Abstract
Background
This study aimed to investigate the renin-angiotensin system (RAS) and cardiometabolic status in mice fed a long-term high-fat diet (HFD).
Material/Methods
C57BL/6J mice were randomly assigned to the control group on a normal diet (ND) (n=15) and the HFD group (n=15). Serum biomarkers were measured, including total cholesterol (TC), triglyceride (TG), insulin, glycated hemoglobin (HbA1c), brain natriuretic peptide (BNP), renin, angiotensin-converting enzyme (ACE), angiotensin II (Ang-II), Ang-II type 1 receptor (AT1R), and aldosterone. Cardiac histology was measured by the cross-sectional area (CSA) of cardiomyocytes and collagen deposition. Levels of myocardial intercalated disc (ICD) proteins and mRNA were analyzed by Western blot and real-time quantitative polymerase chain reaction (RT-qPCR), respectively. The localization of ICD proteins was evaluated by immunohistochemistry (IHC).
Results
Compared with ND, HFD resulted in increased blood glucose, body weight, TC, TG, HbA1c, insulin, and BNP and levels of serum ACE, Ang-II, aldosterone, AT1R, cardiomyocyte CSA, and interstitial collagen in the myocardium compared. Also, HFD significantly down-regulated connexin-43, and upregulated β-catenin, N-cadherin, and plakoglobin in the hearts of HFD mice compared with ND mice. However, the deposition of ICD proteins was not changed in the hearts of HFD mice compared with ND mice.
Conclusions
Long-term HFD in mice resulted in left ventricular hypertrophy, interstitial fibrosis, dysregulation of RAS, and abnormal expression of ICD proteins compared with ND mice, but did not affect the distribution of cardiomyocyte ICD proteins. Long-term HFD resulted in cardiac remodeling and altered expression of ICD proteins through RAS activation.
MeSH Keywords: Cardiomyopathy, Hypertrophic; Diet, High-Fat; Fibrosis; Renin-Angiotensin System
Background
A high-fat diet (HFD) can lead to abdominal obesity, hyperglycemia, insulin resistance, and dyslipidemia and is associated with an increased risk of diabetes mellitus. Chronic HFD is associated with an increased risk of cardiovascular disease and stroke [1,2]. Obesity can also lead to an alteration in cardiac morphology known as cardiac remodeling, including left ventricular hypertrophy and fibrosis [3]. These cardiac changes reduce cardiac reserve and make the heart more susceptible to injury from pressure overload and volume overload and are associated with an increased risk of myocardial ischemia, myocardial infarction, and cardiac failure leading to patient morbidity and mortality.
Increased activity of the renin-angiotensin system (RAS) is associated with the development of left ventricular hypertrophy and cardiac fibrosis [4–6]. Particularly, angiotensin-II (Ang-II) acts on the Ang-II type 1 receptor (AT1R), has been shown to lead to progressive cardiac decompensation, cardiac remodeling, and cardiac failure [4–6]. Also, myocardial intercalated disc (ICD) proteins are important structures that are unique to the myocardium and are involved in the electromechanical transmission, physical structural support, and intercellular communication between cardiomyocytes to maintain structural integrity and to synchronize myocardial contractility [7]. Gap junctions, adherens junctions, and desmosomes are key cell junctional complexes residing at the ICDs in the myocardium.
Connexin 43 (Cx43) is a primary connexin in the ventricular myocardium, which is reduced or redistributed in the cases of cardiac ischemia and hypertrophy and heart failure [8–10]. Also, β-catenin, plakoglobin, and N-cadherin are major components of adherens junctions, and β-catenin has an important role in cell-cell adhesion in the myocardium and acts as a transcriptional activator [7]. Plakoglobin (γ-catenin), a member of desmosomes and adherens junctions, and contributes to the maintenance of normal myocardial structure and function as it localizes to adjacent cardiomyocytes and participates in regulating intercellular adhesion [7,11]. N-cadherin has a role in mediating mechanical and electrical coupling between adjacent cardiomyocytes in the myocardium and is also required for maintaining cell adhesion [12]. Therefore, alteration in the expression and distribution of gap junction and adherens junction proteins can influence the development of cardiac hypertrophy, cardiac arrhythmia, and cardiac dysfunction in response to external factors [7–10,13,14].
However, few studies have been undertaken to investigate the expression of gap junction proteins and adherens junction proteins at the ICDs in cardiac tissue in mice on a long-term HFD. Therefore, this study aimed to investigate whether dysregulation of the RAS activity and ICDs were involved in the development of myocardial remodeling in C57BL/6J mice after long-term exposure to HFD and to correlate the cardiac changes with changes in metabolic factors.
