Abstract
Background
This subgroup analysis of a phase 3 study compares outcomes for eribulin versus dacarbazine in patients with leiomyosarcoma.
Methods
Patients ≥18 years old with advanced liposarcoma or leiomyosarcoma, ECOG PS ≤2, and ≥2 prior treatment regimens were randomly assigned (1:1) to eribulin mesylate (1.4 mg/m² intravenously on day 1 and day 8) or dacarbazine (either 850, 1000, or 1200 mg/m² intravenously) every 21 days until disease progression. The primary end point was OS; additional end points were progression-free survival (PFS) and objective response rate (ORR).
Results
309 Patients with leiomyosarcoma were included (eribulin, n = 157; dacarbazine, n = 152). Median age was 57 years; 42% of patients had uterine disease and 57% had nonuterine disease. Median OS was 12.7 versus 13.0 months for eribulin versus dacarbazine, respectively (hazard ratio [HR] = 0.93 [95% CI 0.71–1.20]; P = 0.57). Median PFS (2.2 vs 2.6 months, HR = 1.07 [95% CI 0.84–1.38]; P = 0.58) and ORR (5% vs 7%) were similar between eribulin- and dacarbazine-treated patients. Grade ≥3 TEAEs occurred in 69% of patients receiving eribulin and 59% of patients receiving dacarbazine.
Conclusions
Efficacy of eribulin in patients with leiomyosarcoma was comparable to that of dacarbazine. Both agents had manageable safety profiles.
Subject terms: Sarcoma, Sarcoma, Sarcoma, Sarcoma
Background
Soft tissue sarcomas (STSs) consist of a heterogeneous group of mesenchymal tumours and comprise numerous histologically distinct subtypes that affect various primary sites. As a group, STS types are rare, with an incidence rate of 3–5 cases per 100,000 persons per year.1,2 Liposarcoma (LPS) and leiomyosarcoma (LMS) are two of the more frequently observed sarcoma subtypes, both of which are further categorised based on distinctive clinical and pathologic features.2–4 LMS represents ~20% of STS cases and forms in smooth muscle, most often in the branches of large veins such as the inferior vena cava, or in the uterus.2,4
Patients with metastatic STS have a poor prognosis, with disease-specific survival rates at 5 years of ~15%.5 For unresectable or metastatic disease, anthracycline-based chemotherapy is the recommended first-line treatment, with variations in practice between use of doxorubicin as a single agent or in combination with other agents (typically with olaratumab, ifosfamide, or dacarbazine).6–8 Dacarbazine has shown single-agent activity and improved efficacy in combination with doxorubicin in patients with LMS.9 In a randomised phase 1b/2 trial in anthracycline-naive patients with advanced STS, the combination of doxorubicin and olaratumab significantly improved overall survival (OS) compared with doxorubicin alone. Results of the confirmatory phase 3 trial are pending.10
Several treatment options, including ifosfamide, trabectedin, and pazopanib, are available for second- or later-line therapy for STS (excluding LPS).11–14 Although not approved for sarcoma, gemcitabine, alone or in combination with either docetaxel or dacarbazine, has demonstrated activity as second-line therapy in STS and is frequently used in clinical practice.15,16 In recent years, subtype-specific sensitivity to drugs has become more apparent and several trials have focused on patient populations with specific STS subtypes. Differing sensitivity between sarcoma subtypes has been observed with other agents, for example, myxoid round cell LPS is particularly sensitive to treatment with trabectedin.11 Recent data suggest that there are different molecular subtypes in LMS and that these subtypes are associated with distinct clinical outcomes.17 This finding suggests potential treatment of LMS using a more targeted approach.
