Abstract
Despite its status as the most commonly mutated oncogene in cancer, Ras has long been considered ‘undruggable’. In 2019, we will see the first clinical trial results for direct mutant Ras inhibitors, a result of persistent cross-disciplinary research that has been informed by a number of previous clinical and biological failures.
Subject terms: Non-small-cell lung cancer, Targeted therapies, Cancer genetics, Tumour biomarkers
Main
For clinicians treating non-small-cell lung cancer (NSCLC), 2018 was peppered with successive high-impact publications that partnered presentations at AACR, ASCO, WCLC and ESMO; developments dominated by new treatment indications such as NTRK fusion, huge therapeutic progress in the application of immunotherapy and tyrosine kinase inhibitors, and a confirmation of clear benefits from low-dose CT screening programmes.1 It is difficult to imagine a lung cancer landscape more different to 5 years ago when the first phase 1 trial of pembrolizumab was being reported.
Amongst this myriad of practice-changing studies, the first direct inhibitors of mutant K-Ras ready for clinical use, AMG 510 and MRTX 849, were presented at the AACR-Ras and general AACR congress over the past 6 months, followed shortly afterwards by a first report of clinical efficacy at ASCO 2019.2,3 To put this development into context, KRAS was first described in 1983 and it has taken 35 years to reach this point, whereas identification of oncogenic BRAF mutations in 2002 was followed by an effective targeted drug in 2009. The main reasons for this delay are (i) Ras is a small GTPase, whose affinity for GTP exponentially exceeds that observed between kinases and ATP, and (ii) it is a small smooth protein, with no good ‘pockets’ for small molecules to hang on to.4
Direct Ras drugs have resulted in particular from a tireless academic pursuit using new insights on the structural biochemistry of mutant K-Ras to iteratively define lead compounds (ARS-853 and ARS-1620), their optimisation, and their in vivo activity.5–7 This success has guided Amgen and Mirati Therapeutics (whose K-Ras drugs are the first on the clinical scene) as well as many other pharma companies who are targeting the Ras pathway.
One crucial aspect of targeting mutant K-Ras is that the developed drugs are covalent inhibitors irreversibly targeted to the cysteine residue of mutant KRAS G12C. Preclinical work has already shown that, as expected, they will not work on other mutant Ras alleles such as G12D and G12V.7 The mutant G12C subtype is most common in NSCLC and thus typically associated with smoking-related C>A genetic transversions. In general, KRAS is the most frequently mutated oncogene in human cancer on account of its per-patient presence in common cancers, such as lung and colorectal adenocarcinoma. Its mutation is not ubiquitous across different cancer types, so it is difficult to imagine how these important drugs will prosper if they do not find success in the increasingly competitive landscape of stage IV lung cancer.
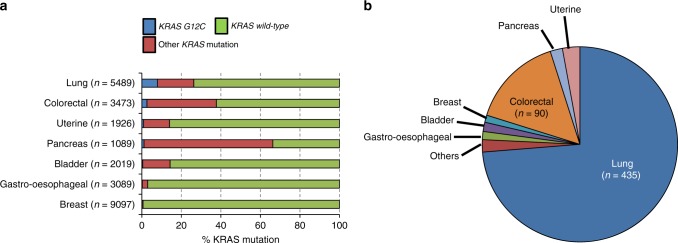
With all of the above in mind, we examined existing sequencing data on KRAS-mutant alleles, noting that we may finally be looking at an ‘actionable’ future for the large KRAS slice of the NSCLC molecular pie chart. A review of large-scale cancer sequencing programmes in cBioPortal confirmed that G12C was most prevalent in lung, colorectal and pancreas cancers, confirming KRAS mutation as a typical feature of recalcitrant epithelial tumours (Fig. 1a). Overall, NSCLC histology was most commonly associated with KRAS G12C mutant cases (70–75%), with colorectal cancer representing the main other significant proportion (Fig. 1b). For an expected raft of G12C drug trials in the coming years, we project that ~9,000–10,000 USA patients are diagnosed each year with stage IV KRAS G12C lung cancer.
Fig. 1.
