Abstract
Background
Atrial fibrillation is the most frequent sustained arrhythmia. Atrial fibrillation often recurs after restoration of normal sinus rhythm. Antiarrhythmic drugs have been widely used to prevent recurrence. This is an update of a review previously published in 2006, 2012 and 2015.
Objectives
To determine the effects of long‐term treatment with antiarrhythmic drugs on death, stroke, drug adverse effects and recurrence of atrial fibrillation in people who had recovered sinus rhythm after having atrial fibrillation.
Search methods
We updated the searches of CENTRAL, MEDLINE and Embase in January 2019, and ClinicalTrials.gov and WHO ICTRP in February 2019. We checked the reference lists of retrieved articles, recent reviews and meta‐analyses.
Selection criteria
Two authors independently selected randomised controlled trials (RCTs) comparing any antiarrhythmic drug with a control (no treatment, placebo, drugs for rate control) or with another antiarrhythmic drug in adults who had atrial fibrillation and in whom sinus rhythm was restored, spontaneously or by any intervention. We excluded postoperative atrial fibrillation.
Data collection and analysis
Two authors independently assessed quality and extracted data. We pooled studies, if appropriate, using Mantel‐Haenszel risk ratios (RR), with 95% confidence intervals (CI). All results were calculated at one year of follow‐up or the nearest time point.
Main results
This update included one new study (100 participants) and excluded one previously included study because of double publication. Finally, we included 59 RCTs comprising 20,981 participants studying quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone and sotalol. Overall, mean follow‐up was 10.2 months.
All‐cause mortality
High‐certainty evidence from five RCTs indicated that treatment with sotalol was associated with a higher all‐cause mortality rate compared with placebo or no treatment (RR 2.23, 95% CI 1.03 to 4.81; participants = 1882). The number need to treat for an additional harmful outcome (NNTH) for sotalol was 102 participants treated for one year to have one additional death. Low‐certainty evidence from six RCTs suggested that risk of mortality may be higher in people taking quinidine (RR 2.01, 95% CI 0.84 to 4.77; participants = 1646). Moderate‐certainty evidence showed increased RR for mortality but with very wide CIs for metoprolol (RR 2.02, 95% CI 0.37 to 11.05, 2 RCTs, participants = 562) and amiodarone (RR 1.66, 95% CI 0.55 to 4.99, 2 RCTs, participants = 444), compared with placebo.
We found little or no difference in mortality with dofetilide (RR 0.98, 95% CI 0.76 to 1.27; moderate‐certainty evidence) or dronedarone (RR 0.86, 95% CI 0.68 to 1.09; high‐certainty evidence) compared to placebo/no treatment. There were few data on mortality for disopyramide, flecainide and propafenone, making impossible a reliable estimation for those drugs.
Withdrawals due to adverse events
All analysed drugs increased withdrawals due to adverse effects compared to placebo or no treatment (quinidine: RR 1.56, 95% CI 0.87 to 2.78; disopyramide: RR 3.68, 95% CI 0.95 to 14.24; propafenone: RR 1.62, 95% CI 1.07 to 2.46; flecainide: RR 15.41, 95% CI 0.91 to 260.19; metoprolol: RR 3.47, 95% CI 1.48 to 8.15; amiodarone: RR 6.70, 95% CI 1.91 to 23.45; dofetilide: RR 1.77, 95% CI 0.75 to 4.18; dronedarone: RR 1.58, 95% CI 1.34 to 1.85; sotalol: RR 1.95, 95% CI 1.23 to 3.11). Certainty of the evidence for this outcome was low for disopyramide, amiodarone, dofetilide and flecainide; moderate to high for the remaining drugs.
Proarrhythmia
Virtually all studied antiarrhythmics showed increased proarrhythmic effects (counting both tachyarrhythmias and bradyarrhythmias attributable to treatment) (quinidine: RR 2.05, 95% CI 0.95 to 4.41; disopyramide: no data; flecainide: RR 4.80, 95% CI 1.30 to 17.77; metoprolol: RR 18.14, 95% CI 2.42 to 135.66; amiodarone: RR 2.22, 95% CI 0.71 to 6.96; dofetilide: RR 5.50, 95% CI 1.33 to 22.76; dronedarone: RR 1.95, 95% CI 0.77 to 4.98; sotalol: RR 3.55, 95% CI 2.16 to 5.83); with the exception of propafenone (RR 1.32, 95% CI 0.39 to 4.47) for which the certainty of evidence was very low and we were uncertain about the effect. Certainty of the evidence for this outcome for the other drugs was moderate to high.
Stroke
Eleven studies reported stroke outcomes with quinidine, disopyramide, flecainide, amiodarone, dronedarone and sotalol. High‐certainty evidence from two RCTs suggested that dronedarone may be associated with reduced risk of stroke (RR 0.66, 95% CI 0.47 to 0.95; participants = 5872). This result is attributed to one study dominating the meta‐analysis and has yet to be reproduced in other studies. There was no apparent effect on stroke rates with the other antiarrhythmics.
