Abstract
Telomeres are long nucleotide repeats and protein complexes at the ends of chromosomes that are essential for maintaining chromosomal stability. They shorten with each cell division and therefore telomere length is a marker for cellular aging and senescence. Epidemiologic research of telomeres investigates the role that these genetic structures have in disease risk and mortality in human populations. This chapter provides an overview of the current telomere epidemiology research and discusses approaches taken in these investigations. We also highlight important methodological considerations that may affect data interpretation.
Keywords: Telomere, telomere length, epidemiology, association studies
Introduction
Telomeres have been an attractive biological marker for longevity and health since the discovery that they shorten with normal cell division (1). Telomere studies in humans have ranged from understanding telomeric structure, function, and regulatory mechanisms, to evaluating their role in aging, disease risk and mortality. At the end of 2013, the search term “telomeres” in PubMed returned more than 17,000 publications.
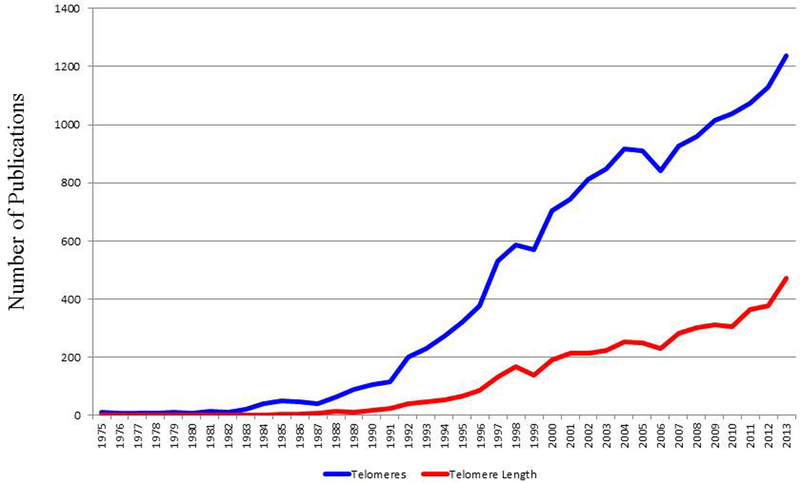
The first (2), published in 1950, reported the existence of a structure that was first hypothesized by Hermann Muller in the 1930s to cap the end of the chromosome. Since then, the number of telomere-related publications has grown rapidly (Figure 1). Telomere studies have been largely conducted in the fields of cancer and aging as noted by the number of publications in those fields. As of early 2014, the search term “telomeres and cancer” returned 5,586 publications and “telomeres and aging” returned 3,340 publications.
Figure 1:
Number of telomere-related PubMed indexed publications and the observed rising trend overtime
Epidemiology is derived from three Greek words: epi, meaning “among”, demos, meaning “people” and logos meaning “study.” In total, they mean “the study of what is among the people,” with a focus on health-related events. The science of epidemiology further expanded from describing distribution and patterns, to identifying etiological factors and evaluating effects of diseases in a defined population.
The epidemiological literature of telomeres over the past decade features studies focused on 1) describing telomere length (TL) variation by demographic characteristics such as age (3), gender or race(4); 2) evaluating the association between rare and common inherited telomeric defects and disease risk or severity(5–7); 3) evaluating the effect of environmental exposures and lifestyle factors on TL (e.g. stress (8, 9), exercise (10), smoking (11, 12), certain chemicals (13), and others); and 4) determining the role telomeres may play in certain outcomes such as mortality (14), or age-related diseases such as cancer (15), cardiovascular diseases (16), or others (17). Few behavioral intervention studies have been implemented to date; they include a study evaluating the role of meditation training on telomerase activity (18) and a study of the role of a lifestyle intervention on TL (19).
In this chapter, we discuss challenges related to study design in telomere epidemiology research, limitations related to telomere measurement methods and surrogate tissue use in most studies. Table 1 presents the definitions of some epidemiological terms (20, 21) introduced in this chapter.
