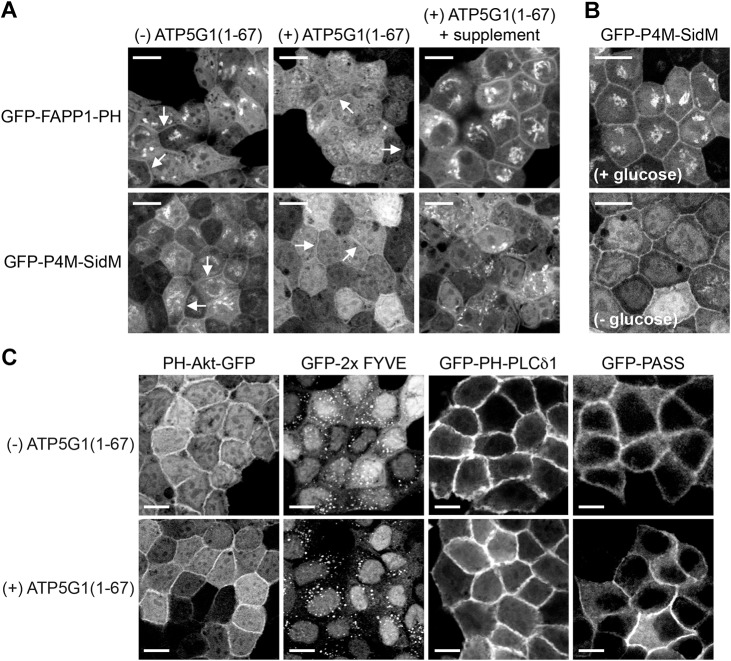
To characterize the effects of ATP5G1(1–67) overexpression on other phospholipids, we examined cellular localization of phospholipid markers in the presence or absence of ATP5G1(1–67). ATP5G1(1–67)–RFP was overexpressed in MDCK cells stably expressing the following mGFP-tagged phospholipid markers and confocal microscopy was performed; the PH domain of FAPP1 (also known as PLEKHA3) (FAPP1-PH) or the P4M domain of SidM (P4M-SidM) for phosphatidylinositol (PI) 4-phosphate (PI4P) (Hammond et al., 2014), PH-Akt for PI(3,4)P2 or PI(3,4,5)P3 (Franke et al., 1997; James et al., 1996), tandem FYVE domains of EEA1 (2×FYVE) for PI3P (Stenmark et al., 1996), PH-PLCδ1 for PI(4,5)P2 (Garcia et al., 1995), and the phosphatidic acid-binding domain of Spo20 (PASS) for phosphatidic acid (Zhang et al., 2014). In the absence of ATP5G1(1–67), PI4P probes were localized to the PM and Golgi, consistent with a previous study (Hammond et al., 2014), whereas Golgi, but not PM (indicated by arrows) localization was perturbed in cells overexpressing ATP5G1(1–67) (Fig. 6A). The Golgi localization of the PI4P probes was restored after cells were incubated with fresh complete growth medium for 2 h (Fig. 6A). To further characterize the effect of glucose depletion on PI4P localization, MDCK cells stably expressing mGFP–P4M-SidM only were incubated in growth medium with or without glucose for 6 h. Our data show that glucose depletion perturbed PI4P content at the Golgi without disrupting PI4P at the PM (Fig. 6B). Cellular localization of other phospholipid markers was unaffected in cells overexpressing ATP5G1(1–67) (Fig. 6C). Taken together with the glucose consumption data (Fig. 4), these data suggest that ATP5G1(1–67) overexpression depletes PI4P at the Golgi by depleting cellular glucose.
Fig. 6.
ATP5G1(1–67) overexpression reduces PI4P at the Golgi. (A) MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–FAPP1-PH or mGFP–4M-SidM were maintained in complete growth medium (DMEM+10% FBS+2 mM L-glutamine). When cells reached full confluence, the growth medium was left unchanged or replaced with fresh complete growth medium and cells were incubated for 2 h (+supplement). Cells were fixed with 4% PFA and imaged using a confocal microscope. As a control, MDCK cells stably expressing mGFP–FAPP1-PH or mGFP–P4M-SidM only [(−) ATP5G1(1–67)] maintained in complete growth medium were imaged. Shown are representative mGFP–FAPP1-PH and mGFP–P4M-SidM images. Arrows indicate the PM localization of FAPP1-PH and P4M-SidM. Scale bars: 10 µm. (B) Fully confluent MDCK cells stably expressing mGFP–P4M-SidM only were incubated in growth medium with (+glucose) or without glucose (−glucose) for 6 h. Cells were fixed with 4% PFA followed by confocal microscopy. Scale bars: 10 µm. (C) MDCK cells stably expressing phospholipid markers PH-Akt–mGFP, mGFP–2×FYVE, mGFP–PH-PLCδ1, or mGFP–PASS with or without ATP5G1(1–67)–RFP were grown to full confluence in complete growth medium, fixed with 4% PFA and imaged using a confocal microscope. Shown are representative images of the phospholipid markers. Scale bars: 10 µm.