Recurrence of atrial fibrillation
Moderate‐ to high‐certainty evidence, with the exception of disopyramide which was low‐certainty evidence, showed that all analysed drugs, including metoprolol, reduced recurrence of atrial fibrillation (quinidine: RR 0.83, 95% CI 0.78 to 0.88; disopyramide: RR 0.77, 95% CI 0.59 to 1.01; propafenone: RR 0.67, 95% CI 0.61 to 0.74; flecainide: RR 0.65, 95% CI 0.55 to 0.77; metoprolol: RR 0.83 95% CI 0.68 to 1.02; amiodarone: RR 0.52, 95% CI 0.46 to 0.58; dofetilide: RR 0.72, 95% CI 0.61 to 0.85; dronedarone: RR 0.85, 95% CI 0.80 to 0.91; sotalol: RR 0.83, 95% CI 0.80 to 0.87). Despite this reduction, atrial fibrillation still recurred in 43% to 67% of people treated with antiarrhythmics.
Authors' conclusions
There is high‐certainty evidence of increased mortality associated with sotalol treatment, and low‐certainty evidence suggesting increased mortality with quinidine, when used for maintaining sinus rhythm in people with atrial fibrillation. We found few data on mortality in people taking disopyramide, flecainide and propafenone, so it was not possible to make a reliable estimation of the mortality risk for these drugs. However, we did find moderate‐certainty evidence of marked increases in proarrhythmia and adverse effects with flecainide.
Overall, there is evidence showing that antiarrhythmic drugs increase adverse events, increase proarrhythmic events and some antiarrhythmics may increase mortality. Conversely, although they reduce recurrences of atrial fibrillation, there is no evidence of any benefit on other clinical outcomes, compared with placebo or no treatment.
Plain language summary
Antiarrhythmics for maintaining sinus rhythm (normal heartbeat) after reversing atrial fibrillation (correcting an irregular heartbeat)
Review question
We reviewed the evidence about the effect of antiarrhythmic medicines on mortality (death), stroke, side effects that cause people to stop taking the medicine and recurrences of irregular heartbeat, in people who had recovered normal heart rhythm after having atrial fibrillation (a type of irregular heartbeat).
Background
Atrial fibrillation is a disease where the heart rhythm is irregular (called arrhythmia) and often, but not always, too fast. Atrial fibrillation may produce complications, either in the heart (heart failure, fainting) or in other organs by causing embolisms. Embolisms are blood clots that form in the cavities of the heart which may then travel to other places, for example the brain.
Atrial fibrillation can be reverted, restoring normal heart rhythm, by using medicines or a controlled electrical shock. However, a major problem is that atrial fibrillation frequently recurs. A variety of medicines have been used to avoid these recurrences and keep the normal heart rhythm.
Study characteristics
This is an update of a review previously published in 2006, 2012 and 2015, and includes results of a search for new studies in January 2019. We found 59 studies testing various antiarrhythmic drugs and involving 20,981 participants. The average age of participants was 65 years. The most frequent diseases were hypertension (high blood pressure) and diseases of the arteries and valves of the heart. We found studies for nine medicines: quinidine, disopyramide, propafenone, flecainide, metoprolol, amiodarone, dofetilide, dronedarone and sotalol.
Key results and certainty of the evidence
High‐certainty evidence from five studies found that deaths from any cause were twice as high in people taking sotalol compared with people taking a placebo (dummy treatment) or no treatment. We calculated that one extra person would die for every 102 people taking sotalol for one year. Evidence for quinidine was low certainty, but the average effect across six studies suggested that people who took quinidine may have a higher risk of death compared with people taking no treatment or placebo. However, the evidence was not strong enough to rule out the possibility that there was no increased risk of death with quinidine. We found few data on mortality for disopyramide, flecainide and propafenone, meaning that we are uncertain of the effect of these drugs on mortality. We found no clear evidence that the other medicines we studied had any effect on risk of death.
We found that people taking any of these medicines were more likely to stop taking them due to side effects, compared with people not taking them. We are less certain of the results for disopyramide, amiodarone, dofetilide and flecainide because the low‐certainty evidence mostly came from small studies with design limitations. Evidence was moderate or high for the other medicines.
One particular side effect of antiarrhythmic medications is proarrhythmia, which means that people have new or more frequent problems with irregular heartbeats. We found high‐certainty evidence that people taking quinidine or metoprolol had a higher risk of proarrhythmia than people taking no treatment or placebo. Moderate‐certainty evidence indicated a similar increased risk for flecainide, amiodarone, dofetilide, dronedarone and sotalol. Evidence from these studies was moderate certainty due to problems with study limitations, smaller size or imprecise results. We are uncertain of the effect of propafenone on proarrhythmia as we only had very low‐certainty evidence for this medicine. None of the disopyramide studies reported how many people had proarrhythmia.
