Abstract
Background
Breast cancer diagnosis may be a teachable moment for lifestyle behaviour change and to prevent adjuvant therapy associated weight gain. We assessed the acceptability and effectiveness of two weight control programmes initiated soon after breast cancer diagnosis to reduce weight amongst overweight or obese women and prevent gains in normal-weight women.
Methods
Overweight or obese (n = 243) and normal weight (n = 166) women were randomised to a three-month unsupervised home (home), a supervised community weight control programme (community) or to standard written advice (control). Primary end points were change in weight and body fat at 12 months. Secondary end points included change in insulin, cardiovascular risk markers, quality of life and cost-effectiveness of the programmes.
Results
Forty-three percent of eligible women were recruited. Both programmes reduced weight and body fat: home vs. control mean (95% CI); weight −2.3 (−3.5, −1.0) kg, body fat −1.6 (−2.6, −0.7) kg, community vs. control; weight −2.4 (−3.6, −1.1) kg, body fat −1.4 (−2.4, −0.5) kg (all p < 0.001). The community group increased physical activity, reduced insulin, cardiovascular disease risk markers, increased QOL and was cost-effective.
Conclusions
The programmes were equally effective for weight control, but the community programme had additional benefits.
Clinical trial registration
ISRCTN68576140
Subject terms: Oncology, Breast cancer
Background
Observational studies indicate that excess weight at breast cancer (BC) diagnosis and significant weight gain (5–10%) thereafter are associated with increased BC specific and all-cause mortality1–4 greater side effects of treatment5,6 and decreased quality of life (QOL).7 Significant numbers of BC patients are overweight (30%) or obese (25%) at diagnosis8 and gain ≥ 5% weight thereafter (30–50%).9 These data suggest that weight loss in overweight/obese women and preventing weight gain in all patients could improve the outcome and wellbeing of women after a diagnosis of BC. A large number of trials amongst overweight or obese BC patients after diagnosis report that weight loss is feasible and safe10,11 however, nearly all of these were initiated long after completion of adjuvant treatment, and after the weight gain associated with diagnosis and treatment had occurred.10,11 Three small randomised studies demonstrate the feasibility and reasonable compliance of weight and exercise programmes based either on clinic visits or telephone interventions during chemotherapy soon after BC diagnosis.12–14
More detailed studies of body composition amongst patients with BC suggest that weight-related adverse effects are specifically associated with increased adiposity and reduced fat-free mass.15–17 Early initiation of weight control is an opportunity prevent deleterious weight gain, gains in fat and reduced fat-free mass which may occur during adjuvant treatment in the months after diagnosis.18 There is a potential teachable moment at diagnosis thus engagement in lifestyle behaviour change soon after diagnosis may be more effective than further down the line after diagnosis.19,20
Both home and supervised community programmes have been found to be effective for behaviour change in the post-treatment setting.10,11 The aim of the study reported here was to compare the acceptability and effectiveness of both types of intervention compared with a control group. We compared a 3-month home phone and mail programme versus a community programme with a control group receiving standard written advice. The interventions were initiated soon after diagnosis, either before or early into adjuvant treatment programmes. The weight control programmes aimed to limit gains in body fat to ≤ 1 kg over the year amongst normal-weight women (body mass index, BMI < 25 kg/m2), and achieve a gradual weight loss of ≥ 5% (i.e. a reduction in body fat of ≥ 3 kg) amongst women who were overweight or obese at diagnosis (BMI ≥ 25 kg/m2). Primary end points were change in weight, body fat and fat-free mass (FFM) at 12 months. Secondary end points included 12-month change in cardiovascular (CVD) markers, insulin sensitivity (homoeostatic model assessment, HOMA), QOL, fitness, and the relative cost-effectiveness of the programmes. We also wished to assess the generalisability of the programme and thus women were recruited from nine breast units across Greater Manchester and Cheshire.
Methods
Study design
A multicentre randomised controlled three-arm trial (1:1:1) within nine breast units in the Greater Manchester and Cheshire Cancer Research Network, UK, coordinated by Manchester University NHS Foundation Trust at Wythenshawe Hospital.
Patient population
Participants were recruited within 12 weeks of surgery for invasive or in-situ primary BC. There were no age, weight or treatment restrictions since we were assessing the general applicability of the interventions in all patients diagnosed with early BC. Women were excluded if they had major physical/psychiatric conditions which would limit compliance to a diet and physical activity (PA) programme, diabetes requiring insulin or regularly taking medication known to affect body composition, e.g. daily glucocorticoids or were treated with neoadjuvant chemotherapy or endocrine therapy. Women were made aware of the trial before surgery or at their initial post-surgery appointment by their breast surgeon or research nurse.
Randomisation and stratification
Randomisation was undertaken in the main recruiting breast unit at Wythenshawe Hospital using a minimisation programme by the trial administrator and was stratified by; chemotherapy or no chemotherapy, BMI ≥ or < 27 kg/m2, axillary node clearance (ANC) versus no ANC, and breast unit.
Study interventions
Standard written advice (‘control’)
This group received a comprehensive booklet which explained the importance of weight control (i.e. ≥ 5% weight loss in overweight/obese and prevention of weight gain in normal-weight subjects) and physical activity (PA) after diagnosis for overall health and wellbeing, and the possible effects on BC outcome. It recommended a healthy Mediterranean type diet (45% energy from low glycaemic index carbohydrates, 30% from fat, 15% monounsaturated, 7% from saturated, 8% from polyunsaturated fat, 25% from lean protein foods, 5–7 portions fruit and vegetables/day) as described previously,21,22 at least 150 min/week of moderate intensity aerobic PA, two sessions of resistance PA per week and arm mobility exercise in accordance with national guidelines,23 and standard advice for dealing with gastrointestinal and fatigue side effects for women receiving chemotherapy.
Home-based phone and mail programme (‘home’)
This group received the written advice described above and individualised diet and PA advice from one of the trial dietitians and the physical activity specialist mainly by telephone after an initial face to face consultation. Diet advice included individualised food portion lists to follow a Mediterranean diet to meet estimated energy requirements for weight maintenance or an energy restriction 25% below estimated energy requirements for weight loss as described previously.22 Physical activity advice promoted a gradual increase towards the above targets for aerobic, resistance and arm mobility exercises which were tailored to the individual. Women were asked to estimate and report the intensity of PA using the rate of perceived exertion scale.24 Initial advice was given face to face in the main recruiting breast unit at Wythenshawe Hospital. The intensive 12 weeks of the programme included six fortnightly 20-min phone calls from their allocated trial dietitian to check compliance to diet and PA targets and address individual problems. This was followed by a mailed summary of goals and recommendations discussed. Women also received six fortnightly mailings which covered the same issues as the community programme. These were received on the weeks between the calls to maintain weekly contact throughout the 12-week programme (Supplementary Table 1).
Supervised community-based group programme (‘community’)
This group received identical written and face to face advice as the ‘home’ group, but were also asked to attend 12 weekly PA and dietary education sessions in one of five different community locations across Greater Manchester. Each session included 30 min of moderate intensity aerobic PA and 10 min of resistance and flexibility PA, followed by a 30-min diet and behaviour change education session (Supplementary Table 1). Women were monitored throughout the class to ensure that they were exercising at a moderate level (50–80% age-adjusted heart rate maximum by pulse checks and rating of perceived exertion). In addition, women were asked to undertake four aerobic and one resistance PA sessions/week at home to meet their weekly goals.
The home and community programmes used established behavioural techniques, i.e. goal setting, self-monitoring of weight and waist (weekly), diet (6 monthly food diaries), PA (daily pedometer), stress and time management, relapse prevention, and overcoming barriers.25 Both groups received booster phone calls from their allocated dietitian to reinforce advice, problem solve and monitor compliance at 4, 6 and 9 months. All study participants including the control group received a three month trial newsletter to encourage retention to the trial.
