Abstract
Background
Signet ring cell carcinoma (SRCC) is a rare subtype of colorectal cancer (CRC). The aim of this study was to characterise the genomic alterations and outcomes of SRCC.
Methods
Medical records of metastatic CRC (mCRC) patients whose tumours were evaluated by NGS analysis were reviewed. SC-mCRC were classified into two groups: SRCC (>50% signet ring cells) and adenocarcinoma (AC) with SC component (≤50% signet ring cells).
Results
Six hundred and sixty-five mCRC patients were included. Of the 93 mCRC cases with SC features, 63 had slides for review. Of those 63 cases, 35 were confirmed SRCC, and 28 were AC with SC component. Compared with AC group, KRAS and PIK3CA mutations (mts) were found in only 11% (OR: 0.13) and 3% (OR: 0.15) of SRCC cases, respectively. In contrast to the 44% rate of APC mts in AC group, only 3% of SRCC patients had APC mts (OR = 0.04).
Conclusions
SRCC has distinct molecular features, including low rates of KRAS, PIK3CA and APC mts. Further study to identify activation pathways and potential therapeutic targets are needed.
Subject terms: Colorectal cancer, Cancer genomics
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer, and the fourth leading cause of cancer death in the world.1 CRC has a variety of histologies, including adenocarcinoma (AC), and rare histologies, such as adenosquamous carcinomas, squamous cell carcinomas, neuroendocrine carcinomas, spindle cell carcinomas and undifferentiated carcinomas.2 Signet ring cell colorectal cancer (SRCC) is a rare subtype of colorectal adenocarcinoma that accounts for 1–2.4% of all CRC.3,4 The histology of SRCC is distinguished from typical adenocarcinoma by an excess amount of intracellular mucin that displaces the nucleus, which results in the formation of signet ring cell (SC) features. SRCC is defined by a greater than 50% presence of signet ring cells, while cases with less than or equal to 50% presence of signet ring cells are noted to have signet ring features without a formal SRCC designation.5 In the previous studies, SRCC has been associated with younger age, advanced tumour stage at presentation and lymph node metastasis,2,6–8 and SRCC has significantly poorer prognosis compared to that of adenocarcinomas.6,9–14 Several studies have suggested a higher rate of microsatellite instability and BRAF gene mutation in SRCC.7,15–17 However, the sample sizes in those studies were generally small, and most evaluated only single gene mutation. A more extensive clinical and molecular characterisation of this subset is needed. Accordingly, the aim of this study was to characterise the genomic alterations, clinicopathological characteristics and outcomes of SRCC.
Methods
This single centre, retrospective cohort study evaluated patients with metastatic CRC (mCRC) who were enrolled in the Assessment of Targeted Therapies Against Colorectal Cancer (ATTACC) program at The University of Texas MD Anderson Cancer Center (UTMDACC) between 13 February 2009 and 18 November 2015, as described previously.18 Additional mCRC cases having signet ring cell features between 1 March 1994 and 31 August 2015 were extracted from the tumour registry at UTMDACC. The protocol for this study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All patients provided written informed consent prior to sequencing of their tumours according to the institutional guideline. The primary objective of this study was to determine the molecular characteristics of SRCC. The secondary objectives were to identify significant associations between SRCC and various clinicopathologic characteristics, and to evaluate their prognostic impact on overall survival (OS).
Pathologic evaluation
Slides from patients whose pathology report documented the presence of signet ring cell histology were obtained and reviewed to confirm the percentage of signet ring cells. Haematoxylin and eosin-stained slides of the tumours were reviewed by an experienced gastrointestinal pathologist (SH). Tumours were classified according to the proportion of signet ring cells, with ≥50% defining SRCC, and ≤50% defining AC with SC component.
Clinical characteristics
As described previously,18 demographic and clinical information, including age, gender, race, primary tumour site, date of diagnosis with stage IV disease, date of last follow-up and date of death, were collected from a review of patient medical records. Right-sided colon cancer was defined as cancer in the region from the cecum to the splenic flexure, while left-sided colon cancer was defined as cancer in the region from the descending colon through the sigmoid colon, and the rectum was considered a separate site. Staging was performed using the American Joint Committee on Cancer/Union for International Cancer Control TMN staging system (version 7, 2010). OS was defined as the interval between the date of diagnosis of metastatic disease and the date of death from any cause. Patients alive at the time of analysis were censored at their last known follow-up date
Molecular characterisation
As described previously,18 DNA was extracted from paraffin-embedded formalin-fixed tumour tissue. Samples were evaluated using a next-generation sequencing platform with 46- or 50-gene panels for the detection of frequently reported point mutations in human malignancies. Complete details of exon and codon coverage in all genes were previously reported.19 DNA testing was performed in a Clinical Laboratory Improvement Amendments-certified molecular diagnostics laboratory that determined the effective lower limit of detection (analytical sensitivity) for single nucleotide variations to be in the range of 5% (one mutant allele in the background of nineteen wild-type alleles) to 10% (one mutant allele in the background of nine wild-type alleles). Details relating to the codons and exons tested are shown in Supplementary Table 1.