Eribulin was approved in 2016 in the United States and European Union for treatment of unresectable or metastatic LPS in patients whose disease had failed to respond to anthracycline chemotherapy.18,19
Eribulin is a structurally modified, synthetic analogue of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai.20 It is a nontaxane inhibitor of microtubule dynamics, with a unique mechanism of action compared with vinca alkaloids.20–22 In preclinical studies, eribulin affected tumour biology, including vascular remodelling, reversal of the epithelial-to-mesenchymal transition, induction of differentiation, and suppression of migration and invasion.23–25
In a phase 2 study of eribulin in STS, 32% of patients with LMS and 47% of patients with LPS met the primary end point of being progression free at 12 weeks.26 Based on these results, eribulin was evaluated in an international phase 3 study of patients with unresectable or metastatic advanced LPS or LMS, in which eribulin significantly improved OS compared with dacarbazine in the total study population (13.5 vs 11.5 months, hazard ratio [HR] 0.77 [0.62–0.95], P = 0.017).18 Here, we present the results of a histology-driven analysis of the efficacy and safety of eribulin in patients with LMS, a randomised and stratified subgroup enrolled in the phase 3 study.
Methods
Study design and participants
The design of the phase 3, randomised, open-label study (NCT01327885) evaluating the effects of eribulin compared to dacarbazine in patients with advanced LPS or LMS has been previously published.18 In brief, patients were randomly assigned (1:1) between March 2011 and May 2013 to treatment groups and stratified by the diagnosis of LPS or LMS, geographic region, and the number of previous chemotherapy regimens. Eligible patients were 18 years or older and had histologically confirmed LPS or LMS with ≥2 prior chemotherapy treatments for advanced disease. Entry criteria also required radiographic evidence of disease progression within 6 months prior to randomisation and the presence of measurable disease according to Response Evaluation Criteria In Solid Tumours version 1.1 (RECIST v1.1).27 The study was conducted at 110 sites in 22 countries under the principles of good clinical practice and the World Medical Association’s Declaration of Helsinki (2008), and was approved by the respective institutional research ethics boards of each participating site (see Supplementary Table 1). All patients provided written informed consent.
Treatment
Eribulin mesylate was given at a dose of 1.4 mg/m2 intravenously (equivalent to 1.23 mg/m2 eribulin [expressed as free base]) on day 1 and day 8 of each 21-day cycle. Dacarbazine was given at a dose of either 850, 1000, or 1200 mg/m2 intravenously on the first day of every 21-day cycle. The dacarbazine starting dose was selected by the investigator prior to randomisation based on each patient’s clinical status and local institutional guidelines. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent.
Tumours were assessed using computed tomography scans or magnetic resonance imaging every 6 weeks for the first 12 weeks and then every 9 weeks thereafter until disease progression. Tumour responses and progression status were determined based on investigator review, following RECIST v1.1, except that chest lesions could not be assessed using X-ray. Safety was assessed by monitoring for treatment-emergent adverse events (TEAEs) and regular evaluation of clinical laboratory test results, vital signs, electrocardiography scans, and physical examinations.
Statistical analysis
The primary end point was OS, and secondary end points included progression-free survival (PFS), progression-free rate at 12 weeks, clinical benefit rate (defined as the proportion of patients whose best overall response was complete response [CR], partial response [PR], or durable stable disease [defined as the proportion of patients who experienced SD for ≥11 weeks]), pharmacokinetics, and safety. Exploratory end points included objective response rate (ORR; defined as the proportion of patients whose best overall response was either CR or PR), and disease control rate (defined as the proportion of patients whose best overall response was CR, PR, or SD). Subgroup analysis based on histologic diagnosis, along with other baseline demographic and disease factors, was prespecified in the study protocol. This study was neither designed nor powered to draw definitive conclusions on the activity of eribulin in histologic subgroups.
OS and PFS were estimated with the Kaplan–Meier product-limit method, and 95% confidence intervals (CIs) were constructed using Greenwood’s formula. HRs were based on a Cox regression model, including treatment as a covariate and stratification factors of geographic region and number of prior chemotherapy regimens. P values were calculated using a two-sided stratified log-rank test with the same stratification factors as for HRs. Efficacy analyses were performed in the intent-to-treat population, comprising all patients who were randomly assigned to treatment. Safety data were summarised descriptively based on all randomised patients who had received at least 1 dose of study treatment and had at least 1 posttreatment safety evaluation.
