Abstract
Talimogene laherparepvec (T-VEC) has demonstrated efficacy for unresectable melanoma. We explored response patterns from a phase 2 study evaluating patients with unresectable stage IIIB–IVM1c malignant melanoma who received T-VEC plus ipilimumab or ipilimumab alone. Patients with objective response per modified irRC were evaluated for pseudo-progression (single ≥25% increase in tumour burden before response). Patients without pseudo-progression were classified by whether they responded within or after 6 months of treatment start; those with pseudo-progression were classified by whether pseudo-progression was due to increase in existing lesions or development of new lesions. Overall, 39% (n = 38/98) in the combination arm and 18% (n = 18/100) in the ipilimumab arm had an objective response. Eight responders (combination, n = 7 [18.4%]; ipilimumab, n = 1 [5.6%]) had pseudo-progression; most occurred by week 12 and were caused by an increase in existing lesions. These data reinforce use of T-VEC through initial progression when combined with checkpoint inhibitors.
Trial Registration NCT01740297 (ClinicalTrials.gov; date of registration, December 4, 2012); 2012-000307-32 (ClinicalTrialsRegister.eu; date of registration, May 13, 2014).
Subject terms: Melanoma, Targeted therapies
Background
Evidence of delayed response or disease progression before response (i.e. pseudo-progression) has been seen with immunotherapies.1–3 Increases in baseline lesions may be attributed to T-cell infiltration rather than tumour cell proliferation,1 resulting in increased tumour size that is not truly tumour growth. Because of this observation, the immune-related response criteria (irRC)1 are frequently used to measure response in lieu of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.4 Talimogene laherparepvec (T-VEC) monotherapy has also been shown to induce perceived tumour progression before response using RECIST version 1.1 criteria.5
Improvements in overall response rates and in lesion-level response rates were observed with T-VEC administered in combination with other therapies.6,7 In a randomised trial, T-VEC plus ipilimumab resulted in a significantly higher objective response rate (ORR) versus ipilimumab alone (odds ratio, 2.9; 95% Cl, 1.5–5.5; P = 0.002) in 198 patients with metastatic unresectable melanoma.7 Similarly, the phase 1 MASTERKEY-265 trial of T-VEC plus pembrolizumab resulted in a confirmed ORR (rate of complete or partial response) of 61.9% (95% CI, 38.4–81.9%) and a complete response (CR) rate of 33.3% (95% CI, 14.6–57.0%) in 21 patients with advanced melanoma.6
To better define clinically meaningful response patterns in patients receiving T-VEC with an immune checkpoint inhibitor, this exploratory analysis evaluated patterns of response in patients with melanoma enrolled in the phase 2 study (ClinicalTrials.gov identifier, NCT01740297) of T-VEC plus ipilimumab versus ipilimumab alone.
Methods
Patients and study design
The primary analysis of this phase 2, randomised, open-label study has been previously reported.7 In the analysis reported here, patients with an objective response were evaluated for pseudo-progression. Patients were randomised (1:1) to receive T-VEC plus ipilimumab or ipilimumab (Fig. S1)7 and stratified by disease stage (stage IIIB/IIIC/IVM1a versus IVM1b/IVM1c) and previous therapy (treatment-naive; previous systemic anticancer immunotherapy; systemic anticancer treatment other than immunotherapy).7 Study procedures were approved by institutional review boards/ethics committees; patients provided informed consent. Additional details are provided in Supplement.
Assessments
Tumour response was assessed at baseline and every 12 weeks after treatment initiation using irRC until documentation of confirmed disease progression, determined as a repeat, consecutive disease progression ≥4 weeks after initial disease progression. Additional details are provided in Supplement.
Statistical analysis
Study weeks were calculated from date of randomisation. Pseudo-progression was defined as disease progression before patient response; however, a confirmation for disease progression per the irRC was not required. Patients with pseudo-progression were further classified according to whether the pseudo-progression was caused by an increase in existing lesions or the development of new measurable lesions.
Patients without pseudo-progression were classified according to whether they responded within 6 months (≤183 days) or after 6 months (>183 days) following treatment start. Timing of response onset was calculated as the date of response onset minus the date of first dose plus one day.