Table 1:
Definitions of some epidemiological and statistical terms
| Term | Definition |
|---|---|
| Confounding | The distortion of the effect of an exposure on an outcome due to the association of another factor (confounder) with the exposure and the outcome. |
| Bias | Systematic deviation of results or inferences from truth |
| Temporal relationship | Exposure always precedes the outcome |
| Biological plausibility | The association is coherent with firmly established knowledge on pathobiological processes |
| Mediator variable | A variable that occurs in the pathway from an exposure to an outcome. |
| Correlation Coefficient (r) | A measure of association that indicates the degree to which two variables have a linear relationship. It ranges between −1 and 1; r=0 means no correlation |
| Coefficient of variation (CV) | A measure of the spread of the data defined as the ratio of the standard deviation to the mean. Usually expressed as a percentage |
| Odds Ratio (OR) | The ratio of the odds of exposure among the cases to the odds of exposure among controls; it is commonly used in case-control studies |
| Hazard Ratio (HR) | The ratio of the hazard rates comparing exposed to unexposed groups. Hazard rate is the number of new cases developing per unit person-time at risk |
| Confidence Interval (CI) | A range of values about a point estimate that indicates the degree of statistical precision that describes the estimate. A wider interval indicates less precision |
Telomere Length in Accessible Tissue as a Surrogate for the Disease Site
Many epidemiological studies measure TL in peripheral blood leukocytes. While leukocyte TL may be the specific biomarker of interest in some studies, in others, it may be a surrogate for a specific tissue. Data comparing TL in peripheral blood leukocytes and in different somatic tissues from the same individual are limited. The use of leukocyte TL as a surrogate for the tissue of interest may be affected by the tissue specific proliferation rate as well as the susceptibility to environmental factors affecting TL regulation in that specific tissue. Leukocytes are a heterogeneous cell population, formed mainly by lymphocytes and granulocytes. Notably, lymphocytes have shorter telomeres than granulocytes (22).
Studies comparing TL in different tissues from normal individuals have found differences in absolute TL between tissues but strong correlations are present. For instance, in a study measuring TL in leukocytes, skeletal muscle, skin, and subcutaneous fat from the same 87 adult individuals, the authors found high degrees of correlation within individuals but noted that leukocytes had the shortest telomeres (23). Similar findings have been reported when comparing TL in leukocytes, skin, and joint tissues in a small study of elderly patients with hip fractures (24). A Japanese study measured TL in cerebral cortex, kidney, liver and myocardium in over 100 autopsies from individuals of different ages, including neonates through centenarians, and also found high intra-individual correlations between different tissue TL measurements (25); myocardial tissue had the longest TL while liver and renal cortex had the shortest. Finally, a study compared average leukocyte TL and buccal TL in young (18–26 years of age) and old (66–75 years of age) adults (26). The authors found that leukocyte TL was significantly shorter than buccal TL in both age groups. As expected, leukocyte TL was significantly shorter in the older group compared with the younger group, but surprisingly there were no differences in buccal TL between the two age groups (26). Of note, other factors, such as increased body mass index (BMI) or tobacco smoking, that have been associated with shortening of leukocyte TL (12), were not accounted for in that study.
Some studies have compared TL measured in different tissues from individuals with specific illnesses. TL was measured in leukocytes, buccal cells and fibroblasts in individuals with dyskeratosis congenita (DC), an inherited telomere biology disorder where telomeres are exceedingly short (below the 1st percentile of normal individuals of the same age) (5, 7). The authors reported strong correlations between the TL in these different types of cells, with leukocytes having the shortest telomeres (27). TL has also been measured in somatic tissues from cancer patients, comparing tumor tissue to adjacent normal in most cases. Telomeres were shorter in prostate cancer cells than in adjacent non-malignant cells (28). Moreover, shorter TL in prostate cancer-associated stromal cells and larger TL variation in prostate cancer cells were associated with poor outcomes. Telomere shortening has also been reported in the early stages of breast carcinogenesis (29) and was correlated with more aggressive subtypes of breast cancer (30). Shorter telomeres have also been observed in tissues near breast tumors compared with distant normal breast tissue (31, 32) suggesting a TL field effect in the adjacent non-malignant breast tissue. Variation in TL in adjacent non-malignant breast tissues was suggested to be a prognostic factor of breast cancer recurrence (33). Finally, findings from a study that measured TL in colon tissues from patients diagnosed with ulcerative colitis, a chronic inflammatory disease of the colon, and healthy controls suggested shorter colonic TL in patients than healthy controls (34). Notably, telomere shortening has also been observed in adenomas and colorectal cancer tissues as compared with normal adjacent or colon distal tissues (35, 36). The field effect suggested in the breast cancer studies did not appear to be present in a colon cancer and adenoma study (36).
Overall, there is evidence of strong correlation between leukocyte TL and TL measured in other somatic tissues from the same individuals, which is not surprising given that TL is highly heritable (37). It is important to note that the absolute TL differs between tissues, and telomeres tend to be shorter in leukocytes compared with other evaluated tissues. This suggests that the dynamics of TL are likely to be tissue-specific, reflecting tissue-specific replicative capacity. Future research is required to evaluate the relationship between TL measured in surrogate tissues and in tissues affected by the disease of interest.
Considerations in Observational Studies of Telomere Length
Association studies of TL and disease have become an important area in molecular and genetic epidemiology. Like other genetic and biological marker studies, telomere epidemiology relies on the common observational study designs, including cross-sectional, case-control, and cohort studies. Table 2 summarizes the purpose and characteristics of each design, which should be carefully considered during study design and data interpretation.
Table 2.
