Abstract
Background
In anterior cervical discectomy and fusion (ACDF) surgery, drilling operation causes a high risk of tissue injury. This study aimed to present a novel feedback system based on sound pressure signals to identify drilling condition during ACDF.
Material/Methods
ACDF surgery was performed on the C4/5 segments of 6 porcine cervical specimens. The annulus fibrosus, endplate cartilage, sub-endplate cortical bone, and posterior longitudinal ligament (PLL) were drilled until penetration using a 2-mm high-speed burr. Sound pressure signals were collected using a microphone and dynamic signal analyzer. The recorded signals of different tissues were proceeded with lifting wavelet transform for extracting harmonic components. The frequencies of harmonic components are 1, 2, 3, 4, and 5 times higher than the motor frequency. The magnitude of harmonic components was calculated to identify different drilling conditions, along a broad spectrum of frequencies (1–5 kHz). For statistical analysis, one-way ANOVA (analysis of variance) and post hoc test (Dunnett’s T3) were performed.
Results
Very good demarcation was found among the signal magnitudes of different drilling conditions. Different drilling conditions do not present the same rate of variation of frequency. Differences in magnitude among all drilling conditions were statistically significant at certain frequency points (p<0.05). In 3 cases, one tissue could not be identified with respect to another (annulus fibrosus and endplate cartilage at 2 kHz, PLL and penetration at 3 kHz, annulus fibrosus and sub-endplate cortical bone at 5 kHz, p>0.05).
Conclusions
Sound pressure signals may provide an auxiliary feedback system for enhancing drilling operation in ACDF surgery, especially in minimally invasive surgery.
MeSH Keywords: Cervical Vertebrae; Decompression; Intraoperative Complications; Sound; Spinal Cord Compression; Surgical Procedures, Minimally Invasive
Background
Anterior cervical discectomy and fusion (ACDF) surgery is a well-established surgical intervention for cervical spondylotic myelopathy, and a high success rate with excellent long-term outcomes has been reported [1–3]. More recently, with the increasing interest in minimally invasive surgery, the approach has been adapted for minimally invasive techniques [4–6].
For traditional and minimally invasive surgery, during ACDF surgery, the drilling process using a high-speed drill is a very common operation and must take place along the narrow intervertebral space to remove the herniated disc [7,8]. Given the limited working space and various important structures adjacent to the PLL, there is a high risk of the burr plunging into the spinal canal during the drilling operation. Failed detection of PLL penetration can cause irreparable damage to the dura mater, spinal cord, and nerve roots [9–12]. These complications may significantly decrease the well-being of patients who may need additional surgeries after the first operation. The drilling tools currently used in orthopedics do not include any feedback system for the detection of drilling conditions, and only radiographic control and the surgeon’s manual skill are used to avoid penetration. Consequently, X-ray examinations are usually performed, which significantly increases the risk of patients and medical staff.
Thus, selecting an appropriate drilling condition detection method for anterior cervical decompression surgery, especially in minimally invasive surgery, is key to avoid the aforementioned issues, and special care should be taken during the drilling operation.
More recently, some studies have reported the application of force and torque feedback in bone drilling or milling operation for identifying the bone cutting status and decreasing potential injury to the surrounding organs after penetration [13–16]. However, these systems cannot be easily integrated with use of high-speed burr, and their feasibility of drilling condition monitoring in clinical research is still unclear.
During orthopedic surgery, drilling sound is closely related to the mechanical characteristic of underlying tissues and could be used to guide drilling motions. Praamsma et al. reported that drilling sound is important for expert orthopedic surgeons to determine bone density and judge drilling states [17]. Previously, studies have reported that bone milling and drilling status can be correctly determined by extracting and analyzing the sound features [18,19]. However, to the best of our knowledge, drilling condition identification based on sound pressure signals during ACDF surgery has never been studied.
Real-time drilling condition identification is critical to facilitate safe decompression during ACDF surgery. To address these challenges, in the present study, a sound pressure signal feedback system was proposed to discriminate between different tissues.
