Abstract
Background:
Older adults have high obesity rates and respond well to evidence-based weight loss programs, such as the Diabetes Prevention Program (DPP) Lifestyle intervention.
Purpose:
To determine whether a lay-health-educator-delivered translation of the DPP Lifestyle program conducted in senior centers is effective in promoting weight loss among older adults.
Design:
A randomized, controlled trial with older adults nested within senior centers. Senior centers identified lay health educators or “coaches” to receive training and deliver the intervention program at the senior center. Senior centers were randomized to DPP Lifestyle program or an attention control intervention (cognitive training).
Setting:
Senior centers (N=15) located throughout Arkansas.
Participants:
Participants (N=228) were obese (BMI=34.5±4.9), older (71.2± 6.6 years) adults able to engage in moderate exercise. Four-month follow-up data were collected on 93% of the original cohort between February 2009 and July 2010.
Intervention:
A 12-session translation of the Diabetes Prevention Lifestyle behavioral weight control program delivered in group sessions by trained lay health educators.
Main Outcome Measure(s):
Body weight was assessed by digital scale. Percent weight loss from baseline and proportion achieving ≥5% and ≥7% weight loss were examined. Analyses were completed in July 2011.
Results:
Participants attending senior centers randomized to Lifestyle lost a significantly greater percent of baseline weight (3.8% , 95% CI, 2.9 – 4.6%) than those in the control senior centers (0.2%, 95% CI, −.6 - .9%) after adjusting for baseline BMI and gender (p < .001). Among participants attending senior centers offering the Lifestyle program, 38% lost ≥ 5% of baseline weight compared with 5% in the control arm (p<.001). Similarly, significantly more participants (24%) in Lifestyle senior centers lost ≥ 7% than did control participants (3%, p=.001).
Conclusions:
A lay-health-educator-delivered behavioral lifestyle weight loss intervention offers a promising vehicle for translation of evidence-based obesity treatment programs in underserved areas.
Obesity rates have risen rapidly in recent decades,1 with accompanying rises in co-morbid conditions, including type 2 diabetes mellitus and cardiovascular disease.2 Older adults are particularly likely to be obese1 and chronic conditions associated with obesity are correspondingly elevated among older age groups.3 The confluence of aging baby boomers and high rates of obesity among older adults has public health experts concerned.4
Weight loss through lifestyle change markedly improves cardiovascular risk and metabolic profile,5–8 and this improvement can be sustainable.9 The success of the Diabetes Prevention Program (DPP) Lifestyle Intervention in achieving a 7% weight loss and forestalling the development of type 2 diabetes mellitus has inspired subsequent behavioral interventions to adapt the DPP program elements for other populations.8, 10 Of particular note in the DPP weight loss outcomes was the efficacy of the Lifestyle intervention across all race-ethnicity groups11 and older adults.12 Thus, the DPP intervention has the potential to significantly impact the health of obese individuals broadly, and older adults are likely to benefit in particular.
Rural regions present challenges for translation and dissemination of evidence-based interventions because of limited access.13 Yet these regions have higher proportion of older adults13 and obese individuals.14, 15 Thus, rural regions are in special need of accessible evidence-based behavioral weight control programs. Interventions involving trained lay people have been suggested as a best-practice strategy for increasing access and disseminating effective health behavior interventions to underserved and high risk communities.16 Lay health educators (LHEs) are community members similar to the target population who offer health-related outreach, system navigation, and/or direct services.17 Lay health educators are also known as community health educators, community health workers, promotoras, peer educators or other names in the literature.16, 18 For the sake of simplicity, we will use the term lay health educator (LHE) throughout this report. LHEs may be particularly helpful in rural areas with sparse health care resources and close knit communities.19 Programs utilizing LHEs to implement behavioral lifestyle interventions have been effective in reducing risk factors for a variety of chronic diseases, including diabetes20 and cardiovascular disease.21 Initial efforts to disseminate the DPP Lifestyle program into clinical22–28 and community settings29–32 have been described, but these adaptations of the DPP program have predominantly utilized health care professionals to implement the intervention. A single uncontrolled study using community members to deliver an adaptation of the DPP reported very modest outcomes.33 Additional exploration of translations of the DPP delivered by LHEs is warranted.