Observational study designs
| Study Design | Purpose | Characteristics |
|---|---|---|
| Cross-sectional |
|
|
| Case-control |
|
|
| Cohort |
|
|
Cross-sectional studies in telomere epidemiology have been used to assess the relationship between TL and demographic characteristics, such as behavioral lifestyles or environmental exposures. For example, cross-sectional study design has been used to evaluate the relationship between TL and chronological age (reviewed in (38)), and its distribution by sex and race (4). Overall, studies have shown that older age (39), White race (4), and male gender (4, 40) are associated with shorter telomeres, but the strength of association varied by participants’ age and method of TL measurement (41). The association between TL and other factors, such as smoking, physical activity or alcohol use, has been inconsistent (11, 12, 42). The inconsistencies found in the published studies may be related to the fact that TL at the time of the study may not reflect the current state of an exposure, as measured in a cross-sectional study (43). Cross-sectional studies are often highly feasible but limited by their inability to determine a temporal relationship between TL and factors of interest (i.e., outcome may have preceded TL changes in this situation). Despite this limitation, cross-sectional studies are valuable tool for hypothesis generation.
Cross-sectional studies are useful in evaluating the relationship between genetic variation in telomere genes and disease risk since an individual germline genome does not change over time. Single nucleotide polymorphisms (SNPs) in the TERT gene locus has been associated with peripheral arterial disease (44) and several cancers (45, 46); variation in the OBFC1 gene predicted cardiovascular mortality in women (47); and variation in both TERT and TEP1 genes were associated with male infertility (48). In these situations, investigators suggest that genetic variation can be thought as a surrogate of TL (49). This is based on a connection between TL and SNPs in several genome-wide association studies that identified associations between genetic variation in some known telomere biology genes (TERT, TERC, and OBFC1) and TL (50–52). However, many more studies are required before making a direct connection between these SNPs and TL.
Case-control and prospective studies are commonly used analytic approaches in evaluating whether TL is a biomarker or risk factor for age-related diseases. The main difference between the two approaches is the timing of TL assessment in relation to the disease of interest. In case-control design, TL is measured after the disease develops and is compared to individuals who are free of the disease (controls). The selection of the control population is crucial; it should be representative to the source population that gave rise to the cases to avoid possible selection bias. Details about control selection processes can be found elsewhere (53). In prospective design (cohort, nested case-control, or case-cohort) studies, TL is measured in disease-free individuals who are followed-up overtime and disease rate is then assessed in relationship to baseline TL. Because classic cohort studies are expensive and usually require long follow-up time, prospective nested case-control and case-cohort studies are valuable because they include advantages present in both case-control and cohort studies (53). In this approach, cases and controls are selected from an existing cohort in whom biological samples have been collected in the past and prior to disease development. Controls in nested case-control design are a random sample from subjects who have not developed the disease at the time of case diagnosis, but are a sample from all cohort participants in case-cohort. The biospecimen collection precedes disease development, thus, TL precedes disease diagnosis in these studies.
Association studies of TL and cancer are an example of why study design is crucial in the conduct and interpretation of TL epidemiology studies. Many studies have suggested that short telomeres are associated with cancer risk (15, 54). However, these data were primarily derived from case-control studies, which could be subject to reverse-causation bias. The meta-analysis by Wentzensen and colleagues showed significant differences between findings of TL and cancer associations in case-control studies compared with cohort studies (odds ratio (OR) =2.90, 95% confidence interval (CI) =1.75–4.80 in case-control studies vs. OR=1.16, 95% CI=0.87–1.54 in prospective studies (15). This observed difference could be, at least partially, explained by a possible reverse-causation bias in case-control studies; i.e., cancer precedes telomere shortening and the observed difference in TL between cases and controls is due to the disease status. This bias is less of a concern in prospective studies. In all prospective studies, it is important to assess the lag-time between sample collection and disease development, since chronic diseases such as heart disease or even cancer can develop over a long period of time. If DNA samples are collected close to the date of diagnosis, the disease could already be present, but not diagnosed, and therefore this relationship will still suffer from possible reverse-causation.
Another example on how the choice of the study design can influence the findings comes from epidemiological studies evaluating age-related telomere attrition rate. Most of these studies have been cross-sectional in nature; only few longitudinal studies have been reported. Cross-sectional studies attempt to estimate such attrition rate based on a single measurement of TL in subjects of different ages. They assume that the telomere attrition rate is constant across different ages and is similar in all individuals. The age range of study participants (telomere erosion varies across ages, with faster erosion during childhood), their gender, race distribution, and survival effect may also affect these estimates (55, 56). Longitudinal studies have calculated the rate of TL attrition based on 2 measurements at a 3 to10-year time interval and suggest that between 38 and 48 base pairs of telomeres are lost per year (38). When cross-sectional and longitudinal assessment of TL attrition rate were simultaneously compared, longitudinal TL attrition varied between individuals with 10% of study participants experiencing TL elongation overtime (57). Whether TL elongation over time is a true phenomenon or an artifact caused by measurement error is yet to be determined (58). Long follow-up periods, large sample sizes, and multiple TL measurement points are needed to thoroughly understand the age-related decline in TL.