Material and Methods
Animal model of a high-fat diet (HFD) in C57BL/6J mice
Female C57BL/6J mice that were 8–10 weeks old were obtained from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). Mice were housed in a controlled environment of 25±2°C and 50±5% humidity, with a 12-hour light and dark cycle. Mice were given free access to food and water. All laboratory animals were housed and cared for in accordance with the guidelines for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (8th edition, revised, 2011). Ethical approval for the animal experiments was given by the Local Ethics Committee of Nantong University (Approval No. 20150305-015). Mice were randomly divided into two groups that included a control group fed a diet of standard rodent chow with 11% fat (ND) (n=15) and a high-fat diet group (HFD) (n=15) fed with a rodent diet containing 60% fat (Cat. No: D12492) (Research Diets Inc., New Brunswick, NJ, USA). The mice in the two study groups were maintained on their diets for 36 weeks.
Biomarker evaluation
Fasting blood samples were analyzed for glucose levels, and mouse body weights were assessed at different time intervals. Fasting whole blood samples were collected before the mice were euthanized at the end of the study and analyzed using enzyme-linked immunosorbent assay (ELISA) kits. Levels of total cholesterol (TC), triglyceride (TG), insulin, glycated hemoglobin A1c (HbA1c), brain natriuretic peptide (BNP), renin, angiotensin-converting enzyme (ACE), angiotensin II (Ang-II), Ang-II type 1 receptor (AT1R), and aldosterone were measured.
Histology of the left ventricle of C57BL/6J mice
The mouse hearts were fixed in 4% formaldehyde. Transverse sections from each heart were taken from the left ventricle at the level of the papillary muscles and embedded in paraffin wax, and tissue sections were cut. Tissue sections were routinely stained with hematoxylin and eosin (H&E), and cardiomyocyte morphology were assessed with the cardiomyocyte cross-sectional area (CSA) of the left ventricle, at a magnification of ×400. Masson’s trichrome (MT) histochemical staining was used to evaluate the degree of cardiac fibrosis, at a magnification of x 200. Light microscopy was performed with a Leica DM2000 light microscope with a digital camera attachment (Leica, Wetzlar, Germany).
Quantification of cardiac and cardiomyocyte morphology by light microscopy was performed using Image J software (National Institutes of Health, Bethesda, MD, USA). The cardiomyocyte cross-sectional area (CSA) was measured in 30 cells from 10 random fields in each tissue section. The quantitative analysis of myocardial collagen deposition was evaluated using AxioVision software (Zeiss GmbH, Oberkochen, Germany) on the ventricular tissue sections stained with Masson’s trichrome. The degree of interstitial fibrosis and perivascular fibrosis was assessed as the percentage are of myocardial interstitial collagen (area of collagen [%]), which was calculated by the blue Masson’s trichrome stained area divided by the sum of the total myocardial area in ten randomly selected fields in each tissue section.
Tissue protein extraction and Western blot
Left ventricular tissue and liver tissue samples from each mouse were homogenized on ice in a tissue homogenizer supplemented with a RIPA buffer (P0013) (Beyotime, Shanghai, China) with phosphatase and protease inhibitors (1: 100). The samples of tissue homogenate were incubated on ice for 10 minutes and were vortexed with every two minutes. Samples were centrifuged at 13,000 rpm for 10 minutes at 4°C. The supernatant was diluted and heated at 95°C for 10 minutes before the samples were stored at −80°C.
Quantitative analysis of total protein was determined using the Pierce 660 nm Protein Assay (#22660) (Thermofisher Scientific, Waltham, MA, USA). The protein samples were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The blotted membranes were incubated in blocking buffer containing 5% dried skimmed milk powder in Tris-buffered saline (TBS) for 30 minutes at room temperature. The membranes were incubated with primary antibodies diluted at 1: 1000 in 5% dried skimmed milk powder in TBS containing 0.1% Tween-20 for 8–12 hours at room temperature. The primary antibodies were anti-GAPDH (sc-25778) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-connexin 43 (Cx43) (#3512) (Cell Signaling Technology, Danvers, MA, USA), anti-β-catenin (#8480) (Cell Signaling Technology, Danvers, MA, USA), anti-N-cadherin (sc-59987) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-plakoglobin (#2309) (Cell Signaling Technology, Danvers, MA, USA), anti-Akt (#9272) (Cell Signaling Technology, Danvers, MA, USA), and anti-phospho-Akt (serine 473, #9271) (Cell Signaling Technology, Danvers, MA, USA). The blotted membranes were washed and incubated with goat anti-rabbit secondary antibody with horseradish peroxidase (HRP)-conjugated IgG diluted 1: 10000 in 5% dried skimmed milk powder in TBS for 2 hours at room temperature. The target blot bands expressing protein-antibody were visualized using Pierce ECL Western blotting substrate (#32106) (Thermofisher Scientific, Waltham, MA, USA) and quantified using Multi Gauge software version 3.0 (Fujifilm, Tokyo, Japan) with adequate exposure duration.