Material and Methods
Specimen preparation
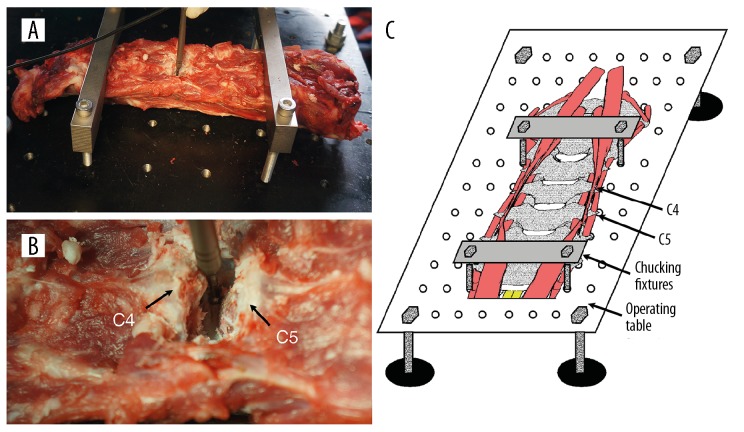
Cervical spine (C2–C7 segments) specimens of 6 immature domestic pigs (mean weight: 37.8 kg, standard deviation: 2.1; 3 females, 3 males) were obtained from a slaughterhouse. The spine specimens were cleared of excess anterior muscle tissues, while all other soft tissues were left intact. Spine specimens were stored frozen at −20°C. Before testing, the spine specimens were thawed at 24°C for 12 h. These 6 specimens were tested in a single session; while one specimen was tested, the other specimens were stored at 4°C. For extra fixation, the specimens were fixed on the operating table by chucking fixtures (Figure 1A, 1C). During preparation and testing, the specimens were kept moist by spraying with saline.
Figure 1.
Preparation of porcine cervical spine specimens (A), the drilling operation during ACDF on the C4/C5 segment (B), schematic representation of the specimen preparation (C).
Surgical procedures
In each specimen, ACDF surgery was performed on the C4/5 segment by an experienced surgeon. The disc was incised and then removed with pituitary rongeurs, bayonetted Kerrisons, and curettes. A high-speed drill with a 2-mm melon burr was applied for drilling the remaining part of the annulus fibrosis, endplate cartilage, sub-endplate cortical bone, and PLL until penetration (Figure 1B). The feeding rate of the drill was controlled to <0.1 mm/s on average. In addition, sufficient and constant cooling irrigation was provided during drilling, and the volume flow rate of the water (4°C) was approximately 20 mL/min.
Sound pressure signal measurement
The global behavior of the measurements on the 6 specimens were managed and analyzed. The experiment comprised measuring the sound pressure signal of different drilling conditions (in vitro) from pigs: annulus fibrosis, endplate cartilage, sub-endplate cortical bone, PLL, and penetration. We repeated the same experiment twice for each type of drilling condition in one specimen. During each drilling process, the burr was controlled to prevent twisting of soft tissues.
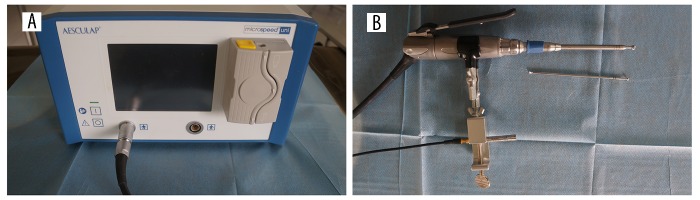
The AESCULAP GD676 (B. Braun Vet Care GmbH, Tuttlingen, Germany) high-speed operation power system was used in the experiments. The maximum and minimum rotation rate of the drill was 10 000 and 80 000 revolutions per minute (rpm). The speed of the motor was set at 60 000 rpm, with motor frequency of 1 kHz. During the drilling process, a 46BE free-field microphone (GRAS, Holte, Denmark) and USB-4431 dynamic signal analyzer (National Instruments, Austin, USA) were used to record the sound pressure signals. The frequency range of the microphone is 0.01–40 kHz and the sensitivity is 4 mV/Pa. The resolution of the analyzer is 24-bit and the sampling frequency is 102.4 kHz, which allow accurate sound pressure signal measurement. The microphone was installed beside the handpiece of the high-speed drill device by a metal clip and was facing the burr. The distance between the microphone and drill bit was 200 mm, so it could maintain synchronous movement with the burr and an accurate sound pressure signal could be recorded (Figure 2).
Figure 2.
The sound pressure signal measurement system. The high-speed drill (A); the 2-mm melon burr and microphone (B).