Epidemiological Evaluation of the Role of Telomeres in Human Health
While it is clear that telomeres play an important role in human health, there is significant variability in the extent to which aberrant telomere biology is connected to disease. For examples patients with the prototypic telomere biology disorder, DC, are at high risk of bone marrow failure, pulmonary and liver fibrosis, immunodeficiency, and certain cancers (59, 60). Patients with DC have TL below the first percentile for age.
In contrast, association studies of TL and disease in the general population evaluate TL that are significantly different between cases and controls but still within the normal range of TL (i.e., not as short as DC). As discussed above, studies of the general population, suggest that short telomeres are associated with mortality (14, 61), cancer (15), cardiovascular diseases (16), infections (17) and inflammatory disorders (62). But similar to all epidemiology research, the question remains “do shorter than average telomeres directly cause health problems in the general population?”
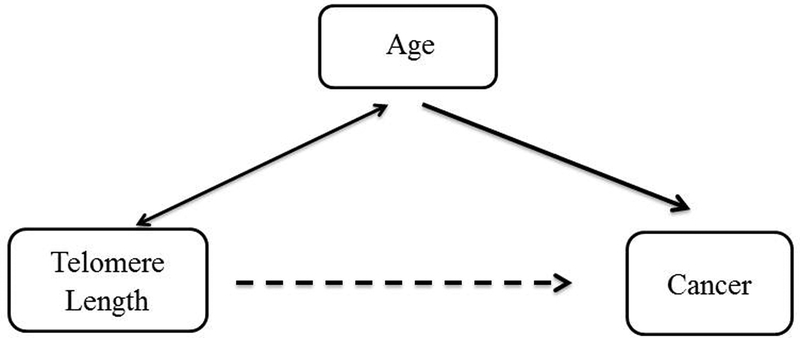
Making a valid causal inference from observational epidemiological studies is complicated mainly by possible bias associated with study design, or the presence of uncontrolled (or unknown) confounding. A confounding factor in an exposure-outcome relationship is a variable that is associated with both the exposure and the outcome (63). Age is an example of a common confounder in observational studies of age-related diseases such as cancer, where the observed association is affected by the unbalanced age distribution between comparison groups. Figure 2 provides a schematic description of the relationship between TL (exposure), age (confounder), and cancer (outcome). In population studies, age only explains a small percentage of the observed inter-individual variation in TL, ranging from 8% (64) to 29% (65) but it is still an important confounder in TL studies. For instance, in a recent study evaluating the role of TL in cancer risk in a large population-based study, age adjustment contributed to a 70% attenuation in the calculated hazard ratio (unadjusted Hazard Ratio; HR=1.74 vs. adjusted HR=0.98) of cancer incidence in individuals with short versus long telomeres (64). TL is also associated with sex (66), race (4), body mass index (67), and tobacco smoking (12), many of which are known risk factor for age-related diseases and therefore have to be accounted for when evaluating the relationship between TL and such diseases.
Figure 2:
A schematic diagram describing confounding. The bidirectional arrow represents a non-causal association, the unidirectional arrow represents a causal association, and the dashed line represents the observed association
While confounding may mislead a true association and should be eliminated, valid interacting factors can be of biological importance. Interaction or effect modification describes a relationship between two or more factors, in which they alter the effect of each other with respect to the outcome (63). Some important examples of interacting factors have been noted in TL epidemiology studies. Fu and colleagues found that liver cirrhosis modified the association between hepatocellular carcinoma (HCC) and leukocyte TL (68). Liver cirrhosis is a strong risk factor for HCC in patients with chronic hepatitis C and B viruses. In that study, significant associations between leukocyte telomeres and HCC risk was restricted to non-cirrhotic patients (OR = 3.54, 95% CI 1.58–7.93 vs. OR = 0.95, 95% CI 0.55–1.64, in patients with liver cirrhosis). Identifying interactions with biological relevance can provide a risk stratification tool that could be valuable for screening or treatment strategies. In another example, Puterman et al. (69) found that physical activity can modify the observed inverse association between TL and perceived stress, suggesting that exercise can protect from the negative biological effect of stress on human cells, as reflected by TL. A subsequent intervention trial suggested that lifestyle changes, including stress management and physical activity, may affect TL over time (19). Testing for interactions is important, but, as in all studies, the biologic plausibility of the interactions and the findings needs to be considered.
