Abstract
Background
In previous studies, higher expression of PREX1 (PtdIns (3,4,5)P3-dependent Rac exchanger 1) has been detected in some subsets of breast cancer, and activation of PREX1 has been associated with tumor progression in vivo. However, an association between PREX1 and breast cancer prognosis has not been examined.
Material/Methods
In this study, we investigated the expression and correlation of PREX1 with important clinical factors and prognosis of patients with breast cancer. Immunohistochemical staining was performed for 121 tumor tissue specimens obtained from primary breast cancer lesions.
Results
We found that 55 tissues exhibited positive staining for PREX1. Moreover, tumors positive for PREX1 were found to have significant association with recurrence rate (P=0.000) and metastasis rate (P=0.001). Univariate and multivariate regression analyses also identified PREX1 expression as an independent variable of disease-free survival. Our analyses indicate that high levels of PREX1 expression were related to longer disease-free survival in patients with breast cancer (P=0.013).
Conclusions
PREX1 is a favorable variable of prognosis for breast cancer patients, these study results need to be confirmed in larger research studies.
MeSH Keywords: 3-Phosphoinositide-Dependent Protein Kinases, Breast Neoplasms, Prognosis
Background
Breast cancer is a malignant tumor that seriously threatens women’s health. It is the most common malignant tumor in women. Worldwide, there are approximately 1.6 million new breast cancer patients each year, and more than 500 000 deaths [1]. Despite the availability of chemotherapy, radiation therapy, hormone therapy, and trastuzumab as treatment options for breast cancer patients, there are a subset of patients still experiencing tumor recurrence or metastasis.
Rac1, Rac2, and Rac3 represent members of the Rho-GTPase family of proteins which are associated with the morphology, motility, and invasion of cancer cells that form distant metastases [2–4]. The most popular mechanism identified for overactivation of Rac in human cancers involves the imbalance of Rac-guanine nucleotide exchange factor (GEF) activity. In particular, PtdIns (3,4,5)P3-dependent Rac exchanger 1 (PREX1) is a subgroup of the Dbl family of Rho-GEFs and has been shown to promote chemotactic agents to stimulate neutrophil chemotaxis and formation of reactive oxygen species [5–7]. When PREX1 is ectopically expressed in vitro, it promotes cell migration, viability, and invasion [8,9]. Similarly, targeting of PREX1 with short-hairpin RNA in breast cancer cells that are positive for ErbB receptors has been reported to lead to a reduction in cell migration, proliferation, and the growth of xenograft tumors [10,11]. In estrogen receptor (ER)-positive luminal breast tumors, both mRNA and protein levels of PREX1 are upregulated, while expression of PREX1 is not detected in normal breast tissue [8,10]. In addition, PREX1 has been found to be amplified in primary breast tumors, while 58% of breast cancers are reported to be positive for PREX1 by immunohistochemistry [10]. Thus, PREX1 has been identified as a putative oncogene [10–14]. Clinically, PREX1 has also been found to be related to poor prognosis in various human cancers, including malignant myeloid diseases [15], ovarian cancer [16], pancreatic endocrine tumors [17], and hereditary prostate cancer [18].
Despite convincing evidence that in some breast cancer subtypes, PREX1 expression is increased and that PREX1 activation is associated with tumor progression in vivo [11], very few data are available regarding the relationship between PREX1 expression and breast cancer prognosis. Therefore, the aim of this study was to retrospectively analyze PREX1 expression in 121 breast cancer tissue samples and investigate a possible correlation between PREX1 expression and breast cancer prognosis.
Material and Methods
Patients with breast cancer and tumor samples
We obtained 121 tumor samples from breast cancer patients. All patients (aged 13–81 years) underwent surgery and other treatments between 2000 and 2007 at Peking Union Medical College Hospital (PUMCH, Beijing, China). The median follow-up time was 29 months. Treatment after surgery included a combination of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), or capecitabine, paclitaxel, anthracycline, radiotherapy, tamoxifen, or aromatase inhibitors. Some patients received a combination of agents for their treatment. In our study, recurrence was the presence of nodules in the chest wall, and metastatic sites were defined as bone, liver, lung, brain, supraclavicular lymph nodes, and contralateral breast.