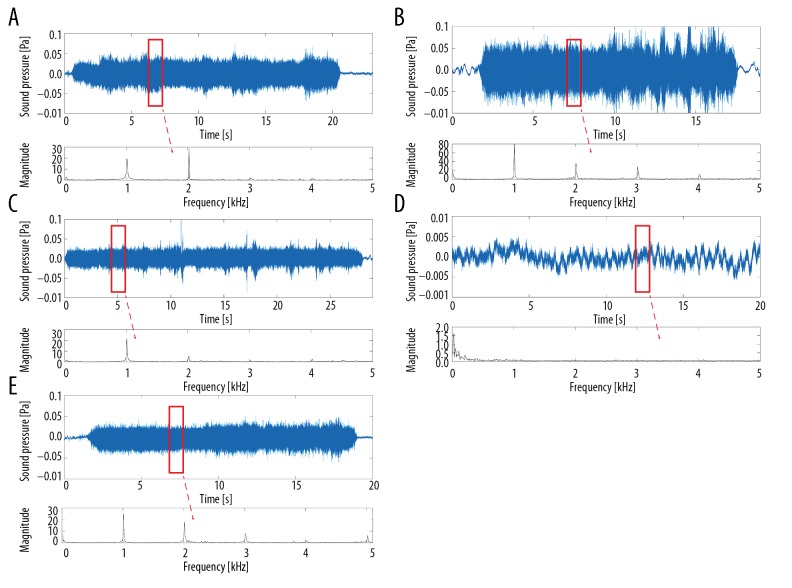
Dai et al. demonstrate that some harmonic components exist in the drilling sound, and their frequencies are 1, 2, 3, 4, and 5 times higher than that of the motor frequency [18]. Therefore, the recorded signals were proceeded using MATLAB 2017a (MathWorks, Natick, MA). The harmonic components at the frequency points of 1, 2, 3, 4, and 5 kHz were extracted by lifting wavelet transform (Figure 3). Finally, for each measurement, at every frequency point, 10 consecutive values of magnitude were obtained for statistical analyses.
Figure 3.
The process of lifting wavelet transforms to extract harmonic components from sound pressure signal files. Annulus fibrosis (A), sub-endplate cortical bone (B), posterior longitudinal ligament (C), penetration (D), endplate cartilage (E).
Statistical analysis
PASW statistics 22 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. Values of magnitude are shown as mean ± standard deviation. To investigate the significance of differences in sound pressure signals between any 2 drilling conditions, one-way ANOVA and post hoc test (Dunnett’s T3) were used to compare magnitudes at every frequency point. Statistical significance was defined as p<0.05.
Results
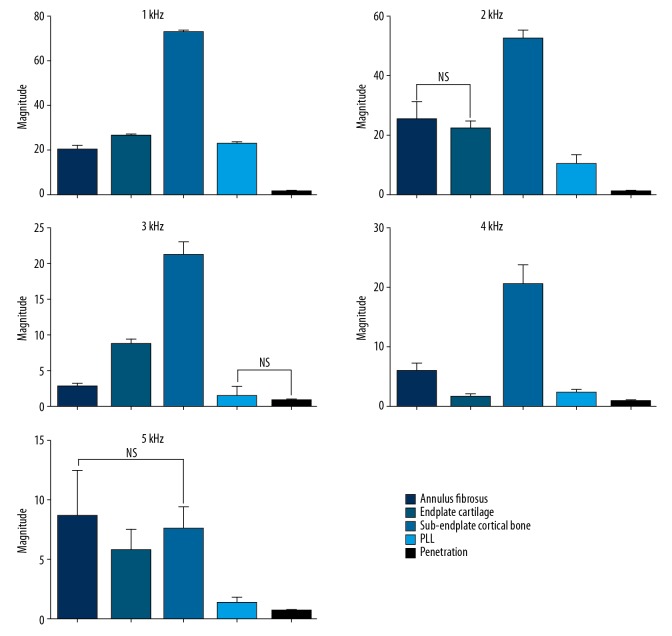
Figure 4 shows the mean value of each drilling condition, with the respective standard deviation of the magnitude of sound pressure signal along the entire frequency spectrum. In general, a clear separation among the drilling conditions was detected. The magnitude values in different tissues did not present the same rate of variation with frequency. For example, the maximum and minimum magnitude values of the annulus fibrosis were recorded at 2 and 3 kHz, those of the endplate cartilage at 1 and 4 kHz, and those of the sub-endplate cortical bone, PLL, and penetration were recorded at 1 and 5 kHz, respectively. The magnitude values in different tissues at every frequency point are shown in Table 1.
Figure 4.