Determine of MMR status
MMR status was determined by analysis of MMR protein expression by immunohistochemistry (IHC) or microsatellite instability (MSI) testing. Deficient mismatch repair (dMMR) was defined as the presence of either high-level MSI (MSI-H) or loss of MMR protein expression. Proficient mismatch repair (pMMR) was defined as the presence of either microsatellite stable (MSS)/low-level MSI (MSI-L) or the presence of normal MMR protein expression. Complete details of IHC analysis of MMR expression and microsatellite instability (MSI) testing were previously published.18
CpG Island Methylation Phenotype (CIMP) panel methylation analysis
As described previously,20 DNA extracted from formalin-fixed, paraffin-embedded tissue was treated with bisulfite to convert unmethylated cytosine to uracil. PCR amplification of unmethylated and methylated MINT1, MINT2 and MINT31 loci, and promoter sequences of p14, p16 and hMLH1 genes was performed, and methylation status was assessed by pyrosequencing. The tumour was considered CIMP High if at least 40% of the markers tested show methylation, and CIMP Low if <40% of the tested markers show methylation.
Statistical analysis
Patient characteristics were compared using descriptive statistics. Pearson’s Chi-square test or Fisher’s exact test was applied to evaluate associations between SRCC and clinicopathological variables, and binary logistic regression was used to calculate odds ratios. Survival was analysed using the Kaplan–Meier method, and comparisons between groups were made using the log-rank test. Cox proportional hazard models were used to estimate the combined influence of clinical and pathologic feature on survival. Median follow-up time was calculated using the reverse Kaplan–Meier method. A two-sided level of significance of 0.05 was applied for all statistical tests. Calculations were performed using SPSS Statistics version 23.0 software (IBM Corp., Armonk, NY, USA).
Results
Six hundred and sixty-five mCRC patients with NGS molecular data were included. Of the 93 mCRC cases with SC features, 63 had available slides for confirmatory review. Of those 63 cases, 35 were confirmed SRCC, and 28 were considered AC with SC component (Supplementary Fig. 1).
The frequency of confirmed SRCC and AC with SC components in the entire cohort was 5.5% (35/635) and 4.4% (28/635), respectively. However, if analysis only the data form ATTACC database, 1.3% (8/604) and 2.3% (14/604) were classified as SRCC and AC with SC components, respectively. The median age was 55 years (range: 15–85), the ratio of males to females was 1.29, and the majority of patients were Caucasian. Patient and tumour characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population, n (%)
| Variable | n | % |
|---|---|---|
| No. of patients | 665 | 100 |
| Age (years), median (range) | 55 (15–85) | |
| Gender | ||
| Female | 291 | 43.8 |
| Male | 374 | 56.2 |
| Age | ||
| 15–39 | 70 | 10.5 |
| 40–49 | 153 | 23.0 |
| ≥50 | 442 | 66.5 |
| Site | ||
| Right-sided | 243 | 36.5 |
| Left-sided | 274 | 41.2 |
| Rectum | 142 | 21.4 |
| No data | 6 | 0.9 |
| Race | ||
| Asian | 34 | 5.1 |
| Black | 60 | 9.0 |
| Hispanic | 61 | 9.2 |
| White | 503 | 75.6 |
| No data | 7 | 1.1 |
| Histology | ||
| Adenocarcinama (AC) | 572 | 86.0 |
| Signet ring cell features (n = 93) | ||
| Confirmed SRCC | 35 | 5.3 |
| AC with SC | 28 | 4.2 |
| No slide reviewed | 30 | 4.5 |
| Differentiated | ||
| Well | 1 | 0.2 |
| Moderately | 449 | 67.5 |
| Poorly | 209 | 31.4 |
| Not available | 6 | 0.9 |
| Liver metastasis | ||
| No | 201 | 30.2 |
| Yes | 464 | 69.8 |
| Lung metastasis | ||
| No | 262 | 39.4 |
| Yes | 403 | 60.6 |
| Peritoneal metastasis | ||
| No | 400 | 60.2 |
| Yes | 265 | 39.8 |
SRCC signet ring cell colorectal cancer, AC with SC adenocarcinoma with signet ring cell component, AC adenocarcinoma
Clinicopathologic features
Compared with the AC group, SRCC was significantly more commonly found in patients with right-sided tumour (odds ratio [OR]: 3.4, p = 0.001), poorly differentiated tumour (p < 0.001) or peritoneal metastasis (OR: 9.8, p < 0.001). In contrast, SRCC was significantly less frequently found in cases with liver metastasis (OR: 0.1, p < 0.001) or lung metastasis (OR: 0.1, p < 0.001). Patients with SRCC were 2.4-fold more likely to be diagnosed before the age of 40 (p = 0.04) (Table 2).