The current study was undertaken to determine whether a LHE-delivered adaptation of the DPP Lifestyle program would be effective in promoting weight loss among obese older adults in rural communities. Senior centers were selected as a well-established community-based venue from which to deliver the intervention because of the potential for broad dissemination due to their presence in all US states,34 the strong encouragement for senior centers to offer evidence-based health and wellness programs,35 and the potential for sustainability given the existing infrastructure. Recent estimates indicate there are nearly 11,000 senior centers,36 providing an extensive platform for building dissemination efforts should the LHE-delivered translation of the DPP intervention prove effective.
Methods
Study Design
This cluster-randomized, controlled trial was conducted in 15 senior centers across the state of Arkansas in which LHEs (or “Coaches”) could be identified from among community volunteers or employees from the senior center. Senior centers were randomized by computer- generated random numbers to either a Lifestyle weight loss program or to a cognitive training program designed to serve as an attention control, matched in contact time, duration and structure. Older adult participants were clustered within senior centers, which were the unit of randomization. Analyses were conducted in Winter 2011. All study procedures followed a written protocol and all Coaches and older adults provided written consent. The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board.
Senior Centers
Senior centers were recruited by mail, phone and personal contact by study investigators at meetings attended by senior center administrators. To participate, senior centers had to agree to be randomized. One of the challenges noted about conducting randomized trials in community settings is that randomization to an assessment-only control group which receives no treatment can provoke concerns.37 To address this challenge and enhance community acceptability, individuals recruited in senior centers randomized to the control arm were offered a cognitive training program, details of which are presented elsewhere.38 In short, this program taught basic information about how the brain functions, memory processes, how aging and other factors affect these processes, and multiple cognitive strategies to enhance memory functioning. Of note, the control program provided no calorie guidelines or physical activity goals, offered no behavioral strategies for weight loss or self-monitoring of dietary intake or exercise and did not weigh participants weekly.
Additionally, senior centers were asked to identify two to three “Coaches” who were willing to be trained and to implement the program at the center. Senior centers also had to be willing to provide space for the group sessions and private data collection visits, and to recruit approximately 18 eligible older adults to participate in the program. Senior centers were not paid for participation; however all intervention materials were provided to facilitate program implementation.
A total of 15 senior centers were recruited and randomized from June 2008 to February 2010. A 16th senior center was recruited but withdrew prior to learning randomization allocation due to last minute staffing changes and prior to enrolling any senor adults. Participating centers provided an average of 2.7 Coaches and represented 8 unique counties throughout the state (11% of the counties in the state). Forty percent of Coaches were community volunteers and the remainder were existing senior center staff. Coaches did not have backgrounds in lifestyle intervention nor were they healthcare professionals; two of the Coaches were cooks in the senior center or at a nursing home, and therefore were knowledgeable about food preparation. Coaches were not paid for delivering the intervention sessions. The majority (90%) were women, with an average age of 59 ± 12 years. Attrition among the Coaches was low, with 95% (all but 2) remaining as Coaches at 4 months; 1 coach moved and 1 withdrew due to change in employment.
Older Adult Participants
Older adult participants were required to be community-dwelling, 60 years of age or older, obese (BMI ≥ 30) and able to engage in moderate physical activity such as walking, swimming or riding a bike. They had to be free of serious memory problems (Mini Mental State Exam39 score ≥23) and available to participate for the duration of the study. Exclusion criteria included significant recent weight loss or concurrent weight loss treatment, and self-report of a recent heart attack, stroke, or other health conditions that would contraindicate participation in a weight loss program. To reach a larger population of overweight individuals who could benefit from weight loss, eligibility was not limited to individuals with prediabetes as in the original DPP.6 Thus, individuals with type 2 diabetes, hypertension and other obesity-related conditions were eligible to enroll. Recruitment efforts were led by the Coaches from the senior center and were supported by a recruitment toolkit provided by research staff. Older adults were offered small incentives upon completion of follow-up data collection (e.g., T-shirt, pillbox).