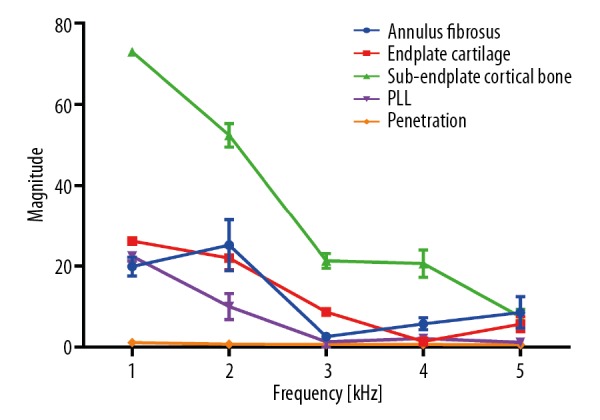
The magnitude of sound pressure signal of different drilling conditions along the entire frequency spectrum, expressed as mean ± standard deviation.
Table 1.
The magnitude of sound pressure signal of different drilling conditions along the whole frequency spectrum (mean ± standard deviation).
| Tissues | Frequency (kHz) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Annulus fibrosus | 19.88±2.31 | 25.17±6.28 | 2.64±0.61 | 5.79±1.5 | 8.6±3.88 |
| Endplate cartilage | 26.19±0.8 | 21.96±2.82 | 8.68±0.86 | 1.43±0.51 | 5.72±1.82 |
| Sub-endplate cortical bone | 72.97±0.59 | 52.4±2.88 | 21.3±1.78 | 20.62±3.36 | 7.49±1.86 |
| PLL | 22.41±1.18 | 10.04±3.19 | 1.38±1.37 | 2.16±0.65 | 1.25±0.53 |
| Penetration | 1.15±0.36 | 0.75±0.16 | 0.68±0.15 | 0.7±0.18 | 0.61±0.12 |
Pairwise post hoc comparisons (Dunnett’s T3 test) indicated that differences between any 2 drilling conditions were statistically significant (p<0.05) at certain frequency points, except in 3 cases: annulus fibrosus and endplate cartilage at 2 kHz, PLL and penetration at 3 kHz, and annulus fibrosus and sub-endplate cortical bone at 5 kHz (p>0.05) (Figure 5). Table 2 presents p values of the pairwise comparisons at each frequency point. Generally, we could discriminate between any 2 drilling conditions at a certain frequency by using the sound pressure signal.
Figure 5.
Comparison between different drilling conditions along the entire frequency spectrum. NS indicates not statistically significant (p>0.05); no symbol indicates statistically significant (p<0.05).
Table 2.
The p values of pairwise comparison among different tissues along the whole frequency spectrum.
| Tissue pairs | Frequency (kHz) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Annulus fibrosus vs. endplate cartilage | S | NS | S | S | S |
| Annulus fibrosus vs. sub-endplate cortical bone | S | S | S | S | NS |
| Annulus fibrosus vs. PLL | S | S | S | S | S |
| Annulus fibrosus vs. penetration | S | S | S | S | S |
| Endplate cartilage vs. sub-endplate cortical bone | S | S | S | S | S |
| Endplate cartilage vs. PLL | S | S | S | S | S |
| Endplate cartilage vs. penetration | S | S | S | S | S |
| Sub-endplate cortical bone vs. PLL | S | S | S | S | S |
| Sub-endplate cortical bone vs. penetration | S | S | S | S | S |
| PLL vs. penetration | S | S | NS | S | S |
Deep blue boxes and NS indicate no statistically significant difference: annulus fibrosus vs. endplate cartilage at 2 kHz (p=0.132), PLL vs. penetration at 3 kHz (p=0.082), and annulus fibrosus vs. sub-endplate cortical bone at 5 kHz (p=0.816). Blue boxes and S indicate a statistically significant difference. All the values in blue boxes are 0.000, except in 2 cases: annulus fibrosus vs. endplate cartilage at 5 kHz (p=0.007) and endplate cartilage vs. sub-endplate cortical bone at 5 kHz (p=0.004).
Discussion
This study investigated the feasibility of applying sound pressure signals to distinguish between different tissues and to improve the success of medullary decompression by providing real-time feedback in ACDF surgery. Our results suggest that it is always possible to discriminate among tissues in terms of magnitude at a certain frequency point.