Table 2.
Clinical characteristics of study population by histology (only reviewed slide cases; n = 635)
| Variable | Histology | p-value* | |||||
|---|---|---|---|---|---|---|---|
| SRCC | % | AC with SC | % | AC | % | ||
| Gender | |||||||
| Female | 18 | 51.4 | 14 | 50.0 | 245 | 42.8 | 0.32 |
| Male | 17 | 48.6 | 14 | 50.0 | 327 | 57.2 | |
| Age | |||||||
| 15–39 | 7 | 20.0 | 2 | 7.1 | 53 | 9.3 | 0.10 |
| 40–49 | 9 | 25.7 | 6 | 21.4 | 136 | 23.8 | |
| ≥50 | 19 | 54.3 | 20 | 71.4 | 383 | 67.0 | |
| Site (n = 602) | |||||||
| Right-sided | 22 | 62.9 | 12 | 42.9 | 189 | 33.3 | 0.001 |
| Left-sided | 13 | 37.1 | 16 | 57.1 | 378 | 66.7 | |
| Race (n = 602) | |||||||
| Asian | 1 | 2.9 | 0 | 0.0 | 32 | 5.6 | 0.43 |
| Black | 4 | 11.8 | 2 | 7.1 | 53 | 9.3 | |
| Hispanic | 2 | 5.9 | 2 | 7.1 | 53 | 9.3 | |
| White | 27 | 79.4 | 24 | 85.7 | 430 | 75.7 | |
| Differentiated (n = 601) | |||||||
| Well-moderately | 0 | 0.0 | 0 | 0.0 | 449 | 79.3 | <0.001 |
| Poorly | 35 | 100 | 28 | 100 | 117 | 20.7 | |
| Liver metastasis | |||||||
| No | 29 | 82.9 | `15 | 53.6 | 132 | 23.1 | <0.001 |
| Yes | 6 | 17.1 | 13 | 46.4 | 440 | 76.9 | |
| Lung metastasis | |||||||
| No | 29 | 82.9 | 22 | 78.6 | 184 | 32.2 | <0.001 |
| Yes | 6 | 17.1 | 6 | 21.4 | 388 | 67.8 | |
| Peritoneal metastasis | |||||||
| No | 6 | 17.1 | 7 | 25.0 | 383 | 67.0 | <0.001 |
| Yes | 29 | 82.9 | 21 | 75.0 | 189 | 33.0 | |
| MMR status (n = 472) | |||||||
| pMMR | 29 | 87.9 | 19 | 90.5 | 404 | 95.1 | 0.10 |
| dMMR | 4 | 12.1 | 2 | 9.5 | 21 | 4.9 | |
| CIMP (n = 215) | |||||||
| CIMP-L/neg | 2 | 66.7 | 5 | 71.4 | 175 | 75.1 | –a |
| CIMP-H | 1 | 33.3 | 2 | 28.6 | 58 | 24.9 | |
The Bold values are statistically significant
A p-value < 0.05 indicates statistical significance
SRCC signet ring cell colorectal cancer, AC with SC adenocarcinoma with signet ring cell component, AC adenocarcinoma
*p-value for SRCC compared with AC
aNo statistical analysis due to small sample size
Molecular characteristics
Two hundred and six cases were tested with the 46-gene panel while 429 cases were tested with the 50-gene panel. Details relating to gene mutation frequencies in the SRCC, AC with SC component, and AC groups are shown in Fig. 1. Compared with the AC group, SRCC was commonly found with KRAS wild-type (wt) (OR: 7.7, 95% CI: 2.7–22.0; p < 0.001), APC wt (OR: 26.8, 95% CI: 3.6–196.9; p = 0.001) and PIK3CA wt (OR: 6.7, 95% CI: 0.9–49.9; p = 0.06). No significant association was observed between SRCC and MSI, NRAS, BRAF, SMAD4, TP53 or FBXW7 status. No difference in gene mutation detection was noted between the two gene panels except in APC gene (46.6% in 50-gene panel vs 29.1% in 46-gene-panel, p < 0.001) (Supplementary Table 2). AC with SC component trended to have an intermediate prevalence of mutation in KRAS, APC and FBXW7 between SRCC and AC group (Fig. 1).
Fig. 1.