Outcome measures
Trial assessments were conducted in the main recruiting breast unit at Wythenshawe Hospital at baseline, 6 and 12 months. Body weight, height, waist and hip circumference, blood pressure, fasting insulin, glucose, HOMA, total, LDL and HDL cholesterol and triglycerides were assessed and estimated using standard methods as described previously.21,22,26 Body fat, FFM (body mass excluding fat mass and bone mineral content) and trunk fat were determined from supine dual energy X-ray absorptiometry (DXA) scans (Hologic Discovery A with Hologic APEX software). Data from the head were excluded from all DXA measures due to the high proportion of bone mineral content known to affect the accuracy of soft‐tissue measures. Unilateral artefacts, i.e. metallic joint replacements, breast implants and lymphoedema were adjusted for by replacing the corresponding contralateral value. Physical/functional capacity was assessed from a 12 min treadmill walking test.27 Quality of life was assessed with the Functional Assessment of Cancer Therapy (FACT) physical wellbeing (PWB), functional wellbeing (FWB), BC specific (BCS), endocrine (ESS) and fatigue (FSS) sub scales reported as the trial outcome indicator (TOI) summary scores, e.g. TOI breast cancer (TOI-BC) = PWB + FWB + BCS; TOI endocrine symptoms (TOI-ES) = PWB + FWB + ESS; TOI fatigue (TOI-F) = PWB + FWB + FSS.28
Adherence at 6 and 12 months
Dietary adherence at 6 and 12 months was assessed from seven-day food diaries in all women and analysed using WISP version 3 (Tinuviel Software, Anglesey, Wales) and levels of moderate and vigorous PA were assessed from the Scottish Physical Activity Questionnaire.29
Economic evaluation
Patient-specific costs were estimated for the three trial arms from patient self-reported health care resource use diaries completed every 3 months and hospital records (hospitalisations, medication, outpatient visits, GP services used, etc) up to 12-months post-randomisation. These data were combined with EQ-5D-3L tariffs collected at baseline, 3, 6 and 12 months to estimate cost-utility.30
The three interventions were compared in terms of their mean total costs and Quality Adjusted Life Years (QALYs) and the incremental cost-effectiveness ratios (ICER) were estimated. This describes the incremental change in costs divided by the incremental change in health outcome. The ICER is compared against the cost-effectiveness threshold. This threshold reflects the maximum amount society is willing to pay for an additional unit of health gain. In the UK, the cost-effectiveness threshold lies between £20,000 and £30,000 per QALY.31
Statistics
The sample size of 131 subjects/group was chosen to detect a 3 kg difference in change in body fat measured with DXA (assuming a common SD of 7.6 kg) between the three groups at 12 months with a two-sided significance level of 2% to adjust for multiple testing. The primary analysis was an intention to treat comparison of body fat and weight between the three groups defined at randomisation. Secondary pre-defined analyses compared body fat and weight at 6 and 12 months in the three groups stratified by whether subjects were normal weight or overweight/obese at baseline and receiving or not receiving adjuvant chemotherapy. We also assessed changes in secondary end points (insulin, HOMA, CVD risk markers, QOL, fitness) between the groups.
Outcomes at 6 and 12 months were analysed using analyses of variance regression models (ANCOVA) incorporating baseline measures as covariates. Specific pairwise comparisons between groups were carried out using Scheffe’s multiple comparison tests. The last observation carried forward (LOCF) method was used for missing outcome data. This is a conservative estimate of the ‘non-random’ missing data, as nonattendance at clinic appointments is considered more likely for those who gained weight.
Results
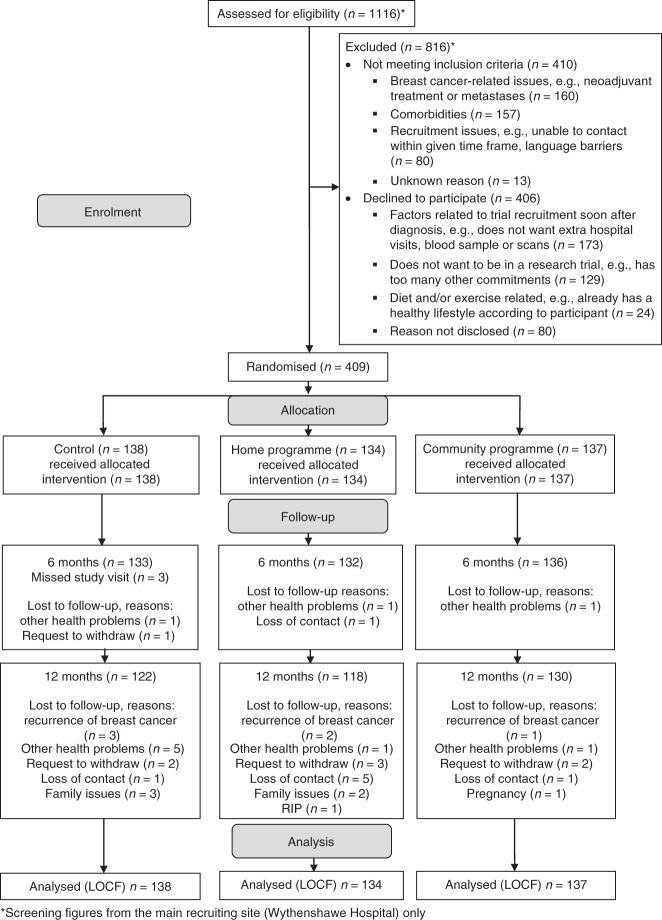
Four hundred and nine women were randomised between August 2008 and February 2011, representing 42% of eligible patients from Wythenshawe Hospital which supplied complete recruitment data as reported previously.32 Women were randomised on average 55 (IQR 39–68) days from the date of their breast surgery.
Sixteen of the control (11.5%), 16 of the home (12%) and 7 of the community group (5%) withdrew from the study due to recurrence of breast cancer (n = 6), other health problems (n = 10), family issues (n = 5), pregnancy (n = 1), request to withdraw (n = 8), patient had died (n = 1) and loss of contact (n = 8) (Fig. 1). At baseline, the three groups were comparable for age, ethnicity, BMI, menopausal status, tumour characteristics, breast surgery, BC treatments and prevalence of co-morbidities, and index of multiple deprivation (Table 1). Fifty-nine percent of the overall cohort were overweight or obese and 41% normal weight. Thirty-eight percent received adjuvant chemotherapy (56% overweight/obese and 44% normal weight). Women who withdrew were of comparable BMI to women who remained in the study, mean (SD) 27.3 (5.5) vs. 27.2 (5.4) kg/m2 P = 0.923, but were significantly younger, 51 (9.0) vs. 55 (10.4) years (P = 0.019) and more likely to be receiving chemotherapy, 13.5% of the chemotherapy group vs. 7.1% of the no chemotherapy group (P = 0.025).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patients recruited to the B-AHEAD trial. *Screening figures are from the main recruiting site (Wythenshawe Hospital) only
Table 1.
Baseline characteristics of patients randomised to the three groups
| Control | Home | Community | |
|---|---|---|---|
| (n = 138) | (n = 134) | (n = 137) | |
| Age (years) | 55.3 (10.5) | 54.6 (11.2) | 54.0 (9.2) |
| BMI (kg/m²) | 27.6 (6.1) | 26.9 (4.8) | 27.0 (5.1) |
| BMI Category | |||
| Normal weight (18.5–24.99 kg/m2) | 58 (42.0%) | 50 (37.3%) | 55 (40.1%) |
| Overweight (≥ 25–29.99 kg/m2) | 38 (27.5%) | 56 (41.8%) | 49 (35.8%) |
| Obese (≥ 30 kg/m2) | 42 (30.4%) | 28 (20.9%) | 33 (24.1%) |
| Pre/peri-menopausal | 56 (40.6%) | 55 (41.0%) | 49 (35.8%) |
| Post-menopausal | 82 (59.4%) | 79 (59.0%) | 88 (64.2%) |
| Days between final breast surgery and randomisation | 50 (39–68) | 55 (36–66) | 54 (40–68) |
| Current smoker | 15 (10.9%) | 17 (12.7%) | 9 (6.6%) |
| Ethnicity | |||
| White | 132 (95.7%) | 126 (94.0%) | 129 (94.2%) |
| Black | 2 (1.4%) | 4 (3.0%) | 4 (2.9%) |
| Asian | 3 (2.2%) | 4 (3.0%) | 3 (2.2%) |
| Mixed | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) |
| Social circumstances | |||
| Married or cohabiting | 106 (76.8%) | 94 (70.0%) | 102 (74.5%) |
| Educated to degree level or higher | 64 (46%) | 59 (44%) | 67 (49%) |
| Index of multiple deprivation | |||
| Greater Manchester Quintile | |||
| 1 (least deprived) | 66 (47.8%) | 46 (34.3%) | 48 (35.0%) |
| 2 | 28 (20.3%) | 31 (23.1%) | 31 (22.6%) |
| 3 | 15 (10.9%) | 26 (19.4%) | 24 (17.5%) |
| 4 | 12 (8.7%) | 15 (11.2%) | 17 (12.4%) |
| 5 (most deprived) | 17 (12.3%) | 16 (11.9%) | 17 (12.4%) |
| Co-morbidities | |||
| Respiratory, e.g. asthma, COPD | 19 (13.8%) | 15 (11.2%) | 15 (10.9%) |
| Psychiatric, e.g. anxiety, depression | 14 (10.1%) | 16 (11.9%) | 19 (13.9%) |
| Cardiovascular disease | 12 (8.6%) | 10 (7.4%) | 11 (8.0%) |
| Arthritis, back or joint problems | 41 (29.0%) | 32 (24.0%) | 29 (21.6%) |
| Type 2 diabetes | 3 (2.2%) | 7 (5.2%) | 4 (2.9%) |
| Previous breast cancer | 8 (5.8%) | 7 (5.2%) | 3 (2.2%) |
| Tumour characteristics | |||
| DCIS/LCIS | 13 (9.4%) | 18 (13.4%) | 18 (13.2%) |
| Invasive tumour Grade 1 | 25 (18.0%) | 23 (17.2%) | 21 (15.3%) |
| Invasive tumour Grade 2 | 61 (44.2%) | 56 (41.8%) | 62 (45.3%) |
| Invasive tumour Grade 3 | 40 (28.9%) | 38 (28.4%) | 34 (24.8%) |
| Surgery | |||
| Mastectomy | 45 (32.6%) | 47 (35.1%) | 48 (35.0%) |
| Axillary node clearance (ANC) | 34 (24.6%) | 34 (25.4%) | 33 (24.1%) |
| Screen detected breast cancer | 52 (51.0%) | 53 (54.6%) | 61 (60.4%) |
| Adjuvant treatment a | |||
| Chemotherapy | 52 (37.7%) | 52 (38.8%) | 51 (37.2%) |
| Anthracycline only | 29 (21.0%) | 33 (24.6%) | 27 (20.0%) |
| Anthracyline & taxane | 23 (16.7%) | 19 (14.2%) | 24 (17.5%) |
| Radiotherapy | 98 (71.0%) | 91 (67.9%) | 104 (75.9%) |
| Herceptin | 19 (13.8%) | 13 (9.7%) | 11 (8.0%) |
| Tamoxifen | 68 (49.3%) | 75 (56.0%) | 78 (56.9%) |
| Aromatase inhibitor | 36 (26.1%) | 34 (25.4%) | 34 (24.8%) |
| No adjuvant treatment (no chemothetherapy, radiotherapy or other endocrine treatment) | 5 (3.6%) | 10 (7.5%) | 8 (5.8%) |
BMI body mass index, COPD chronic obstructive pulmonary disease, DCIS ductal carcinoma in situ, LCIS lobular carcinoma in situ
Mean (SD) n (%) median (interquartile range)
aPatients were recruited between September 2008 and November 2010
Participation in the home and community group programmes
During the initial 12-week phase of the programmes, women in the home group received mean (interquartile range) 85 (83–100) % of their six scheduled home phone calls and were sent 100% of the mailings, whilst women in the community group attended 64 (50–75)% of the 12 scheduled weekly group classes. Four- and nine-month booster calls were received respectively by 84 and 80% of the home and 83 and 82% of the community groups.