Immunohistochemical staining of the cardiac tissue in C57BL/6J mice
To investigate proteins in the mouse ventricle, sections were deparaffinized in xylene, rehydrated in ethanol, and the endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide. Antigen retrieval was performed using 10 mmol/L citrate buffer. After blocking the tissue sections in normal goat serum, the sections were incubated in primary antibodies, including anti-Cx43, anti-β-catenin, anti-N-cadherin, and anti-plakoglobin, which were diluted at 1: 100, and incubated the mouse tissue sections of the left ventricle for 2 hours at 37°C. Then, each tissue section was washed and incubated in the secondary antibody for 30 min at 37°C, and developed with 3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin. Light microscopy images were captured at ×400 using a Leica DM2000 microscope with a digital camera attachment (Leica, Wetzlar, Germany).
RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR) of cardiac tissue from C57BL/6J mice
Total RNA of the mouse left ventricle tissue samples was extracted using an E.Z.N.A.® Total RNA Kit (R6834) (Omega Bio-tek Inc., Norcross GA, USA), according to the manufacturer’s instructions. The cDNA was obtained by reverse transcription using a SuperScript First-Strand cDNA Synthesis kit (BU-304-01) (Biouniquer Technology, Beijing, China). RT-qPCR was performed using SYBR Green I PCR Master Mix with the LightCycle® 96 Instrument (Roche, Basel, Switzerland). The relative mRNA expression level was normalized by the level of glyceraldehyde phosphate dehydrogenase (GAPDH) transcripts and was calculated by the 2(−ΔΔCT) method. All primer sequences used for RT-qPCR were synthesized by Shenggong Biotech (Shanghai, China) and were shown in Table 1.
Table 1.
Primer sequences used for real-time quantitative polymerase chain reaction (RT-qPCR).
| Forward primer (5′-3′) | Reverse primer (3′-5′) | |
|---|---|---|
| CX43 | TGCTTACTTCAATGGCTGCTCCTC | TCGCTGGCTTGCTTGTTGTAATTG |
| β-catenin | AGGAATGAAGGCGTGGCAACATAC | GGCACCAATGTCCAGTCCAAGATC |
| Plakoglobin | GCTGTCCTGTTCCGCATCTCTG | GAGGCACATCGCTGGAGTACATG |
| N-cadherin | AGGCGTCTGTGGAGGCTTCTG | TGCCGTCCTCGTCCACCTTG |
| GAPDH | AGGTCGGTGT GAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (IBM, Chicago, IL, USA) and GraphPad Prism version 5.01 software (GraphPad Software, La Jolla, CA, USA). The unpaired independent samples t-test and Spearman’s correlation test were used. The data were presented as the mean ± standard deviation (SD) for continuous variables based on the results of normality tests. Statistical tests were two-sided and statistical significance was defined as P<0.05.
Results
Body weight, levels of blood glucose, and levels of biomarkers in C57BL/6J mice fed a long-term high-fat diet (HFD)
C57BL/6J mice that were randomly assigned to the control group on a normal diet (ND) (n=15) and the high-fat diet (HFD) group (n=15) showed no significance in body weight and blood glucose at the beginning of the study (Table 2). The C57BL/6J HFD mice had a significant increase in body weight and blood glucose after 8 weeks, 24 weeks, and 36 weeks of feeding compared with C57BL/6J mice in the ND group (Table 2). Also, the mice fed a HFD had significantly increased levels of TC, TG, insulin, and HbA1c than the mice in the ND group (Table 3), indicating the development of metabolic disorders associated with a chronic HFD.
Table 2.
Body mass and levels of blood glucose of C57BL/6J mice fed a normal diet (ND) and a high-fat diet (HFD).
| Group | Body mass (gm) | Blood glucose (mmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 weeks | 8 weeks | 24 weeks | 36 weeks | 0 weeks | 8 weeks | 24 weeks | 36 weeks | |
| ND | 21.09±1.11 | 23.25±0.99 | 24.35±3.37 | 25.93±3.89 | 5.75±0.69 | 7.41±1.24 | 6.41±1.20 | 5.19±1.05 |
| HFD | 21.70±1.78 | 28.57±3.72* | 48.57±6.39* | 56.03±6.22* | 6.00±0.68 | 9.08±0.50* | 8.86±0.65* | 8.36±0.91* |
P<0.001 vs. the normal diet (ND) group.
Table 3.