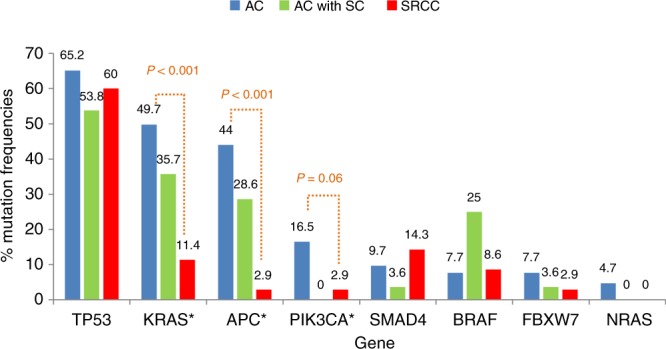
AC molecular characteristics of adenocarcinoma, AC with SC adenocarcinoma with signet ring cell component and SRCC signet ring cell colorectal cancer
Patient outcomes
The median follow-up time was 27 months. Univariate analysis by Kaplan–Meier survival analysis and log-rank test was performed using several factors, including age, primary tumour site, histological grade, histological type, KRAS, BRAF, PIK3CA and MSI status. Factors found to be significantly associated with worse OS were age 15–39 years (p = 0.002), right-sided tumour (p < 0.001), poor differentiation (p < 0.001), signet ring cell feature (p < 0.001), KRAS mutation (p = 0.013), BRAF mutation (p < 0.001) and pMMR (p = 0.016). Patients with SRCC tumours had significantly worse OS than patients with AC-mCRC (median OS: 16.4 months, 95% CI: 11.3–21.5 vs. median OS 47.2 months, 95% CI: 43.6-50.9, respectively; p < 0.001) (Table 3, Fig. 2).
Table 3.
Survival analysesa
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Median survival (mo) | 95% CI | p-value | n | HR | 95% CI | p-value | |
| Age (n = 662) | ||||||||
| 15–39 years | 70 | 36.8 | 27.0–46.6 | 0.002 | 52 | 1.57 | 0.98–2.53 | 0.060 |
| 40–49 years | 152 | 41.2 | 32.4–49.9 | 123 | 1.34 | 0.97–1.86 | 0.077 | |
| ≥50 years | 440 | 46.2 | 42.2–50.2 | 293 | Ref. | |||
| Site (n = 656) | ||||||||
| Right-sided | 242 | 35.7 | 30.6–40.8 | <0.001 | 163 | 1.28 | 0.94–1.74 | 0.12 |
| Left-sided | 414 | 48.8 | 44.9–52.7 | 305 | Ref. | |||
| Differentiated (n = 656) | ||||||||
| Well-moderately | 450 | 48.5 | 44.5–52.4 | <0.001 | 331 | Ref. | ||
| Poorly | 206 | 31.6 | 25.0–38.1 | 137 | 1.35 | 0.96–1.90 | 0.088 | |
| KRAS (n = 661) | ||||||||
| Wild-type | 358 | 48.2 | 43.1–53.4 | 0.013 | 259 | Ref. | ||
| Mutant | 303 | 40.7 | 35.1–46.4 | 209 | 1.51 | 1.17–1.95 | 0.002 | |
| BRAF (n = 662) | ||||||||
| Wild-type | 602 | 45.7 | 42.1–49.3 | <0.001 | 425 | Ref. | ||
| Mutant | 60 | 35.9 | 15.8–56.0 | 43 | 1.86 | 1.20–2.89 | 0.005 | |
| PIK3CA (n = 658) | ||||||||
| Wild-type | 560 | 45.6 | 41.6–49.6 | 0.111 | ||||
| Mutant | 98 | 42.4 | 33.6–51.1 | |||||
| MMR status (n = 498) | ||||||||
| Proficient | 470 | 44.8 | 40.3–49.4 | 0.016 | 442 | Ref. | 1.20–3.90 | 0.010 |
| Deficient | 28 | 35.9 | 8.5–63.4 | 26 | 2.17 | |||
| Histology (n = 633) | ||||||||
| AC | 572 | 47.2 | 43.6–50.9 | <0.001 | 416 | Ref. | ||
| AC with SC | 26 | 19.3 | 10.7–27.8 | 19 | 2.63 | 1.30–5.33 | 0.007 | |
| SRCC | 35 | 16.4 | 11.3–21.5 | 33 | 3.11 | 1.73–5.60 | <0.001 | |
The Bold values are statistically significant
A p-value < 0.05 indicates statistical significance
CI confidence interval, HR hazard ratio, Ref. reference, SRCC signet ring cell colorectal cancer, AC with SC adenocarcinoma with signet ring cell component, AC adenocarcinoma
aOnly conducted among patients with available data
Fig. 2.