Diabetes Prevention Program (DPP) Lifestyle Intervention
The intervention delivered was an adaptation of the publically-available DPP Lifestyle Intervention40 modified so that it was appropriate for delivery in a group setting in twelve weekly sessions, following the methods of other adaptations of the DPP Lifestyle intervention for delivery by health care professionals.25 Program goals included 7% weight loss, calorie restriction with ≤25% of calories from fat, and graded physical activity goals progressing to 150 min/week of moderate to vigorous exercise (i.e., walking). Pedometers were provided to assist in increasing walking, and self-monitoring diaries were provided with the instruction to record all dietary intake and physical activity. Diaries were reviewed by Coaches weekly and returned to older adults with feedback to reinforce behavior changes and identify targets for additional modification. Behavioral strategies to support habit change were introduced each week and included self-monitoring, stimulus control, problem solving, goal setting, and relapse prevention. All sessions followed a structured protocol that outlined the material to be covered by the Coaches, provided a script that Coaches could follow, and were accompanied by lesson handouts for older adults. Group sessions lasted 60 minutes and were delivered by the Coaches, who weighed participants prior to beginning the intervention session. In contrast with the original DPP Lifestyle Intervention, the current translation did not include the toolbox funds to reduce barriers and promote dietary and physical activity change ($100 per participant per year).40
Training to Deliver Lifestyle Intervention
Coaches were trained in all aspects of delivering the Lifestyle program by research staff. Training was skills-based and followed a structured protocol which focused on the evidence base for the program, the key elements of a behavioral weight control approach and the importance of protocol fidelity. Intervention goals were introduced and behavioral strategies to achieve these goals were discussed, with particular attention paid to giving feedback on self-monitoring diaries as self-monitoring has been consistently associated with weight loss in programs delivered by professionals.41 Coaches were trained in recruitment methods and techniques for conducting effective group sessions, and individual session materials were reviewed and rehearsed. A total of 32 hours of face-to-face training was provided to prepare Coaches to deliver the twelve weekly sessions and to participate in the responsible conduct of research (all Coaches obtained human subjects protection certification). Once group sessions at a senior center were underway, a weekly technical support conference call between a research team member and the Coaches reviewed the group process, problem solved difficulties that surfaced, and monitored attendance at groups and weight losses of participants. In addition, in vivo observation of program delivery by a research team member offered an opportunity to monitor fidelity to protocol and provide constructive feedback or augment training.
Measures
Body weight was measured in street clothes without shoes using a calibrated digital scale (Tanita BWB 800) at baseline and 4-month follow-up (after the 12-week program). Height was measured at baseline using a stadiometer (Seca Corporation, Hanover, MD). Body mass index was calculated as weight (kg)/ height squared (m2). Demographic characteristics were obtained by self-report questionnaire at baseline. Coaches recorded participant attendance at group sessions and self-monitoring diary submission on process logs which were submitted to the research team weekly.
Statistical Analysis
Treatment group comparisons between Lifestyle and control senior centers were conducted using general linear mixed models, with the covariance structure accounting for variability between clusters (senior centers). Percent weight reduction from baseline visit to 4-month follow-up was the primary outcome. A similar generalized linear mixed model approach for binary outcomes was used to test the equality of proportions achieving a clinically-significant weight loss (≥5%) between the two treatment arms, after adjusting for baseline weight. To avoid introducing bias associated with attrition due to failure to lose weight, missing weight values were imputed conservatively assuming no weight change from baseline among participants lost to follow-up. Baseline characteristics were analyzed for differences between the two treatment arms, and between those lost to follow up and those retained. A similar approach was used for modeling the relationship between treatment adherence parameters (attendance and completion of self-monitoring diaries) and weight loss.
The a priori sample size calculations were powered to detect a difference of 3.5 kg between the group means with a standard deviation of 7 and an intracluster correlation of 0.02 using a two-sided t-test with a significance level of 0.05. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC). Alpha was set at .05.