Primary end points: change in weight and body composition
DXA data were analysed from 389 participants; 4 had no DXA scan and 16 had their DXA data omitted as they had bilateral high-density artefacts. At 12 months the home and community groups both significantly reduced weight and body fat whilst these increased in the control group. Weight reduction in the home group compared with controls was mean (95% confidence interval) −2.3 (−3.5, −1.0) kg, and body fat reduction was −1.6 (−2.6, −0.7) kg (Table 2). Weight reduction in the community group compared with controls was −2.4 (−3.6, −1.1) kg, and body fat reduction was −1.4 (−2.4, −0.5) kg (all p < 0.001). There were small but statistically significant reductions in FFM in the home and community groups and a modest increase in the control group. The control group experienced gains in weight and body fat between 6 and 12 months, whilst the home and community groups respectively maintained or had further reductions of weight in this period which was 3–9 months after the initial intensive 12-week programme (Table 2).
Table 2.
Changes in weight and body composition at six and twelve months for the overall cohort
| Change over timea | Group differenceb | ||||||
|---|---|---|---|---|---|---|---|
| Control | Home | Community | Home vs Control | Community vs Control | Community vs Home | ||
| Weight (kg) | Baseline |
(n = 138) 72.5 (16.1) |
(n = 134) 71.0 (13.9) |
(n = 137) 71.9 (13.5) |
|||
| Change at 6 months |
0.3 (−0.4, 1.0) |
−1.4 (−2.1, −0.7) |
−1.1 (−1.8, −0.5) |
−1.7 (−2.8, −0.6) p = 0.001 |
−1.4 (−2.6, −0.3) p = 0.008 |
0.3 (−0.9, 1.4) p = 1.000 |
|
| Change at 12 months |
0.8 (0.1, 1.50) |
−1.5 (−2.2, −0.8) |
−1.6 (−2.3, −0.9) |
−2.3 (−3.5, −1.0) p < 0.001 |
−2.4 (−3.6, −1.1) p < 0.001 |
−0.1 (−1.3, 1.2) p = 1.000 |
|
| DXA Body fat (kg) | Baseline |
(n = 134) 27.9 (10.1) |
(n = 128) 27.3 (8.1) |
(n = 127) 27.7 (8.5) |
|||
| Change at 6 months |
0.1 (−0.4, 0.5) |
−1.2 (−1.7, −0.7) |
−0.9 (−1.3, −0.4) |
−1.3 (−2.0, −0.5) p = 0.001 |
−0.9 (−1.7, −0.1) p = 0.016 |
0.3 (−0.5, 1.1) p = 0.980 |
|
| Change at 12 months |
0.5 (−0.7, 1.0) |
−1.2 (−1.7, −0.6) |
−0.9 (−1.5, −0.4) |
−1.6 (−2.6, −0.7) p < 0.001 |
−1.4 (−2.4, −0.5) p = 0.001 |
0.2 (−0.8, 1.2) p = 1.000 |
|
| DXA Fat free mass (kg) | Baseline |
(n = 134) 39.1 (6.3) |
(n = 128) 38.5 (0.6) |
(n = 127) 39.2 (5.9) |
|||
| Change at 6 months |
0.3 (0.0, 0.5) |
−0.1 (−0.4, 0.2) |
−0.2 (−0.4, 0.1) |
−0.4 (−0.9, 0.1) p = 0.200 |
−0.4(−0.9, 0.1) p = 0.140 |
0.0 (−0.5, 0.5) p = 1.000 |
|
| Change at 12 months |
0.4 (0.1, 0. 7) |
−0.3 (−0.6, 0.0) |
−0.3 (−0.6, 0.0) |
−0.7 (−1.2, −0.2) p = 0.005 |
−0.7 (−1.2, −0.1) p = 0.008 |
0.0 (−0.5, 0.6) p = 1.000 |
|
Mean (SD)
aANCOVA, Mean (95% CI)
bANCOVA with Bonferroni adjustment, Mean (95% CI)
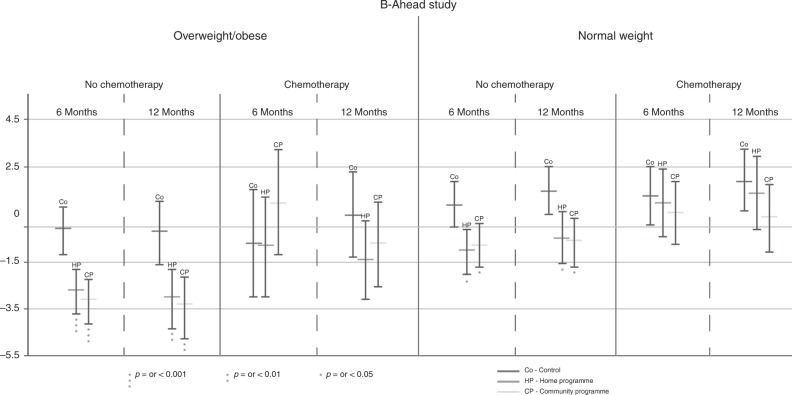
Pre-specified subgroup analyses indicated that weight and body composition results differed according to BMI category and whether patients were treated with chemotherapy (Table 3, Fig. 2). Both programmes induced weight loss amongst overweight/obese women who were not receiving chemotherapy, whilst weight was maintained in controls. However, the programmes did not induce weight loss amongst overweight/obese patients who were receiving chemotherapy for whom weight was maintained.
Table 3.