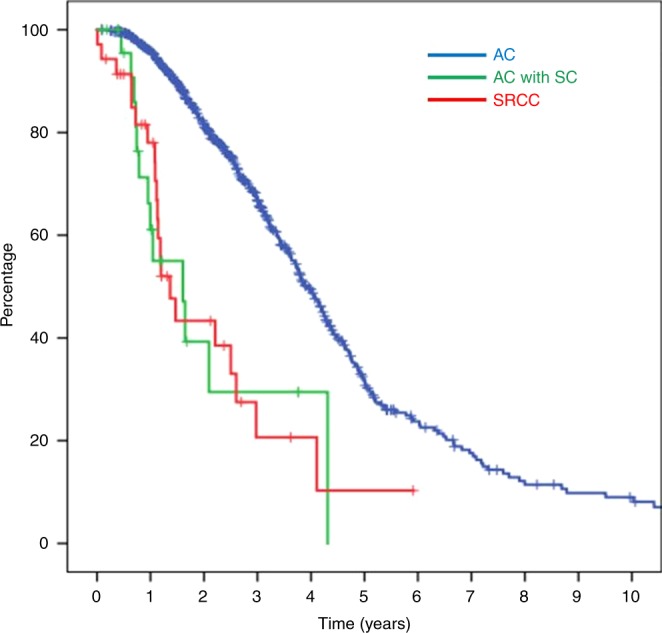
Kaplan–Meier survival curve of CRC patients according to signet ring cell histology. Patients with SRCC had significantly worse OS (median overall survival [OS]: 16.4 mo, 95% confidence interval [CI]: 11.3–21.5) than patients with AC (median OS: 47.2 mo, 95% CI: 43.6–50.9) (p < 0.001)
Multivariate Cox proportional hazard regression analysis revealed KRAS mutation (HR: 1.51, 95% CI: 1.17–1.95; p = 0.002), BRAF mutation (HR: 1.86, 95% CI: 1.20–2.89; p = 0.005) and pMMR (HR: 2.17, 95% CI: 1.20–3.90; p = 0.010) as independent predictors of worse outcome. After adjusting for all of these features, SRCC remained a significant prognostic factor for poor OS (HR: 3.11, 95% CI: 1.73–5.6; p < 0.001) (Table 3).
Discussion
This study identified the molecular characteristics of SRCC, which is a rare subtype of colorectal adenocarcinoma. We demonstrated associations between SRCC and KRASwt, PIK3CAwt and APCwt. This study also confirmed that SRCC is associated with young onset, right-sided tumour, peritoneal metastasis and poor outcome.
Adenocarcinoma is the most common histologic type of CRC, accounting for more than 90% of CRC cases. Mucinous adenocarcinoma (MAC) and SRCC are less commonly observed subtypes, with MAC accounting for 10% of cases, and SRCC accounting for 1% of cases.8,9,21–23 SRCC is defined by the presence of >50% of tumour cells with intracytoplasmic mucin, whereas MAC is defined as carcinoma with >50% of the tumour volume showing extracellular mucin.24 Due to the rarity of this subtype and the difficulties associated with characterising it molecularly, genomic alteration data in SRCC are scarce. A summary of previously reported molecular alterations in SRCC are provided in Table 4.
Table 4.
Clinical studies that investigating molecular features, MSI-H and CIMP-H in signet ring cell colorectal cancer
| Study | n | Stage | Histology | KRAS (n, %) | NRAS (n, %) | BRAF (n, %) | PIK3CA (n, %) | FBXW7 (n, %) | APC (n, %) | TP53 (n, %) | SMAD4 (n, %) | MSI-H (n, %) | CIMP-H (n, %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | 35 | IV | Confirmed ≥ 50% SC | 4/35 (11.4%) | 0/35 (0.0%) | 3/35 (8.6%) | 1/35 (2.9%) | 1/35 (2.9%) | 1/35 (2.9%) | 21/35 (60.0%) | 5/35 (14.3%) | 4/33 (12.1%) | 1/3 (33.3%) |
| Kawabata Y, 199924 | 10 | II–IV | Not confirmed | 1/9 (11.0%) | – | – | – | – | – | – | – | 3/10 (30.0%) | – |
| Kakar S, 20054 | 45 | I–IV | Confirmed ≥ 50% SC | – | – | – | – | – | – | – | – | 12/45 (26.7%) | – |
| Ogino S, 200625 | 9 | NA | Confirmed ≥ 50% SC | 0/8 (0.0%) | – | 2/9 (22.0%) | – | – | – | – | – | 2/8 (25.0%) | – |
| Kakar S, 201217 | 33 | I–IV | Confirmed ≥ 50% SC | 16/30 (53.3%) | – | 9/27 (33.3%) | – | – | – | – | – | 8/33 (24.2%) | 16/33 (48.5%) |
| Hartman DJ, 201316 | 53 | I–IV | Confirmed ≥ 50% SC | – | – | 16/50 (32.0%) | – | – | – | – | – | 23/53 (43.4%) | – |
| Inamura K, 201515 | 20 | I–IV | Confirmed ≥ 50% SC | 1/17 (5.9%) | – | 6/17 (35.3%) | 1/16 (6.2%) | – | – | – | – | 5/17 (29.4%) | 5/17 (29.4%) |
| Nitsche U, 201612 | 160 | I–IV | Not confirmed | – | – | – | – | – | – | – | – | 1/5 (20.0%) | – |
| Wei Q, 20167 | 39 | I–IV | Confirmed ≥ 50% SC | 5/33 (15.2%) | – | 1/33 (3.0%) | – | – | – | – | – | – | – |
| Yalcin S, 201728 | 9 | II–IV | Confirmed ≥ 50% SC | – | – | 4/9 (36.4%) | – | – | – | – | – | – | – |
| Nam JY, 201827 | 5 | NA | Confirmed > 50% SC | 2/5 (40.0%) | 0/5 (0.0%) | – | 0/5 (0.0%) | 1/5 (20.0%) | 1/5 (20.0%) | 2/5 (40.0%) | 1/5 (20.0%) | – | – |