KRAS G12C mutation in cancer. a Proportions of G12C and non-G12C KRAS mutations in seven cancers with the most KRAS G12C cases. b Percentage contribution of each cancer type to KRAS G12C cancer
How can success be optimised for G12C drugs in a molecular subset of cancer which is notorious for its unmet need? Beyond ensuring that drug pharmacokinetics and pharmacodynamics are appropriate, toxicity is anticipated to be a first small hurdle: RAS mutation should offer a therapeutic window, avoiding unwanted effects on healthy cells. Second, phase 3 trial design will be of crucial importance, a key lesson from the failed SELECT-1 phase 3 trial where the potential benefits of selumetinib in KRAS-mutant NSCLC were overestimated in an earlier phase 2 study.8 In the current NSCLC landscape, it is impossible to imagine a clinical line of sight that does not involve early assessment of combinations with checkpoint inhibitors. Toxicity in this setting may be of more concern, given the unpredictable side-effect profiles observed so far with dual immunotherapy/TKI treatments: while severe hepatic toxicity has been observed with nivolumab/crizotinib and pembrolizumab/gefitinib combinations, similar concerns have not been realised in early studies of alectinib/pembrolizumab and pembrolizumab/erlotinib.9 Third, if toxicity or efficacy does prove challenging in the first-line immunotherapy space, can RAS inhibitors establish their role as a monotherapy or combination optimised for a rapidly evolving second-line treatment landscape in NSCLC? Encouraging new phase I trial results from ASCO 2019 suggest that this should be the minimum target, with AMG 510 reporting a favourable toxicity profile and partial responses in 50% of NSCLC cases (5/10 patients, 1 PR unconfirmed).10
The application of these drugs to the minority of gastrointestinal cancers with G12C mutation could also still hold value. For example, colorectal cancer (CRC, ~4% with G12C) is the main cancer where upfront KRAS status is considered a prerequisite for treatment, with RAS/RAF mutation predicting lack of benefit from EGFR inhibitors such as cetuximab. The relative simplicity of identifying KRAS G12C in CRC is reflected by a phase I report showing that it was the main histological subtype recruited so far,10 although previous unexpected resistance to BRAF-mutant inhibition in CRC suggests reasons to be cautious. The dismal prognosis and paucity of treatment options for pancreas cancer (~2% with G12C) potentially confer a more amenable treatment landscape for breakthroughs to prosper, perhaps via recruitment with other G12C cancer types in pan-disease basket studies.
The Ras field has been here before. Other than selumetinib, failed studies of farnesyl transferase inhibitors offered a cautionary tale 10–15 years ago. Waterfall plots in early-phase trials should therefore be noted with a pause for further data, at least until survival advantages are confirmed by large-scale randomised trials. Most importantly, the iterative process of Ras research should not be deterred if these drugs do not succeed initially. On-treatment biopsies hold the key to understanding their mechanisms of resistance, which can inform subsequent drug development, clinical trials and research.
Acknowledgements
The authors have no acknowledgements.
Author contributions
Design and writing—both C.L. and F.B.
Competing interests
Professor Blackhall reports previous research grants from AstraZeneca, Novartis, Pfizer, Amgen and BMS; Advisory board for Regeneron, Medivation, AbbVie, Takeda, Roche and Ibsen. Non-financial support from CellMedica, MSD. Speaker bureau from Boehringer Ingelheim. None of these funds related to submitted work.
Ethics approval and consent to participate
Not applicable.
Funding
This work is supported by Cancer Research UK via funding to the CRUK Manchester Institute (Grant number A25254) and the CRUK Lung Cancer Centre of Excellence (Grant number A20465).
Data availability
The data appearing in Figure 1 were obtained from cBioPortal for Cancer Genomics (https://www.cbioportal.org/).11,12
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carlisle JW, Ramalingam SS. A banner year for immunotherapy and targeted therapy. Nat Rev Clin Oncol. 2019;16:79–80. doi: 10.1038/s41571-018-0138-4. [DOI] [PubMed] [Google Scholar]
- 2.https://clinicaltrials.gov/ct2/show/NCT03600883
- 3.https://clinicaltrials.gov/ct2/show/NCT03785249?term=G12C&rank=1
- 4.Lindsay CR, Jamal-Hanjani M, Forster M, Blackhall F. KRAS: Reasons for optimism in lung cancer. Eur J Cancer. 2018;99:20–27. doi: 10.1016/j.ejca.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patricelli MP, Janes MR, Li LS, Hansen R, Peters U, Kessler LV, et al. Selective Inhibition of Oncogenic KRAS Output with Small Molecules Targeting the Inactive State. Cancer Discov. 2016;6:316–329. doi: 10.1158/2159-8290.CD-15-1105. [DOI] [PubMed] [Google Scholar]
- 7.Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell. 2018;172:578–589. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA. 2017;317:1844–1853. doi: 10.1001/jama.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spigel DR, Reynolds C, Waterhouse D, Garon EB, Chandler J, Babu S, et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370) J Thorac Oncol. 2018;13:682–688. doi: 10.1016/j.jtho.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Fakih Marwan, O'Neil Bert, Price Timothy Jay, Falchook Gerald Steven, Desai Jayesh, Kuo James, Govindan Ramaswamy, Rasmussen Erik, Morrow Phuong Khanh H., Ngang Jude, Henary Haby A., Hong David S. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. Journal of Clinical Oncology. 2019;37(15_suppl):3003–3003. doi: 10.1200/JCO.2019.37.15_suppl.3003. [DOI] [Google Scholar]
- 11.Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Science Signaling. 2013;6(269):pl1–pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerami, E., Gao, J., Dogrusoz, U., Gross, B.E., Sumer, S.O., Aksoy, B.A. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov.2, 401–404 (2012). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data appearing in Figure 1 were obtained from cBioPortal for Cancer Genomics (https://www.cbioportal.org/).11,12