Results
Older Adult Participants
A total of 228 older adults were recruited and enrolled. On average, each senior center recruited 15.2 ± 3.8 older adult participants (range = 8 – 21). The majority were female and moderately to severely obese as reflected in an average BMI over 36, with more women and heavier participants in the Lifestyle arm (Table 1). Therefore, all subsequent analyses controlled for these variables. There were no other significant baseline differences between the Lifestyle and control conditions.
Table 1:
Baseline Characteristics of Enrolled Older Adults
| Total Sample (N=228) |
Lifestyle Arm (N=116) |
Controls (N=112) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Age, mean (sd) | 71.2 | (6.6) | 70.6 | (6.6) | 71.9 | (6.6) | 0.257 |
| Gender, % female | 84 | 91 | 77 | 0.004 | |||
| Race, % White | 92 | 92 | 92 | 0.878 | |||
| Completed High School, % | 89 | 91 | 88 | 0.741 | |||
| Married, % | 49 | 45 | 53 | 0.507 | |||
| Living with another adult in household, % | 55 | 53 | 56 | 0.790 | |||
| Children living in household with senior adult (1 or more), % | 4.4 | 1.7 | 7.1 | 0.083 | |||
| Current employment status, % | 12.7 | 13.8 | 11.6 | 0.642 | |||
| Currently employed (full, part, self) | |||||||
| Retired or disabled | 80.3 | 81.9 | 78.6 | ||||
| Other (Volunteer, homemaker) | 7.0 | 4.3 | 9.8 | ||||
| Body Mass Index, mean (sd) | 36.1 | (5.1) | 37.1 | (5.7) | 35.0 | (4.2) | 0.003 |
| Baseline Body Weight (kg), mean (sd) | 94.1 | (15.4) | 95.0 | (17.0) | 93.1 | (17.0) | 0.385 |
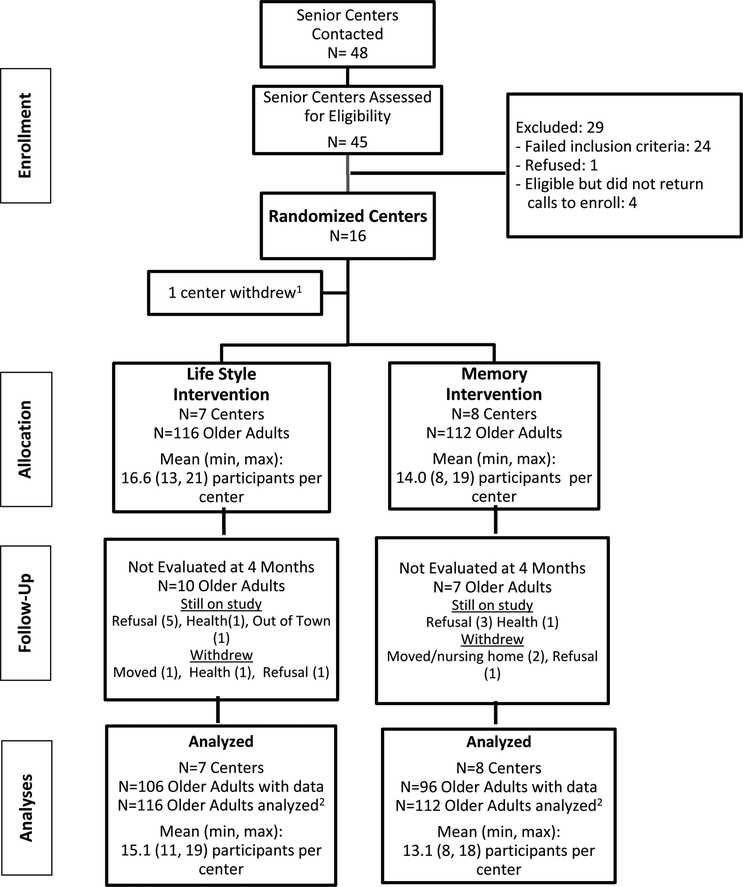
Follow-up assessments were conducted with 211 (93%) older adults at four months (Figure 1). The baseline characteristics of those who provided follow-up data were similar to those who did not. Further, older adults from Lifestyle senior centers were no more likely to be missing from follow-up assessment than controls (9% vs. 6%, respectively).