Our study identified a low rate of KRASmt (11.4%) in SRCC, which is comparable to the rates reported in many, but not all previous studies of SRCC.7,17,25–28 However, and importantly, the larger sample size in our study, our attention to confirmation of the histologic diagnosis, and use of NGS in a clinical lab should influence increased confidence in our results. The rate of MSI-H in this study was lower than previously reported in SRCC.4,12,15–17,25,26,29 This is likely due to the fact that our population was limited to stage IV disease, which normally has lower rate MSI-H compared to earlier stage disease.
Previous studies by Kakar S et al.17 and Inamura K et al.15 found BRAF V600E mutation and CIMP-positive status to be more common in SRCC, and proposed that SRCC might be related to the serrated pathway, based on the increased prevalence of BRAF V600Emt and CIIMP-positive status in a majority of serrated polyps.30 However, in metastatic disease, the findings of our study suggest that this association may not be as clear, and suggests the involvement of alternate carcinogenesis pathways.
Phosphatidylinositol-4,5-biphosphonate 3-kionase (PIK3CA) mutations have been reported in 10–20% of all CRC.31 However, no detectable PIK3CA mutation was found in SRCC in this study, which is consistent with the findings of Inamura et al. who reported a prevalence of only 6.2% (1/16 cases) in SRCC.15 Adenomatous polyposis coli (APC) mutations are the most commonly acquired mutation in sporadic colon cancer, and are considered the initial genetic alteration in CRC tumorigenesis.32 Interestingly, our study found APCmt in only 3% of SRCC compared to 44% in AC group.
In the present study, we also reported the gene mutation frequencies in AC with SC component mCRC. Interestingly, the frequencies in most of the genes were similar to those observed in non-SC mCRC, while the frequencies in the other genes were varied between those observed in SRCC and those observed in non-SC mCRC (Fig. 2). This could be influenced by mixed component of SRCC and conventional adenocarcinoma during the tissue selection process. It is, therefore, strongly encouraged to define the patient as either SRCC or AC with signet ring cell component.
When compared with conventional adenocarcinoma, SRCC has distinct clinicopathological characteristics. SRCC was reported to be predominately observed in younger onset group (especially in patients aged less than 40 years), and to be associated with right-sided tumour, advanced stage at presentation, and poor prognosis.8–10,12,33–35 The reported 5-year survival in the literature was 28.6–33%,17,21 but only 4.5% in stage IV disease.21 Peritoneal carcinomatosis is the most common site of metastasis.3 In our study, we found SRCC to be more commonly found in younger aged patients (especially age 15–39 years), right-sided tumour, poorly differentiated histology, more frequently with peritoneal metastasis over lung or liver metastasis and significantly inferior OS compared with non-SRCC tumours. We also found the worse prognosis of SC histology to be similar between SRCC and AC with SC component. We, therefore, conclude that any presence of signet ring cells of any proportion in CRC leads to poorer clinical outcomes.
This study has some mentionable limitations. First and consistent with the retrospective nature of this study, some patient data may have been missing or incomplete. Second, the data included in this study was from a single centre. Third, the sequencing panels that were used were limited to hotspot regions of several tumour suppressor genes. Therefore, the presence of other mutations outside of these regions cannot be excluded. Finally, the small number of included samples due to the rarity of SRCC may have given our study insufficient statistical power to identify all significant associations and differences. However, to the best of our knowledge, this is the largest study to evaluate the molecular profiles of SRCC. Further study to determine the key mechanism of tumour development is needed to improve treatments and patient outcomes.
