Abstract
Background
Nivolumab is approved for the treatment of advanced renal cell carcinoma (RCC). However, traditional overall survival (OS) or progression-free survival (PFS) do not reflect patient prognosis after initial management. Therefore, this study aimed to evaluate conditional overall survival (COS) and conditional progression-free survival (CPFS) in patients with advanced RCC treated with nivolumab.
Material/Methods
There were 847 patients with advanced RCC treated with first-line nivolumab plus ipilimumab (n=425) and sunitinib (n=422), and 821 patients were treated with second-line nivolumab (n=410) and everolimus (n=411). Primary endpoints were COS and CPFS. Individual patient data of PFS and OS were digitally reconstructed from two large randomized controlled trials (CheckMate 025 and CheckMate 214).
Results
In first-line treatment, compared with sunitinib, improvement of one-year CPFS for the nivolumab plus ipilimumab group after living for 0.5 and 0.75 years were 14% (from 53.0% to 67.0%) and 16% (from 57.0% to 73.0%) higher than the one-year PFS of 6.5% (from 42.9% to 49.4%), with similar results for one-year COS following first-line treatment. For second-line treatment, compared with everolimus, the improvement of one-year CPFS for the nivolumab group after living for 0.5 and 0.75 years were 19% (from 25.0% to 44.0%) and 19% (from 27.0% to 46.0%) and significantly higher than the one-year PFS of 4.5% (from 18.5% to 23.0%).
Conclusions
Survival benefit for patients with advanced RCC from nivolumab (plus ipilimumab) compared with sunitinib was more evident from conditional survival (CS) analysis of first-line treatment.
MeSH Keywords: Carcinoma, Renal Cell; Immunotherapy; Survival
Background
Renal cell carcinoma (RCC) is the most common primary kidney cancer and is a heterogeneous disease. Many patients have metastases at the time of initial diagnosis. Also, RCC is characterized by a high degree of resistance to chemotherapy. Although many targeted therapies have been approved for the treatment of RCC, including sorafenib, they bring limited benefits to overall survival (OS).
Recently, immunotherapy has changed the landscape of treatment of several cancers. Nivolumab, a human IgG4 immunoglobulin, is a PD-1 binding immune checkpoint inhibitor [1]. Nivolumab has been shown to induce a significant survival benefit in patients with several advanced cancers, including non-small cell lung cancer (NSCLC), melanoma, and urothelial carcinoma [2–4]. Nivolumab has also been evaluated in the treatment of advanced renal cell carcinoma (RCC) as a first-line or second-line treatment. However, traditional overall survival (OS) or progression-free survival (PFS) may not reflect prognosis accurately after initial disease management.
Conditional survival (CS), derived from the concept of conditional probability, could provide more relevant prognostic information at each follow-up period [5,6]. For example, a patient may wish to know the expected survival for another two years after living five years from initial diagnosis, which is different from the initial seven-year survival after diagnosis and is best answered as two-year conditional survival at five years. Previous studies have suggested that CS may offer more accurate estimates for these patients.
Therefore, the present study aimed to evaluate conditional OS (COS) and conditional PFS (CPFS) in patients with advanced RCC treated with nivolumab as a first-line or second-line therapy, based on two large randomized controlled trials (RCTs), CheckMate 025 and CheckMate 214 [7,8]. The use of CS may have important implications for patient counseling and planning patient surveillance.
Material and Methods
Individual patient data on progression-free survival (PFS) and overall survival (OS) were digitally reconstructed from the CheckMate 025 and CheckMate 214 randomized controlled trials (RCTs) [7,8], using R and DigitizeIt software (Figure 1). Previous studies described the steps and the corresponding computer programs that are publicly available to enable further statistical methodology research [9]. The methods were widely used in previous studies [10,11] We used the method and the available R code to obtain individual patient data. The data included individual treatment type and possibly censored time to event data consistent with a published Kaplan-Meier curve.
Figure 1.