Inhibition of the Golgi-localized PI4 kinases mislocalizes K-Ras and PtdSer from the PM
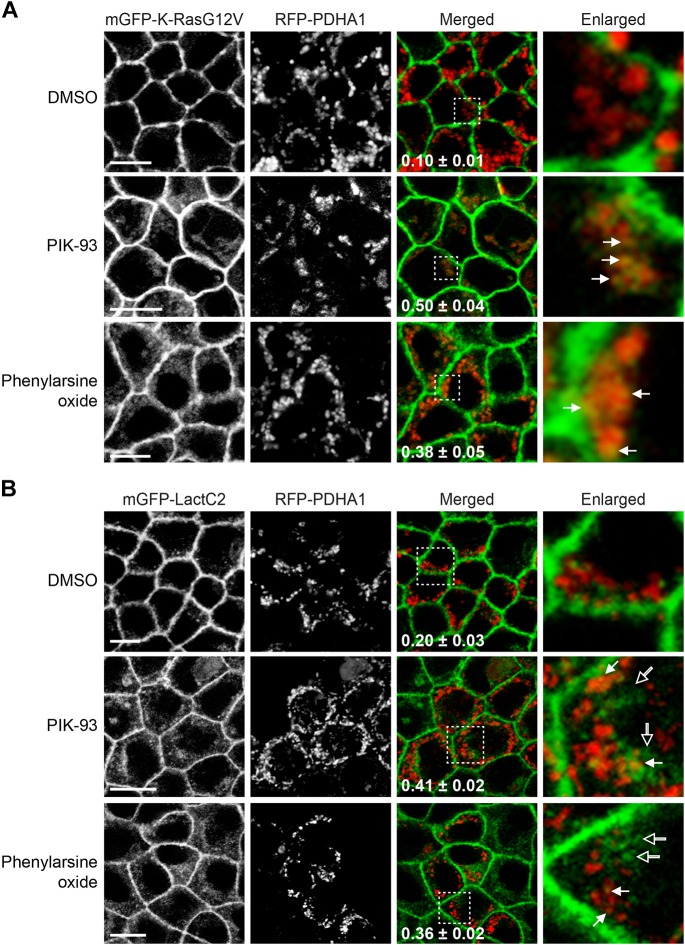
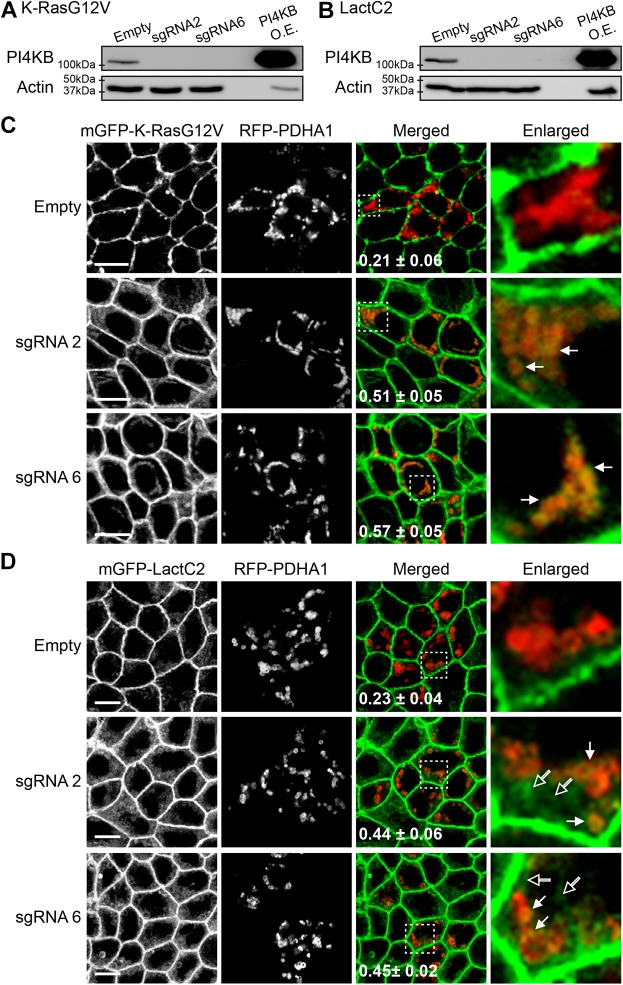
PI4P is synthesized from PI or PI(4,5)P2 by PI4 kinases (PI4Ks) or inositol 5-phosphatases, respectively (Hammond et al., 2012; Varnai et al., 2006). Recent studies reported that PM PI4P depletion through inhibition of PM-localized PI4KA mislocalizes PtdSer from the PM and translocates K-Ras to the Golgi (Chung et al., 2015; Gulyás et al., 2017; Moser von Filseck et al., 2015). We show that ATP5G1(1–67) overexpression depletes PI4P at the Golgi without altering the PM PI4P and PI(4,5)P2 distribution, and mislocalizes PtdSer and K-Ras from the PM (Figs 1, 2 and 6). Taken together, these results led us to hypothesize that PI4P depletion at the Golgi redistributes PtdSer to mitochondria and other endomembranes, which translocates K-Ras to mitochondria. To test this, we examined K-RasG12V and LactC2 localization after inhibition of Golgi-localized PI4Ks. There are four PI4K isoforms: PI4K2A, PI4K2B, PI4KA and PI4KB. Of those, PI4K2A and PI4KB are localized predominantly at the Golgi complex (Wang et al., 2003; Weixel et al., 2005). Synthetic inhibitor PIK-93 specifically inhibits PI4KB (Knight et al., 2006; Tóth et al., 2006), whereas phenylarsine oxide blocks PI4K2A/B activities (Sorensen et al., 1998; Wiedemann et al., 1996). MDCK cells stably expressing mGFP–K-RasG12V or mGFP–LactC2 were incubated with baculovirus expressing RFP–PDHA1 and the PI4K inhibitors for 48 h. Cell images were taken using a confocal microscope and Manders' coefficients for RFP–PDHA1 colocalization with K-RasG12V or LacC2 were calculated to quantitate their mitochondrial localization. Our data show that while K-RasG12V did not colocalize with RFP–PDHA1 in vehicle-treated control cells, it colocalized with RFP–PDHA1 after treatment with the PI4K inhibitors at a concentration that depleted PI4P at the Golgi, but not at the PM (Fig. 7A; Fig. S3). In cells expressing mGFP–LactC2, the PI4K inhibitors redistributed LactC2 to mitochondria (indicated by closed arrows) and other endomembranes (indicated by open arrows) (Fig. 7B). The same observation was made when cells treated with the PI4K inhibitors were stained with MitoTracker (Fig. S4). These data suggest that inhibition of the Golgi-localized PI4Ks mislocalizes K-Ras and PtdSer from the PM to mitochondria and endomembranes, respectively. To further validate the role of Golgi-localized PI4Ks, we knocked out PI4KB in MDCK cells stably expressing mGFP–K-RasG12V or mGFP–LactC2 using CRISPR-Cas9 technology, and studied the cellular localization of K-RasG12V and LactC2. Confocal microscopy data show that when PI4KB expression was completely abolished (Fig. 8A,B; Fig. S5), K-RasG12V and LactC2 translocated to mitochondria and endomembranes, respectively (Fig. 8C,D). Taken together with the PI4K inhibitor data, our data suggest that depletion of Golgi-localized PI4P by inhibition or knockout of the Golgi-localized PI4Ks translocates K-Ras and PtdSer to mitochondria and endomembranes, respectively.
Fig. 7.