Figure 1.
Study Flow Diagram
1 Unable to participate due to last minute staffing changes prior to learning randomization allocation
2 Missing 4-month assessments imputed as no change
Weight Loss
Participants attending senior centers randomized to Lifestyle lost a significantly greater percentage of baseline weight than those in the control senior centers (Table 2). The intraclass correlation was .02. Participants in the Lifestyle arm had achieved an average weight loss of 3.7 kg compared with an average of 0.3 kg in the control arm. To further explore weight loss outcomes, the proportions of older adults achieving a clinically-significant weight loss were examined (Table 2). After adjusting for baseline weight, BMI, and gender, participants in the Lifestyle arm had a 9.7 times higher odds (95% CI, 3.5–26.8) of achieving ≥5% weight reduction as compared to those in the control arm (p<.001). We also examined weight loss outcomes using the DPP goal of ≥ 7% reduction from baseline weight loss and found that those in the Lifestyle arm had a 10.0 times greater odds of achieving this benchmark than those in the control arm (95% CI, 2.5–40.3, p=0.001).
Table 2:
Weight Change at 4-month Follow-up
| Lifestyle (N=116) |
Control (N=112) |
Adjusted p-value* | |
|---|---|---|---|
| Weight Change (kg), mean (SD) Range | −3.7±3.7 −15.3 to +4.3 |
−0.3± 2.4 kg −8.0 to +5.1 |
<.001 |
| Percent Baseline Weight Change, mean (SD) Range | −3.9 (3.8) −15.0 to +5.4 |
−0.3% −10 to +4.9 |
<.001 |
| Proportion Losing ≥ 7% | 24% | 3% | .001 |
| Proportion Losing ≥ 5% | 38% | 5% | <.001 |
Adjusting for baseline weight, baseline BMI, and gender and accounting for clustering of seniors in centers using baseline observation carry forward for 17 missing 4-month values.
Treatment Program Adherence
Older adults offered the Lifestyle program attended 9.1 ± 3.3 of the 12 Lifestyle sessions, with a majority (86%) attending at least 50% of the sessions. An average of 8.3 ± 3.4 self-monitoring diaries were submitted over the 12-week intervention. Weight loss was associated with both attendance at group sessions (Pearson r = −0.40, p<0.001) and number of self-monitoring diaries submitted (Pearson r = −0.46, p<0.001). After accounting for clustering within centers, each self-monitoring diary submitted was associated with .5% (95% CI, .3-.7%) weight loss (p<0.001), and each session attended was associated with .5% (95% CI, .3-.6%) loss (p<0.001).
Predictors of Treatment Success
Baseline characteristics and program adherence were examined to determine what factors might be associated with success in achieving ≥5% weight loss. Baseline characteristics included age, gender, education, marital status, and employment status. Because there were so few non-whites in the sample, race was not considered. None of these characteristics were associated with clinically-significant weight loss. However, intervention adherence was associated with achieving clinically-significant weight loss, even after accounting for demographic factors. After adjusting for participant characteristics, those who submitted ≥ 50% of self-monitoring diaries had 7.6 (95% CI, 1.6–35.7) times higher odds of losing ≥ 5% at 4 months as compared to those who submitted less than half of their self-monitoring diaries (p=0.011). In an analogous model, those who attended 50% or more of sessions had 11.6 (95% CI, 1.4–100.0) higher odds of losing 5% or more at 4 months as compared to those who did not (p=0.025).