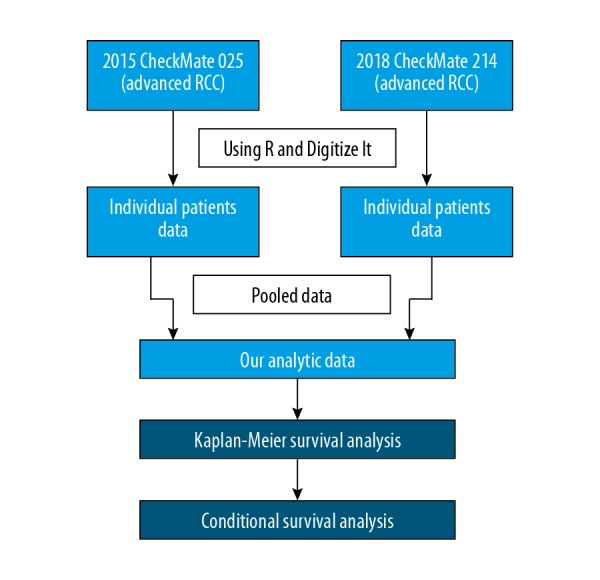
Flowchart showing individual patient data of progression-free survival (PFS) and overall survival (OS) that were digitally reconstructed from the CheckMate 025 and CheckMate 214 studies [7,8], using R and DigitizeIt software.
Conditional survival described the proportion of patients who survived. For example, three additional years was represented by the following equation: when S(t) is overall survival at time t, conditional survival was represented by: S(x +3)/S(x).
Standardized differences (d) were used to assess the differences in CS between subgroups based on the method described by Cucchetti et al. [12]. The standardized difference in proportions was calculated as: (P2–P1)/√ [P(1–P)] where P was the weighted mean of P1 and P2. The primary endpoints in this study were conditional overall survival (COS) and conditional progression-free survival (CPFS). CPFS or COS were estimated from individual patient data using the multiplicative law of probability. The differences in conditional survival (CS) between groups were compared with the calculation of the d-value, as previously described [13].
Results
There were 847 patients with advanced RCC treated with first-line nivolumab plus ipilimumab (n=425) and sunitinib (n=422), and 821 patients were treated with second-line nivolumab (n=410) and everolimus (n=411). Primary endpoints were conditional overall survival (COS) and conditional progression-free survival (CPFS). Individual patient data of PFS and OS were digitally reconstructed from two large randomized controlled trials, CheckMate 025 and CheckMate 214 [7,8]. CPFS and COS at various time points for advanced RCC patients treated with nivolumab as a first-line and second-line treatment are shown in Table 1.
Table 1.
Conditional progression-free survival (CPFS) and conditional overall survival (COS) at various time points in patients with advanced renal cell carcinoma (RCC) treated with nivolumab as a first-line and second-line treatment.
| Overall survival (OS) | Progression-free survival (PFS) | |||||||
|---|---|---|---|---|---|---|---|---|
| Observed survival (%) | One-year conditional OS (COS) (%) | Observed survival (%) | One-year conditional PFS (CPFS) (%) | |||||
| First-line | Nivolumab + Ipilimumab | Sunitinib | Nivolumab + Ipilimumab | Sunitinib | Nivolumab + Ipilimumab | Sunitinib | Nivolumab + Ipilimumab | Sunitinib |
| 3 months | 95.70 | 94.60 | 80.00 | 68.00 | 80.70 | 77.80 | 59.00 | 48.00 |
| 6 months | 89.70 | 86.20 | 83.00 | 70.00 | 64.10 | 61.00 | 67.00 | 53.00 |
| 9 months | 84.40 | 78.60 | 83.00 | 71.00 | 54.80 | 49.00 | 73.00 | 57.00 |
| 12 months | 80.30 | 72.30 | 81.00 | 73.00 | 49.40 | 42.90 | 62.00 | 55.00 |
| Second-line | Nivolumab | Everolimus | Nivolumab | Everolimus | Nivolumab | Everolimus | Nivolumab | Everolimus |
| 3 months | 95.40 | 91.80 | 72.00 | 68.00 | 59.50 | 63.30 | 33.00 | 25.00 |
| 6 months | 88.90 | 81.10 | 71.00 | 67.00 | 38.80 | 39.20 | 44.00 | 25.00 |
| 9 months | 83.70 | 71.90 | 67.00 | 65.00 | 32.10 | 29.90 | 46.00 | 27.00 |
| 12 months | 75.70 | 66.40 | 69.00 | 67.00 | 23.00 | 18.50 | 59.00 | 28.00 |
PFS – progression-free survival; OS – overall survival; CPFS – conditional progression-free survival; COS – conditional overall survival; RCC – renal cell carcinoma.