Golgi-localized PI4K inhibition mislocalizes K-RasG12V and PtdSer to mitochondria and endomembrane, respectively. (A,B) MDCK cells stably expressing mGFP–K-RasG12V (A) or mGFP–LactC2 (B) were treated with vehicle (DMSO), 1 µM PIK-93 or 50 nM phenylarsine oxide for 48 h in the presence of modified baculovirus encoding RFP–PDHA1. Cells were fixed with 4% PFA and imaged using a confocal microscope. Inserted values are mean±s.e.m. of the fraction of RFP–PDHA1 colocalized with mGFP–K-RasG12V or mGFP–LactC2, calculated by Manders' coefficient from three independent experiments. Selected regions indicated by the white squares are shown at a higher magnification. mGFP–K-RasG12V and mGFP–LactC2 colocalized and not colocalized with RFP–PDHA1 are indicated by closed and open arrows, respectively. Scale bars: 10 µm.
Fig. 8.
Knockout of PI4KB translocates K-Ras and PtdSer to mitochondria and endomembranes, respectively. Cell lysates from MDCK cells with PI4KB knocked out by means of CRISPR-Cas9 (sgRNA2, sgRNA6), and stably expressing mGFP–K-RasG12V (A) or mGFP–LactC2 (B) were immunoblotted with anti-PI4KB antibody, with lysates from MDCK cells transfected with an empty vector as control. Cell lysates from HEK293T cells overexpressing human PI4KB (PI4KB O.E.) were used as a positive control for the antibody. A representative blot is shown from three independent experiments, and an actin blot is shown as a loading control. (C,D) PI4K knockout MDCK cells stably expressing mGFP–K-RasG12V (C) or mGFP–LactC2 (D) were incubated with baculovirus encoding RFP–PDHA1 for 48 h. Cells were fixed with 4% PFA and imaged using a confocal microscope. Inserted values are mean±s.e.m. of the fraction of RFP–PDHA1 colocalized with mGFP–K-RasG12V or mGFP–LactC2 calculated by Manders' coefficient from three independent experiments. Selected regions indicated by the white squares are shown at a higher magnification. mGFP–K-Ras and mGFP–LactC2 colocalized and not colocalized with RFP–PDHA1 are indicated by closed and open arrows, respectively. Scale bars: 10 µm.
DISCUSSION
In this study, we show that overexpression of the mitochondrial targeting sequence and the leading six amino acid residues of the catalytic domain (amino acids 1–67) of ATP5G1, a subunit of mitochondrial ATP synthase, elevates cellular glucose consumption and blocks glycolysis without disrupting mitochondrial respiration. ATP5G1(1–67) overexpression also depletes PI4P at the Golgi, and redistributes PtdSer to mitochondria and other endomembranes, resulting in K-Ras translocation to mitochondria. These effects are reversed by glucose supplementation. We further show that glucose depletion reduces PI4P contents at the Golgi, but not the PM, and that PI4P depletion at the Golgi by inhibition or knockout of Golgi-localized PI4Ks also translocates K-Ras and PtdSer to mitochondria and endomembranes, respectively. Taken together, our study demonstrates that PI4P content at the Golgi is sensitive to cellular glucose levels, and that PI4P content at the Golgi regulates the PM localization of PtdSer and K-Ras. PI4KB, one of the Golgi-localized PI4Ks, and Pik1p, the yeast homolog of PI4KB, have been reported to localize to the Golgi and the nucleus (de Graaf et al., 2002; Strahl et al., 2005). Under glucose depletion, Pik1p dissociates from the Golgi and accumulates in the nucleus by interacting with 14-3-3 proteins, with this response reversed upon glucose supplementation (Demmel et al., 2008). Based on these studies and our own observations, we propose that glucose depletion translocates PI4KB from the Golgi to the nucleus, which, in turn, depletes PI4P content at the Golgi, leading to the PM mislocalization of PtdSer and K-Ras. Upon glucose supplementation, PI4KB returns to and produces PI4P at the Golgi, resulting in the return of PtdSer and K-Ras to the PM.
It is unclear how ATP5G1(1–67) overexpression promotes cellular glucose consumption while inhibiting the glycolytic process. The pentose phosphate pathway (PPP) uses glucose-6-phosphate (G-6-P) converted from glucose to ultimately produce ribose-5-phosphate for nucleic acid synthesis, and NADPH, important in anti-oxidant defense against reactive oxygen species (ROS) (Patra and Hay, 2014; Stanton, 2012). It is plausible that overexpression of a truncated mutant subunit in the mitochondrial ATP synthase complex may elevate ROS by stressing the mitochondrial reparatory chain. This, in turn, promotes cells to utilize glucose preferentially for the PPP over glycolysis to generate sufficient NADPH to defend against the elevated ROS.
PI4P metabolism is important for PtdSer localization at the inner PM leaflet. Studies in mammalian cells and yeast identified that oxysterol-binding protein-related proteins 5 and 8 (ORP5, ORP8, also known as OSBPL5 and OSBPL8) exchange newly synthesized PtdSer from the ER for PI4P from the PM (Chung et al., 2015; Moser von Filseck et al., 2015). This exchange is driven by the synthesis of PI4P at the PM by PI4KA and the concomitant hydrolysis of PI4P by Sac1 phosphatase in the ER to maintain a PI4P gradient across the PM and ER. PI(4,5)P2 at the PM further promotes the recruitment of ORP8 to the PM for transporting PI4P to the ER. Eliminating any component of this mechanism depletes PtdSer content in the inner PM leaflet (Chung et al., 2015; Moser von Filseck et al., 2015; Sohn et al., 2018). PI4P at the Golgi is also transported to the ER by oxysterol binding protein (OSBP) at ER–Golgi membrane contact sites. OSBP exchanges Golgi PI4P for sterol from the ER, and OSBP dissociates from the Golgi when Golgi PI4P levels are low (Mesmin et al., 2013). Taking these studies together with our data, we propose that when Golgi PI4P is depleted by inhibition of Golgi-localized PI4Ks, OSBP is no longer recruited to ER–Golgi membrane contact sites, resulting in reduced PI4P content in the ER. This, in turn, perturbs the PI4P gradient necessary for the ORP5/8 machinery, resulting in PtdSer redistribution.