Levels of total cholesterol (TC), triglyceride (TG), glycated hemoglobin (HbA1c), and insulin of C57BL/6J mice C57BL/6J mice fed a normal diet (ND) and a high-fat diet (HFD).
| Group | TC (mmol/L) | TG (mmol/L) | HbA1c | Insulin (ng/L) |
|---|---|---|---|---|
| ND | 11.19±2.81 | 7.15±1.30 | 4.55±1.42 | 0.47±0.31 |
| HFD | 33.20±11.68* | 16.71±5.30* | 7.24±1.77* | 1.14±0.73* |
TC – total cholesterol; TG – triglyceride; HbA1c – glycated hemoglobin.
P<0.001 vs. the normal diet (ND) group.
Activated renin-angiotensin system (RAS) and increased insulin resistance in C57BL/6J mice fed a long-term HFD
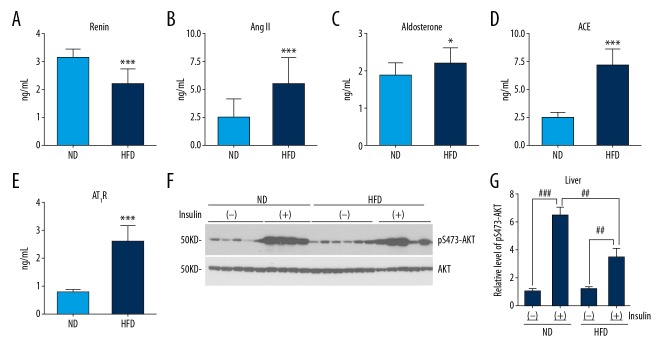
To determine the activity of RAS of mice in the HFD group, we analyzed the levels of renin, aldosterone, Ang-II, and AT1R. In the HFD group, there were significantly increased levels of ACE, Ang-II, and aldosterone, as well as circulating AT1R compared with the ND group. The levels of renin were significantly reduced in C57BL/6J mice in the HFD group compared with mice in the ND group (Figure 1A–1E). These results indicated that a chronic HFD resulted in an activated RAS in mice.
Figure 1.
Effects of a high-fat diet (HFD) on the renin-angiotensin system (RAS) and peripheral insulin resistance in C57BL/6J mice. (A–E) A high-fat diet (HFD) upregulated the activity of the RAS and the level of angiotensin II (Ang-II) type 1 receptors (AT1R) measured by enzyme-linked immunosorbent assay (ELISA). (F, G) HFD inhibited increased phosphorylation of AKT at Ser473 induced by insulin after quantitative densitometry of representative Western blots of the mouse liver. Results are presented as the mean ±SD. * P<0.05, ** P<0.01, *** P<0.001 vs. the normal diet (ND) group. # P<0.05, ## P<0.01, ### P<0.001.
At the end of the experiment, we randomly selected 4 or 5 mice per group that were injected with 3 IU/kg of insulin by intraperitoneal injection before being euthanized. Liver tissue was homogenized and total proteins extracted for Western blot. After insulin injection, phosphorylation of AKT at residue Ser473, normalized by total AKT, was significantly increased in both the HFD and ND groups, with a significantly lower level of phosphorylation in the HFD group than in the ND group, shown by quantitative densitometry analysis of Western blots (Figure 1F, 1G), indicating peripheral insulin resistance in the HFD group. Therefore, we found a long-term exposure to a HFD resulted in significant increases in blood glucose and body weight and resulted in hyperlipidemia, hyperinsulinemia, peripheral insulin resistance, and increased RAS activity.
Cardiac remodeling and dysfunction in C57BL/6J mice fed a long-term HFD
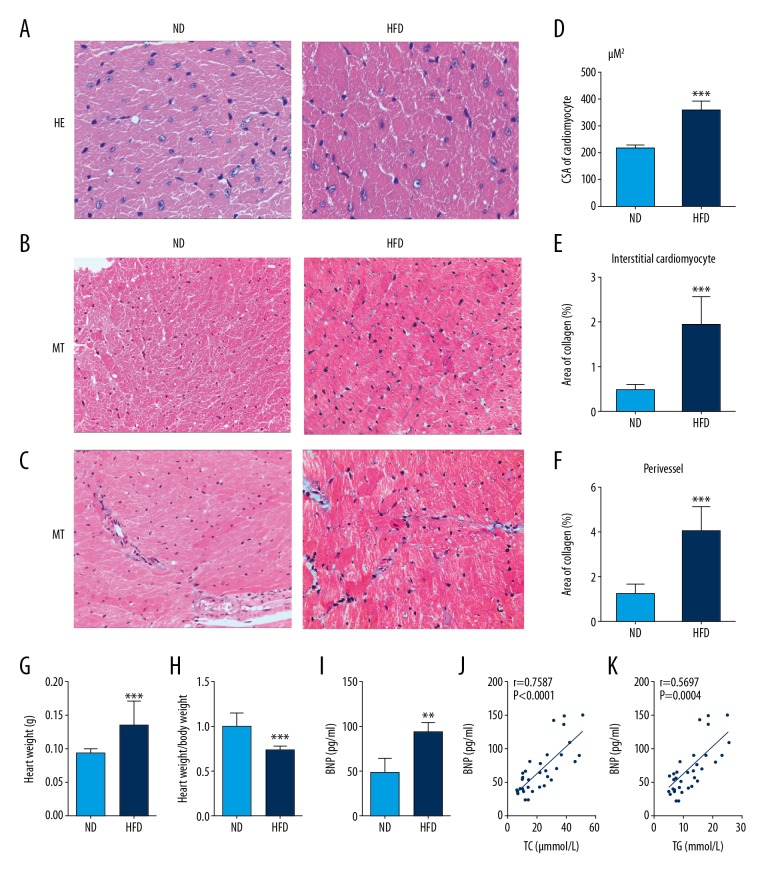
Light microscopy with routine H&E staining and MT staining for myocardial fibrosis showed a significant increase in cardiomyocyte CSA, and the percentage area of collagen in the myocardial interstitium and the perivascular regions of the myocardium in the HFD group when compared with the ND group. These results suggested that mice fed a HFD had significant myocyte hypertrophy and increased myocardial collagen associated with the pathologic changes in the hearts in HFD mouse group (Figure 2A–2F).