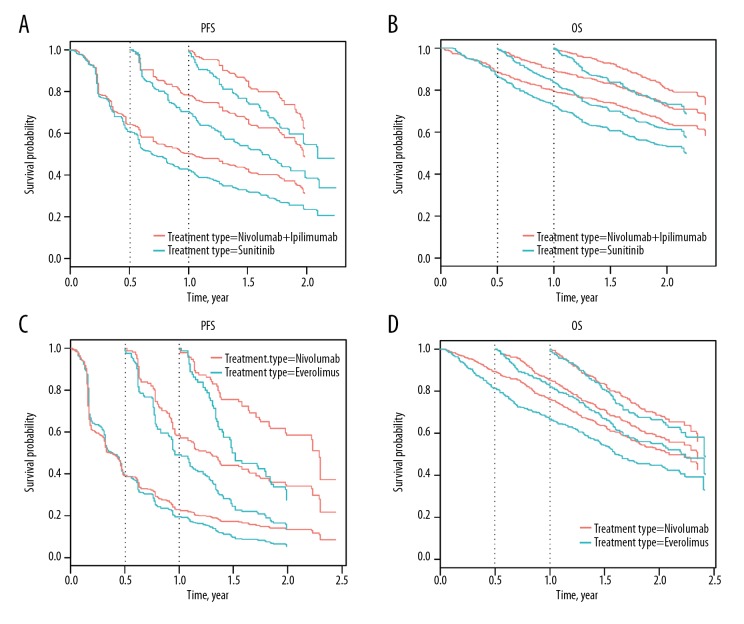
In first-line treatment, compared with sunitinib, improvement of one-year CPFS for the nivolumab plus ipilimumab group after living for 0.5 and 0.75 years were 14% (from 53.0% to 67.0%) and 16% (from 57.0% to 73.0%) higher than the one-year PFS of 6.5% (from 42.9% to 49.4%), with similar results for one-year COS following first-line treatment (Figure 2A). The improvement of one-year COS after treatment with nivolumab and ipilimumab compared with sunitinib for 0.5 and 0.75 year were 13% (from 70.0% to 83.0%) and 12% (from 71.0% to 83.0%) higher than that of one-year OS (8.0%, from 72.3% to 80.3%) (Figure 2B).
Figure 2.
Conditional progression-free survival (CPFS) and conditional overall survival (COS) curves for patients with advanced renal cell carcinoma (RCC) treated with nivolumab, ipilimumab, or sunitinib. (A) Conditional progression-free survival (CPFS) curves of patients with advanced renal cell carcinoma (RCC) treated with nivolumab (plus ipilimumab) or sunitinib according to the number of years after randomization. (B) Conditional overall survival (COS) curves of patients with advanced RCC treated with nivolumab (plus ipilimumab) or sunitinib. (C) CPFS curves of patients with advanced RCC treated with nivolumab or everolimus. (D) COS curves of patients with advanced RCC treated with nivolumab or everolimus. Traditional Kaplan-Meier estimates of PFS/OS (the starting point of the X axis=0) overlaid by conditional CS estimates at 0.5 yr (the starting point of the X axis=0.5), 0.75 yr (the starting point of the X axis=0.75) and 1 yr (the starting point of the X axis=1) are shown from the time of randomization.
For second-line treatment, compared with everolimus, the improvement of one-year CPFS for the nivolumab group after living for 0.5 and 0.75 years were 19% (from 25.0% to 44.0%) and 19% (from 27.0% to 46.0%) and significantly higher than the one-year PFS of 4.5% (from 18.5% to 23.0% (Figure 2C). However, the one-year COS improvement were 4.0% (from 67.0% to 71.0%) and 2.0% (from 65.0% to 67.0%) lower than that of the one-year OS of 9.3% (from 66.4% to 75.7% (Figure 2D).
Discussion
Patients with advanced renal cell carcinoma (RCC) have a poor prognosis, and there is an urgent requirement for effective treatment. Standard treatment includes targeted treatments, including tyrosine kinase inhibitors (sorafenib, sunitinib), mammalian targets of rapamycin (mTOR) inhibitors (temsirolimus, everolimus), and anti-angiogenic antibodies (bevacizumab). However, these treatments may not be effective. Immunotherapy is a growing field in cancer treatment. Recently, and nivolumab has been tested on patients with RCC in two randomized controlled trials (RCTs), CheckMate 025 and CheckMate 214 [7,8].
Nivolumab blocks the interaction between PD-1 and PD-1 ligand 1 (PD-L1) and 2 (PD-L2) to potentiate immune responses and antitumor activity. There have been several RCTs that have investigated the safety and efficacy of nivolumab in several types of cancer. Nivolumab is superior to chemotherapy in patients with metastatic melanoma or advanced non-small cell lung cancer (NSCLC). CheckMate 214 showed that nivolumab combined with ipilimumab could prolong overall survival (OS) in previously untreated patients when compared with sunitinib [9]. The results of the CheckMate 025 trial showed that nivolumab could improve OS but not progression-free survival (PFS) in previously treated patients.