Results
Patients
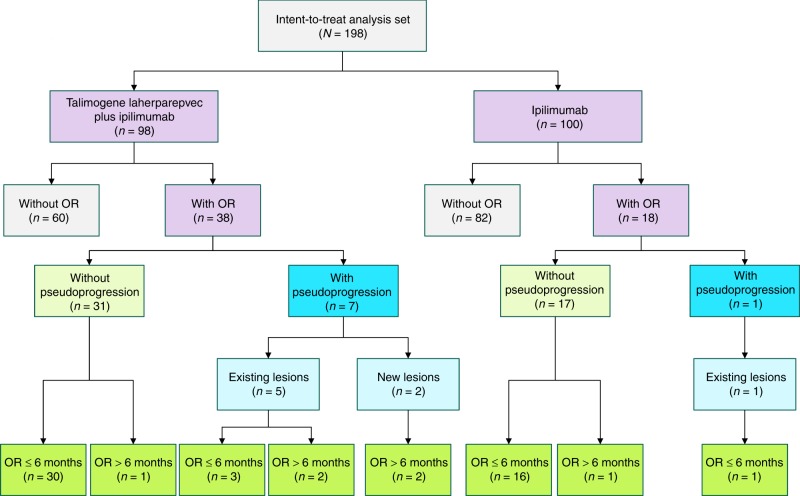
Of 198 patients enrolled (T-VEC plus ipilimumab, n = 98; ipilimumab alone, n = 100), 56 (28%) had a confirmed objective response (T-VEC plus ipilimumab, n = 38 [39%]; ipilimumab, n = 18 [18%]; Fig. 1) and were included in the patterns of response analysis. Patient demographics and baseline clinical characteristics for patients with an objective response are shown in Table S1.
Fig. 1.
Patient incidence of pseudo-progression. OR objective response
Patterns of response
Seven responders (18%) in the T-VEC plus ipilimumab arm and one responder (6%) in the ipilimumab alone arm had pseudo-progression (Table S2). Most cases of pseudo-progression were observed at the first tumour assessment at week 12 (n = 7/8; 88%). Of the eight patients with pseudo-progression, six experienced progression due to an increase in the size of existing lesions (T-VEC plus ipilimumab, n = 5; ipilimumab, n = 1; Fig. S2A, Fig. S2B), and two experienced progression due to the development of new measurable lesions leading to a total burden increase exceeding the 25% threshold for disease progression (T-VEC plus ipilimumab, n = 2; Fig. S2C). For all patients with pseudo-progression, median (range) duration of response (DOR) was 36.5 (12.0–123.7) weeks; median (range) DOR was 36.1 (12.0–101.0) weeks for T-VEC plus ipilimumab and 123.7 weeks for the patient in the ipilimumab arm. The patient in the ipilimumab arm received treatment for ~2 months and then underwent on-protocol surgery 2 months later. No subsequent anticancer therapy was received after surgery.
Forty-eight patients responded without pseudo-progression; 46 (96%) had an objective response within 6 months (T-VEC plus ipilimumab, n = 30; ipilimumab, n = 16; Fig. S3A, S3B) and two (4%) had an objective response after 6 months (T-VEC plus ipilimumab, n = 1; ipilimumab, n = 1; Fig. S3C, S3D). For these 46 patients, median (range) DOR was 47.9 (4.3–136.1) weeks; median (range) DOR was 49.0 (4.3–136.1) weeks for T-VEC plus ipilimumab and 46.7 (12.3–129.0) for ipilimumab alone. Patient response images are depicted in the Supplement (Fig. S4).
Discussion
From our analysis, we identified 18% of patients receiving T-VEC plus ipilimumab and 6% of patients receiving ipilimumab alone who had pseudo-progression and eventually achieved a response. The incidence of pseudo-progression was higher in the combination arm and was associated with a higher ORR versus the control arm, 39% versus 18%, respectively. Because 18% of patients in the combination arm experienced pseudo-progression, we believe that T-VEC plus immune checkpoint inhibitors should be administered even in the setting of signs of progression to provide patients with the greatest opportunity to respond.
The incidence of pseudo-progression with T-VEC plus ipilimumab (18%) in our study was lower than observed with T-VEC monotherapy in OPTiM (48%),5 but higher than rates reported with other checkpoint inhibitor monotherapies (ipilimumab, 10%; pembrolizumab, 7%).1,3 Pseudo-progression rates in this study were higher than seen in an ipilimumab-nivolumab combination study8 and comparable to those observed in previous immunotherapy trials.1–3 DOR in this analysis was longer for patients without pseudo-progression versus those with pseudo-progression; median DOR was not reached for either group in the T-VEC monotherapy study.5 In most instances, pseudo-progression developed before week 12; however, cases of delayed pseudo-progression (e.g. after week 12) also occurred in this study (one of eight responders; 13%) and in previous trials.1,3 The timing of pseudo-progression does not appear to affect treatment response after progression.1,3 These data indicate that pseudo-progression is common with immune checkpoint inhibitor therapies, T-VEC, and T-VEC plus ipilimumab. Oncologists who administer these agents should be aware of this likely immune-mediated phenomenon and consider continuing treatment for ≥3 months assuming no clinical deterioration is observed.