Results
Patients
This is a histology-driven subgroup analysis from a large, prospective, randomised, phase 3 trial of eribulin in which 452 patients with advanced LPS or LMS in the intent-to-treat population (Supplementary Fig. 1) were randomly assigned to receive eribulin (n = 228) or dacarbazine (n = 224). A total of 309 patients with LMS were included in this analysis; 157 patients were treated with eribulin and 152 were treated with dacarbazine (Table 1). Patients with LMS composed 68% of the overall patient population in the phase 3 study. Within the LMS subgroup, 131 (42%) patients had uterine disease and 177 (57%) patients had nonuterine disease (Table 1). Baseline demographic and disease characteristics were generally well balanced between treatment arms.
Table 1.
Demographic, baseline, and disease characteristics of patients with leiomyosarcoma
| Characteristic | Eribulin (n = 157) | Dacarbazine (n = 152) | Total (N = 309) |
|---|---|---|---|
| Median age (minimum, maximum), years | 57 (28, 76) | 56 (24, 77) | 57 (24, 77) |
| Age group, n (%), years | |||
| <65 | 123 (78) | 124 (82) | 247 (80) |
| ≥65 | 34 (22) | 28 (18) | 62 (20) |
| Sex, n (%) | |||
| Male | 29 (18) | 31 (20) | 60 (19) |
| Female | 128 (82) | 121 (80) | 249 (81) |
| Race, n (%) | |||
| White | 110 (70) | 117 (77) | 227 (74) |
| African American | 6 (4) | 4 (3) | 10 (3) |
| Asiana | 14 (9) | 12 (8) | 26 (8) |
| Otherb | 27 (17) | 19 (12) | 46 (15) |
| ECOG PS, n (%) | |||
| 0 | 76 (48) | 66 (43) | 142 (46) |
| 1 | 80 (51) | 79 (52) | 159 (52) |
| 2 | 1 (1) | 7 (5) | 8 (3) |
| Histology subcategory, n (%) | |||
| Uterine | 68 (43) | 63 (41) | 131 (42) |
| Nonuterine | 88 (56) | 89 (59) | 177 (57) |
| Tumour grade, n (%) | |||
| High | 112 (71) | 113 (74) | 225 (73) |
| Intermediate | 45 (29) | 37 (24) | 82 (27) |
| Not done | 0 | 2 (1) | 2 (1) |
| Geographic region, n (%) | |||
| USA and Canada | 62 (40) | 61 (40) | 123 (40) |
| Western Europe, Australasia, Israel | 70 (45) | 68 (45) | 138 (45) |
| Eastern Europe, Latin America, Asia | 25 (16) | 23 (15) | 48 (16) |
| Median age at diagnosis (minimum, maximum), years | 53.0 (24, 75) | 52.5 (23, 75) | 53.0 (23, 75) |
| Previous anticancer therapy,c n (%) | |||
| 0 | 0 | 0 | 0 |
| 1 | 2 (1) | 1 (1) | 3 (1) |
| 2 | 76 (48) | 70 (46) | 146 (47) |
| 3 | 44 (28) | 44 (29) | 88 (28) |
| 4 | 17 (11) | 25 (16) | 42 (14) |
| >4 | 18 (12) | 12 (8) | 30 (10) |
ECOG PS Eastern Cooperative Oncology Group performance status
aIncludes Japanese, Chinese, and other Asian
bIncludes American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, other, and not applicable
cExcludes radiotherapy and surgery
Efficacy
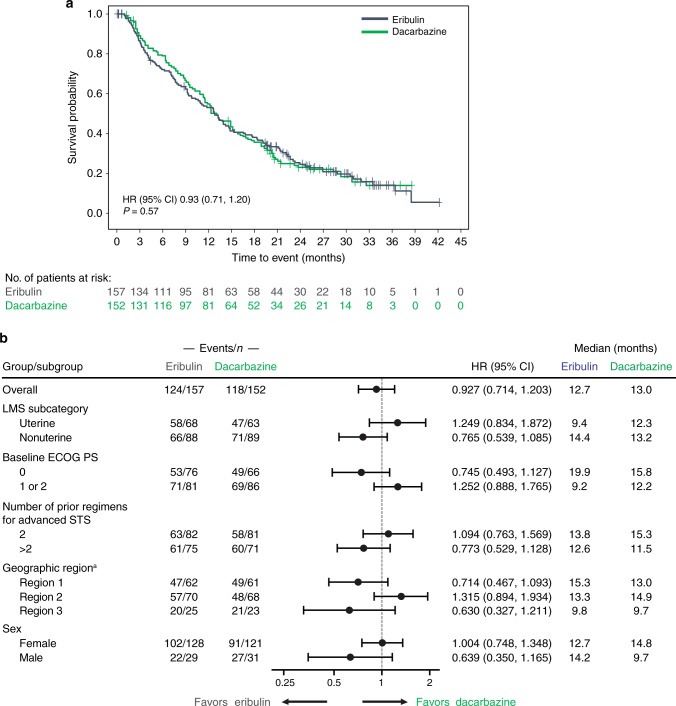