Ethics approval and consent to participate
Peking Union Medical College Hospital ethics committee reviewed the study protocol and deemed the study exempt from full review (approval no. S-K609, dated November 5, 2018). All patients in our study provided written informed consent for participation.
Availability of data and materials
The datasets generated during and/or analyzed in the current study are not publicly available but are available from the corresponding author on reasonable request.
Immunohistochemical (IHC) staining and analysis
Collected tissues were paraffin embedded after fixing in neutral buffered formalin (10%) and tumor sections (4 um thickness) were accomplished by adhesion slides. According to normal protocols, immunohistochemical (IHC) staining was performed with standard autostaining protocols (Ventana Benchmark XT autostainer system). Isotype antibody and control tissue were included in positive and negative controls, according to the manufacturer’s recommendations. Two pathologists evaluated each IHC slide respectively.
IHC analyses of ER, progesterone (PR), and human epidermal growth factor receptor 2 (HER2) were analyzed in the clinical laboratory at PUMCH. The sections were cut at 5 μm thickness and mounted on silicified slides. The expression of ER, PR, and HER2 were determined according to routine methods. All the samples were stained with hematoxylin and eosin, and histological analyses were performed according to classifications established by the World Health Organization [19,20].
From pathological reports, tumor size and number of metastatic lymph nodes were derived. Metastases were confirmed by biopsy and the locations were detected by imaging examinations. In IHC staining, negative expression of ER, PR, and HER2 were defined as basal-like features. If at least 1% of tumor cells were stained, ER and PR expression were considered positive. If >30% of the invasive tumor cells were stained in intense membrane by IHC, more than 6 HER-2 gene copies per nucleus were revealed by fluorescence in situ hybridization (FISH), or HER2 signal compared to chromosome 17 signal was >2.2 with FISH ratio, HER-2 expression was considered positive.
Statistical analyses
Statistical analyses were performed by SPSS software (version 21.0). The χ2 test and Mann-Whitney U test were applied to IHC results and various clinicopathologic parameters. The latter were also evaluated in relation to disease-free survival (DFS) with Mann-Whitney U test, χ2 test, and logistic regression. The log-rank test was used to determine the significance of associations observed between PREX1 and DFS in Kaplan-Meier survival analyses. All of the statistical tests performed were 2-sided and P-values less than 0.05 were considered significant.
Results
Clinicopathological characteristics and survival data
A total of 121 breast cancer patients were examined in this study (Table 1). Most cases involved invasive breast ductal carcinoma (102 out of 121 cases, 84.4%) and 109 out of 121 patients (90.1%) underwent modified radical mastectomy. The median age of this cohort at the time of surgery was 50 years (range, 13–81 years). Postsurgical treatment included CMF, or various combinations of anthracycline, paclitaxel, tamoxifen, aromatase inhibitors, or radiotherapy. Among the cases with adjuvant treatment information available, 98.3% (119 out of 121 cases) included chemotherapy and 49.6% (61 out of 121 cases) included radiation. The median follow-up time was 29 months (range, 2–91 months) and the DFS 5-years was 40.4%.
Table 1.
Baseline clinicopathological characteristics and treatments of the cohort.