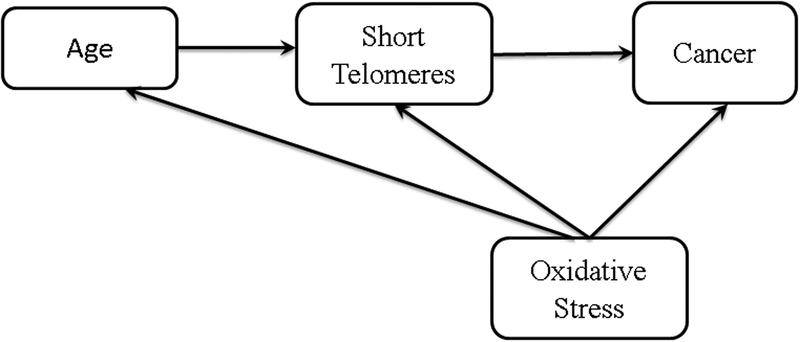
Evaluating the relationship between TL, risk factors, and disease in a multidimensional way is attractive for an in-depth understanding of the actual role TL plays in human diseases. One example is through mediation analysis (70). The mediation model identifies factors that are in the causal pathway between the study variable and outcome. Through it, a researcher can quantify the total effect of an exposure on the outcome of interest, and can calculate how much of this association is through the mediator of interest. Figure 3 illustrates a theoretical example in which TL is in the causal pathway between age and cancer, and extends this to show that this model can accommodate multiple factors (e.g. oxidative stress). Several investigators have suggested that telomere dysfunction may causally mediate the association between psychological stress and cardiovascular diseases (71), or lifestyle factors and risk of type 2 diabetes (72). Mediation analysis have been used in few small studies to evaluate if TL could explain some of the epidemiological observations that are not yet well understood including the relationship between childhood socioeconomic status and the high risk of respiratory infections in adulthood (73) and a possible connection between consanguinity and mental illnesses (74).
Figure 3:
A schematic diagram describing mediation where short telomeres are in the causal pathway of age and cancer
The Effect of Telomere Length Method of Measurement on Study Results
Several methods have been used to measure TL, each with its strengths and limitations reviewed in detail elsewhere (75–77). In this section, we focus on issues that may impact validity of TL-disease association studies comparing the three most commonly used TL measurement methods in epidemiological and clinical research. Southern blots of terminal restriction fragments (TRF) are considered the gold standard for TL measurement and are used to calibrate other methods (78). TL has also been successfully measured using real-time quantitative polymerase chain reaction (qPCR) (79, 80). Both southern blot and qPCR techniques measure TL in extracted DNA, and therefore, can be used for stored samples commonly available for epidemiological studies. However, qPCR is more attractive for large-scale studies because it is high throughput, needs small amounts of DNA, and is relatively inexpensive. Both methods are limited by their reported lack of sensitivity to detect very short telomeres, and by the fact that their TL measurement is an average across population of cells per sample (77, 81). On the other hand, flow cytometry with fluorescent in situ hybridization (flow FISH) TL measurement of leukocytes is very accurate and provides cell population-specific measure (82), but its utility in population-based studies is limited by its need for viable cells.
The correlation of TL values between these three measurement methods is generally modest. In an epidemiological study of 681 elderly individuals, the correlation coefficient (r) between TL data generated by Southern blot and qPCR was weak (r=0.52) (41). This is significantly weaker than reported correlations between TL measured by the two methods in the initial validation studies of either qPCR monoplex assay (r=0.82) (79), or qPCR monochrome multiplex assay (r=0.91) (80). Our data comparing TL measured by qPCR and flow FISH in 52 normal individuals showed similar result (r=0.47) (83). No data are available for the correlation between flow FISH and Southern blot TL beyond the strong correlation reported in the initial flow FISH study (r=0.9) (82).
The weak correlations between methods may partially explain some of the inconsistencies observed in the published literature when different TL measurement methods are used. For example, a large cohort study measuring relative TL using qPCR reported significant excess in cancer mortality associated with short telomeres (n=787, HR=11.1 in the shortest versus longest tertile) (84). However, a second study (85) measuring TL using Southern blot analysis reported no association between TL and cancer mortality (n=1,136; HR=1.2, in the shortest versus longest quartile). In a meta-analysis including TL-obesity studies the authors reported that the results from studies that used the Southern blot assay to measure TL were more consistent than those that used qPCR (67). Similar inconsistencies between studies evaluating the association between qPCR relative TL and breast cancer has been observed (two showed an association with long telomeres, three with short telomeres, and three showed no association) (15). Of interest, this variation existed across different study designs (case-control and prospective studies) (15), reflecting a possible role for measurement variability between laboratories, and highlighting the need to standardize qPCR laboratory procedure in relative TL measurement. In a study comparing blinded duplicates blood samples for Southern blot TL and qPCR relative TL, results from Southern blot assay were found to be more consistent than that of qPCR (coefficient of variation (CV) for Southern blot =1.7% vs. 6.5% for qPCR) (65). Of note, qPCR relative TL measurements may be sensitive to DNA quality, quantity, and extraction method (86).