Here, we show that K-Ras translocation to mitochondria is induced by PtdSer accumulated at mitochondria in cells overexpressing ATP5G1(1–67) (Fig. 5). It is not fully understood why K-Ras is accumulated predominantly at mitochondria, while PtdSer is redistributed to mitochondria and other endomembranes. We speculate that the outer mitochondrial membranes with increased PtdSer content upon inhibition of Golgi-localized PI4Ks provide a lipid environment necessary for recruitment of and interaction with the polybasic domain and farnesyl-anchor of K-Ras. When PtdSer at mitochondria is depleted by PISD overexpression, the outer mitochondrial membranes no longer provide the lipid environment, resulting in K-Ras redistribution to other endomembranes. Intriguingly, while depletion of PM PI4P redistributes PtdSer from the PM, it translocates K-Ras predominantly to the Golgi, which is diminished by the concomitant depletion of Golgi-localized PI4P (Gulyás et al., 2017). Taking these studies together with our data, it suggests that PI4P content at the PM and Golgi regulate K-Ras trafficking via molecular mechanisms that are different but inter-related.
In summary, we demonstrate that PI4P depletion at the Golgi redistributes PtdSer from the PM to mitochondria and other endomembranes, resulting in K-Ras translocation to mitochondria. Our study proposes newly discovered roles for Golgi-localized PI4P in regulating cellular trafficking of PtdSer and K-Ras.
MATERIALS AND METHODS
Plasmids and reagents
Staurosporine (STS) (BIA-S1086) was purchased from BioAustralis. Antibodies for detecting PI4KB (13247-1-AP; 1:1000), β-actin (60008-1-Ig; 1:5000) and GFP (66002-1-lg; 1:4000) were purchased from Proteintech. Anti-cleaved caspase 3 (9661; 1:1000), anti-cleaved caspase 8 (9496; 1:1000), anti-total Akt (2920; 1:1000), anti-total ERK (4696; 1:1000), anti-pAkt (S473) (4060; 1:1000), and anti-ppERK (4370; 1:3000) antibodies for immunoblotting were from Cell Signaling Technology. Goat anti-mouse IgG (G21040; 1:2000) and anti-rabbit IgG secondary antibodies (G21234; 1:5000) were purchased from Invitrogen. Molecular Probes CellLight Mitochondria–RFP BacMam 2.0 (C10601), MitoTracker Red FM (M2225), and sodium pyruvate (11-360-070) were from Invitrogen. Dextrose (BDH9230) was from VWR. pLV-mitoDsRed was deposited by Pantelis Tsoulfas (Addgene plasmid #44386) (Kitay et al., 2013). pSpCas9(BB)-2A-Puro(PX459) V2.0 was deposited by Feng Zhang (Addgene plasmid #62988). GFP-P4M-SidM was deposited by Tamas Balla (Addgene plasmid #51469). pDONR223-PI4KB was deposited by William Hahn and David Root (Addgene plasmid #23839). GFP–evectin-2-PH was a gift from Tomohiko Taguchi (Tohoku University, Japan). Human phosphatidylserine decarboxylase (PISD) cDNA was purchased from GenScript (OHu25313D).
Cell lines
Madin–Darby canine kidney [MDCK, ATCC (CCL-34)], human embryonic kidney [HEK293T, ATCC (CRL-11268)], and baby hamster kidney [BHK, ATCC (CCL-10)] cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (10569-010, Invitrogen) or glucose/pyruvate-free DMEM (11966-025, Invitrogen). DMEM was supplemented with 2 mM L-glutamine (CA009-010, GenDepot), and 10% FBS (16000044, Life Technologies). All cell lines were grown at 37°C in 5% CO2. All cell lines have been frequently tested for mycoplasma using MycoAlert Mycoplasma Detection Kit (Lonza, LT07-318).
Growth medium treatment
On day 1, 3×105 MDCK cells co-expressing ATP5G1(1–67)–RFP and mGFP–K-RasG12V or mGFP–LactC2 were seeded onto coverslips in complete growth medium (DMEM, 10% FBS, 2 mM L-glutamine) on a 12-well plate. On day 2, cells were washed twice with 1× PBS and incubated with fresh complete growth medium. On day 4, cells were incubated with complete growth medium, glucose/pyruvate-free DMEM with 10% FBS and 2 mM L-glutamine [(−)glucose/pyruvate], glucose/pyruvate-free DMEM with 25 mM dextrose, 10% FBS and 2 mM L-glutamine [(−)pyruvate], glucose/pyruvate-free DMEM with 1 mM sodium pyruvate, 10% FBS and 2 mM L-glutamine [(−)glucose], or glucose/pyruvate-free DMEM with 25 mM dextrose, 1 mM sodium pyruvate, 10% FBS and 2 mM L-glutamine [(+)glucose/pyruvate] for indicated time points.
Western blotting
Cells were washed twice with ice-cold 1× PBS. Cells were harvested in lysis buffer B containing 50 mM Tris-Cl (pH 7.5), 75 mM NaCl, 25 mM NaF, 5 mM MgCl2, 5 mM EGTA, 1 mM DTT, 100 μM NaVO4, 1% NP-40 plus protease and phosphatase inhibitors. SDS-PAGE and immunoblotting were generally performed using 20 μg of lysate from each sample group. Signals were detected by enhanced chemiluminescence (PI34578, Thermo Fisher Scientific) and imaged using an Amersham Imager 600 (GE Healthcare). ImageJ software (v1.51k) was used to quantitate band intensity.
Annexin V binding assay
MDCK cells were incubated with FITC-conjugated Annexin V (BMS500FI, Invitrogen) according to the manufacturer's instructions. Annexin V-positive cells were counted using a BD AccuriC6 Analyzer with a 533/30 nm filter.