| Characteristics | Values |
|---|---|
| Age at diagnosis | |
| Median range (years) | 50 (13–81) |
| <40 | 18 (14.9%) |
| 40~59 | 67 (55.4%) |
| ≥60 | 36 (29.6%) |
| Surgery | |
| MRM | 109 (90.1%) |
| BCS | 9 (7.4%) |
| Others | 3 (2.5%) |
| Histology subtype | |
| IDC | 102 (84.4%) |
| Others | 19 (18.6%) |
| Tumor size, cm | |
| ≤2 | 41 (33.9%) |
| 2~5 | 64 (52.9%) |
| >5 | 16 (13.2%) |
| Lymph node involvement | |
| LN(−) | 24 (19.8%) |
| LN(+) | 97 (80.2%) |
| Number of positive lymph nodes | |
| 0 | 24 (19.8%) |
| 1~3 | 38 (31.4%) |
| 4~9 | 29 (24.0%) |
| ≥10 | 30 (24.8%) |
| Stage | |
| I | 12 (9.9%) |
| II | 48 (39.7%) |
| III | 61 (50.4%) |
| Lymphovascular invasion | |
| No | 0 (100%) |
| Yes | 121 (0%) |
| Molecular subtype | |
| Basal-like TNBC | 30 (24.8%) |
| Non basal-like TNBC | 91 (75.2%) |
| Chemotherapy | |
| No | 2 (1.7%) |
| Yes | 119 (98.3%) |
| Chemotherapy regimen | |
| None | 2 (1.7%) |
| A-based | 12 (9.9%) |
| AT-based | 71 (58.7%) |
| Capecitabine-based | 9 (7.4%) |
| CMF | 7 (5.8%) |
| Others | 20 (16.5%) |
| Radiation | |
| No | 45 (37.2%) |
| Yes | 60 (49.6%) |
| NA | 16 (13.2%) |
| Hormone therapy | |
| No | 65 (53.7%) |
| Yes | 46 (38.0%) |
| NA | 10 (8.3%) |
MRM – modified radical mastectomy; BCS – breast-conserving surgery; IDC – invasive ductal carcinoma; NA – not available; A – anthrocycline;T – taxanes.
IHC detection of PREX1
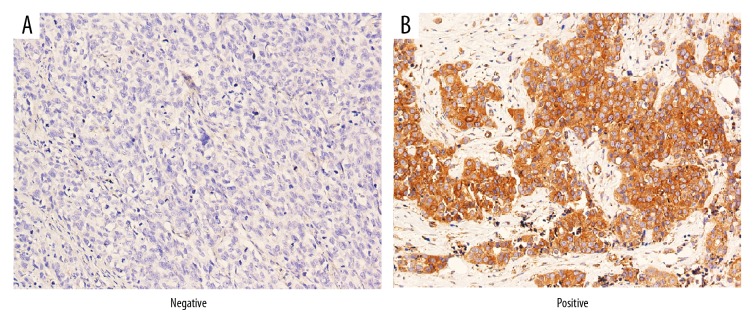
Consequently, total PREX1 expression was analyzed in 121 human breast cancer tissue sections. Representative negative and positive IHC staining for PREX1 are shown in Figure 1. IHC staining results of epithelial cells, and nonstromal cells, were classified as: 3+ (tumor cells were stained positive at least 50%), 2+ (tumor cells were stained positive in 10–50%), 1+ (tumor cells were stained positive in 1–10%), and negative (tumor cells were stained positive <1%).
Figure 1.
Immunohistochemical staining of PREX1 in representative breast tumor sections. Negative (A) and positive (B) staining for PREX1 are shown at 400× magnification.
Associations of PREX1 with clinicopathologic features of breast cancer patients
Table 2 summarizes the associations identified between the clinicopathological characteristics of our cohort and PREX1 expression. No significant associations were detected between PREX1 expression and patient age, histology subtype, tumor size, lymph node involvement, or pathologic stage. In contrast, PREX1-negative tumors were significantly associated with tumor recurrence (P=0.000) and metastasis (P=0.001). Associations between DFS and tumor size, basal-like features, and PREX1 status were also examined (Table 3). In univariate regression analyses, basal-like features, hormone therapy, and PREX1 expression exhibited significant associations with DFS. In multivariate regression analyses, PREX1 expression, and hormone therapy were identified as independent predictors of DFS.
Table 2.