In patients with LMS, the median OS was 12.7 vs 13.0 months for eribulin and dacarbazine, respectively (HR = 0.93 [95% CI 0.71–1.20]; P = 0.57) (Fig. 1a, b). HRs for OS favoured eribulin treatment, but were not statistically different from dacarbazine treatment, in patients with LMS who had >2 prior chemotherapy treatments for advanced disease, had nonuterine disease, had a baseline Eastern Cooperative Oncology Group performance status of 0, were enrolled in geographic region 1 (North America) or geographic region 3 (Eastern Europe, Latin America, and Asia), or were male (Fig. 1b). Median OS for patients with uterine LMS was 9.4 versus 12.3 months for eribulin and dacarbazine, respectively, which was not statistically significantly different (HR = 1.25 [95% CI 0.83–1.87]) (Fig. 1b, Supplementary Fig. 2a). For patients with nonuterine disease, median OS was 14.4 versus 13.2 months for eribulin and dacarbazine, respectively, again not statistically significantly different (HR = 0.77 [95% CI 0.54–1.09]) (Fig. 1b, Supplementary Fig. 2a). Male patients treated with eribulin had numerically longer OS compared with male patients taking dacarbazine (14.2 vs 9.7 months, respectively) (Fig. 1b, Supplementary Fig. 3a), but this difference was not statistically significant.
Fig. 1.
Kaplan–Meier curve of overall survival (a) and hazard ratios of overall survival (b) in patients with leiomyosarcoma. CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance score, HR hazard ratio (eribulin to dacarbazine), based on a Cox regression model including treatment as a covariate and stratification factors of geographic region and number of prior chemotherapy regimens, LMS leiomyosarcoma, STS soft tissue sarcoma. a Region 1 = USA and Canada; Region 2 = Western Europe, Australasia, and Israel; Region 3 = Eastern Europe, Latin America, and Asia
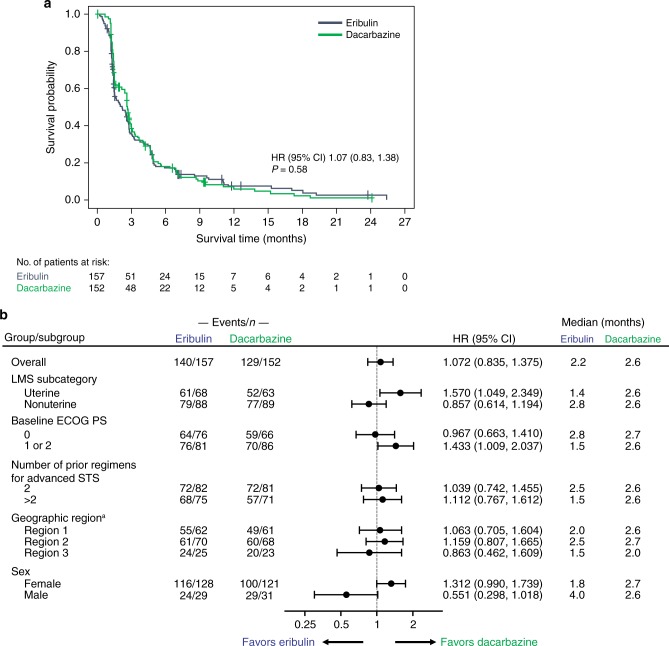
Median PFS in patients with LMS was similar between treatment arms (2.2 vs 2.6 months with eribulin and dacarbazine, respectively, HR = 1.07 [95% CI 0.84–1.38]; P = 0.58) (Fig. 2a, b). In patients with uterine LMS, median PFS was 1.4 vs 2.6 months for eribulin and dacarbazine, respectively (HR = 1.57 [95% CI 1.05–2.35]) (Fig. 2b, Supplementary Fig. 2b). In patients with nonuterine LMS, median PFS was 2.8 vs 2.6 months for eribulin and dacarbazine, respectively; this difference was not statistically significant (HR = 0.86 [95% CI 0.61–1.19]) (Fig. 2b, Supplementary Fig. 2b). Male patients treated with eribulin had numerically (but not statistically significantly) longer PFS compared with male patients taking dacarbazine (4.0 vs 2.6 months, respectively) (Fig. 2b, Supplementary Fig. 3b).