Glycolytic rate and mitochondrial respiration assays
On day 1, MDCK cells were seeded in 250 µl complete DMEM growth medium per well on XF24 cell culture plates (100777-004, Agilent). On day 2, cells were incubated with fresh complete growth medium. On day 4, cells were grown to full confluence and cell culture medium was replaced with 500 µl XF medium (103575-100, Agilent) supplemented with 2 mM L-glutamine only. Cells were incubated in a non-CO2 37°C incubator for 45–60 min, and OCR and ECAR were measured as readouts for the mitochondrial respiration and glycolytic rate, respectively, using a XF24-3 Seahorse Analyzer with the XF24 Extracellular Flux Assay kit (100850-001, Agilent). For mitochondrial respiration, measurements were taken at four different time points: (1) before injection, (2) after injection of oligomycin (final 1 µM) (AAJ61898MA, Alfa Aesar), (3) after injection of FCCP (final 0.5 µM) (NC0904863, Cayman Chemical), and (4) after injection of rotenone (final 0.5 µM) (NC0779735, Cayman Chemical) and antimycin A (final 0.5 µM) (89149-958, Enzo Life Sciences). For glycolytic rate, measurements were taken at four different time points: (1) before injection, (2) after injection of glucose (final 10 mM) (BDH9230, VWR), (3) after injection of oligomycin (final 1 µM) (AAJ61898MA, Alfa Aesar), and (4) after injection of 2-deoxy-D-glucose (final 50 mM) (AC111980010, Thermo Fisher Scientific). For both assays, results were normalized to cell number as quantified by NucLight Live Cell Stain (R37605, Life Technologies) and cell counting using a Biotek Cytation 5 imaging reader. Raw data were exported to graphs using Seahorse Wave Desktop software (Version 2.6.0, Agilent) and Seahorse XF Cell Mito or Glycolysis Stress Test Report Generator (v 3.0.6, Agilent).
Glucose consumption measurement
On day 1, 2×105 MDCK cells were seeded in complete growth medium in a 12-well plate. On day 2, cells were incubated with fresh complete growth medium. On day 4, cells were incubated with fresh complete growth medium for 2 h. The culture medium harvested before and after the incubation was analyzed using a Glucose Colorimetric Detection Kit (EIAGLUC, Invitrogen) as per the manufacturer's protocol, and the numbers of cells per well were counted. In brief, medium sample was mixed with 1× HRP solution, substrate, and 1× glucose oxidase in wells of a clear half-area 96-well plate and incubated at room temp for 30 min. Absorbance was read at 560 nm. Results were normalized to number of cells.
Cellular ATP measurement
On day 1, 2×105 of MDCK cells were seeded in complete growth medium in a 12-well plate. On day 2, cells were supplemented with fresh complete growth medium. On day 4, cells were incubated with fresh complete growth medium for 2 h. Cells were washed twice with ice-cold 1× PBS, and harvested in 1× lysis buffer (25 mM Tris-phosphate pH 7.8, 2 mM DTT, 2 mM DCTA, 10% glycerol and 1% Triton X-100). Samples were microcentrifuged at 12,000 g for 5 min at 4°C and the supernatant was collected, followed by determining protein concentrations by BCA assay. Samples were diluted 1:20 in 1× lysis buffer for use in the ATP assay. As per the ATP Determination Kit manufacturer's instructions (A22066, Invitrogen), 95 µl of standard reaction solution, which contains luciferin and firefly luciferase, and 5 µl of each diluted sample was added to the wells of an opaque white 96-well plate. The plate was incubated at room temp for 15 min and then luminescence read. Results are normalized to protein concentration.
Cellular ADP/ATP ratio measurement
On day 1, 2×105 of MDCK cells were seeded in complete growth medium in a 12-well plate. On day 2, cells were supplemented with fresh complete growth medium. On day 4, cells were incubated with fresh complete growth medium for 2 h. ADP/ATP ratio was measured using the EnzyLight ADP/ATP Ratio Assay Kit (75878-114, Bioassay Systems) as per the manufacturer's instructions. In brief, cells were washed twice with ice-cold 1× PBS and harvested in 1× lysis buffer (25 mM Tris-phosphate pH 7.8, 2 mM DTT, 2 mM DCTA, 10% glycerol and 1% Triton X-100). Protein concentrations were measured by means of BCA assay. 10 µg protein per sample was mixed with ATP reagent in a white opaque 96-well plate, and luminescence was read after 1 min [relative luminescence unit (RLU) A]. 10 min after reading RLU A, luminescence was read again (RLU B). Immediately after reading RLU B, ADP reagent was added to each well and luminescence read after 1 min (RLU C). The ADP/ATP ratio was determined by subtracting RLU B from RLU C, then dividing by RLU A.
Phospholipid supplementation
Phospholipids were prepared and supplemented to cells as described previously (Cho et al., 2015). Briefly, brain phosphatidylserine (PtdSer; 830032C) and phosphatidylcholine (PtdCho; 840053C, both from Avanti Polar Lipids) were dried under a vacuum in a glass vial to remove the solvent, reconstituted in growth medium without glucose (DMEM without glucose/pyruvate, 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate) using a water sonicator, and diluted to a final concentration of 10 µM. For confocal microscopy, 3.0×105 MDCK cells stably co-expressing ATP5G1(1–67)–RFP with mGFP–K-RasG12V or mGFP–LactC2 were seeded onto a glass coverslip in a 12-well plate and maintained in complete growth medium (DMDM, 10% FBS, 2 mM L-glutamine) for 72 h. The culture medium was replaced with phospholipid-containing growth medium and further incubated at 37°C in 5% CO2 for indicated time points. Cells were fixed with 4% PFA and imaged using a confocal microscope.
CRISPR-Cas9-mediated knockout of phosphatidylinositol 4-kinase B and validation
A previously reported protocol was used for CRISPR-Cas9-mediated gene knockout (Ran et al., 2013). Briefly, single-guide (sg) RNAs were designed against the canine PI4KB cDNA sequence (NCBI reference sequence: XM_022404993.1) using Benchling algorithm (https://www.benchling.com/crispr/). PI4KB sgRNA2 and sgRNA6 target nucleotide positions 101 and 267, respectively, in exon 1. The sequence of sgRNA2 primers are: forward 5′-CACCGcttgctaggcgtcatcacag-3′ and reverse 5′-AAACctgtgatgacgcctagcaagC-3′. The sequence of sgRNA6 primers are: forward 5′-CACCGtaggtggatcatccaggcaa-3′ and reverse 5′-AAACttgcctggatgatccacctaC-3′ (uppercase letters depict overhangings compatible with a Bbs1 sticky end). sgRNA2 and sgRNA6 were cloned into a CRISPR-Cas9 vector, pSpCas9(BB)-2A-Puro (PX459). MDCK cells stably expressing mGFP–K-RasG12V or mGFP–LactC2 were transfected with the plasmid and monoclonal selection was performed using 2 µg/ml puromycin. After selection, cells were cultured in 1 µg/ml puromycin. Cell lysates were prepared and immunoblotted to validate PI4KB knockout. An antibody against human PI4KB amino acid residues 449–801 was used to detect endogenous PI4KB. For the immunoblot assay, cell lysates from HEK293T cells overexpressing untagged human PI4KB cDNA were used as a positive control for the antibody. To verify CRISPR-Cas9-mediated mutation, cDNA was generated from the PI4KB knockout MDCK cells stably expressing mGFP–K-RasG12V and amplified by PCR using a forward primer: 5′-ATGGGAGACATGGTGGTG-3′ and reverse 5′-GGCAACGATGGACTATGTAGGG-3′. The PCR products were resolved using agarose gel electrophoresis, purified and sequenced.