Relationships between PREX1 cells with clinicopathologic features.
| All cases | PREX1 expression in tumor cells | P | |
|---|---|---|---|
| Negative | Positive | ||
| Age | 0.061 | ||
| <40 | 13 (19.7%) | 5 (9.1%) | |
| 40~59 | 37 (56.1%) | 30 (54.5%) | |
| ≥60 | 16 (24.2%) | 20 (36.4%) | |
| Histology subtype | 0.856 | ||
| IDC | 56 (84.8%) | 46 (83.6%) | |
| Others | 10 (15.2%) | 9 (16.4%) | |
| Tumor size | 0.686 | ||
| ≤2 | 22 (33.3%) | 19 (34.5%) | |
| 2~5 | 34 (51.5%) | 30 (54.5%) | |
| >5 | 10 (15.2%) | 6 (10.9%) | |
| Lymph node involvement | 0.340 | ||
| LN(−) | 11 (16.7%) | 13 (23.6%) | |
| LN(+) | 55 (83.3%) | 42 (76.4%) | |
| Number of positive lymph nodes | 0.020 | ||
| 0 | 11 (16.7%) | 13 (23.6%) | |
| 1~3 | 16 (24.2%) | 22 (40.0%) | |
| 4~9 | 18 (27.3%) | 11 (20.0%) | |
| ≥10 | 21 (31.8%) | 9 (16.4%) | |
| Stage | 0.057 | ||
| I | 6 (9.1%) | 6 (10.9%) | |
| II | 21 (31.8%) | 27 (49.1%) | |
| III | 39 (59.1%) | 22 (40.0%) | |
| Basal-like features | 0.267 | ||
| Present | 19 (28.8%) | 11 (20.0%) | |
| Absent | 47 (71.2%) | 44 (80.0%) | |
| Recurrence | 0.000 | ||
| Absent | 21 (51.2%) | 36 (83.7%) | |
| Present | 20 (48.8%) | 7 (16.3%) | |
| Metastasis | 0.001 | ||
| Absent | 21 (45.7%) | 36 (75.0%) | |
| Present | 25 (54.3%) | 12 (25.0%) | |
Table 3.
Univariate and multivariate analyses of various predictors of disease-free survival.
| Variable | No.patients | No.events (%) | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| P | P | |||
| Age | 0.145 | |||
| <40 | 18 | 8 (44.4%) | ||
| 40~59 | 67 | 35 (52.2%) | ||
| ≥60 | 36 | 23 (63.9%) | ||
| Operation method | 0.427 | |||
| MRM | 109 | 60 (55.0%) | ||
| BCS | 9 | 3 (33.3%) | ||
| Others | 3 | 3 (100.0%) | ||
| Tumor size | 0.165 | |||
| ≤2 | 41 | 18 (43.9%) | ||
| 2~5 | 64 | 39 (60.9%) | ||
| >5 | 16 | 9 (56.3%) | ||
| Lymph node involvement | 0.967 | |||
| LN(−) | 24 | 13 (54.2%) | ||
| LN(+) | 97 | 53 (54.6%) | ||
| Number of positive lymph nodes | 0.672 | |||
| 0 | 24 | 13 (54.2%) | ||
| 1~3 | 38 | 20 (52.6%) | ||
| 4~9 | 29 | 15 (51.7%) | ||
| ≥10 | 30 | 18 (60.0%) | ||
| Pathologic stage | 0.641 | |||
| I | 12 | 7 (58.3%) | ||
| II | 48 | 24 (50.0%) | ||
| III | 61 | 35 (57.4%) | ||
| HR | 0.208 | |||
| (−) | 56 | 34 (60.7%) | ||
| (+) | 65 | 32 (49.2%) | ||
| Chemotherapy | 0.195 | |||
| No | 2 | 2 (100.0%) | ||
| Yes | 119 | 64 (53.8%) | ||
| Radiation | 0.608 | |||
| No | 45 | 25 (55.6%) | ||
| Yes | 60 | 34 (56.7%) | ||
| NA | 16 | 7 (43.8%) | ||
| PREX1 | 0.000 | 0.000 | ||
| Negative | 66 | 47 (71.2%) | ||
| Positive | 55 | 19 (34.5%) | ||
| Basal-like feature | 0.018 | 0.174 | ||
| Present | 30 | 22 (73.3%) | ||
| Absent | 81 | 44 (48.4%) | ||
| Hormone therapy | 0.001 | 0.009 | ||
| No | 65 | 44 (67.7%) | ||
| Yes | 46 | 20 (43.5%) | ||
| NA | 10 | 2 (20.0%) | ||
Associations of PREX1 expression with metastasis and recurrence
The rate of metastasis or recurrence was 53.7% among the 121 patients examined. The associations between PREX1-positive tumors with both recurrence rate (P=0.000; Figure 2) and metastasis rate (P=0.001; Figure 3) were significant. Kaplan-Meier survival curves for DFS versus PREX1 expression are shown in Figure 4. PREX1 expression exhibit a significant association with DFS (P=0.013).