These differences in TL measurement methods illustrate the importance of minimizing technique-related measurement error, and thus limit misclassification bias. Simple measures that can help with this include 1) distributing samples from both comparisons groups (case/control, exposed/unexposed) on the same plate; 2) avoiding the use of DNA samples of unknown source or unknown extraction method; 3) using a single method of DNA extraction or, at least, match the cases and controls on the extraction method.
Of note, recent research suggest a need to switch from measuring average TL to a more biologically relevant measurement of TL based on the proportion of short telomeres in cells (77). In vitro and animal studies suggest that this measurement may be a better indicator of cell aging than the average TL, which can be effected by the presence of very long telomeres in some cells (87, 88).
Analytical Considerations in Telomere Length Epidemiology Studies
Determining the number of individuals included in a study is one of the important early decisions made during study design. Things to be taken in account when calculating sample size include (89): study design, scale of measurement of the response variables (i.e., are we comparing means or proportions?), the difference we want to detect (larger sample sizes are needed to detect smaller differences), the population inter-individual variability of the measure of interest (the more homogeneous the population, the smaller the number of individuals needed), the desired level of statistical significance (the smaller the p-value the larger the sample size needed), and study power (i.e., probability to detect differences when exist; the higher the desired power, the larger the sample size needed).
For TL studies, a large enough sample size is especially important because inter-individual TL heterogeneity and age variability must be included in the calculations. Using simulations, it was shown that longitudinal designs may have more power to observe TL association with age-related diseases, because they can capture the actual TL attrition rate (43).
Adjusting for confounders is crucial in epidemiological studies. Several methods are used, some at the design level and others are used during the analysis. At the design level, comparison groups (e.g., cases and controls) are often matched by potential confounders (e.g., age). A disadvantage of a matched design is that strict matching (one-to-one) limits our ability to evaluate the relationship of the matched variable with the study outcome. At the analysis level, regression models are the most commonly used method to control for confounding. However it is important to note that limitations may exist (reviewed in detail in (90)) including: 1) the adjusted effect size can be invalid if the distribution of the potential confounder greatly differs between studied groups (e.g., when certain age groups are not presented in the control groups, usually very young or very old); 2) multivariable model misspecification can lead to biased results, such as when the model doesn’t account for some important nonlinearities. Nonlinearity has been observed in TL relationship with age in several studies (39, 91). For instance, a study of 835 individuals found that a piecewise linear curve with breakpoints hinged at 1 and 18 years of age provided the best fit to the data as compared with linear, quadratic, or cubic curves (39).
Another widely used method to control for confounding, mostly to adjust for age, is calculating age-standardized TL using an independent population (5, 7). Again selecting a large enough reference population for each age category is important to ensure capturing the TL variability.
Conclusions
Epidemiological studies are crucial in understanding the role of telomere biology in human aging and disease. In this chapter we have summarized some important epidemiologic concepts and issues that can affect the interpretation of the results from epidemiological studies of TL. Careful selection of an appropriate study design, an adequate sample size, and an accurate TL measurements assay are important factors that can significantly affect the findings of the study. Future research to compare TL measurement from different assays in large studies is needed. This will aid the development of more reliable assays for large epidemiological studies. It is also paramount to measure TL from multiple samples collected in longitudinal epidemiological studies with long follow-up times to precisely estimate the age-related telomere attrition rate and how it relates to disease risk. Including large enough sample from different age groups is necessary to enhance our understanding of telomere dynamics during the life span. Telomere measurement in somatic tissues will provide information the organ-specific aging rate. Additionally, standardization of available measurement methods is crucial to allow comparing between studies. Recent research calls for new methods that measure the proportion of short telomeres within cells rather than the current average TL measurement. Such an assay could provide many research opportunities and help to better understand the role of telomeres in health and disease.
In summary, proper epidemiological research has the potential to clarify the role telomeres play in disease risk and may eventually guide preventive strategies, identify modifiable targets, and aid in the development of diagnostic and prognostic biomarkers.