However, traditional survival estimates do not apply to patients who have survived a period of time after initial diagnosis or treatment. Therefore, this study was designed to assess conditional progression-free survival (CPFS) and conditional overall survival (COS) in patients with advanced RCC treated with nivolumab. The analysis of two large prospective trials give convincing and contemporary data for novel immunotherapy, as the mean follow-up after immunotherapy in both trials was <26 months, the one-year COS was chosen for our analysis. Our results suggested that a survival benefit from nivolumab (plus ipilimumab) over sunitinib was more apparent in the areas of CS in first-line treatment. A previous study showed that the one-year COS of patients with metastatic RCC treated with vascular endothelial growth factor (VEGF)-targeted therapy ranged from 66–70% after surviving 1.5 years [6]. In our study, one-year COS of patients treated with sunitinib was similar to previous results. Therefore, CS may be a useful prognostic measure that can be used for patient counseling, such as decisions would be the use of immunotherapy, predicting survival, especially for patients treated with nivolumab as the first-line treatment. This study also supported the latest recommendation from the European Association of Urology Renal Cell Cancer Guidelines Panel and the International Kidney Cancer Coalition based on the improved use of CS [10]. These guidelines recommend nivolumab and ipilimumab for patients with an International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score with features associated with intermediate risk and poor risk. However, studies with long-term follow-up are still required.
This study had several limitations. The present study was based on survival plot analysis from previous publications. Therefore, the grouping of patients was pre-specified, and a comprehensive subgroup analysis could not be performed.
Conclusions
The findings from this study provided contemporary data of conditional progression-free survival (CPFS), and conditional overall survival (COS) in patients with advanced renal cell carcinoma (RCC) treated with nivolumab as first-line or second-line therapy. The survival benefit from nivolumab (plus ipilimumab) over sunitinib was more apparent in the areas of conditional survival (CS) in first-line treatment. CS may be a useful measure permitting more accurate prognosis information for immunotherapy. This study also supported the recommendations to offer nivolumab (plus ipilimumab) to patients with advanced RCC based on the marked improvement in CS. The CS information of nivolumab has important implications for patient counseling and treatment decisions.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81370073 for Yao Zhu and 81472377, 81672544 for Dingwei Ye)
Conflict of interest
None.
References
- 1.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430–37. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higuchi M, Owada Y, Inoue T, et al. FDG-PET in the evaluation of response to nivolumab in recurrent non-small-cell lung cancer. World J Surg Oncol. 2016;14(1):238. doi: 10.1186/s12957-016-0998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traylor JI, Kuo JS. Combined nivolumab and ipilimumab is an effective treatment for melanoma brain metastases. Neurosurgery. 2019;84(3):E134–35. doi: 10.1093/neuros/nyy619. [DOI] [PubMed] [Google Scholar]
- 4.Rui X, Gu TT, Pan HF, Zhang HZ. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int Immunopharmacol. 2018;67:378–85. doi: 10.1016/j.intimp.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Zamboni BA, Yothers G, Choi M, et al. Conditional survival and the choice of conditioning set for patients with colon cancer: An analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010;28(15):2544–48. doi: 10.1200/JCO.2009.23.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harshman LC, Xie W, Bjarnason GA, et al. Conditional survival of patients with metastatic renal cell carcinoma treated with VEGF-targeted therapy: A population-based study. Lancet Oncol. 2012;13(9):927–35. doi: 10.1016/S1470-2045(12)70285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satagopan JM, Iasonos A, Kanik JG. A reconstructed melanoma data set for evaluating differential treatment benefit according to biomarker subgroups. Data Brief. 2017;12:667–75. doi: 10.1016/j.dib.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bex A, Albiges L, Staehler M, et al. A joint statement from the European Association of Urology Renal Cell Cancer Guidelines Panel and the International Kidney Cancer Coalition: The rejection of ipilimumab and nivolumab for renal cancer by the Committee for Medicinal Products for Human Use Does not Change Evidence-based Guideline Recommendations. Eur Urol. 2018;74(6):849–51. doi: 10.1016/j.eururo.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Aguiar PN, Jr, Haaland B, Park W, et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 2018;4(8):1080–84. doi: 10.1001/jamaoncol.2018.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucchetti A, Piscaglia F, Cescon M, et al. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res. 2012;18(16):4397–405. doi: 10.1158/1078-0432.CCR-11-2663. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Margonis GA, Prescott JD, et al. Curative surgical resection of adrenocortical carcinoma: Determining long-term outcome based on conditional disease-free probability. Ann Surg. 2017;265(1):197–204. doi: 10.1097/SLA.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]