Figure 2.
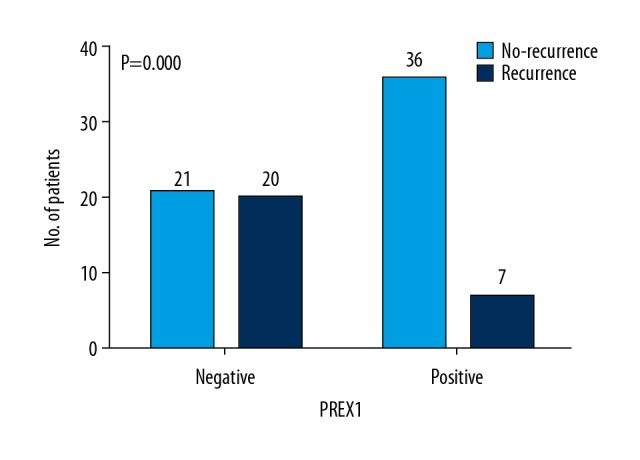
Relationship between PREX1 expression and recurrence. The number of patients with and without recurrence according to PREX1 expression. P-values are indicated.
Figure 3.
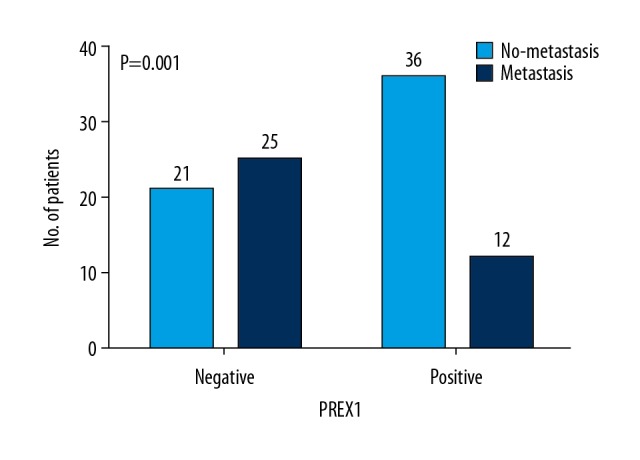
Relationship between PREX1 expression and metastasis. The number of patients with and without metastasis according to PREX1 expression. P-values are indicated.
Figure 4.
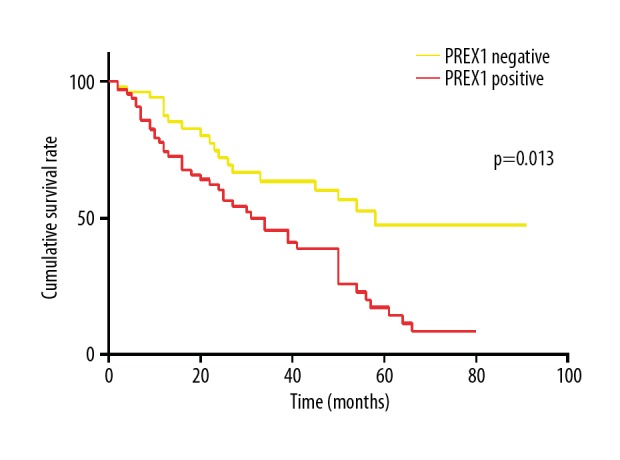
Disease free survival according to PREX1 expression.