Conclusion
Colorectal SRCC has distinct molecular features, including low rates of KRAS, PIK3CA and APC mutations. Its unique clinical features and association with early age of disease onset necessitate further study to identify activation pathways and potential therapeutic targets.
Supplementary information
Acknowledgements
I would like to express my very great appreciation to Dr Sarppasit Ariyapanya for his help in preparing the figures.
Author contributions
K.K.: conceptualisation, methodology, investigation, writing original draft, writing-reviewing and editing and visualisation. V.M., J.S.D., M.J.O., D.R.F., B.K.K, A.D., K.P.S.R., I.S., R.A.W., C.E and D.G.M.: writing-review and editing, and supervision. M.T., and S.H.: methodology, investigation, reviewing and editing. S.K.: conceptualisation, writing-reviewing and editing, and visualisation and supervision.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The protocol for this study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All patients provided written informed consent prior to sequencing of their tumours according to the institutional guideline. The study was performed in accordance with the Declaration of Helsinki.
Funding
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672 and R01CA187238.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-019-0548-9.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013;258:775–782. doi: 10.1097/SLA.0b013e3182a69f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann. Surg. Oncol. 1996;3:344–348. doi: 10.1007/BF02305663. [DOI] [PubMed] [Google Scholar]
- 4.Kakar S, Smyrk TC. Signet ring cell carcinoma of the colorectum: correlations between microsatellite instability, clinicopathologic features and survival. Mod. Pathol. 2005;18:244–249. doi: 10.1038/modpathol.3800298. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton S. R., Bosman F. T., Boffetta P., et al. Carcinoma of the colon and rectum. In: WHO Classification of Tumours of the Digestive System (eds Bosman F. T., Carneiro F., Hruban R. H., Theise N. D.) 134–146 (IARC Press, Lyon, 2010).
- 6.Börger ME, Gosens MJEM, Jeuken JWM, van Kempen LCLT, van de Velde CJH, van Krieken JHJM, Nagtegaal ID. Signet ring cell differentiation in mucinous colorectal carcinoma. The Journal of Pathology. 2007;212(3):278–286. doi: 10.1002/path.2181. [DOI] [PubMed] [Google Scholar]
- 7.Wei Q, Wang X, Gao J, Li J, Qi C, Li Y, et al. Clinicopathologic and molecular features of colorectal adenocarcinoma with signet-ring cell component. PLoS One. 2016;11:e0156659. doi: 10.1371/journal.pone.0156659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann. Surg. Oncol. 2012;19:2814–2821. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew MH, Yeo SA, Ng ZP, Lim KH, Koh PK, Ng KH, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J. Colorectal Dis. 2010;25:1221–1229. doi: 10.1007/s00384-010-1033-3. [DOI] [PubMed] [Google Scholar]
- 10.Barresi Valeria, Reggiani Bonetti Luca, Domati Federica, Baron Luigi. Prognostic relevance of histopathological features in signet ring cell carcinoma of the colorectum. Virchows Archiv. 2016;469(3):267–275. doi: 10.1007/s00428-016-1983-0. [DOI] [PubMed] [Google Scholar]
- 11.Jung SH, Kim SH, Kim JH. Prognostic impact of microsatellite instability in colorectal cancer presenting with mucinous, signet-ring, and poorly differentiated cells. Ann. Coloproctol. 2016;32:58–65. doi: 10.3393/ac.2016.32.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitsche Ulrich, Friess Helmut, Agha Ayman, Angele Martin, Eckel Renate, Heitland Wolf, Jauch Karl-Walter, Krenz Detlef, Nüssler Natascha C., Rau Horst-Günter, Ruppert Reinhard, Schubert-Fritschle Gabriele, Wilhelm Dirk, Werner Jens, Engel Jutta. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. Journal of Cancer Research and Clinical Oncology. 2016;142(11):2357–2366. doi: 10.1007/s00432-016-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugen N, Verhoeven RH, Lemmens VE, van Aart CJ, Elferink MA, Radema SA, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int. J. Cancer. 2015;136:333–339. doi: 10.1002/ijc.28981. [DOI] [PubMed] [Google Scholar]
- 14.Kermanshahi Taher Reza, Magge Deepa, Choudry Haroon, Ramalingam Leksmi, Zhu Benjamin, Pingpank James, Ahrendt Steven, Holtzman Matthew, Zeh Herbert, Bartlett David, Zureikat Amer, Pai Reetesh K. Mucinous and Signet Ring Cell Differentiation Affect Patterns of Metastasis in Colorectal Carcinoma and Influence Survival. International Journal of Surgical Pathology. 2016;25(2):108–117. doi: 10.1177/1066896916664990. [DOI] [PubMed] [Google Scholar]