Figure 2.
A high-fat diet (HFD) increased cardiac hypertrophy and myocardial fibrosis in C57BL/6J mice. (A) Representative photomicrographs of the light microscopy show the cross-sectional area (CSA) in cardiomyocytes. Hematoxylin and eosin (H&E). Magnification ×400. (B, C) Representative photomicrographs of the light microscopy show myocardial fibrosis in the left ventricles. Masson’s trichrome. Magnification × 200. (D–F) Quantitative analysis of cardiomyocyte CSA, and the percentage area of collagen in the cardiac interstitium and around myocardial vessels in the left ventricle. (G, H) HFD is associated with a significant increase in heart weight and levels of brain natriuretic peptide (BNP). (I, J, K) BNP is positively correlated with serum lipid levels in mice (analyzed by Spearman correlation). Results are presented as the mean ±SD. * P<0.05, ** P<0.01, *** P<0.001 vs. ND group.
There was also a significant increase in heart weight in the C57BL/6J mice in the HFD group and a significantly reduced ratio of heart weight to body weight in the HFD group compared with the ND group (Figure 2G, 2H). BNP, mainly generated by the heart in response to myocardial stress, is an indicator of the severity of heart disease and is a clinical prognostic indicator in heart failure [15]. When compared with the ND group, the BNP level was significantly increased in the HFD group, as measured by ELISA (Figure 2I). Also, the BNP level was significantly correlated with the level of TC and TG, using linear regression analysis (Figure 2J, 2K). These findings suggest that in the mouse model, HFD induced metabolic cardiomyopathy and cardiac dysfunction.
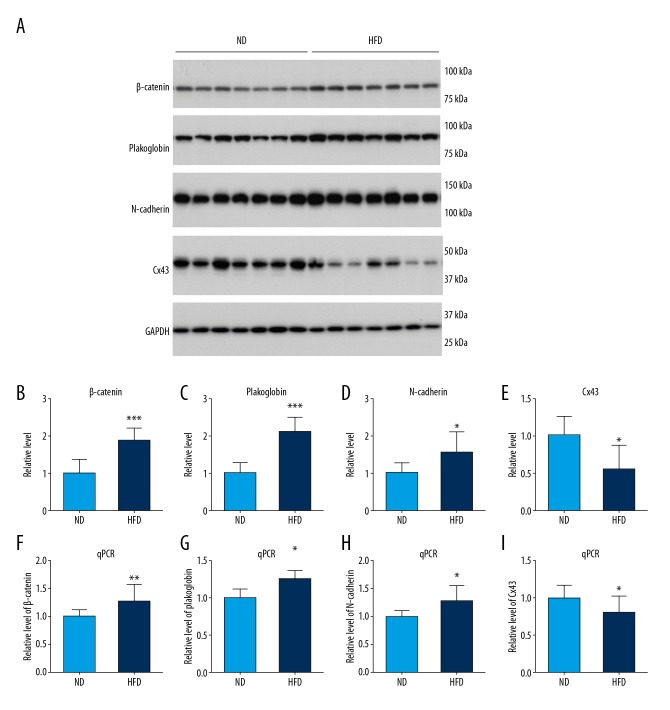
Altered expression of junction proteins and mRNA associated with intercalated discs (ICDs) in C57BL/6J mice fed a long-term HFD
The possible mechanisms for ventricular remodeling and whether the HFD modulated the expression levels of intercalated disc (ICD) proteins in C57BL/6J mice were studied. The results showed significantly increased expression of β-catenin, plakoglobin, and N-cadherin in the left ventricles of mice in HFD group when compared with the ND group, identified by Western blot (Figure 3A–3D). Analysis of mRNA levels by real-time quantitative polymerase chain reaction (RT-qPCR) showed that the HFD induced an increase in mRNA levels of β-catenin, plakoglobin, and N-cadherin (Figure 3F–3H). There was also a significantly reduced expression of level of Cx43 protein and its mRNA level in mice in the HFD group compared with the ND group (Figures 3A, 3E, and 3I). The altered expression level of gap junction Cx43 and adherens junction protein suggested that a chronic HFD in the mouse model could affect cardiac remodeling, which may be the basis for cardiac dysfunction.