Confocal microscopy
Cells were grown on coverslips and fixed with 4% paraformaldehyde, followed by 50 mM NH4Cl treatment, and imaged by confocal microscopy (Olympus FV1000) using a 60× objective.
Statistical analysis
Prism (v7.0, GraphPad Software) was used for one-way ANOVA tests and Student's t-tests.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.-J.C.; Methodology: K.-J.C.; Validation: T.E.M., K.M.H., K.-J.C.; Formal analysis: T.E.M., M.H., K.-J.C.; Investigation: T.E.M., K.M.H., A.T.S., K.-J.C.; Resources: M.H., R.S., S.M.H., K.-J.C.; Writing - original draft: T.E.M., K.-J.C.; Visualization: K.-J.C.; Supervision: K.-J.C.; Funding acquisition: K.-J.C.
Funding
This work was supported by the National Cancer Institute [R00-CA188593 to K.-J.C.] and the National Institutes of Health [R21NS100077 and 1R01NS089815 to A.T.S.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.231886.supplemental
Reference
- Apolloni A., Prior I. A., Lindsay M., Parton R. G. and Hancock J. F. (2000). H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 20, 2475-2487. 10.1128/MCB.20.7.2475-2487.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian K., Mirnikjoo B. and Schroit A. J. (2007). Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J. Biol. Chem. 282, 18357-18364. 10.1074/jbc.M700202200 [DOI] [PubMed] [Google Scholar]
- Bivona T. G., Quatela S. E., Bodemann B. O., Ahearn I. M., Soskis M. J., Mor A., Miura J., Wiener H. H., Wright L., Saba S. G. et al. (2006). PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell 21, 481-493. 10.1016/j.molcel.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Boyer P. D. (2000). Catalytic site forms and controls in ATP synthase catalysis. Biochim. Biophys. Acta 1458, 252-262. 10.1016/S0005-2728(00)00077-3 [DOI] [PubMed] [Google Scholar]
- Chandra A., Grecco H. E., Pisupati V., Perera D., Cassidy L., Skoulidis F., Ismail S. A., Hedberg C., Hanzal-Bayer M., Venkitaraman A. R. et al. (2012). The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat. Cell Biol. 14, 148-158. 10.1038/ncb2394 [DOI] [PubMed] [Google Scholar]
- Chen Z., Otto J. C., Bergo M. O., Young S. G. and Casey P. J. (2000). The C-terminal polylysine region and methylation of K-Ras are critical for the interaction between K-Ras and microtubules. J. Biol. Chem. 275, 41251-41257. 10.1074/jbc.M006687200 [DOI] [PubMed] [Google Scholar]
- Cho K.-J., Park J.-H., Piggott A. M., Salim A. A., Gorfe A. A., Parton R. G., Capon R. J., Lacey E. and Hancock J. F. (2012). Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J. Biol. Chem. 287, 43573-43584. 10.1074/jbc.M112.424457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. J., van der Hoeven D., Zhou Y., Maekawa M., Ma X., Chen W., Fairn G. D. and Hancock J. F. (2015). Inhibition of acid sphingomyelinase depletes cellular phosphatidylserine and mislocalizes K-Ras from the plasma membrane. Mol. Cell. Biol. 36, 363-374. 10.1128/MCB.00719-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.-J., Casteel D. E., Prakash P., Tan L., van der Hoeven D., Salim A. A., Kim C., Capon R. J., Lacey E., Cunha S. R. et al. (2016). AMPK and endothelial nitric oxide synthase signaling regulates K-Ras plasma membrane interactions via cyclic GMP-dependent protein kinase 2. Mol. Cell. Biol. 36, 3086-3099. 10.1128/MCB.00365-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E. and Philips M. R. (1999). Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98, 69-80. 10.1016/S0092-8674(00)80607-8 [DOI] [PubMed] [Google Scholar]
- Chung J., Torta F., Masai K., Lucast L., Czapla H., Tanner L. B., Narayanaswamy P., Wenk M. R., Nakatsu F. and De Camilli P. (2015). Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts . Science 349, 428-432. 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J. and Stock J. (1988). Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc. Natl. Acad. Sci. USA 85, 4643-4647. 10.1073/pnas.85.13.4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf P., Klapisz E. E., Schulz T. K., Cremers A. F., Verkleij A. J. and van Bergen en Henegouwen P. M. (2002). Nuclear localization of phosphatidylinositol 4-kinase beta. J. Cell Sci. 115, 1769-1775. [DOI] [PubMed] [Google Scholar]
- Demmel L., Beck M., Klose C., Schlaitz A.-L., Gloor Y., Hsu P. P., Havlis J., Shevchenko A., Krause E., Kalaidzidis Y. et al. (2008). Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase Pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol. Biol. Cell 19, 1046-1061. 10.1091/mbc.e07-02-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M. R. and Walker J. E. (1993). Sequences of members of the human gene family for the c subunit of mitochondrial ATP synthase. Biochem. J. 293, 51-64. 10.1042/bj2930051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M. R., Gay N. J. and Walker J. E. (1989). DNA sequences of a bovine gene and of two related pseudogenes for the proteolipid subunit of mitochondrial ATP synthase. Biochem. J. 260, 249-258. 10.1042/bj2600249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke T. F., Kaplan D. R., Cantley L. C. and Toker A. (1997). Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275, 665-668. 10.1126/science.275.5300.665 [DOI] [PubMed] [Google Scholar]
- Garcia P., Gupta R., Shah S., Morris A. J., Rudge S. A., Scarlata S., Petrova V., McLaughlin S. and Rebecchi M. J. (1995). The pleckstrin homology domain of phospholipase C-delta 1 binds with high affinity to phosphatidylinositol 4,5-bisphosphate in bilayer membranes. Biochemistry 34, 16228-16234. 10.1021/bi00049a039 [DOI] [PubMed] [Google Scholar]
- Gulyás G., Radvánszki G., Matuska R., Balla A., Hunyady L., Balla T. and Várnai P. (2017). Plasma membrane phosphatidylinositol 4-phosphate and 4,5-bisphosphate determine the distribution and function of K-Ras4B but not H-Ras proteins. J. Biol. Chem. 292, 18862-18877. 10.1074/jbc.M117.806679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J. and Hancock J. F. (1989). Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 8, 1093-1098. 10.1002/j.1460-2075.1989.tb03478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J., Qi X., Pallotti F., Schon E. A., Manfredi G., Carelli V., Martinuzzi A., Hauswirth W. W. and Lewin A. S. (2002). Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann. Neurol. 52, 534-542. 10.1002/ana.10354 [DOI] [PubMed] [Google Scholar]