Fig. 2.
Kaplan–Meier curve of progression-free survival (a) and hazard ratios of progression-free survival (b) in patients with leiomyosarcoma. CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance score, HR hazard ratio (eribulin to dacarbazine), based on a Cox regression model including treatment as a covariate and stratification factors of geographic region and number of prior chemotherapy regimens, LMS leiomyosarcoma, STS soft tissue sarcoma. a Region 1 = USA and Canada; Region 2 = Western Europe, Australasia, and Israel; Region 3 = Eastern Europe, Latin America, and Asia
ORR was similar between eribulin-treated and dacarbazine-treated patients (5% vs 7% respectively). The exploratory tumour response end points, disease control rate and durable stable disease rate (defined as the proportion of patients with SD ≥11 weeks) were numerically higher in patients treated with dacarbazine than in those receiving eribulin (57% [95% CI 48–64%] vs 52% [95% CI 44–60%] and 45% [95% CI 37–54%] vs 36% [95% CI 28–44%], respectively) (Table 2); these differences were not statistically significant.
Table 2.
Summary of tumour responses by investigator assessment
| Parameter | Eribulin (n = 157) | Dacarbazine (n = 152) |
|---|---|---|
| Best overall response | ||
| CR, n (%) | 0 | 0 |
| PR, n (%) | 8 (5) | 11 (7) |
| SD, n (%) | 73 (47) | 75 (49) |
| PD, n (%) | 69 (44) | 56 (37) |
| Not evaluable, n (%) | 2 (1) | 1 (1) |
| Unknown, n (%) | 5 (3) | 9 (6) |
| Objective response rate | ||
| ORR (95% CI) | 5 (2, 10) | 7 (4, 13) |
| Disease control rate | ||
| DCR (95% CI) | 52 (44, 60) | 57 (48, 64) |
| Durable stable disease rate | ||
| dSD (95% CI) | 36 (28, 44) | 45 (37, 54) |
CI confidence interval, CR complete response, DCR disease control rate (defined as proportion of PR+CR+SD), dSD durable stable disease (defined as the proportion with stable disease for ≥11 weeks), HR hazard ratio, ORR objective response rate (defined as the proportion of CR + PR), PFS progression-free survival, PD progressive disease, PR partial response, SD stable disease
Safety
In patients with LMS, the five most frequent TEAEs with eribulin were neutropenia (46%), fatigue (46%), nausea (41%), alopecia (33%), and constipation (33%). The five most frequent TEAEs with dacarbazine were nausea (49%), fatigue (41%), thrombocytopenia (31%), anaemia (29%), and constipation (27%) (Table 3). Grade ≥3 TEAEs were reported in 69% of patients in the eribulin arm versus 59% of patients in the dacarbazine arm. Grade ≥3 neutropenia and leukopenia occurred more frequently in patients treated with eribulin, whereas grade ≥3 anaemia and thrombocytopenia occurred more frequently in patients treated with dacarbazine (Table 3).
Table 3.