Acknowledgment
This work is supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health
References
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–60. [DOI] [PubMed] [Google Scholar]
- 2.Warters M, Griffen AB. The telomeres of Drosophila. J Hered. 1950; 41:182–90. [PubMed] [Google Scholar]
- 3.Frenck RW Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. ProcNatlAcadSciUSA. 1998; 95:5607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008; 7:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007; 110:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011; 53:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012; 97:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drury SS, Theall K, Gleason MM, Smyke AT, De VI, Wong JY, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. MolPsychiatry. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. ProcNatlAcadSciUSA. 2004; 101:17312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krauss J, Farzaneh-Far R, Puterman E, Na B, Lin J, Epel E, et al. Physical fitness and telomere length in patients with coronary heart disease: findings from the Heart and Soul Study. PLoS One. 2011; 6:e26983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009; 8:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005; 366:662–4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Postgrad Med J. 2013; 89:722–8. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003; 361:393–5. [DOI] [PubMed] [Google Scholar]
- 15.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011; 20:1238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007; 165:14–21. [DOI] [PubMed] [Google Scholar]
- 17.Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PLoSOne. 2008; 3:e2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011; 36:664–81. [DOI] [PubMed] [Google Scholar]
- 19.Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013; 14:1112–20. [DOI] [PubMed] [Google Scholar]
- 20.Rothman K Epidemiology an introduction: Oxford University Press; 2002. [Google Scholar]
- 21.A dictionary of epidemiology. 5th ed. New York: Oxford University Press; 2008. [Google Scholar]
- 22.Aubert G, Lansdorp PM. Telomeres and Aging. Physiological Reviews. 2008; 88:557–79. [DOI] [PubMed] [Google Scholar]
- 23.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013; 4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrich U, Griese E-U, Schwab M, Fritz P, Thon K-P, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000; 119:89–99. [DOI] [PubMed] [Google Scholar]
- 25.Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, et al. Telomere lengths are characteristic in each human individual. Experimental Gerontology. 2002; 37:523–31. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, O’ Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008; 129:183–90. [DOI] [PubMed] [Google Scholar]
- 27.Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY). 2010; 2:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannucci E, et al. Prostate Cancer Cell Telomere Length Variability and Stromal Cell Telomere Length as Prognostic Markers for Metastasis and Death. Cancer Discovery. 2013; 3:1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P. Telomere Shortening Occurs in Subsets of Normal Breast Epithelium as well as in Situ and Invasive Carcinoma. Am J Pathol. 2004; 164:925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heaphy CM, Subhawong AP, Gross AL, Konishi Y, Kouprina N, Argani P, et al. Shorter telomeres in luminal B, HER-2 and triple-negative breast cancer subtypes. Mod Pathol. 2011; 24:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaphy CM, Bisoffi M, Fordyce CA, Haaland CM, Hines WC, Joste NE, et al. Telomere DNA content and allelic imbalance demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2006; 119:108–16. [DOI] [PubMed] [Google Scholar]
- 32.Kurabayashi R, Takubo K, Aida J, Honma N, Poon SSS, Kammori M, et al. Luminal and cancer cells in the breast show more rapid telomere shortening than myoepithelial cells and fibroblasts. Hum Pathol. 2008; 39:1647–55. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Meeker AK, Makambi KH, Kosti O, Kallakury BV, Sidawy MK, et al. Telomere length variation in normal epithelial cells adjacent to tumor: potential biomarker for breast cancer local recurrence. Carcinogenesis. 2012; 33:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen W-T, Emerson S, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002; 32:280–4. [DOI] [PubMed] [Google Scholar]
- 35.Rampazzo E, Bertorelle R, Serra L, Terrin L, Candiotto C, Pucciarelli S, et al. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer. 2010; 102:1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Sullivan J, Risques RA, Mandelson MT, Chen L, Brentnall TA, Bronner MP, et al. Telomere length in the colon declines with age: a relation to colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2006; 15:573–7. [DOI] [PubMed] [Google Scholar]
- 37.Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013; 21:1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders JL, Newman AB. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? EpidemiolRev. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012; 8:e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller P, Mayer S, Mattfeldt T, Muller K, Wiegand P, Bruderlein S. Sex-related differences in length and erosion dynamics of human telomeres favor females. Aging (AlbanyNY). 2009; 1:733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elbers CC, Garcia ME, Kimura M, Cummings SR, Nalls MA, Newman AB, et al. Comparison Between Southern Blots and qPCR Analysis of Leukocyte Telomere Length in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010; 91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006; 35:1424–9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Chen Y, Yang X, Fan J, Mi X, Wang J, et al. Functional haplotypes of the hTERT gene, leukocyte telomere length shortening, and the risk of peripheral arterial disease. PLoS One. 2012; 7:e47029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013; 45:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kote-Jarai Z, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Dadaev T, Jugurnauth-Little S, et al. Fine-mapping identifies multiple prostate cancer risk loci at 5p15, one of which associates with TERT expression. Human Molecular Genetics. 2013; 22:2520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnett-Hartman AN, Fitzpatrick AL, Kronmal RA, Psaty BM, Jenny NS, Bis JC, et al. Telomere-associated polymorphisms correlate with cardiovascular disease mortality in Caucasian women: the Cardiovascular Health Study. Mech Ageing Dev. 2012; 133:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan L, Wu S, Zhang S, Ji G, Gu A. Genetic variants in telomerase reverse transcriptase (TERT) and telomerase-associated protein 1 (TEP1) and the risk of male infertility. Gene. 2014; 534:139–43. [DOI] [PubMed] [Google Scholar]