- Hammond G. R. V., Fischer M. J., Anderson K. E., Holdich J., Koteci A., Balla T. and Irvine R. F. (2012). PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727-730. 10.1126/science.1222483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. R. V., Machner M. P. and Balla T. (2014). A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 205, 113-126. 10.1083/jcb.201312072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F. (2003). Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 4, 373-384. 10.1038/nrm1105 [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E. and Marshall C. J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167-1177. 10.1016/0092-8674(89)90054-8 [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H. and Marshall C. J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133-139. 10.1016/0092-8674(90)90294-O [DOI] [PubMed] [Google Scholar]
- Heit B., Yeung T. and Grinstein S. (2011). Changes in mitochondrial surface charge mediate recruitment of signaling molecules during apoptosis. Am. J. Physiol. Cell Physiol. 300, C33-C41. 10.1152/ajpcell.00139.2010 [DOI] [PubMed] [Google Scholar]
- Higuti T., Kawamura Y., Kuroiwa K., Miyazaki S. and Tsujita H. (1993). Molecular cloning and sequence of two cDNAs for human subunit c of H+-ATP synthase in mitochondria. Biochim. Biophys. Acta 1173, 87-90. 10.1016/0167-4781(93)90249-D [DOI] [PubMed] [Google Scholar]
- James S. R., Downes C. P., Gigg R., Grove S. J., Holmes A. B. and Alessi D. R. (1996). Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem. J. 315, 709-713. 10.1042/bj3150709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckheere A. I., Smeitink J. A. M. and Rodenburg R. J. T. (2012). Mitochondrial ATP synthase: architecture, function and pathology. J. Inherit. Metab. Dis. 35, 211-225. 10.1007/s10545-011-9382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay B. M., McCormack R., Wang Y., Tsoulfas P. and Zhai R. G. (2013). Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLD(S)-mediated axon protection independent of axonal mitochondria. Hum. Mol. Genet. 22, 1601-1614. 10.1093/hmg/ddt009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Tóth B. et al. (2006). A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733-747. 10.1016/j.cell.2006.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. T. S. and Conibear E. (2015). ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife 4, e11306 10.7554/eLife.11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G. and Kroemer G. (2013). Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 23, 1247-1248. 10.1038/cr.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Smith D. H., Chen E. Y., Seeburg P. H., Goeddel D. V. and Levinson A. D. (1983). Structure and organization of the human Ki-ras proto-oncogene and a related processed pseudogene. Nature 304, 501-506. 10.1038/304501a0 [DOI] [PubMed] [Google Scholar]
- Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G. and Antonny B. (2013). A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155, 830-843. 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]
- Mookerjee S. A., Nicholls D. G. and Brand M. D. (2016). Determining maximum glycolytic capacity using extracellular flux measurements. PLoS ONE 11, e0152016 10.1371/journal.pone.0152016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser von Filseck J., Copic A., Delfosse V., Vanni S., Jackson C. L., Bourguet W. and Drin G. (2015). Intracellular transport. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate . Science 349, 432-436. 10.1126/science.aab1346 [DOI] [PubMed] [Google Scholar]
- Oca-Cossio J., Kenyon L., Hao H. and Moraes C. T. (2003). Limitations of allotopic expression of mitochondrial genes in mammalian cells. Genetics 165, 707-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra K. C. and Hay N. (2014). The pentose phosphate pathway and cancer. Trends Biochem. Sci. 39, 347-354. 10.1016/j.tibs.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy A. K., Moore J. F., Carson M. A. and Waechter C. J. (1983). Characterization of brain phosphatidylserine decarboxylase: localization in the mitochondrial inner membrane. Arch. Biochem. Biophys. 223, 484-494. 10.1016/0003-9861(83)90613-6 [DOI] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A. and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281-2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A. and Bastiaens P. I. (2005). An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746-1752. 10.1126/science.1105654 [DOI] [PubMed] [Google Scholar]
- Rocks O., Gerauer M., Vartak N., Koch S., Huang Z.-P., Pechlivanis M., Kuhlmann J., Brunsveld L., Chandra A., Ellinger B. et al. (2010). The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141, 458-471. 10.1016/j.cell.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Roy S., Plowman S., Rotblat B., Prior I. A., Muncke C., Grainger S., Parton R. G., Henis Y. I., Kloog Y. and Hancock J. F. (2005). Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25, 6722-6733. 10.1128/MCB.25.15.6722-6733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim A. A., Cho K.-J., Tan L., Quezada M., Lacey E., Hancock J. F. and Capon R. J. (2014a). Rare streptomyces N-formyl amino-salicylamides inhibit oncogenic K-Ras. Org. Lett. 16, 5036-5039. 10.1021/ol502376e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim A. A., Xiao X., Cho K.-J., Piggott A. M., Lacey E., Hancock J. F. and Capon R. J. (2014b). Rare Streptomyces sp. polyketides as modulators of K-Ras localisation. Org. Biomol. Chem. 12, 4872-4878. 10.1039/C4OB00745J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim A. A., Tan L., Huang X.-C., Cho K.-J., Lacey E., Hancock J. F. and Capon R. J. (2015). Oligomycins as inhibitors of K-Ras plasma membrane localisation. Org. Biomol. Chem. 14, 711-715. 10.1039/C5OB02020D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmick M., Vartak N., Papke B., Kovacevic M., Truxius D. C., Rossmannek L. and Bastiaens P. I. (2014). KRas localizes to the plasma membrane by spatial cycles of solubilization, trapping and vesicular transport. Cell 157, 459-471. 10.1016/j.cell.2014.02.051 [DOI] [PubMed] [Google Scholar]