Treatment-emergent adverse events ≥10% (all grades, either arm) in patients with leiomyosarcoma
| TEAE category, n (%) | Eribulin (n = 156) | Dacarbazine (n = 152) | ||||
|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Neutropenia | 72 (46) | 36 (23) | 25 (16) | 40 (26) | 16 (11) | 8 (5) |
| Fatigue | 71 (46) | 6 (4) | 0 | 63 (41) | 3 (2) | 0 |
| Nausea | 64 (41) | 1 (1) | 0 | 74 (49) | 1 (1) | 0 |
| Alopecia | 51 (33) | 1 (1) | 0 | 5 (3) | 0 | 0 |
| Constipation | 51 (33) | 2 (1) | 0 | 41 (27) | 1 (1) | 0 |
| Anaemia | 49 (31) | 10 (6) | 2 (1) | 44 (29) | 15 (10) | 4 (3) |
| Pyrexia | 46 (30) | 2 (1) | 0 | 20 (13) | 0 | 0 |
| Asthenia | 35 (22) | 2 (1) | 0 | 34 (22) | 4 (3) | 0 |
| Cough | 34 (22) | 0 | 0 | 24 (16) | 0 | 0 |
| Headache | 32 (21) | 0 | 0 | 17 (11) | 0 | 0 |
| Peripheral sensory neuropathy | 30 (19) | 2 (1) | 0 | 6 (4) | 0 | 0 |
| Vomiting | 30 (19) | 2 (1) | 0 | 34 (22) | 0 | 0 |
| Abdominal pain | 28 (18) | 3 (2) | 1 (1) | 21 (14) | 6 (4) | 0 |
| Decreased appetite | 27 (17) | 1 (1) | 0 | 21 (14) | 0 | 0 |
| Dyspnoea | 27 (17) | 3 (2) | 1 (1) | 25 (16) | 3 (2) | 2 (1) |
| Back pain | 26 (17) | 2 (1) | 0 | 23 (15) | 3 (2) | 0 |
| Diarrhoea | 25 (16) | 0 | 0 | 22 (15) | 0 | 0 |
| Leukopenia | 25 (16) | 12 (8) | 4 (3) | 20 (13) | 5 (3) | 3 (2) |
| Stomatitis | 22 (14) | 2 (1) | 0 | 4 (3) | 1 (1) | 0 |
| Oedema peripheral | 21 (14) | 0 | 0 | 11 (7) | 1 (1) | 0 |
| Abdominal pain, upper | 17 (11) | 0 | 0 | 8 (5) | 1 (1) | 0 |
| Hypokalemia | 17 (11) | 4 (3) | 0 | 7 (5) | 2 (1) | 0 |
| Aspartate aminotransferase increased | 16 (10) | 1 (1) | 0 | 5 (3) | 2 (1) | 0 |
| Myalgia | 16 (10) | 0 | 0 | 16 (11) | 0 | 0 |
| Urinary tract infection | 16 (10) | 2 (1) | 0 | 10 (7) | 0 | 0 |
| Thrombocytopenia | 10 (6) | 1 (1) | 0 | 47 (31) | 13 (9) | 13 (9) |
TEAE treatment-emergent adverse event
Nonfatal serious adverse events occurred in 33% of patients in the eribulin arm and 32% of patients in the dacarbazine arm (Supplementary Table 2). TEAEs leading to treatment discontinuation occurred in 8% of patients in the eribulin arm and 5% of patients in the dacarbazine arm, and 16% of patients in the dacarbazine arm required a dose reduction because of TEAEs compared with 28% of patients in the eribulin arm (Supplementary Table 2). However, the frequency of dose interruptions was similar between eribulin- and dacarbazine-treated patients (34% each). A total of 10 patients in the LMS subgroup died from TEAEs, including seven patients in the eribulin arm and three patients in the dacarbazine arm (Supplementary Table 2). In the eribulin arm, only a single event—neutropenic sepsis—was considered possibly related to treatment. None of the three deaths in the dacarbazine arm were considered by the investigators to be treatment related.
Discussion
We present the results from a histology-driven subgroup analysis of patients with LMS from a large-scale, prospective, randomised, controlled, phase 3 study comparing the efficacy and safety of eribulin to dacarbazine in previously treated patients with advanced STS of two histologically distinct types: LMS and LPS.18 The study design was randomised and stratified based on these histological subtypes; however, the study was neither designed nor powered to draw definitive conclusions on the activity of eribulin in histologic subgroups. The phase 3 study was also not designed to be a noninferiority trial.