The most severe complication in ACDF surgery is spinal cord injury during decompression [12,20]. Localization of the motor burr is important for safe decompression and for the surgeon during robot-assisted surgery [21]. Nanda et al., in their series of 1576 patients, reported that the incidence of dural tear and cerebrospinal fluid leak during ACDF was 1.3% [22]. The spinal cord is at risk for injury throughout all phases of anterior cervical spine surgery, with the reported incidence of acute iatrogenic spinal cord injury ranging from 0.2% to 0.9% [23,24]. Clear identification of the annulus fibrosus and PLL helps prevent iatrogenic cord injury. In this study, we observed significant differences in sound pressure signals between the annulus fibrosus and PLL. Therefore, when the burr contacted with the PLL, the proposed feedback system could alert the surgeons to stop drilling in time to prevent injury.
In addition, we detected the sound pressure signal characteristics of PLL penetration. If the feeding rate is slow enough, the burr can be controlled by the feedback system, avoiding contact with the dura. However, a more sensitive multiparameter feedback system needs to be developed to prevent cord or nerve root injury.
Cage subsidence after ACDF surgery aggravates kyphosis, with decrease in the foraminal volume and recurrence of spinal canal stenosis [20,25]. A previous study found that interruption of the sub-endplate cortical bone often results in cage subsidence and kyphosis. Preserving the integrity of the sub-endplate cortical bone is crucial to maintain good mechanical condition of implants and to maintain intervertebral height [26,27]. Hence, the endplates should be carefully cleaned and not penetrated. Our study demonstrated a statistically significant difference in the sound pressure signals between the endplate cartilage and sub-endplate cortical bone at every frequency point, indicating that sound pressure detection can decrease the neurological damage during and after surgery.
Several limitations of this study should be mentioned. First, using a porcine model provides a homogeneous specimen population and assists with controlling factors such as age, diet, weight, and level of activity, but limits the direct applicability of the results in humans. In the future, we will evaluate the feasibility of sound pressure signal in clinical or cadaver experiments. Second, we only explored the sound pressure signal for tissue identification in ACDF surgery. Other feedback systems combined with sound pressure signal should be studied to improve decompression safely. Finally, to realize the automation of the surgical drilling process, a computer-controlled system matched with sound pressure signal should be developed in the next step.
Conclusions
During the drilling process, differences in the sound pressure signals among annulus fibrosus, endplate cartilage, sub-endplate cortical bone, PLL, and penetration are detectable. Use of sound pressure signals may be a potential candidate for building a feedback system to facilitate safe decompression during ACDF surgery. In this study, the noncontact feature of sound pressure signal measurement makes it suitable for integration with use of a high-speed burr.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (grant number: 81871124, 81471403, 30973024, and 61773223), and the Natural Science Foundation of Tianjin (grant number: 18JCYBJC18800)
Conflict of interest
None.
References
- 1.Li J, Zheng Q, Guo X, et al. Anterior surgical options for the treatment of cervical spondylotic myelopathy in a long-term follow-up study. Arch Orthop Trauma Surg. 2013;133(6):745–51. doi: 10.1007/s00402-013-1719-4. [DOI] [PubMed] [Google Scholar]
- 2.Finn MA, MacDonald JD. C2–3 anterior cervical fusion: Technical report. Clin Spine Surg. 2016;29(10):E536–41. doi: 10.1097/BSD.0b013e318292b3ca. [DOI] [PubMed] [Google Scholar]
- 3.Wen Z, Lu T, Wang Y, et al. Anterior cervical corpectomy and fusion and anterior cervical discectomy and fusion using titanium mesh cages for treatment of degenerative cervical pathologies: A literature review. Med Sci Monit. 2018;24:6398–404. doi: 10.12659/MSM.910269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergara P, Timofeev I. Minimally invasive anterior cervical discectomy and fusion: A valid alternative to open techniques. Acta Neurochir (Wien) 2018;160(12):2467–71. doi: 10.1007/s00701-018-3719-1. [DOI] [PubMed] [Google Scholar]
- 5.Cai R-Z, Wang Y-Q, Wang R, et al. Microscope-assisted anterior cervical discectomy and fusion combined with posterior minimally invasive surgery through tubular retractors for multisegmental cervical spondylotic myelopathy. Medicine. 2017;96(35):e7965. doi: 10.1097/MD.0000000000007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn Y. Percutaneous endoscopic cervical discectomy using working channel endoscopes. Expert Rev Med Devices. 2016;13(6):601–10. doi: 10.1080/17434440.2016.1180245. [DOI] [PubMed] [Google Scholar]