Conclusions
To our knowledge, few published studies have conducted a randomized controlled trial to test the effectiveness of an evidence-based weight loss intervention as delivered by LHEs in community settings, and those that have been published have not utilized the successful DPP as the intervention model. The current study demonstrates that trained LHEs can successfully implement a behavioral lifestyle weight loss program adapted from the DPP to older adults in a community setting, and at 4-months participants in senior centers randomized to the Lifestyle program achieved significantly greater average weight losses than participants from control senior centers. Of note, over a third of older adults in senior centers delivering the Lifestyle program achieved a weight loss of 5% or greater, a degree of weight loss associated with clinical improvements.42 Further, the outcomes in the current study compare well with studies translating the DPP into community settings in programs delivered by wellness or health care professionals which report about a 4% weight loss30, 31 or achieve at least a 5% weight loss in 39% of participants.32
Older adults responded well to the LHE-delivered program offered at their local senior center. Most participants (86%) attended at least half of the sessions provided, comparing favorably to reports of attendance among other studies implementing a DPP translation in community settings. For example, the DEPLOY study, which offered a 16-session, group-based behavioral weight control program in a YMCA, reported an average attendance of 57% of available sessions.30
In the current study, session attendance and self-monitoring predicted greater weight loss. These are identical to weight loss predictors for participants in behavioral weight control programs delivered by trained healthcare professionals in randomized clinical trials.43 Further, studies translating the DPP using health care professionals to implement the weight loss program23, 24 have similarly found greater self-monitoring to be associated with greater weight loss. This suggests that the group implementation process is similar between LHEs in the community and health care professionals, and provides confidence that LHEs embedded in community settings are a tenable vehicle for disseminating evidence-based weight control interventions.
These findings are consistent with previous studies demonstrating that lay health workers can contribute to improvements in lifestyle behaviors to reduce risk for chronic diseases associated with obesity.21, 44–46 However, the results of LHE-led interventions for chronic disease are mixed and conclusions are limited by multiple methodological problems.46 The present study addresses many of these issues by testing an evidence-based intervention, using a randomized controlled design and an appropriate sample size, and including an “attention control” group with equivalent structure and contact time.
There are substantive differences between the original DPP and the intervention implemented in the current study which should be noted. This community-based translation was developed with considerations of dissemination to underserved areas and sustainability and therefore featured fewer sessions, a group delivery format, and none of the toolbox funds utilized in the DPP to encourage behavior change. Eligibility criteria differed slightly as well, with participation open to overweight individuals who did not have prediabetes. Adaptations made in the current intervention are very similar to those made by others translating the DPP into practice and community settings. However, other factors such as the reduced number of sessions, and the lack of free meal replacements or toolbox funds to facilitate habit change, as well as the use of lay health educators rather than dietitians or masters-level health professionals may account for, at least in part, the smaller weight losses observed in the current study than achieved in the original DPP study.
The study has limitations that merit consideration. Substantially more women were enrolled than men, as might be expected from the demographics of this age group47 and the population which tends to frequent senior centers.48 Further study of LHE-delivered behavioral weight control is warranted before generalization to older men should be made. In addition, mean weight losses achieved at 4-months after a 12-session program (−3.67 kg) were lower than the 7.05 kg weight loss achieved at 6-months in the DPP after 16-sessions,11 although weight losses may increase in the current cohort as participants continue to apply the behavioral skills learned in the program. However, there is good reason to believe that weight re-gain will occur once regular treatment sessions are discontinued.49 Longer-term outcomes for the LHE-delivered program will provide insight regarding this.
Implications
Trained LHEs hold significant promise for the translation of the DPP into community settings, with senior centers offering a promising venue for delivery given the success in the current study and the existing infrastructure provided in rural settings by senior centers.
Acknowledgements
This research was supported in part by a research grant from the Centers for Disease Prevention and Control (R18 DP001145).
None of the authors has any financial disclosures.