- 15.Inamura K, Yamauchi M, Nishihara R, Kim SA, Mima K, Sukawa Y, et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann. Surg. Oncol. 2015;22:1226–1235. doi: 10.1245/s10434-014-4159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman DJ, Nikiforova MN, Chang DT, Chu E, Bahary N, Brand RE, et al. Signet ring cell colorectal carcinoma: a distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am. J. Surg. Pathol. 2013;37:969–977. doi: 10.1097/PAS.0b013e3182851e2b. [DOI] [PubMed] [Google Scholar]
- 17.Kakar S, Deng G, Smyrk TC, Cun L, Sahai V, Kim YS. Loss of heterozygosity, aberrant methylation, BRAF mutation and KRAS mutation in colorectal signet ring cell carcinoma. Mod. Pathol. 2012;25:1040–1047. doi: 10.1038/modpathol.2012.44. [DOI] [PubMed] [Google Scholar]
- 18.Korphaisarn K, Morris VK, Overman MJ, Fogelman DR, Kee BK, Raghav KPS, et al. FBXW7 missense mutation: a novel negative prognostic factor in metastatic colorectal adenocarcinoma. Oncotarget. 2017;8:39268–39279. doi: 10.18632/oncotarget.16848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B, et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J. Mol. Diagn. 2013;15:607–622. doi: 10.1016/j.jmoldx.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Korphaisarn K, Loree JM, Nguyen V, Coulson R, Holla V, Litzenburger BC, et al. Genomic analysis of exceptional responder to regorafenib in treatment-refractory metastatic rectal cancer: a case report and review of the literature. Oncotarget. 2017;8:57882–57888. doi: 10.18632/oncotarget.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis. Colon Rectum. 2005;48:1161–1168. doi: 10.1007/s10350-004-0932-1. [DOI] [PubMed] [Google Scholar]
- 22.Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J. Clin. Pathol. 2012;65:381–388. doi: 10.1136/jclinpath-2011-200340. [DOI] [PubMed] [Google Scholar]
- 23.Gopalan V, Smith RA, Ho YH, Lam AK. Signet-ring cell carcinoma of colorectum–current perspectives and molecular biology. Int J. Colorectal Dis. 2011;26:127–133. doi: 10.1007/s00384-010-1037-z. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton SR. BRAF mutation and microsatellite instability status in colonic and rectal carcinoma: context really does matter. J. Natl. Cancer Inst. 2013;105:1075–1077. doi: 10.1093/jnci/djt189. [DOI] [PubMed] [Google Scholar]
- 25.Kawabata Y, Tomita N, Monden T, Ohue M, Ohnishi T, Sasaki M, et al. Molecular characteristics of poorly differentiated adenocarcinoma and signet-ring-cell carcinoma of colorectum. Int. J. Cancer. 1999;84:33–38. doi: 10.1002/(SICI)1097-0215(19990219)84:1<33::AID-IJC7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod. Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 27.Imamura Y, Morikawa T, Liao X, Lochhead P, Kuchiba A, Yamauchi M, et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin. Cancer Res. 2012;18:4753–4763. doi: 10.1158/1078-0432.CCR-11-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam JY, Oh BY, Hong HK, Bae JS, Kim TW, Ha SY, et al. Molecular characterization of colorectal signet-ring cell carcinoma using whole-exome and RNA sequencing. Transl. Oncol. 2018;11:836–844. doi: 10.1016/j.tranon.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalcin S, Onguru O. BRAF mutation in colorectal carcinomas with signet ring cell component. Cancer Biol. Med. 2017;14:287–292. doi: 10.20892/j.issn.2095-3941.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YH, Kakar S, Cun L, Deng G, Kim YS. Distinct CpG island methylation profiles and BRAF mutation status in serrated and adenomatous colorectal polyps. Int. J. Cancer. 2008;123:2587–2593. doi: 10.1002/ijc.23840. [DOI] [PubMed] [Google Scholar]
- 31.Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin. Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 33.Fu KI, Sano Y, Kato S, Saito H, Ochiai A, Fujimori T, et al. Primary signet-ring cell carcinoma of the colon at early stage: a case report and a review of the literature. World J. Gastroenterol. 2006;12:3446–3449. doi: 10.3748/wjg.v12.i21.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am. J. Gastroenterol. 1996;91:2195–2199. [PubMed] [Google Scholar]
- 35.Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J. Surg. Oncol. 2000;75:103–107. doi: 10.1002/1096-9098(200010)75:2<103::AID-JSO6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