Figure 3.
Abnormal expression of intercalated discs proteins and mRNA in the myocardium of mice in response to a high-fat diet (HFD). (A) Representative Western blots show the expression levels of β-catenin, plakoglobin, N cadherin, and connexin-43 (Cx43) in mouse heart tissue. (B–E) Densitometry analysis of β-catenin, plakoglobin, N-cadherin, and Cx43 expression from the Western blots. (F–I) Real-time quantitative polymerase chain reaction (RT-qPCR) results show the relative mRNA levels of β-catenin, plakoglobin, N-cadherin, and Cx43 in the mouse myocardium. Results are presented as the mean ±SD. * P<0.05, ** P<0.01, *** P<0.001 vs. the normal diet (ND) group.
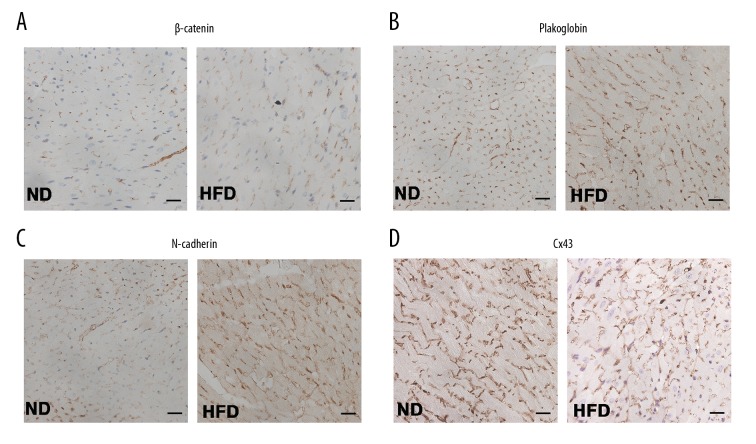
The distribution of ICD proteins in cardiac tissue of C57BL/6J mice fed a long-term HFD was unaltered
Immunohistochemistry was used to investigate the localization of ICD proteins in mice heart sections and showed that β-catenin, plakoglobin, and N-cadherin were expressed on the surface of cardiomyocytes and appeared to be in regions of cardiomyocyte cell-cell adhesion in the ND group and HFD group. Finally, the distribution of Cx43 proteins was evaluated (Figure 4A–4C). Accumulation of Cx43 was particular in the ICDs in the left ventricles in C57BL/6J mice in both the HFD and ND groups (Figure 4D). Although the ICD protein levels were significantly altered in the HFD mouse group, the distribution of ICDs was detected at cell-cell junctions and was unaltered after exposure to a chronic HFD when compared with the ND group of C57BL/6J mice.
Figure 4.
Distribution of the intercalated disc proteins in the myocardium of the high-fat diet (HFD) mouse model. Representative photomicrographs of the immunohistochemistry for expression of β-catenin (A), plakoglobin (B), N-cadherin (C), and connexin 43 (Cx43) protein (D) in heart tissue of the two study groups of mice fed a high-fat diet (HFD) and a normal diet (ND). Magnification ×400.
Discussion
Patients with obesity are at much higher risk for ischemic heart disease and cardiac failure, including diastolic cardiac insufficiency. This study aimed to investigate the RAS activity and cardiometabolic status in C57BL/6J mice fed a long-term high-fat diet (HFD), and to determine whether long-term HFD would induce cardiac remodeling and further reduce cardiac function. The findings showed that in mice in the HFD group, there was increased activity of RAS and abnormal expression of myocardial intercalated disc (ICD) proteins and mRNA in the left ventricular tissue. These findings require further study as they may indicate the underlying mechanism of obesity-associated cardiac morphological changes and cardiovascular functional injury.