Changes in weight and body composition for normal and overweight and chemotherapy sub-groups
| Change over timea | Group differenceb | ||||||
|---|---|---|---|---|---|---|---|
| Control | Home | Community | Home vs. control | Community vs. control | Community vs. home | ||
| Weight | |||||||
| BMI ≥ 25 kg/m2 | Baseline |
(n = 51) 83.0 (16.0) |
(n = 52) 78.6 (12.0) |
(n = 53) 78.7 (12.5) |
|||
| No chemotherapy | Change at 6 months | −0.1 (−1.1, 0.8) | −2.7 (−3.7, −1.8) | −3.1 (−4.1, −2.2) |
−2.6 (−4.2, −1.0) p = 0.001 |
−3.0(−4.6, −1.4) p = 0.001 |
−0.4 (−2.0, 1.2) p = 1.000 |
| Change at 12 months | −0.2 (−1.6, 1.0) | −3.0 (−4.3, −1.8) | −3.3 (−4.7, −2.1) |
−2.8 (−5.0, −0.5) p = 0.009 |
−3.1 (−5.4, −0.9) p = 0.003 |
0.3 (−2.6, 1.9) p = 1.000 |
|
| BMI ≥ 25 kg/m2 | Baseline |
(n = 27) 79.5 (13.4) |
(n = 32) 77.2 (11.3) |
(n = 28) 80.7 (8.9) |
|||
| Chemotherapy | Change at 6 months | −0.7 (−2.9, 1.5) | −0.8 (−2.9, 1.0) | 1.0 (−1.2, 3.2) |
−0.1 (−3.8, 3.6) p = 1.000 |
1.7 (−2.1, 5.5) p = 0.840 |
1.8 (−1.9, 5.5) p = 0.700 |
| Change at 12 months | 0.5 (−1.3, 2.3) | −1.4 (−3.1, 0.2) | −0.7 (−2.5, 1.0) |
−1.9 (−4.9, 1.1) p = 0.370 |
−1.2 (−4.3, 1.9) p =p = 0.990 |
−0.7 (−2.3, 3.7) p = 1.000 |
|
| BMI < 25 kg/m2 | Baseline |
(n = 35) 59.5 (4.7) |
(n = 30) 60.2 (8.1) |
(n = 33) 60.6 (9.3) |
|||
| No chemotherapy | Change at 6 months | 0.9 (0.0, 1.7) | −1.0 (−2.0, −0.1) | −0.8 (−1.7, 0.1) |
−1.9 (−3.5, −0.3) p = 0.011 |
−1.7 (−3.3, −0.2) p = 0.023 |
0.2 (−1.4, 1.8) p = 1.000 |
| Change at 12 months | 1.5 (0.5, 2.5) | −0.5 (−1.6, 0.6) | −0.6 (−1.7, 0.4) |
−2.0 (−3.9, −0.2) p = 0.028 |
−2.1 (−4.0, −0.3) p = 0.015 |
0.1 (−2.0, 1.8) p = 1.000 |
|
| BMI < 25 kg/m2 | Baseline |
(n = 25) 62.0 (8.2) |
(n = 20) 57.4 (7.2) |
(n = 23) 62.0 (5.7) |
|||
| Chemotherapy | Change at 6 months | 1.3 (0.1, 2.5) | 1.0 (−0.4, 2.4) | 0.6 (−0.7, 1.8) |
−0.3 (−2.6, 2.0) p = 1.000 |
−0.7 (−2.9, 1.4) p = 1.000 |
0.4 (−2.8, 1.9) p = 1.000 |
| Change at 12 months | 1.9 (0.6, 3.2) | 1.4 (−0.1, 0.6) | 0.4 (−0.4, 2.4) |
−0.5 (−3.0, 2.0) p = 1.000 |
−1.5 (− 3.8, 2.0) p = 0.340 |
−1.0 (−3.5, 1.5) p = 0.980 |
|
| Body fat | |||||||
| BMI ≥ 25 kg/m2 | Baseline |
34.8 (9.4) (n = 49) |
32.6 (6.3) (n = 50) |
32.4 (8.2) (n = 49) |
|||
| No chemotherapy | Change at 6 months | −0.1 (−0.1, 0.6) | −2.3 (−3.0, −1.6) | −1.9 (−2.5, −1.2) |
−2.2 (−3.4, −1.0) p < 0.001 |
−1.7 (−3.0, −0.5) p = 0.002 |
0.4 (−0.8, 1.6) p = 1.000 |
| Change at 12 months | −0.3 (−1.3, 0.6) | −2.2 (−3.1, −1.3) | −2.1 (−3.1, −1.2) |
−1.9 (−3.5, −0.21) p = 0.022 |
−1.8 (−3.4, −0.1) p = 0.033 |
0.09(−1.6, 1.7) p = 1.000 |
|
| BMI ≥ 25 kg/m2 | Baseline |
32.5 (8.7) (n = 27) |
30.3 (6.1) (n = 31) |
31.8 (5.6) (n = 27) |
|||
| Chemotherapy | Change at 6 months |
−0.9 (−2.3, 0.3) (n = 49) |
−0.7 (−1.9, 0.6) (n = 50) |
−0.1 (−1.4, 1.3) (n = 49) |
0.3 (−1.9, 2.5) p = 1.000 |
0.9 (−1.4, 3.2) p = 1.000 |
0.6 (−1.6, 2.8) p = 1.000 |
| Change at 12 months | 0.1 (−1.4, 1.6) | −1.3 (−2.7, 0.1) | 0.1 (−1.4, 1.6) |
−1.4 (−3.9, 1.1) p = 0.530 |
−0.(−2.7, 2.5) p = 1.000 |
1.4 (−1.2, 3.8) p = 0.590 |
|
| BMI < 25 kg/m2 | Baseline |
19.6 (3.5) (n = 33) |
20.0 (5.0) (n = 28) |
21.8 (5.5) (n = 30) |
|||
| No chemotherapy | Change at 6 months |
0.6 (0.0, 1.3) (n = 25) |
−1.3 (−2.0, −0.6) (n = 19) |
−0.9(−1.6, −0.2) (n = 21) |
−1.9 (−3.1, −0.7) p = 0.001 |
−1.5 (−2.7, 0.3) p = 0.010 |
0.4 (−0.8, 1.7) p = 1.000 |
| Change at 12 months | 1.3 (0.5, 2.1) | −0.8 (−1.7, 0.0) | −0.9 (−1.7, 0.0) |
−2.1 (−3.6, −0.7) p = 0.002 |
−2.1 (−3.6, −0.7) p = 0.002 |
0.0 (−1.5, 1.5) p = 1.000 |
|
| BMI < 25 kg/m2 | Baseline | 20.0 (3.6) | 19.2 (3.3) | 19.9 (4.2) | |||
| Chemotherapy | Change at 6 months | 0.6 (−0.4, 1.6) | 0.9 (−0.3, 2.0) | 0.7 (−0.4, 1.7) |
0.3 (−1.6, 2.1) p = 1.000 |
0.1 (−1.7, 1.9) p = 1.000 |
−0.2 (−2.1, 1.8) p = 1.000 |
| Change at 12 months | 1.3 (0.2, 2.3) | 1.3 (0.0, 2.5) | 0.7 (−0.5, 1.9) |
0.03 (−2.1, 2.0) p = 1.000 |
−0.6 (−2.6, 1.4) p = 1.000 |
−0.5 (−2.7, 1.6) p = 1.000 |
|
| Fat free mass | |||||||
| BMI ≥ 25 kg/m2 | Baseline |
42.0 (6.4) (n = 49) |
41.0 (6.3) (n = 50) |
40.3 (5.7) (n = 49) |
|||
| No chemotherapy | Change at 6 months | 0.2 (−0.3, 5.8) | −0.3 (−0.7, 0.1) | −0.8 (−1.2, 0.4) |
−0.5 (−1.2, 0.2) p = 0.280 |
−0.9 (−1.6, −0.3) p = 0.005 |
−0.4 (−1.2, 0.3) p = 0.370 |
| Change at 12 months | 0.4 (−0.2, 0.9) | −0.5 (−1.1, 0.0) | −0.6 (−1.2, −0.1) |
−0.9 (−1.8, 0.1) p = 0.072 |
−1.0 (−2.0, 0.1) p = 0.033 |
−0.1 (−1.1, 0.8) p = 1.0 |
|
| BMI ≥ 25 kg/m2 | Baseline |
41.6 (6.1) (n = 27) |
41 (6.2) (n = 31) |
43 (4.8) (n = 27) |
|||
| Chemotherapy | Change at 6 months | 0.2 (−0.7,1.0) | −0.8 (−0.9, 7.2) | 0.1 (−0.8, 1.0) |
−0.2 (−1.7, 1.2) p = 1.000 |
−0.1 (−1.2, 1.4) p = 1.000 |
0.2 (−1.3, 1.6) p = 1.000 |
| Change at 12 months | 0.3 (−0.4, 1.0) | −0.5 (−1.2, 0.2) | −0.7 (−1.5, −0.1) |
−0.8 (−2.0, 0.4) p = 0.310 |
−1.1 (−2.3, 0.2) p = 0.130 |
−0.3 (−1.5, 1.0) p = 1.000 |
|
| BMI < 25 kg/m2 | Baseline |
34.3 (3.4) (n = 49) |
35.6 (4.4) (n = 50) |
35.4 (5.7) (n = 49) |
|||
| No chemotherapy | Change at 6 months | 0.2 (−0.3, 0.6) | 0.2 (−0.3, 0.7) | 0.4 (−0.1, 0.9) |
0.0 (−0.8, 0.8) p = 1.000 |
0.2 (−5.9, 1.1) p = 1.000 |
0.2 (−0.6, 1.1) p = 1.000 |
| Change at 12 months | 0.4 (−1.8, 0.9) | 0.1 (−0.5, 0.6) | 0.6 (0.1, 1.2) |
−0.3 (−1.2, 0.7) p = 1.000 |
0.3 (−0.7, 1.2) p = 1.000 |
0.6 (−0.4, 1.6) p = 0.540 |
|
| BMI < 25 kg/m2 | Baseline |
37.1 (4.8) (n = 25) |
33.4 (4.7) (n = 19) |
37.0 (4.0) (n = 21) |
|||
| Chemotherapy | Change at 6 months | 0.6 (−0.05, 1.1) | 0.0 (−0.7, 0.7) | 0.0 (−0.7, 0.6) |
−0.6 (−1.7, 0.6) p = 0.750 |
−0.6 (−1.6, 0.5) p = 0.590 |
−0.01 (−1.2, 1.2) p = 1.000 |
| Change at 12 months | 0.4 (−0.2, 1.0) | 0.0 (−0.7, 0.7) | −0.1 (−0.7, 0.5) |
−0.4 (−1.5, 0.7) p = 1.000 |
−0.5 (−1.5, 0.5) p = 0.660 |
−0.1 (−1.3, 1.0) p = 1.000 |
|
Mean (SD)
a ANCOVA, m ean (95% CI)
b ANCOVA with Bonferroni adjustment, m ean (95% CI)
Fig. 2.