References
- 1.Ogden C, Carroll M, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association 2006;295:1549–1555. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen N, Magno C, Lane K, Hinojosa M, Lane J. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. Journal of the American College of Surgeons 2008;207:928–34. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, the Merck Company Foundation. The state of aging and health in America 2007. In. Whithouse Station, NJ: The Merck Company Foundation; 2007. [Google Scholar]
- 4.Arterburn D, Crane P, Sullivan S. The coming epidemic of obesity in elderly Americans. Journal of the American Geriatric Society 2004;52:1907–12. [DOI] [PubMed] [Google Scholar]
- 5.Whelton P, Appel L, Espeland M, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled Trial of Nonpharmacologic Interventions in the Elderly (TONE). Journal of the American Medical Association 1998;279:839–46. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 2005;28:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Look AHEAD research group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2007;30(6):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look AHEAD Research Group. Long-term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes Mellitus. Arch Intern Med 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subak L, Wing R, West D, Franklin F, Vittinghoff E, Creasman J, et al. Randomized trial of a behavioral weight loss program for urinary incontinence in overweight and obese women. New England Journal of Medicine 2009;360:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of Black, White, and Hispanic men and women in the Diabetes Prevention Program (DPP). Obesity 2008;16:1413–1420. [DOI] [PubMed] [Google Scholar]
- 12.Wing RR, Hamman R, Bray G, Delahanty L, Edelstein S, Hill J, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obesity Research 2004;12:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goins RT, Krout JA, editors. Service Delivery to Rural Older Adults: Research, Policy and Practice. New York, NY: Springer Publishing Company; 2006. [Google Scholar]
- 14.Jackson J, Doescher M, Jerant A, Hart L. A national study of obesity prevalence and trends by type of rural county. Journal of Rural Health 2005;21:140–8. [DOI] [PubMed] [Google Scholar]
- 15.Patterson P, Moore C, Probst J, Shinogle J. Obesity and physical inactivity in rural America. Journal of Rural Health 2004;20:151–159. [DOI] [PubMed] [Google Scholar]
- 16.Smedley BD, Stith AY, Nelson AR, Institute of Medicine (U.S.). Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care Unequal treatment : confronting racial and ethnic disparities in health care. Washington, D.C.: National Academy Press; 2003. [PubMed] [Google Scholar]
- 17.HRSA. Community Health Worker National Workforce Study. In: USDHHS, editor. Washington, DC; 2007. [Google Scholar]
- 18.Quinn MT. Training lay health educators to conduct a church-based weight-loss program for African American women. Diabetes Educator 2001;27(2):231–238. [DOI] [PubMed] [Google Scholar]
- 19.Raczynski JM, Cornell CE, Stalker V, Phillips M, Dignan M, Pulley L, et al. Developing community capacity and improving health in African American communities. American Journal of the Medical Sciences 2001;322(5):294–300. [PubMed] [Google Scholar]
- 20.Norris S, Chowdhury F, Van Let K, Horsley T, Bronstein N, Zhang X, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabetic Medicine 2006;23:544–556. [DOI] [PubMed] [Google Scholar]
- 21.Brownstein J, Bone L, Dennison C, Hill M, Kim M, Levine D. Community health workers as interventionists in the prevention and control of heart disease and stroke. American Journal of Preventive Medicine 2005;29(5S1):128–133. [DOI] [PubMed] [Google Scholar]
- 22.Mcbride PE, Einerson JA, Grant H, Sargent C, Underbakke G, Vitcenda M, et al. Putting the Diabetes Prevention Program into Practice: A Program for Weight Loss and Cardiovascular Risk Reduction for Patients with Metabolic Syndrome or Type 2 Diabetes Mellitus. Journal of Nutrition Health & Aging 2008;12(10):745s–749s. [DOI] [PubMed] [Google Scholar]
- 23.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma YS. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychology 2008;27(1):S91–S98. [DOI] [PubMed] [Google Scholar]
- 24.Amundson HA, Butcher MK, Gohdes D, Hall TO, Harwell TS, Helgerson SD, et al. Translating the Diabetes Prevention Program Into Practice in the General Community Findings From the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educator 2009;35(2):209–223. [DOI] [PubMed] [Google Scholar]