The findings of the present study showed that in the mouse model a chronic high fat diet could lead to obesity, hyperglycemia, hyperlipidemia, peripheral insulin resistance, as well as an increase in heart weight, indicating that HFD might cause heart hypertrophy. The heart weight normalized to body weight was lower in the HFD group compared with the controls with a normal diet (ND), which was likely due to the relative increase in visceral fat and adipose tissue in HFD mice. In this mouse model, a chronic high fat diet resulted in the pathological changes of cardiomyocyte hypertrophy and cardiac fibrosis, identified by histological staining. Cardiac hypertrophy is an adaptation of the heart for increased workload, which may ultimately lead to heart failure. The transition from compensated hypertrophy to cardiac failure involves a complex series of events that include cardiomyocyte hypertrophy and disarray, alterations in the myocardial interstitium, the expression of ICD proteins, and re-expression of fetal genes. The adaptive changes found in the obese mice in the present study included myocyte hypertrophy, interstitial fibrosis, phenotypic myocyte changes, and fetal gene re-expression.
Patients with obesity can develop heart failure with preserved ejection fraction [16], with myocyte hypertrophy and fibrosis. In this study, we found that chronic HFD caused a significant increase in the brain natriuretic peptide (BNP) levels in mice compared with the control ND mice, and the BNP level was correlated with lipid levels. It is possible that mice fed a HFD could be more likely to develop heart failure when exposed to myocardial stressors, such as inflammation, infection, and fever. The definitive mechanisms of myocardial hypertrophy and fibrosis in mice fed a HFD remain to be determined. The effect of HFD on cardiac hypertrophy and fibrosis might partly contribute to loss in NAD-dependent deacetylase sirtuin-3 or SIRT3 [17]. A previous study showed that HFD led to the inhibition of glycogen synthase kinase 3 beta (GSK3β), and indicated that the GSK3β-β-catenin-YAP pathway mediated the process of myocardial remodeling [18]. Also, disorders in the nervous system and endocrine system are associated with myocardial remodeling and cardiac failure [4–6,19]. However, it remains unclear whether obesity can block the recovery of homeostasis of internal RAS due to an inadequate diet. In the current study, mice fed a chronic HFD showed increased expression of Ang-II, ACE, and aldosterone, indicating increased activity of RAS. An abnormally high level of Ang-II reflects the activation of the RAS and can cause cardiac inflammation, influence the process of cardiac hypertrophy and fibrosis, and deteriorate cardiac function [4–6,19]. The upregulated expression of the RAS, in turn, acts on AT1R, which was also over-expressed in the HFD group, and led to an increase in aldosterone, which have key roles in vasoconstriction, cardiomyocytes hypertrophy, and cardiac fibrosis in HFD-fed mice. It is likely that RAS influenced the process of cardiac hypertrophy and fibrosis in mice fed a HFD.
ICD proteins impact cardiac electromechanical coupling and chemical communications to maintain structural support among adjacent cardiomyocytes to achieve the regular contraction which is essential for cardiac function [7,20,21]. Changes in the expression of Cx43 was associated with cardiac remodeling in the present study, which was in accordance with previous findings [8–10,22]. Our results indicated that Cx43 expression was significantly down-regulated and accompanied by left ventricular hypertrophy and increased collagen deposition in mouse hearts in the HFD group. Abnormal expression of Cx43 and an increase in interstitial fibrosis may decrease the conduction velocity and impair or destroy synchronous contraction [22], as Cx43 is the main connexin protein responsible for synchronous contraction by forming the myocyte-to-myocyte pathways and spreading a regular electrical excitation wave [20,22]. Therefore, the degree of change in Cx43 might contribute to the pathologic changes in mice fed a HFD. We further explored the impact of HFD on the expression of other ICDs in cardiac hypertrophy and cardiac fibrosis. It has previously been shown that β-catenin, plakoglobin, and N-cadherin are main adhesion factors in the heart, associated with myocyte-to-myocyte adhesion that contribute to maintaining normal cardiac structure and function [7,11,12,14]. Abnormal expression and distribution of adhesion proteins in the heart were previously shown to be associated with cardiomyopathy and impaired cardiac function [7,11,13,14,23]. In support of these previous findings, we found a significant increase in β-catenin, plakoglobin, N-cadherin protein and related mRNA levels in the HFD mice, but there was no difference in the distribution of these proteins between the hypertrophic hearts of the HFD mice and the ND mice. Upregulation of β-catenin may regulate the downstream signal transduction and transcriptional activity of several target genes, which are known to be involved in the remodeling of cardiomyocytes [7,23]. However, the increased expression levels of plakoglobin and N-cadherin protein may be requires to maintain the normal structure of the ICDs and the intercellular adhesion between cardiomyocytes [11,13]. Based on observations, it may be hypothesized that upregulation of β-catenin, plakoglobin, and N-cadherin proteins contribute to a compensatory mechanism for the loss of Cx43, to preserve cardiac phenotype, function, and mechanical and electrical activity in hearts of obese mice fed a HFD.