Changes in weight in overweight/ obese, normal weight and chemotherapy sub groups shown as mean 95% confidence intervals)
Both programmes prevented weight gain amongst normal-weight women who were not receiving chemotherapy, whilst weight increased in controls. In contrast, the programmes did not prevent weight gain amongst normal-weight women receiving chemotherapy. In this normal weight group, patients receiving chemotherapy gained comparable amounts of weight to the non-chemotherapy patients at 12 months (Table 3, Fig. 2).
Changes in body fat were comparable to changes in weight in these subgroups. Overweight/obese non-chemotherapy patients experienced small reductions in FFM alongside weight loss, whilst FFM was maintained in the overweight/obese chemotherapy group and the chemotherapy and non– chemotherapy normal weight sub groups (Table 3).
Secondary end points
Biochemistry: The community and home groups were similar with respect to weight control but significant reductions in total and LDL cholesterol, systolic blood pressure, serum insulin, HOMA, and triglycerides at 12 months were only detected in the community group (Table 4). The home group had smaller reductions in these parameters which were not significantly different from controls. Distance walked on the 12-minute walk test (a measure of fitness) increased above baseline in all groups. At 6 months the community group had significantly greater increases compared to the controls and home groups. The difference between the groups was attenuated at 12 months.
Table 4.
Changes in secondary end points over 12 months for the overall cohort
| Change over timea | Group differenceb | ||||||
|---|---|---|---|---|---|---|---|
| Control | Home | Community | Home vs. | Community vs. | Community vs. | ||
| Control | Control | Home | |||||
| Waist (cm) | Baseline |
(n = 138) 95.6 (15.6) |
(n = 134) 94.2 (13.3) |
(n = 137) 94.7 (12.9) |
|||
| Change at 6 months | 0.1 (−0.6, 0.8) | −1.5 (−2.2, −0.8) | −2.6 (−3.3, −1.9) |
−1.6 (−2.8, −0.4) p = 0.006 |
−2.7 (−3.9, −1.4) p < 0.001 |
−1.1 (−2.3,0.2) p = 0.110 |
|
| Change at 12 months | 0.4 (−0.5, 1.2) | −1.7 (−2.6, −0.8) | −2.1 (−3.0, −1.3) |
−2.1 (−3.6, −0.5) p = 0.003 |
−1.7 (−2.6, −0.8) p < 0.001 |
−0.5 (−2.0, 1.0) p = 1.000 |
|
| Hip (cm) | Baseline |
(n = 138) 104.5 (11.2) |
(n = 134) 102.3 (8.6) |
(n = 137) 103.7 (9.8) |
|||
| Change at 6 months | 0.3 (−0.4, 0.9) | −1.2 (−1.8, −0.5) | −1.6 (−2.2, −1.0) |
−1.4 (−2.6, −0.3) p = 0.006 |
−1.8 (−3.0, −0.7) p < 0.001 |
−0.4 (−1.5, 0.7) p = 1.000 |
|
| Change at 12 months | 0.9 (0.2, 1.6) | −1.1 (−1.8, −0.4) | −1.4 (−2.1, −0.7) |
−2.0 (−3.2, −0.8) p < 0.001 |
−2.4 (−3.6, −1.1) p < 0.001 |
−0.4 (−1.6, 0.9) p = 1.000 |
|
| DXA trunk fat (kg) | Baseline |
(n = 134) 13.1 (5.3) |
(n = 129) 12.9 (4.7) |
(n = 128) 13.2 (4.7) |
|||
| Change at 6 months | 0.0 (−0.3, 0.2) | −0.7 (−0.9, −0.4) | −0.5 (−0.75, −0.25) |
−0.7 (−1.1, −0.2) p = 0.001 |
−0.5 (−0.9, 0.0) p = 0.019 |
0.2 (−0.3, 0.6) p = 1.000 |
|
| Change at 12 months | 0.2 (0.0, 0.5) | −0.7 (−1.0, −0.4) | −0.5 (−0.9, −0.3) |
−0.9 (−1.4, −0.4) p < 0.001 |
−0.8 (−1.3, −0.3) p = 0.001 |
0.2 (−0.4, 0.7) p = 1.000 |
|
| Glucosec (mmol/L) | Baseline d |
(n = 134) 5.1 (3.2, 7.6) |
(n = 124) 5.2 (4.1, 16.0) |
(n = 134) 4.93 (4.0, 6.3) |
|||
| Ratio of change at 6 months |
1.008 (0.994, 1.021) |
0.994 (0.980, 1.009) |
0.988 (0.974, 1.002) |
0.986 (0.963, 1.011) p = 0.540 |
0.98 (0.958, 1.004) p = 0.140 |
0.994 (0.970, 1.018) p = 1.000 |
|
| Ratio of change at 12 months |
1.007 (0.993, 1.022) |
0.99 (0.974, 1.005) |
0.988 (0.973, 1.003) |
0.982 (0.957, 1.009) p = 0.320 |
0.98 (0.955, 1.006) p = 0.190 |
0.998 (0.971, 1.024) p = 1.000 |
|
| Insulinc (pmol/L) | Baseline d |
(n = 131) 51.8 (15.4, 195.7) |
(n = 124) 56.6 (21.2, 183.9) |
(n = 134) 53.6 (15.8, 276.8) |
|||
| Ratio of change at 6 months |
1.004 (0.953, 1.058) |
0.958 (0.908, 1.011) |
0.93 (0.883, 0.979) |
0.955 (0.871, 1.047) p = 0.680 |
0.926 (0.846, 1.013) p = 0.120 |
0.97 (0.886, 1.062) p = 1.000 |
|
| Ratio of change at 12 months |
1.020 (0.962, 1.082) |
0.954 (0.899, 1.014) |
0.908 (0.858, 0.962) |
0.936 (0.844, 1.037) p = 0.370 |
0.891 (0.806, 0.985) p = 0.018 |
0.952 (0.860, 1.054) p = 0.730 |
|
| HOMAc | Baseline d |
(n = 131) 1.7 (0.5, 8.0) |
(n = 122) 1.7 (0.4, 6.0) |
(n = 133) 1.7 (0.6, 8.7) |
|||
| Ratio of change at 6 months |
1.011 (0.954, 1.073) |
0.956 (0.900, 1.016) |
0.918 (0.867, 0.973) |
0.946 (0.852, 1.049) p = 0.590 |
0.908 (0.821, 1.004) p = 0.066 |
0.96 (0.866, 1.064) p = 1.000 |
|
| Ratio of change at 12 months |
1.03 (0.965, 1.100) |
0.95 (0.888, 1.016) |
0.900 (0.844, 0.959) |
0.922 (0.822, 1.034) p = 0.270 |
0.873 (0.781, 0.977) p = 0.011 |
0.947 (0.845, 1.062) p = 0.760 |
|
| Total cholesterol (mmol/L) | Baseline |
(n = 134) 5.3 (1.2) |
(n = 128) 5.4 (1.2) |
(n = 135) 5.5 (1.1) |
|||
| Change at 6 months |
0.06 (−0.06, 0.18) |
−0.13 (−0.25, −0.01) |
−0.17 (−0.29, −0.05) |
−0.18 (−0.39, 0.02) p = 0.1000 |
−0.23 (−0.43, −0.02) p = 0.026 |
−0.04 (−0.25, 0.17) p = 1.000 |
|
| Change at 12 months |
0.09 (−0.05, 0.23) |
−0.10 (−0.24, 0.04) |
−0.18 (−0.32, −0.04) |
−0.19 (−0.43, 0.05) p = 0.180 |
−0.28 (−0.52, −0.04) p = 0.018 |
−0.08 (−0.33, 0.16) p = 1.000 |
|
| LDLc cholesterol (mmol/ L) | Baseline |
(n = 134) 3.2 (0.9) |
(n = 126) 3.3 (0.9) |
(n = 134) 3.3 (1.0) |
|||
| Change at 6 months |
0.02 (−0.12, 0.15) |
−0.17 (−0.31, −0.03) |
−0.22 (−0.36, −0.09) |
−0.19 (−0.42, 0.05) p = 0.170 |
−0.24 (−0.47, −0.01) p = 0.040 |
−0.05 (−0.29, 0.18) p = 1.00 |
|
| Change at 12 months |
−0.02 (−0.16, 0.13) |
−0.10 (−0.24, 0.05) |
−0.30 (−0.45, −0.16) |
−0.08 (−0.33, 0.18) p = 1.00 |
−0.28 (−0.54, −0.04) p = 0.020 |
−0.21 (−0.46, 0.05) p = 0.150 |
|
| HDL cholesterol (mmol/ L) | Baseline d |