Discussion
In recent studies, PREX1 has been shown to be an important mediator of tumor cell migration [8–10], the human PREX1 gene has been related with poor prognosis in cancer [11,21], and hypomethylation of the PREX1 promoter was identified as a prognostic marker of poor patient survival [22]. In the present study, PREX1 expression was not found to be significantly related to tumor size, pathological stage, hormone receptor (HR) expression, or basal-like features. These results are inconsistent with previous findings that PREX1 are upregulated in estrogen receptor positive breast tumors [8,10] and that PREX1 expression is highest in ER+ breast tumors compared with other cancer subtypes [8]. In contrast, the significant association that was identified between PREX1 and tumor recurrence in the present study is inconsistent with the results of previous in vitro and in vivo studies [10,11]. Moreover, univariate and multivariate regression analyses revealed that PREX1 is an independent predictor of DFS, and according to Kaplan-Meier survival curves, higher expression of PREX1 was associated with longer DFS in breast cancer.
In a recent study, PREX1-Rac-GEF activity was shown to be critical for cell growth and the growth of xenograft tumors. Thus, PREX1, Rac, and GEF are likely to be therapeutic targets for breast cancers with high expression of PREX1 [23]. In another study, a significant association between levels of phosphorylated Rex1 (P-Rex1) in human luminal breast cancer and poor prognosis was observed [21].
Downregulation of phosphorylated Rex1 (P-Rex1) was also reported to increase sensitivity of prostate tumors to bevacizumab [21]. Accordingly, VEGF/VEGFR-targeted therapy in combination with inhibition of P-Rex1 or Rac1 may improve the efficacy of these therapies significantly [24]. Overall, downregulation or upregulation of PREX1 has been found to reduce or promote the proliferation, migration, and invasive capacity of breast cancer cells, respectively [8–11]. The results of the present study indicate that lower levels of PREX1 expression are associated with poor prognosis in breast cancer patients, which is contrary to previous research conclusions. Our results remain to be confirmed in a larger study.
There were several limitations associated with the present study. First, the retrospective and single-institution samples analysis each represented limitations. Evaluation of PREX1 expression without standard method is also a disadvantage in this present study. Furthermore, multicollinearity existed between tumor size, number of metastatic lymph nodes, and TNM stage among the clinicopathological information, although these features were analyzed independently with regression models. Finally, we observed that PREX1 is associated with breast cancer recurrence and metastasis, and PREX1 was identified as an independent prognostic factor of DFS. It is possible that the short follow-up time and limited sample size of the present study account for this inconsistency with other research. Accordingly, a prospective multi-institutional study with larger number of patient samples are needed to prove the prognostic role of PREX1. Given the importance of PREX1 in breast cancer, it is additionally possible that PREX1 represents a valuable target in diagnosis and treatment for breast cancer.
Conclusions
The present study demonstrated that PREX1 expression was associated with recurrence rate and metastasis rate. Univariate and multivariate regression analyses also identified PREX1 expression as an independent predictor of DFS. While the present findings remain to be verified in a studies with a larger number of samples, our results suggested that PREX1 expression is an independent favorable predictor for breast cancer patients.
Acknowledgements
We would like to thank all of our patients for participating in this study and everyone who contributed towards the article.