In this subgroup analysis of patients with LMS, median OS was comparable between patients treated with eribulin versus dacarbazine (HR = 0.93; 95% CI [0.71–1.20]). Secondary outcomes of PFS (HR = 1.07; 95% CI [0.84–1.38]) and ORR (5% vs 7%, respectively) were also comparable between eribulin and dacarbazine treatment groups. Historical data suggest that dacarbazine is more active in LMS than in other sarcomas. In a randomised trial of doxorubicin every 3 weeks versus weekly doxorubicin versus doxorubicin plus dacarbazine for metastatic STS, the combination demonstrated a significant improvement in response rates in patients with LMS compared with doxorubicin alone (44% vs 17% [weekly] vs 20% [every 3 weeks], respectively).9 That trial included 99 (36%) of 275 evaluable patients with LMS. By comparison, this phase 3 trial enrolled >300 (309/452; 68%) patients with LMS.
This subgroup analysis supports the hypothesis that treatment outcomes for a particular sarcoma histology will differ as a function of its primary anatomic site.2,28 In patients with nonuterine LMS, HRs for OS and PFS favoured eribulin, whereas in patients with uterine LMS, HRs for OS and PFS favoured dacarbazine. However, further research is required to determine if various primary LMS sites have molecular differences that would suggest differential antitumor activity of eribulin between uterine and nonuterine LMS and between male and female patients. Emerging data suggest that there are differences in molecular profiles between uterine and nonuterine LMS.17 Differing sensitivity between sarcoma subtypes has been seen with other agents, for example, myxoid round cell LPS is particularly sensitive to treatment with trabectedin, whereas all LPS subtypes are equally sensitive to doxorubicin.11 These results may highlight a need to refine stratification criteria in STS trials to include not only disease histology, but also disease site and molecular biology.
Dacarbazine and eribulin each had different TEAE profiles but, overall, the toxicity profiles of both chemotherapy agents in patients with LMS were manageable. Adverse events did not often lead to study-drug discontinuation. No new safety signals were identified, and adverse events associated with eribulin were similar to previous findings.26
In summary, this subgroup analysis suggests that the effects of eribulin treatment in patients with LMS are comparable to those of dacarbazine. This contrasts with the results of a subgroup analysis of patients with LPS, in which an OS benefit of eribulin vs dacarbazine was evident.29 Outcome heterogeneity within the LMS subgroup may represent biologic differences in this sarcoma subtype. To further understand unique sarcoma subtypes, future prospective trials in STS should aim to examine treatment outcomes as a function of histology, molecular alteration, anatomic site, and gender. The LMS subtype encompasses different disease types based on clinical and pathologic features, and future research should benefit from additional stratification criteria. Such research may contribute to further improvement in outcomes for patients with LMS.
Supplementary information
Acknowledgements
This work was supported by Eisai Inc., Woodcliff Lake, NJ, USA. Eli Berdougo of Oxford PharmaGenesis Inc., Newtown, PA, USA, assisted with preparation of this manuscript and this assistance was funded by Eisai Inc.
Author contributions
Study design: J.-Y.B., P.S., S.B., A.K.-H., C.B., D.D. and R.M.; Data collection: J.-Y.B., P.S., S.B., A.K.-H., C.B. and R.M.; Manuscript preparation: J.-Y.B., P.S., S.B., A.K.-H., C.B., D.D., Y.J. and R.M.; Data analysis & interpretation: J.-Y.B., P.S., S.B., A.K.-H., C.B., D.D. and R.M.; Statistical analysis & data interpretation: Y.J. In addition, all authors confirm they contributed to manuscript reviews—revising it critically for important intellectual content—and read and approved the final draft for submission. All authors are also responsible for the manuscript content.
Competing interests
J.-Y.B. has received research support and honoraria from Eisai. P.S. has received translational research support, travel support and honoraria for advisory and educational functions from Eisai. S.B. has received honoraria from Novartis and participated in an advisory board; has received honoraria from PharmaMar; grants and personal fees from Blueprint Medicines; has participated in advisory boards for Eli Lilly and Deciphera; and has received grants from Incyte. D.R.D. and Y.J. are employees of Eisai Inc. R.G.M. has received research support from Eisai Inc. The remaining authors declare no competing interests.