(n = 134) 1.5 (0.7, 3.5) |
(n = 127) 1.5 (0.8, 3.6) |
(n = 135) 1.47 (0.8, 3.0) |
|||
| Ratio of change at 6 months |
1.019 (0.993, 1.046) |
1.019 (0.992, 1.047) |
1.013 (0.987, 1.040) |
1 (0.955, 1.046) p = 1.000 |
0.994 (0.950, 1.039) p = 1.000 |
0.994 (0.949, 1.040) p = 1.000 |
|
| Ratio of change at 12 months |
1.053 (1.026, 1.082) |
1.061 (1.032, 1.090) |
1.049 (1.022, 1.077) |
1.006 (0.961, 1.053) p = 1.000 |
0.996 (0.951, 1.042) p = 1.000 |
0.99 (0.946, 1.037) p = 1.000 |
|
| Triglyceridesc (mmol/ L) | Baselined |
(n = 134) 1.12 (0.4, 3.3) |
(n = 127) 1.16 (0.4, 6.5) |
(n = 135) 1.20 (0.4, 5.8) |
|||
| Ratio of change at 6 months |
1.040 (0.985, 1.097) |
0.981 (0.929, 1.037) |
0.967 (0.916, 1.019) |
0.944 (0.859, 1.037) p = 0.420 |
0.930 (0.847, 1.020) p = 0.180 |
0.985 (0.897, 1.082) p = 1.000 |
|
| Ratio of change at 12 months |
1.051 (0.995, 1.111) |
0.971 (0.918, 1.027) |
0.954 (0.897, 1.008) |
0.924 (0.839, 1.107) p = 0.150 |
0.908 (0.826, 0.998) p = 0.045 |
0.982 (0.892, 1.081) p = 1.000 |
|
| Systolic blood pressure (mmHg) | Baseline |
(n = 137) 124.7 (19.8) |
(n = 137) 122.4 (20.8) |
(n = 137) 126.5 (18.5) |
|||
| 6 months | 1.3 (−1.0, 3.7) | −2.2 (−4.7, 0.2) | −4.1 (−6.2, −2.1) |
−4.1 (−7.9, −0.4) p = 0.023 |
−5.0 (−8.7, −1.3) p = 0.004 |
−0.8 (−4.6, 2.9) p = 1.000 |
|
| 12 months | 1.1 (−1.5, 3.8) | −1.3 (−3.7, 1.1) | −3.8 (−6.3, −1.3) |
−3.1 (−7.1, 0.9) p = 0.197 |
−4.4 (−8.4, −0.4) p = 0.025 |
−1.3 (−5.3, 2.7) p = 1.000 |
|
| 12 -min walk test—distance walked (m) | Baseline |
(n = 125) 927 (208) |
(n = 127) 901 (218) |
(n = 128) 928 (190) |
|||
| 6 months | 59 (39, 79) | 74 (52, 95) | 108 (86, 130) |
15 (−25, 45) p = 1.000 |
49 (15, 84) p = 0.002 |
34 (5, 75) p = 0.020 |
|
| 12 months | 92 (69, 11) | 103 (78, 127) | 131 (105, 156) |
11 (−34, 45) p = 1.000 |
39 (0, 79) p = 0.050 |
28 (−5, 73) p = 0.115 |
|
| FACT TOI-F scoree | Baseline |
(n = 132) 81.3 (17.1) |
(n = 128) 78.7 (18.8) |
(n = 131) 79.3 (18.2) |
|||
| 6 months | 3.3 (0.5, 6.0) | 4.1 (1.6, 6.7) | 7.4 (4.4, 10.3) |
0.8 (−4.4, 4.3) p = 1.000 |
3.1 (−1.0, 7.7) p = 0.194 |
3.3 (−0.9, 7.8) p = 0.176 |
|
| 12 months | 5.1 (2.5, 7.6) | 6.7 (4.0, 9.4) | 9.4 (6.6, 12.1) |
1.6 (−3.5, 4.7) p = 1.000 |
4.3 (−0.5, 7.5) p = 0.114 |
2.7(−1.2, 6.9) p = 0.266 |
|
| FACT TOI-ES scoree | Baseline |
(n = 130) 101.7 (15.6) |
(n = 128) 98.9 (16.5) |
(n = 130) 100.0 (15.1) |
|||
| 6 months | −0.4 (−2.7, 2.0) | 2.6 (0.6, 4.6) | 3.6 (1.1, 6.0) |
3.0 (−1.5, 5.9) p = 0.460 |
4.0 (−0.2, 7.1) p = 0.069 |
1.0 (−2.4, 5.0) p = 1.000 |
|
| 12 months | 0.9 (−1.4, 3.2) | 3.1 (0.9, 5.1) | 4.5 (2.1, 6.7) |
2.2 (−2.3, 4.9) p = 1.000 |
3.6 (−0.6, 6.6) p = 0.139 |
1.4 (−1.9, 5.3) p = 0.799 |
|
| FACT TOI-BC scoree | Baseline |
(n = 130) 66.0 (13.3) |
(n = 127) 64.1 (13.7) |
(n = 130) 65.3 (12.9) |
|||
| 6 months | 2.9 (1.0, 4.7) | 4.0 (2.3, 5.6) | 6.2 (4.2, 8.1) |
1.1 (−2.3, 3.5) p = 1.000 |
3.3 (0.2, 6.0) p = 0.029 |
2.2 (−0.39, 5.4) p = 0.113 |
|
| 12 months | 4.7 (2.9, 6.5) | 6.4 (4.5, 8.3) | 8.3 (6.6, 10.0) |
1.7 (−1.7,3.8) p = 1.000 |
3.6 (0.6, 6.1) p = 0.011 |
1.9 (−1.8, 6.4) p = 0.138 |
|
Mean (SD)
a ANCOVA, Mean (95% CI)
b ANCOVA with Bonferroni adjustment, mean (95% CI)
c Patients with fasting baseline values were included into summaries and analyses
d Geometric mean (range)
e FACT analysis: TOI = Trial outcome indicator for fatigue = PWB + FWB + FSS
TOI-ES = Trial outcome indicator for endocrine system = PWB + FWB + ESS
TOI-BC = Trial outcome indicator for breast cancer = PWB + FWB + BC
Quality of life: There were numerical improvements in the scores for QOL (FACT TOI for fatigue, endocrine symptoms and BC) in all three groups at 6 and 12 months. The improvement reached significance using the FACT TOI BC at 6 and 12 months for the community group compared with controls (both p < 0.05) but not the home group compared with controls (Table 4).
Changes in dietary intake and physical activity: All three groups reduced energy intake (Supplementary Table 2). The greatest numerical reductions were reported by the home group; mean (95% CI) difference in the home vs. control group was −153 (−239, −68) kcal/day (P = 0.001) at 6 months and −161 (−261, −61) kcal/day at 12 months (p < 0.001), whilst mean (95% CI) difference in energy intake in the community vs. control group was −82 (−185, + 20) kcal/day at 6 months (P = 0.166) and −107 (−205, −9) kcal/day at 12 months (P = 0.027). Reported levels of PA increased in all three groups over the year (Supplementary Table 2). At 12 months the community group had significantly greater increases compared to the control and home groups; mean (95% CI) difference community vs. control 119 (6, 230) minutes/week (P = 0.035) and mean (95% CI) difference community vs. home group 151 (36 to 265) minutes/week (P = 0.005).
Economic evaluation
Disaggregated and mean total health care costs and mean QALYs of the three interventions are reported in Supplementary Tables 3 and 4. The home group (£7737) and the community group (£7914) had reduced patient costs compared with the control group (£8547) mainly related to decreased usage of medications to treat treatment toxicity, e.g. granulocyte-colony stimulating factor (GCSF) used in chemotherapy patients who had become neutropenic, decreased accident and emergency visits and physiotherapy contacts. The three interventions had equivalent QALY scores of ~0.8. Comparing the difference in costs and difference in effects between the home and community groups gives an incremental cost-effectiveness ratio (ICER) of £9381.45. For a threshold of £20,000 per QALY there is a 52% probability that the community group is cost-effective; this increases to 60% for a £30,000 per QALY threshold (Supplementary Fig. 1).