However, in the present study, it was not possible to identify the underlying mechanisms for the abnormal expression of ICD proteins or the increased susceptibility to cardiac injury due to the HFD. There have been several previous studies that may provide information regarding the possible mechanisms involved. A previous study has shown that overexpression of cardiac-specific angiotensin-converting enzyme (ACE) was associated with the activation of RAS and down-regulated ventricular Cx43 expression by affecting transcriptional and post-transcriptional regulation of connexins [24]. In the present study, decreased expression of ventricular Cx43 might be due to the upregulated activity of circulating RAS following exposure to a HFD in mice. RAS has previously been shown to be associated with the activation of kinases and phosphatases, which may modulate the degradation of cardiac connexin and gap junctions [25].
Conclusions
This study aimed to investigate the renin-angiotensin system (RAS) and cardiometabolic status in C57BL/6J mice fed a long-term high-fat diet (HFD). The findings were that long-term HFD in mice resulted in left ventricular hypertrophy, interstitial fibrosis, dysregulation of RAS, and abnormal expression of ICD proteins compared with ND mice, but did not affect the distribution of cardiomyocyte ICD proteins. Altered expression of ICDs may augment RAS activity associated with a HFD, and cardiometabolic changes may mediate the development of cardiac hypertrophy, fibrosis, and heart failure. These findings raise the possibility that angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers might be better therapeutic options to mitigate the adverse effect of HFD-induced cardiac remodeling and cardiac dysfunction.
Footnotes
Source of support: This study was supported by the Cadre Healthcare Ward of Jiangsu Province (BJ15010)
References
- 1.Parto P, Lavie CJ. Obesity and cardiovascular diseases. Curr Probl Cardiol. 2017;42(11):376–94. doi: 10.1016/j.cpcardiol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–67. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 3.Wong RC, Tan KB. Asymmetric left ventricular hypertrophy associated with morbid obesity mimicking familial hypertrophic cardiomyopathy. Singapore Med J. 2014;55(12):e201–4. doi: 10.11622/smedj.2014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaufarb IS, Sonnenblick EH. The renin-angiotensin system in left ventricular remodeling. Am J Cardiol. 1996;77(13):8C–16C. doi: 10.1016/s0002-9149(96)00183-x. [DOI] [PubMed] [Google Scholar]
- 5.Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II: Role of an intracardiac renin-angiotensin system. Annu Rev Physiol. 1992;54:227–41. doi: 10.1146/annurev.ph.54.030192.001303. [DOI] [PubMed] [Google Scholar]
- 6.Qi GM, Jia LX, Li YL, et al. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155(6):2254–65. doi: 10.1210/en.2013-2011. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009;19(6):182–90. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996;76(5):412–17. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88(3):864–75. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 10.dos Santos DO, Blefari V, Prado FP, et al. Reduced expression of adherens and gap junction proteins can have a fundamental role in the development of heart failure following cardiac hypertrophy in rats. Exp Mol Pathol. 2016;100(1):167–76. doi: 10.1016/j.yexmp.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Qu J, Yi XP, et al. Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;292(1):H270–76. doi: 10.1152/ajpheart.00576.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro L, Fannon AM, Kwong PD, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374(6520):327–37. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 13.Kostetskii I, Li J, Xiong Y, et al. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96(3):346–54. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 14.Masuelli L, Bei R, Sacchetti P, et al. Beta-catenin accumulates in intercalated disks of hypertrophic cardiomyopathic hearts. Cardiovasc Res. 2003;60(2):376–87. doi: 10.1016/j.cardiores.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med. 2001;39(7):571–88. doi: 10.1515/CCLM.2001.093. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Zeng H, Vaka VR, He X, et al. High-fat diet induces cardiac remodelling and dysfunction: Assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19(8):1847–56. doi: 10.1111/jcmm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Li L, Zhao H, et al. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism. 2015;64(8):917–25. doi: 10.1016/j.metabol.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haq S, Choukroun G, Lim H, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103(5):670–77. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 20.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62(2):228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Lin JL, Wu KH, et al. Xin proteins and intercalated disc maturation, signaling and diseases. Front Biosci. 2012;17:2566–93. doi: 10.2741/4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontes MS, van Veen TA, de Bakker JM, van Rijen HV. Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Acta. 2012;8(9):2020–29. doi: 10.1016/j.bbamem.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Q, Chen P, Xu Z, et al. Expression and redistribution of β-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. J Mol Histol. 2013;44(5):565–73. doi: 10.1007/s10735-013-9507-6. [DOI] [PubMed] [Google Scholar]
- 24.Kasi VS, Xiao HD, Shang LL, et al. Cardiac-restricted angiotensin-converting enzyme overexpression causes conduction defects and connexin dysregulation. Am J Physiol Heart Circ Physiol. 2007;293(1):H182–92. doi: 10.1152/ajpheart.00684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62(2):256–67. doi: 10.1016/j.cardiores.2003.12.021. [DOI] [PubMed] [Google Scholar]