- 49.Hartwig FP. Telomere Length and Telomere-related Genetic Variations in Epidemiology: Getting the Context Right. J Genet Syndr Gene Ther. 2013; 4. [Google Scholar]
- 50.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nature genetics. 2013; 45:371–84, 84e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet. 2013; 22:5056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013; 45:422–7, 7e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman KJ. Types of epidemiologyic study In: Rothman K, editor. Epidemiology: an introduction. New York: Oxford University Press Inc; 2002. p. 78–84. [Google Scholar]
- 54.Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer Lett. 2012; 319:130–5. [DOI] [PubMed] [Google Scholar]
- 55.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte Telomere Dynamics: Longitudinal Findings Among Young Adults in the Bogalusa Heart Study. American Journal of Epidemiology. 2009; 169:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus Cross-sectional Evaluations of Leukocyte Telomere Length Dynamics: Age-Dependent Telomere Shortening is the Rule. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011; 66A:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011; 66:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 2013; 41:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vulliamy T, Dokal I. Dyskeratosis congenita. SeminHematol. 2006; 43:157–66. [DOI] [PubMed] [Google Scholar]
- 60.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. ExpertRevHematol. 2013; 6:327–37. [DOI] [PubMed] [Google Scholar]
- 61.Zhong R, Liu L, Zou L, Zhu Y, Chen W, Zhu B, et al. Genetic variations in TERT-CLPTM1L locus are associated with risk of lung cancer in Chinese population. Mol Carcinog. 2013; 52 Suppl 1:E118–26. [DOI] [PubMed] [Google Scholar]
- 62.Kinouchi Y, Hiwatashi N, Chida M, Nagashima F, Takagi S, Maekawa H, et al. Telomere shortening in the colonic mucosa of patients with ulcerative colitis. J Gastroenterol. 1998; 33:343–8. [DOI] [PubMed] [Google Scholar]
- 63.Gordis L Epidemiology. 2nd ed. ed. Pennsylvania: W.B. Saunders Company; 2000. [Google Scholar]
- 64.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013; 105:459–68. [DOI] [PubMed] [Google Scholar]
- 65.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011; 39:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: Systematic review and meta-analysis. Exp Gerontol. 2013; 51C:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2013. [DOI] [PubMed] [Google Scholar]
- 68.Fu X, Wan S, Hann HW, Myers RE, Hann RS, Au J, et al. Relative telomere length: a novel non-invasive biomarker for the risk of non-cirrhotic hepatocellular carcinoma in patients with chronic hepatitis B infection. Eur J Cancer. 2012; 48:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010; 5:e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013. [DOI] [PubMed] [Google Scholar]
- 71.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006; 31:277–87. [DOI] [PubMed] [Google Scholar]
- 72.Elks CE, Scott RA. The long and short of telomere length and diabetes. Diabetes. 2014; 63:65–7. [DOI] [PubMed] [Google Scholar]
- 73.Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, Li-Korotky HS, et al. Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain Behav Immun. 2013; 34:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mansour H, Chowdari K, Fathi W, Elassy M, Ibrahim I, Wood J, et al. Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res. 2011; 188:129–32. [DOI] [PubMed] [Google Scholar]
- 75.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012; 730:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin KW, Yan J. The telomere length dynamic and methods of its assessment. J Cell Mol Med. 2005; 9:977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vera E, Blasco MA. Beyond average: potential for measurement of short telomeres. Aging (Albany NY). 2012; 4:379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aviv A Commentary: Raising the bar on telomere epidemiology. Int J Epidemiol. 2009; 38:1735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002; 30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009; 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horn T, Robertson BC, Gemmell NJ. The use of telomere length in ecology and evolutionary biology. Heredity (Edinb). 2010; 105:497–506. [DOI] [PubMed] [Google Scholar]
- 82.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. NatBiotechnol. 1998; 16:743–7. [DOI] [PubMed] [Google Scholar]
- 83.Khincha PP, Gadalla SM, Katki H, Giri N, Wong J, Spellman S, et al. Evaluating the utility of telomere length measurement by qPCR as a diagnostic test for dyskeratosis congenita. Blood. 2013; 122:3700. [Google Scholar]
- 84.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010; 304:69–75. [DOI] [PubMed] [Google Scholar]
- 85.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011; 66:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cunningham JM, Johnson RA, Litzelman K, Skinner HG, Seo S, Engelman CD, et al. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2013; 22:2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berardinelli F, Nieri D, Sgura A, Tanzarella C, Antoccia A. Telomere loss, not average telomere length, confers radiosensitivity to TK6-irradiated cells. Mutat Res. 2012; 740:13–20. [DOI] [PubMed] [Google Scholar]
- 88.Vera E, Bernardes de Jesus B, Foronda M, Flores JM, Blasco MA. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2012; 2:732–7. [DOI] [PubMed] [Google Scholar]
- 89.Rigby AS, Vail A. Statistical methods in epidemiology. II: A commonsense approach to sample size estimation. Disabil Rehabil. 1998; 20:405–10. [DOI] [PubMed] [Google Scholar]
- 90.Martin W Making valid causal inferences from observational data. Prev Vet Med. 2013. [DOI] [PubMed] [Google Scholar]
- 91.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Research. 2011; 39:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]