Discussion
The three-month dietary and PA weight control programmes initiated soon after surgery produced sustained weight reduction in overweight women and prevented weight gain in normal-weight women during the 12 months of the study. Although equally effective for weight control, the community programme was more effective for increasing PA, whilst the home programme appeared more effective for dietary change and reducing energy intake. The greater PA in the community group probably accounts for the greater reductions in insulin, lipids33 and blood pressure,34 and improvements in fitness and QOL.35 Neither programme was effective for inducing weight loss or preventing weight gain amongst patients receiving chemotherapy.
Long term behaviour change is pivotal to the effectiveness of any weight control programme. Beneficial changes in diet and PA behaviours, weight, CVD disease biomarkers, and QOL were observed at 12 months, i.e. 9 months after completion of the initial 3-month phone and community programmes. Some evidence suggests that uptake of interventions and maintenance of behaviours within studies are greater when were commenced nearer to the time of diagnosis compared with those initiated later although there are no randomised trials comparing early versus later interventions.36 Few studies have assessed the maintenance of diet and PA behaviour change and weight loss beyond the end of the intervention.36 The respective maintained and continued weight loss in the home and supervised programmes between 6 and 12 months (i.e. 3–9 months after the intensive 12-week intervention) is an important finding. Both groups did receive minimal ongoing contact via booster calls at 4 and 9 months. This ongoing albeit minimal contact is likely to have contributed to their maintained lifestyle behaviour change. Average weight loss in the overweight/obese non-chemotherapy patients was 3 kg (4%) when assessed 9 months after the end of the active intervention. This compares favourably with reported 12-month weight loss at the end of a 12-month active intervention amongst women who joined the programmes 9–60 months after diagnosis summarised by Chlebowski and Reeves et al. which ranged from 3.7–5 kg (4–5%).10 This level of weight loss may be clinically important for BC patients. A large randomised trial of low-fat dietary intervention (Women’s Intervention Nutrition Study, WINS)37 reported a 24% reduction in relapse where women lost on average 2.3 kg (3%).
Home based home diet and PA programmes have been shown to be equivalent to face to face versions for weight loss amongst BC patients after adjuvant treatment,38 and amongst other patient groups.39,40 Home based mail and web programmes are effective for changing diet and weight, but they only have limited effects on PA as reported here.41 PA increases more within supervised programmes42 as these overcome common barriers including women’s concerns about safety and low self-efficacy for physical activity.43 The independent effects of PA on toxicity44 and possibly BC outcome45 mean that programmes need to impact on PA as well as diet and weight. Future trials should test home based programmes which include initial supervised PA sessions and more intensive self-monitoring and feedback, which have proven to successfully promote PA in home based CVD rehabilitation and heart failure programmes.46,47
Weight gain was observed in the normal weight but not the overweight controls which is consistent with previous reports in the literature.18 The weight control advice was effective for preventing these gains amongst patients who were not receiving chemotherapy. It is important to note that none of the normal-weight patients reduced weight to a BMI of < 18.5 kg/m2 and so did not require weight gain advice.
Chemotherapy patients were keen to enter the programmes, and were equally likely to attend the community sessions or receive calls, but slightly more likely to drop out. Both programmes appeared to confer some benefits to the chemotherapy patients in terms of reduced costs of toxicity related medication (e.g. GCSF) and accident and emergency admissions, but they were ineffective for weight control during chemotherapy and in the post-treatment phase up to 12 months. The limited success of home12,13 and group48 weight control programmes during adjuvant chemotherapy has been reported previously in BC patients, with the exception of an intensive dietary intervention which involved twice-weekly community cookery classes and group meals.49 However, Goodwin et al. reported that women who had previously received chemotherapy achieved comparable successful weight loss to women who had not previously received chemotherapy in a home-based phone weight loss programme initiated at a median of 9 months from diagnosis and at least 1 month after completion of adjuvant chemotherapy.50 Future studies should test modified, more achievable approaches amongst chemotherapy patients, e.g. intermittent energy restriction which is effective for weight loss in the non-cancer setting.51
The combined diet and PA community programme was the most cost-effective. There are few data on the cost-effectiveness of programmes amongst early BC patients.52 Two earlier studies failed to demonstrate the cost-effectiveness of PA only programmes during adjuvant treatments which were home53 or community54 based.
The strengths of this study include random allocation to the three groups, DXA assessment of body composition, and 12 months follow up. We have previously reported the good uptake to the trial and that our cohort is representative of newly diagnosed early-stage BC patients,32 whilst the low drop-out provides reliable follow up data without making assumptions about missing data. We tested 12-week programmes. The optimal length of programme for sustained behaviour change for weight loss is not known. Some guidelines advocate a minimum of 16 contacts over a 6-month period as used in the Diabetes Prevention Programme,55 whilst others advocate a minimum of 12 weeks.56 It is estimated to take 10 weeks to form a habit.57 Both of our groups involved weekly contacts. However, half of the contacts in the home-based group were mailings rather than direct patient contact, hence this group had reduced contact with their allocated dietitian which may have limited the effectiveness of the programme. The reduced effects on physical activity in the home-based group is likely to reflect the home-based modality rather than the level of contact. Poor collective results of home-based PA interventions have been reported amongst patients with BC regardless of the length of intensity of the intervention.58
Limitations include that the sample size may not be sufficiently powered for the subgroup analyses in chemotherapy and non-chemotherapy patients and the use of self-report rather than objective measurements of PA such as accelerometry. Our metrics for assessing adherence were based on retention to the study and attendance to classes and receipt of the calls. Future studies should evaluate more detailed adherence to the diet and PA prescriptions which would give a more meaningful evaluation of engagement with the programmes.
We have shown significant numbers of BC patients are interested and motivated to enter and adhere to home and community-based diet and PA weight control programmes soon after diagnosis. Lifestyle programmes in current oncology practice are mainly focussed at the end of active treatment.59,60 This has been identified as a time of need amongst patients,61 but it means that programmes are initiated after women may have already gained weight as a result of the psychological and physical effects of BC diagnosis and treatment. Research should focus on developing cost-effective interventions for women soon after diagnosis to utilise this potential teachable moment. Such programmes are likely to improve the future health of women affected by BC by reducing future weight-related illness and improving QOL. Ongoing randomised trials62 will inform the potential effectiveness of weight control programmes for improving BC specific outcomes for BC patients long after diagnosis and treatment.
Supplementary information
Acknowledgements
We thank the participants and the research teams in the recruiting centres.
Author contributions
M.H., A.H., N.B., J.W., A.C., and J.M. were involved in the design of the study. M.H., M.P., D.M., K.L., J.A., and J.W. performed data acquisition. J.M., M.H., D.M., M.P., S.H., and A.H. were involved in the statistical analysis and interpretation of data. N.B. enrolled patients. J.W., H.C., and E.L. undertook the health economic analysis. J.A. performed the interpretation of the radiological images. M.H., J.W., and J.A. wrote the draft manuscript. A.H. and M.P. helped with the manuscript editing. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the North West 8 Research Ethics Committee—Greater Manchester East, reference 08/H1013/45. Informed consent was obtained from all subjects and the study was performed in accordance with the Declaration of Helsinki.
Funding
This project was funded by the NIHR RFPB (PB-PG-0407-12313) and Prevent Breast Cancer Limited (Registered Charity Number: 1109839, award GA08-006) and supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Consent to publish
Not applicable.
Data availability
All datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Judith Adams
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-019-0522-6.