In this analysis, ~15% of patients with an objective response overall had pseudo-progression, mostly due to an increase in existing lesion size. Because of this apparent tumour enlargement, RECIST version 1.1 (a ≥30% decrease in tumour burden measured in one dimension, longest diameter versus baseline4 would have captured this pseudo-progression as true progression; and the eight patients with pseudo-progression would likely have stopped T-VEC prematurely and their ultimate objective response would not have occurred. Thus, the data reported in this trial encourage the continued use of the revised irRC for evaluating immunotherapies and support the additional monitoring and treatment of patients through initial tumour progression.
Overall, these findings support the continued use of T-VEC, particularly when administered in combination with ipilimumab, through initial tumour progression in patients with melanoma, and use of irRC when evaluating response to immunotherapies to make the right treatment decisions for patients receiving chronic or maintenance administration of T-VEC and/or immune checkpoint inhibitors.
Supplementary information
Acknowledgements
The authors thank Meghan Johnson, PhD (Complete Healthcare Communications, LLC, North Wales, PA, a CHC Group Company), whose work was funded by Amgen Inc., for medical writing assistance in the preparation of this manuscript. We also thank Stacy Baum and Katie Golladay for providing essential clinical research support related to data collection at the James Graham Brown Cancer Center, University of Louisville.
Author contributions
J.C., I.P., F.C., M.M., A.H., C.G., P.S. and J.M. contributed to patient data collection/acquisition. L.C. contributed to the conception of the study design. All authors contributed to the analysis and interpretation of data, and to the drafting and revision of the manuscript.
Competing interests
J.C. has received honoraria, consulting and/or advisory fees, and travel and accommodations expenses from Amgen; research funding from Amgen, Iovance, and Bristol-Myers Squibb; and has received reimbursement for service on a scientific advisory panel for Replimune. I.P. has received honoraria, consulting and/or advisory fees, and travel and accommodations expenses from Amgen. F.C. has received consulting fees from Amgen through a consulting agreement that ended in 2018, and institutional research funding from Amgen and Novartis. M.M. has served on the GIST advisory board for Blueprint Solutions and on an advisory board for Amgen. A.H. has received research funding, consulting fees, and honoraria from Novartis, Amgen, Bristol-Myers Squibb, MSD/Merck, Pierre Fabre, Provectus, and Roche; research funding and consulting fees from Merck Serono, Philogen, and Regeneron; consulting fees and speakers’ honoraria from Sanofi-Aventis, and consulting fees from OncoSec and Philogen. L.C. and A.S. are employees of and own stock in Amgen. C.G. has received honoraria and consulting and/or advisory fees from Amgen, Bristol-Myers Squibb, Merck Sharpe & Dohme, Novartis, and Roche; research funding from Bristol-Myers Squibb, Novartis, and Roche; and travel and accommodations expenses from Amgen, Bristol-Myers Squibb, Merck Sharpe & Dohme, and Novartis. P.S. has nothing to disclose. J.M. has received honoraria and consulting and/or advisory fees from Boehringer Ingelheim, EMD Serono, Pfizer, Merck, and Amgen; research funding from Amgen, Novartis, Merck, Polynoma, AstraZeneca, Immunocore, Macrogenics, and Incyte; and travel and accommodation expenses from Merck, EMD Serono, and Boehringer Ingelheim.
Ethics approval and consent to participate
All patients provided written informed consent; study procedures were approved by the institutional review boards/ethics committees at each site (for a listing of the names for all participating sites see Table S3). The study was performed in accordance with the Declaration of Helsinki.
Funding
This study was sponsored by Amgen Inc.
Consent to publish
Patient consent was obtained for all images.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-019-0530-6.
References
- 1.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECISTv1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Andtbacka RH, Ross M, Puzanov I, Milhem M, Collichio F, Delman KA, et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 2016;23:4169–4177. doi: 10.1245/s10434-016-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.