Abbreviations
- IDC
invasive ductal carcinoma
- TNBC
triple-negative breast cancer
- N/A
not available
- A
anthrocycline
- T
taxane
- CMF
cyclophosphamide, methotrexate, and 5-fluorouracil
- MRM
modified radical mastectomy
- BCS
breast-conserving surgery
Footnotes
Source of support: This work was supported by Beijing Science and Technology Commission 2016: Science and Technology Project (Optimization of Breast Cancer Screening Program for the Right Age Women in Beijing)
Conflict of interests
None.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Ann Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 3.Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadok A, Marshall CJ. Rho GTPases: Masters of cell migration. Small GTPases. 2014;5:e29710. doi: 10.4161/sgtp.29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch HC, Coadwell WJ, Ellson CD, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108(6):809–21. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 6.Welch HC, Condliffe AM, Milne LJ, et al. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15(20):1867–73. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Lawson CD, Donald S, Anderson KE, et al. P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. J Immunol. 2011;186(3):1467–76. doi: 10.4049/jimmunol.1002738. [DOI] [PubMed] [Google Scholar]
- 8.Dillon LM, Bean JR, Yang W, et al. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene. 2015;34(30):3968–76. doi: 10.1038/onc.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell AD, Lawn S, McGarry LC, et al. P-Rex1 cooperates with PDGFRbeta to drive cellular migration in 3D microenvironments. PLoS One. 2013;8(1):e53982. doi: 10.1371/journal.pone.0053982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosa MS, Lopez-Haber C, Yang C, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell. 2010;40(6):877–92. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero JC, Seoane S, Ocana A, Pandiella A. P-Rex1 participates in Neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer. Oncogene. 2011;30(9):1059–71. doi: 10.1038/onc.2010.489. [DOI] [PubMed] [Google Scholar]
- 12.Fine B, Hodakoski C, Koujak S, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325(5945):1261–65. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsay CR, Lawn S, Campbell AD, et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun. 2011;2:555. doi: 10.1038/ncomms1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrio-Real L, Kazanietz MG. Rho GEFs and cancer: Linking gene expression and metastatic dissemination. Sci Signal. 2012;5(244):e43. doi: 10.1126/scisignal.2003543. [DOI] [PubMed] [Google Scholar]
- 15.Asimakopoulos FA, Green AR. Deletions of chromosome 20q and the pathogenesis of myeloproliferative disorders. Br J Haematol. 1996;95(2):219–26. doi: 10.1046/j.1365-2141.1996.d01-1896.x. [DOI] [PubMed] [Google Scholar]
- 16.Larramendy ML, Lushnikova T, Bjorkqvist AM, et al. Comparative genomic hybridization reveals complex genetic changes in primary breast cancer tumors and their cell lines. Cancer Genet Cytogenet. 2000;119(2):132–38. doi: 10.1016/s0165-4608(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 17.Stumpf E, Aalto Y, Hoog A, et al. Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer. 2000;29(1):83–87. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1011>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Berry R, Schroeder JJ, French AJ, et al. Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am J Hum Genet. 2000;67(1):82–91. doi: 10.1086/302994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134(7):e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 21.Barrio-Real L, Wertheimer E, Garg R, et al. Characterization of a P-Rex1 gene signature in breast cancer cells. Oncotarget. 2016;7(32):51335–48. doi: 10.18632/oncotarget.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrio-Real L, Benedetti LG, Engel N, et al. Subtype-specific overexpression of the Rac-GEF P-REX1 in breast cancer is associated with promoter hypomethylation. Breast Cancer Res. 2014;16(5):441. doi: 10.1186/s13058-014-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu HJ, Ooms LM, Srijakotre N, et al. PtdIns(3,4,5)P3-dependent Rac exchanger 1 (PREX1) Rac-guanine nucleotide exchange factor (GEF) activity promotes breast cancer cell proliferation and tumor growth via activation of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. J Biol Chem. 2016;291(33):17258–70. doi: 10.1074/jbc.M116.743401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goel HL, Pursell B, Shultz LD, et al. P-Rex1 promotes resistance to VEGF/VEGFR-targeted therapy in prostate cancer. Cell Rep. 2016;14(9):2193–208. doi: 10.1016/j.celrep.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are not publicly available but are available from the corresponding author on reasonable request.