References
- 1.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J. Natl. Cancer Inst. 2015;107:djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nechuta S, Chen WY, Cai H, Poole EM, Kwan ML, Flatt SW, et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int. J. Cancer. 2016;138:2088–2097. doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutschler NS, Scholz C, Friedl TWP, Zwingers T, Fasching PA, Beckmann MW, et al. Prognostic impact of weight change during adjuvant chemotherapy in patients with high-risk early breast cancer: results from the ADEBAR study. Clin. Breast Cancer. 2018;18:175–183. doi: 10.1016/j.clbc.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Guenancia Charles, Lefebvre Annick, Cardinale Daniela, Yu Anthony F., Ladoire Sylvain, Ghiringhelli François, Zeller Marianne, Rochette Luc, Cottin Yves, Vergely Catherine. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Oncology. 2016;34(26):3157–3165. doi: 10.1200/JCO.2016.67.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su HI, Sammel MD, Springer E, Freeman EW, DeMichele A, Mao JJ. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res. Treat. 2010;124:205–211. doi: 10.1007/s10549-010-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips SM, McAuley E. Associations between self-reported post-diagnosis physical activity changes, body weight changes, and psychosocial well-being in breast cancer survivors. Support Care Cancer. 2015;23:159–167. doi: 10.1007/s00520-014-2346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–1328. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renehan AG, Harvie M, Cutress RI, Leitzmann M, Pischon T, Howell S, et al. How to manage the obese patient with cancer. J. Clin. Oncol. 2016;34:4284–4294. doi: 10.1200/JCO.2016.69.1899. [DOI] [PubMed] [Google Scholar]
- 10.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes. Rev. 2014;15:749–768. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 11.Playdon M, Thomas G, Sanft T, Harrigan M, Ligibel J, Irwin M. Weight loss intervention for breast cancer survivors: a systematic review. Curr. Breast Cancer Rep. 2013;5:222–246. doi: 10.1007/s12609-013-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin. Breast Cancer. 2008;8:70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 13.Djuric Z, Ellsworth JS, Weldon AL, Ren J, Richardson CR, Resnicow K, et al. A diet and exercise intervention during chemotherapy for breast cancer. Open Obes. J. 2011;3:87–97. doi: 10.2174/1876823701103010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarini A, Pasanisi P, Raimondi M, Gargano G, Bruno E, Morelli D, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast Cancer Res. Treat. 2012;135:581–589. doi: 10.1007/s10549-012-2184-4. [DOI] [PubMed] [Google Scholar]
- 15.James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI. Obesity in breast cancer-what is the risk factor? Eur. J. Cancer. 2015;51:705–720. doi: 10.1016/j.ejca.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Liu LN, Lin YC, Miaskowski C, Chen SC, Chen ML. Association between changes in body fat and disease progression after breast cancer surgery is moderated by menopausal status. BMC Cancer. 2017;17:863. doi: 10.1186/s12885-017-3869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J. Clin. Oncol. 2014;5:272–282. doi: 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluethmann Shirley M., Basen-Engquist Karen, Vernon Sally W., Cox Matthew, Gabriel Kelley Pettee, Stansberry Sandra A., Carmack Cindy L., Blalock Janice A., Demark-Wahnefried Wendy. Grasping the ‘teachable moment’: time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psycho-Oncology. 2015;24(10):1250–1257. doi: 10.1002/pon.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013;110:1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell A, Stevinson C, Crank H. The BASES expert statement on exercise and cancer survivorship. J. Sports. Sci. 2012;30:949–952. doi: 10.1080/02640414.2012.671953. [DOI] [PubMed] [Google Scholar]
- 24.Borg G. Borg’s perceived exertion and pain scales. Human Kinetics, Illinois, USA, 1998
- 25.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol. Health. 2011;26:1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Stevens D, Elpern E, Sharma K, Szidon P, Ankin M, Kesten S. Comparison of hallway and treadmill six-minute walk tests. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 1):1540–1543. doi: 10.1164/ajrccm.160.5.9808139. [DOI] [PubMed] [Google Scholar]
- 28.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J. Clin. Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 29.Lowther M, Mutrie N, Loughlan C, McFarlane C. Development of a Scottish physical activity questionnaire: a tool for use in physical activity interventions. Br. J. Sports Med. 1999;33:244–249. doi: 10.1136/bjsm.33.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ. 1996;5:141–154. doi: 10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–452. doi: 10.1002/hec.864. [DOI] [PubMed] [Google Scholar]
- 32.Pegington Mary, Adams Judith E., Bundred Nigel J., Campbell Anna M., Howell Anthony, Howell Sacha J., Speed Shaun, Wolstenholme Jane, Harvie Michelle N. Recruitment to the “Breast—Activity and Healthy Eating After Diagnosis” (B-AHEAD) Randomized Controlled Trial. Integrative Cancer Therapies. 2017;17(1):131–137. doi: 10.1177/1534735416687850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18-65 years old) who are overfat, or obese; systematic review and meta-analysis. J. Diabetes Metab. Disord. 2015;14:31. doi: 10.1186/s40200-015-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–316. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]
- 35.van Gemert WA, van der Palen J, Monninkhof EM, Rozeboom A, Peters R, Wittink H, et al. Quality of life after diet or exercise-induced weight loss in overweight to obese postmenopausal women: the SHAPE-2 randomised controlled trial. PLoS One. 2015;10:e0127520. doi: 10.1371/journal.pone.0127520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. J. Cancer Surviv. 2013;7:74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 38.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J. Clin. Oncol. 2016;34:669–676. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev. Med. 2017;100:194–207. doi: 10.1016/j.ypmed.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutton GR, Laitner MH, Perri MG. Lifestyle interventions for cardiovascular disease risk reduction: a systematic review of the effects of diet composition, food provision, and treatment modality on weight loss. Curr. Atheroscler. Rep. 2014;16:442. doi: 10.1007/s11883-014-0442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goode AD, Lawler SP, Brakenridge CL, Reeves MM, Eakin EG. Telephone, print, and web-based interventions for physical activity, diet, and weight control among cancer survivors: a systematic review. J. Cancer. Surviv. 2015;9:660–682. doi: 10.1007/s11764-015-0442-2. [DOI] [PubMed] [Google Scholar]
- 42.van WH, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J. Clin. Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 43.Henriksson A, Arving C, Johansson B, Igelstrom H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient. Educ. Couns. 2016;99:1220–1226. doi: 10.1016/j.pec.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Lipsett A, Barrett S, Haruna F, Mustian K, O’Donovan A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: A systematic review and meta-analysis. Breast. 2017;32:144–155. doi: 10.1016/j.breast.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta. Oncol. 2015;54:635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 46.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst. Rev. 2017;6:CD007130. doi: 10.1002/14651858.CD007130.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Back M, Jivegard L, Johansson A, Nordanstig J, Svanberg T, Adania UW, et al. Home-based supervised exercise versus hospital-based supervised exercise or unsupervised walk advice as treatment for intermittent claudication: a systematic review. J. Rehabil .Med. 2015;47:801–808. doi: 10.2340/16501977-2012. [DOI] [PubMed] [Google Scholar]
- 48.Goodwin P, Esplen MJ, Butler K, Winocur J, Pritchard K, Brazel S, et al. Multidisciplinary weight management in locoregional breast cancer: results of a phase II study. Breast Cancer Res. Treat. 1998;48:53–64. doi: 10.1023/A:1005942017626. [DOI] [PubMed] [Google Scholar]
- 49.Villarini A, Pasanisi P, Raimondi M, Gargano G, Bruno E, Morelli D, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast Cancer Res. Treat. 2012;135:581–589. doi: 10.1007/s10549-012-2184-4. [DOI] [PubMed] [Google Scholar]
- 50.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J. Clin. Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 51.Harvie Michelle, Howell Anthony. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects—A Narrative Review of Human and Animal Evidence. Behavioral Sciences. 2017;7(4):4. doi: 10.3390/bs7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng KKF, Lim YTE, Koh ZM, Tam WWS. Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst. Rev. 2017;8:CD011152. doi: 10.1002/14651858.CD011152.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res. Treat. 2010;124:163–175. doi: 10.1007/s10549-010-1126-2. [DOI] [PubMed] [Google Scholar]
- 54.May AM, Bosch MJ, Velthuis MJ, van der Wall E, Steins Bisschop CN, Los M, et al. Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and colon cancer undergoing adjuvant chemotherapy: the randomised PACT study. BMJ Open. 2017;7:e012187. doi: 10.1136/bmjopen-2016-012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.NICE. Obesity: identification, assessment and management Clinical guideline [CG189. National Institute of Clinical Excellence [serial online] 2014; Accessed 28.3.2019.
- 57.Gardner B, Lally P, Wardle J. Making health habitual: the psychology of ‘habit-formation’ and general practice. Br. J. Gen. Pract. 2012;62:664–666. doi: 10.3399/bjgp12X659466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013-2018): We still haven’t found what we’re looking for. Cancer Treat. Rev. 2018;69:188–203. doi: 10.1016/j.ctrv.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin. J. Oncol. Nurs. 2014;18(Suppl:):15–22. doi: 10.1188/14.CJON.S1.15-22. [DOI] [PubMed] [Google Scholar]
- 60.Department of Health. Living With and Beyond Cancer: Taking Action to Improve Outcomes. 2-3-2013. 7-1-2016.
- 61.Maria Hewitt, Sheldon Greenfield, and Ellen Stovall, (eds). From cancer patient to cancer survivor: Lost in transition Committee on Cancer Survivorship: Improving Care and Quality of Life The National Academies Press Washington, D.C.
- 62.Goodwin PJ. Obesity and breast cancer outcomes: how much evidence is needed to change practice? J. Clin. Oncol. 2016;34:646–648. doi: 10.1200/JCO.2015.64.7503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets used and analysed during the current study are available from the corresponding author on reasonable request.