- 25.Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: A Comprehensive Model for Prevention Training and Program Delivery. American Journal of Preventive Medicine 2009;37:505–511. [DOI] [PubMed] [Google Scholar]
- 26.Kramer MK, Kriska AM, Venditti EM, Semler LN, Miller RG, McDonald T, et al. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res Clin Pract 2010;90(3):e60–3. [DOI] [PubMed] [Google Scholar]
- 27.McTigue KM, Conroy MB, Hess R, Bryce CL, Fiorillo AB, Fischer GS, et al. Using the Internet to Translate an Evidence-Based Lifestyle Intervention into Practice. Telemedicine Journal and E-Health 2009;15(9):851–858. [DOI] [PubMed] [Google Scholar]
- 28.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the Diabetes Prevention Program to Primary Care A Pilot Study. Nursing Research 2009;58(1):2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldana SG, Barlow M, Smith R, Yanowitz FG, Adams T, Loveday L, et al. The diabetes prevention program: a worksite experience. AAOHN J 2005;53(11):499–505; quiz 506–7. [PubMed] [Google Scholar]
- 30.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35(4):357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: Results of translational research. Journal of Public Health Management and Practice 2008;14(1):29–32. [DOI] [PubMed] [Google Scholar]
- 32.Matvienko OA, Hoehns JD. A Lifestyle Intervention Study in Patients with Diabetes or Impaired Glucose Tolerance: Translation of a Research Intervention into Practice. Journal of the American Board of Family Medicine 2009;22(5):535–543. [DOI] [PubMed] [Google Scholar]
- 33.Mau MK, Keawe’aimoku Kaholokula J, West MR, Leake A, Efird JT, Rose C, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Pilot project. Prog Community Health Partnersh 2010;4(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Administration on Aging. Table 10. Focal Points and Senior Centers, Fiscal Year: 10/01/2007–09/30/2008. 2010. [cited 2010 12/20/2010]; Available from: available at http://www.aoa.gov/AoARoot/Program_Results/SPR/2008/Index.aspx#state
- 35.Wiener JM, Gage B, Rabiner DJ, Brown DW, , , Maier J, Mitchell N, et al. Assessment of the Title III-D of the Older Americans Act: Disease Prevention and Health Promotion Services. In. Waltham, MA: RTI International; 2006. [Google Scholar]
- 36.Administration on Aging. FY 2008 Profile of State AOA Programs. 2009. 12/20/2010]; Available from: http://www.aoa.gov/AoARoot/Program_Results/SPR/2008/Index.aspx
- 37.Israel B, Schulz E, Parker E, Becker A, Allen A, Guzman J. Critical Issues In Developing and Following CBPR Principles In: Minkler M, Wallerstein N, editors. Community Based Participatory Research for Health From Process to Outcomes. Second ed: Jossey-Bass; 2008. p. 47–66. [Google Scholar]
- 38.Beck C, Bursac Z, Cornell C, Felix HC, Fausett J, Krukowski R, et al. A Community-based, Lay Health Educator Delivered Cognitive Intervention for Obese Senior Adults. In: University of Arkansas for Medical Sciences; 2010. [Google Scholar]
- 39.Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 40.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadden T, West D, Delahanty L, Jakicic J, Rejeski W, Williamson D, et al. The Look AHEAD Study: A description of the lifestyle intervention and the evidence supporting it. Obesity 2006;14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NHLBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obesity Research 1998;6:51S–209S. [PubMed] [Google Scholar]
- 43.Wadden T, West D, Neiberg R, Wing R, Ryan D, Johnson K, et al. One-year weight losses in the Look AHEAD Study: Factors associated with success. Obesity 2009;17:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabetic Medicine 2006;23(5):544–556. [DOI] [PubMed] [Google Scholar]
- 45.Foster G, Taylor S, Eldridge S, Ramsay J, Griffiths C. Self-Management education programmes by lay leaders for people with chronic conditions (Review). Cochrane Database of Systematic Reviews 2009(1). [DOI] [PubMed] [Google Scholar]
- 46.Viswanathan M, Kraschnewski JL, Nishikawa B, Morgan LC, Honeycutt AA, Thieda P, et al. Outcomes and Costs of Community Health Worker Interventions A Systematic Review. Medical Care 2010;48(9):792–808. [DOI] [PubMed] [Google Scholar]
- 47.Administration on Aging. A Profile of Older Americans: 2009 In: USDHHS; 2009. [Google Scholar]
- 48.Administration on Aging. National Survey of Older Americans Programs, Congregate Meal Participants Demographic Characteristics. In: Available at: http://www.gpra.net/nationalsurvey/NS-CGM.asp; 2004.
- 49.Perri M, McAdoo W, McAllister D, et al. Effects of peer support and therapist contact on long-term weight loss. Journal of Consulting and Clinical Psychology 1987;55:615–617. [DOI] [PubMed] [Google Scholar]