- 7.Leimert M, Bostelmann R, Juratli TA, et al. A newly developed drill with a polished tip for the anterior cervical approach in spinal canal stenosis: A technical note. Eur Spine J. 2013;22(4):809–12. doi: 10.1007/s00586-012-2572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guven M, Ceylan D, Aras AB, et al. Effect of using high-speed drill in anterior cervical discectomy and fusion. Turk Neurosurg. 2016;26(1):97–104. doi: 10.5137/1019-5149.JTN.11182-14.2. [DOI] [PubMed] [Google Scholar]
- 9.Cammisa FP, Jr, Girardi FP, Sangani PK, et al. Incidental durotomy in spine surgery. Spine (Phila Pa 1976) 2000;25(20):2663–67. doi: 10.1097/00007632-200010150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Fountas KN, Kapsalaki EZ, Johnston KW. Cerebrospinal fluid fistula secondary to dural tear in anterior cervical discectomy and fusion: Case report. Spine (Phila Pa 1976) 2005;30(10):E277–80. doi: 10.1097/01.brs.0000162399.93992.5c. [DOI] [PubMed] [Google Scholar]
- 11.Feng F, Ruan W, Liu Z, et al. Anterior versus posterior approach for the treatment of cervical compressive myelopathy due to ossification of the posterior longitudinal ligament: A systematic review and meta-analysis. Int J Surg. 2016;27:26–33. doi: 10.1016/j.ijsu.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Daniels AH, Riew KD, Yoo JU, et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16(12):729–38. doi: 10.5435/00124635-200812000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Lee W-Y, Shih C-L. Force control and breakthrough detection of a bone drilling system. Ieee-Asme Transactions on Mechatronics. 2003;9(1):20–29. [Google Scholar]
- 14.Allotta B, Belmonte F, Bosio L, Dario P. Study on a mechatronic tool for drilling in the osteosynthesis of long bones: Tool/bone interaction, modeling and experiments. Mechatronics. 1996;6(4):447–59. [Google Scholar]
- 15.Cao T, Li X, Gao Z, et al. A method for identifying otological drill milling through bone tissue wall. Int J Med Robot. 2011;7(2):148–55. doi: 10.1002/rcs.382. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Jin H, Zhang L, et al. State recognition of pedicle drilling with force sensing in a robotic spinal surgical system. IEEE/ASME Transactions on Mechatronics. 2014;19(1):357–65. [Google Scholar]
- 17.Praamsma M, Carnahan H, Backstein D, et al. Drilling sounds are used by surgeons and intermediate residents, but not novice orthopedic trainees, to guide drilling motions. Can J Surg. 2008;51(6):442–46. [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Y, Xue Y, Zhang J. Condition monitoring based on sound feature extraction during bone drilling process. Proceedings of the 33rd Chinese Control Conference; 2014 July 28–30. [Google Scholar]
- 19.Dai Y, Xue Y, Zhang J. State identification based on sound analysis during surgical milling process. 2015 IEEE International Conference on Robotics and Biomimetics (ROBIO); 2015 Dec 6–9. [Google Scholar]
- 20.Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32(21):2310–17. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt M, Bast P, Lauer W, et al. Manual vs. robotic milling parameters for development of a new robotic system in cranial surgery. International Congress Series. 2004;1268:533–38. [Google Scholar]
- 22.Nanda A, Sharma M, Sonig A, Ambekar S, Bollam P. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: A single surgeon’s experience of 1,576 patients. World Neurosurg. 2014;82(6):1380–87. doi: 10.1016/j.wneu.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80(7):941–51. doi: 10.2106/00004623-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hilibrand AS, Schwartz DM, Sethuraman V, et al. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86-A(6):1248–53. doi: 10.2106/00004623-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Li S, Miao D, et al. Risk factors of cage subsidence in patients with ossification of posterior longitudinal ligament (OPLL) after anterior cervical discectomy and fusion. Med Sci Monit. 2018;24:4753–59. doi: 10.12659/MSM.910964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartels RH, Donk RD, Feuth T. Subsidence of stand-alone cervical carbon fiber cages. Neurosurgery. 2006;58(3):502–8. doi: 10.1227/01.NEU.0000197258.30821.50. discussion 502–8. [DOI] [PubMed] [Google Scholar]
- 27.Lim TH, Kwon H, Jeon CH, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine (Phila Pa 1976) 2001;26(8):951–56. doi: 10.1097/00007632-200104150-00021. [DOI] [PubMed] [Google Scholar]