Consent for publication
There is no identifying patient information in this article. In addition, all authors confirm that this manuscript can be considered for publication.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and guidelines of the International Conference for Harmonization/Good Clinical Practice and was approved by the respective institutional research ethics boards of each participating site (see Supplementary Table 1). All patients provided written informed consent prior to undergoing any study-related procedure.
Data availability
The data of this study are considered commercially proprietary and are not stored for unrestricted access. All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-019-0462-1.
References
- 1.Mastrangelo G, Coindre JM, Ducimetière F, Dei Tos AP, Fadda E, Blay JY, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer. 2012;118:5339–5348. doi: 10.1002/cncr.27555. [DOI] [PubMed] [Google Scholar]
- 2.Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur. J. Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari A, Sultan I, Huang TT, Rodriguez-Galindo C, Shehadeh A, Meazza C, et al. Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pedia. Blood Cancer. 2011;57:943–949. doi: 10.1002/pbc.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int. J. Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 5.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:739–747. doi: 10.1016/S0360-3016(03)00714-4. [DOI] [PubMed] [Google Scholar]
- 6.ESMO Guidelines Committee. eUpdate – Soft Tissue Sarcoma Treatment Recommendations. http://www.esmo.org/Guidelines/Sarcoma-and-GIST/Soft-Tissue-and-Visceral-Sarcomas/eUpdate-Treatment-Recommendations.
- 7.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Soft Tissue Sarcoma. Version 1.2018, https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.
- 8.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(Suppl 3):iii102–iii112. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 9.Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J. Clin. Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 10.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Liberal J, Alam S, Constantinidou A, Fisher C, Khabra K, Messiou C, et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma. 2013;2013:868973. doi: 10.1155/2013/868973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 14.Schöffski P, van Cann T, Cornillie J. Treatment options for anthracycline-resistant, advanced soft-tissue sarcoma: the role of eribulin. Expert. Opin. Orphan Drugs. 2017;5:445–453. doi: 10.1080/21678707.2017.1316190. [DOI] [Google Scholar]
- 15.Garcia-Del-Muro X, Lopez-Pousa A, Maurel J, Martin J, Martinez-Trufero J, Casado A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011;29:2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 16.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J. Clin. Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Jo VY, Mills AM, Zhu SX, Lee CH, Espinosa I, et al. Clinically relevant molecular subtypes in leiomyosarcoma. Clin. Cancer Res. 2015;21:3501–3511. doi: 10.1158/1078-0432.CCR-14-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 19.Halaven (eribulin mesylate) injection [prescribing information]. Woodcliff Lake, NJ: Eisai Inc., 2016.
- 20.Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 21.Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol. Cancer Ther. 2008;7:2003–2011. doi: 10.1158/1535-7163.MCT-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105:1334–1342. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano S, Asano M, Adachi Y, Matsui J. Antimitotic and non-mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Res. 2016;36:1553–1561. [PubMed] [Google Scholar]
- 25.Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br. J. Cancer. 2014;110:1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schöffski P, Ray-Coquard IL, Cioffi A, Bui NB, Bauer S, Hartmann JT, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol. 2011;12:1045–1052. doi: 10.1016/S1470-2045(11)70230-3. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Cho YJ, Kim SH, Rha SY, Ahn JB, Yang WI, et al. Leiomyosarcoma: investigation of prognostic factors for risk-stratification model. Int. J. Clin. Oncol. 2015;20:1226–1232. doi: 10.1007/s10147-015-0847-y. [DOI] [PubMed] [Google Scholar]
- 29.Demetri GD, Schoffski P, Grignani G, Blay JY, Maki RG, Van Tine BA, et al. Activity of Eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of Eribulin versus Dacarbazine. J. Clin. Oncol. 2017;35:3433–3439. doi: 10.1200/JCO.2016.71.6605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are considered commercially proprietary and are not stored for unrestricted access. All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.