- Segawa K., Kurata S., Yanagihashi Y., Brummelkamp T. R., Matsuda F. and Nagata S. (2014). Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164-1168. 10.1126/science.1252809 [DOI] [PubMed] [Google Scholar]
- Sohn M., Korzeniowski M., Zewe J. P., Wills R. C., Hammond G. R. V., Humpolickova J., Vrzal L., Chalupska D., Veverka V., Fairn G. D. et al. (2018). PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J. Cell Biol. 217, 1797-1813. 10.1083/jcb.201710095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen S. D., Linseman D. A., McEwen E. L., Heacock A. M. and Fisher S. K. (1998). A role for a wortmannin-sensitive phosphatidylinositol-4-kinase in the endocytosis of muscarinic cholinergic receptors. Mol. Pharmacol. 53, 827-836. [PubMed] [Google Scholar]
- Stanton R. C. (2012). Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 64, 362-369. 10.1002/iub.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Aasland R., Toh B.-H. and D'Arrigo A. (1996). Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271, 24048-24054. 10.1074/jbc.271.39.24048 [DOI] [PubMed] [Google Scholar]
- Strahl T., Hama H., DeWald D. B. and Thorner J. (2005). Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J. Cell Biol. 171, 967-979. 10.1083/jcb.200504104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G., Kinnaird A., Dromparis P., Paulin R., Stenson T. H., Haromy A., Hashimoto K., Zhang N., Flaim E. and Michelakis E. D. (2014). A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84-97. 10.1016/j.cell.2014.04.046 [DOI] [PubMed] [Google Scholar]
- Swarthout J. T., Lobo S., Farh L., Croke M. R., Greentree W. K., Deschenes R. J. and Linder M. E. (2005). DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280, 31141-31148. 10.1074/jbc.M504113200 [DOI] [PubMed] [Google Scholar]
- Tan L., Cho K. J., Neupane P., Capon R. J. and Hancock J. F. (2018). An oxanthroquinone derivative that disrupts RAS plasma membrane localization inhibits cancer cell growth. J. Biol. Chem. 293, 13696-13706. 10.1074/jbc.RA118.003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen J. A., Gross J. M., Subramanian K., Meyer T. and Casey P. J. (1997). Prenylation-dependent association of Ki-Ras with microtubules. Evidence for a role in subcellular trafficking. J. Biol. Chem. 272, 30362-30370. 10.1074/jbc.272.48.30362 [DOI] [PubMed] [Google Scholar]
- Tóth B., Balla A., Ma H., Knight Z. A., Shokat K. M. and Balla T. (2006). Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 281, 36369-36377. 10.1074/jbc.M604935200 [DOI] [PubMed] [Google Scholar]
- Tsai F. D., Lopes M. S., Zhou M., Court H., Ponce O., Fiordalisi J. J., Gierut J. J., Cox A. D., Haigis K. M. and Philips M. R. (2015). K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc. Natl. Acad. Sci. USA 112, 779-784. 10.1073/pnas.1412811112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Hasegawa J., Chinnapen D., Inoue T., Okazaki S., Kato R., Wakatsuki S., Misaki R., Koike M., Uchiyama Y. et al. (2011). Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc. Natl. Acad. Sci. USA 108, 15846-15851. 10.1073/pnas.1109101108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D., Cho K. J., Ma X., Chigurupati S., Parton R. G. and Hancock J. F. (2013). Fendiline inhibits K-Ras plasma membrane localization and blocks K-Ras signal transmission. Mol. Cell. Biol. 33, 237-251. 10.1128/MCB.00884-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D., Cho K. J., Zhou Y., Ma X., Chen W., Naji A., Montufar-Solis D., Zuo Y., Kovar S. E., Levental K. R. et al. (2017). Sphingomyelin metabolism is a regulator of K-Ras function. Mol. Cell. Biol. 38, e00373-17 10.1128/MCB.00373-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P., Thyagarajan B., Rohacs T. and Balla T. (2006). Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377-382. 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C., Magrane J., Andreu A. L. and Manfredi G. (2010). Novel role of ATPase subunit C targeting peptides beyond mitochondrial protein import. Mol. Biol. Cell 21, 131-139. 10.1091/mbc.e09-06-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. and Deschenes R. J. (2006). Plasma membrane localization of Ras requires class C Vps proteins and functional mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 3243-3255. 10.1128/MCB.26.8.3243-3255.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., Kirchhausen T., Albanesi J. P., Roth M. G. and Yin H. L. (2003). Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299-310. 10.1016/S0092-8674(03)00603-2 [DOI] [PubMed] [Google Scholar]
- Weixel K. M., Blumental-Perry A., Watkins S. C., Aridor M. and Weisz O. A. (2005). Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J. Biol. Chem. 280, 10501-10508. 10.1074/jbc.M414304200 [DOI] [PubMed] [Google Scholar]
- Wiedemann C., Schäfer T. and Burger M. M. (1996). Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 15, 2094-2101. 10.1002/j.1460-2075.1996.tb00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G. and Lowy D. R. (1984). The p21 ras C-terminus is required for transformation and membrane association. Nature 310, 583-586. 10.1038/310583a0 [DOI] [PubMed] [Google Scholar]
- Yan W. L., Lerner T. J., Haines J. L. and Gusella J. F. (1994). Sequence analysis and mapping of a novel human mitochondrial ATP synthase subunit 9 cDNA (ATP5G3). Genomics 24, 375-377. 10.1006/geno.1994.1631 [DOI] [PubMed] [Google Scholar]
- Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A. and Grinstein S. (2008). Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210-213. 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang Z., Lu M., Yonekubo Y., Liang X., Zhang Y., Wu P., Zhou Y., Grinstein S., Hancock J. F. et al. (2014). Temporal production of the signaling lipid phosphatidic acid by phospholipase D2 determines the output of extracellular signal-regulated kinase signaling in cancer cells. Mol. Cell. Biol. 34, 84-95. 10.1128/MCB.00987-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Prakash P., Liang H., Cho K. J., Gorfe A. A. and Hancock J. F. (2017). Lipid-sorting specificity encoded in K-Ras membrane anchor regulates signal output. Cell 168, 239-251.e16. 10.1016/j.cell.2016.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.