We found high‐certainty evidence that dronedarone may reduce the risk of stroke. There was no evidence of an effect of sotalol (moderate‐certainty evidence); amiodarone, flecainide, quinidine (all low‐certainty evidence) or disopyramide (very low‐certainty evidence) on risk of stroke. No studies reported risk of stroke with propafenone, metoprolol or dofetilide.
Moderate‐ to high‐certainty evidence, except disopyramide which was low certainty, showed that all the medicines we assessed reduced recurrence of atrial fibrillation, compared with not taking any treatment or taking a placebo. However, atrial fibrillation still recurred in about half of participants (43% to 67%) treated with antiarrhythmics.
Overall, It is unclear whether long‐term treatment with antiarrhythmic medicines carries benefits that outweigh their risks for this group of people.
Summary of findings
Background
Description of the condition
Atrial fibrillation is the most common sustained arrhythmia and its incidence increases substantially with age (Go 2001; Knuiman 2014; Ruigomez 2002). Atrial fibrillation is associated with increased morbidity and mortality, due to stroke, other embolic complications and heart failure (Benjamin 1998; Heeringa 2006; Krahn 1995; Stewart 2002). In high‐income countries, atrial fibrillation has grown progressively since the 1990s as a contributing cause of hospitalisation and death (Ayala 2003; Chugh 2014; Wattigney 2003).
In people who have atrial fibrillation, normal sinus rhythm is interrupted by periods of atrial fibrillation that may be either symptomatic or asymptomatic. Symptoms can be mild (e.g. palpitations, breathlessness or reduced effort capacity) or severe, causing syncope, heart failure or acute coronary syndrome. Many of the symptoms caused by atrial fibrillation are related to the degree of tachycardia and can be improved by either controlling heart rate (rate control strategy) or converting atrial fibrillation to normal sinus rhythm by electrical or pharmacological means (rhythm control strategy).
Most patients alternate between atrial fibrillation and sinus rhythm. The frequency and duration of atrial fibrillation are highly variable, both within patients and between patients, and are employed to classify this arrhythmia (AHA/ACC/HRS 2014; ESC 2016; NICE 2014). If the arrhythmia terminates spontaneously, atrial fibrillation is designated as 'paroxysmal', and it may or may not recur. When atrial fibrillation is sustained beyond seven days, it is designated as 'persistent'. Termination with pharmacological or electrical intervention does not change the designation. When atrial fibrillation is first detected, and it is not known if it will resolve or persist, it is designated 'recent‐onset' or simply 'first‐detected' atrial fibrillation. Finally, 'permanent' atrial fibrillation refers to persistent atrial fibrillation where cardioversion has failed or has not been attempted because it is considered that there is no possibility to restore sinus rhythm. An individual patient can show different classes of atrial fibrillation over time.
Description of the intervention
Many patients recover sinus rhythm spontaneously after an episode of recent‐onset atrial fibrillation, as many as 70% in some studies (Geleris 2001). Electrical and pharmacological cardioversion are very effective in restoring sinus rhythm, even in long‐standing persistent atrial fibrillation. However, one major problem is that recurrence of atrial fibrillation occurs frequently. The risk of recurrence of atrial fibrillation is dependent on age, duration of the atrial fibrillation, and the existence and severity of underlying heart disease (Flaker 1995; Frick 2001). The overall rate of recurrence of atrial fibrillation without treatment is high; of patients who have converted to sinus rhythm, only 20% to 30% will have remained in sinus rhythm one year later (AFFIRM 2002; Golzari 1996; Van Gelder 1996).
Long‐term antiarrhythmic therapy has been widely used to prevent the recurrence of atrial fibrillation. Antiarrhythmic drugs are usually grouped into four classes following the classification by Vaughan Williams (Vaughan Williams 1984). Class I drugs are those with a direct membrane action (sodium channel blockade), subdivided to Ia, Ib and Ic depending on specific effects on conduction and repolarisation; class II drugs are beta‐blockers; class III drugs are those that prolong repolarisation; and class IV drugs are calcium channel blockers. There is evidence that several class I, class III and maybe class II antiarrhythmic drugs are more effective than placebo for maintaining sinus rhythm (Miller 2000; Nichol 2002). However, some questions remain concerning the long‐term use of antiarrhythmic drugs.
How the intervention might work
It has been assumed that keeping patients in sinus rhythm would improve their quality of life and reduce the risks of embolism, stroke, heart failure or increased mortality that are associated with atrial fibrillation (Anter 2009). However, this has not been confirmed and, unfortunately, many of the trials with antiarrhythmic drugs have focused only on maintenance of sinus rhythm and have not assessed other relevant outcomes (Connolly 2000). Overall, rhythm control strategy, using antiarrhythmics to maintain sinus rhythm, has shown no clear benefit on clinical outcomes (e.g. mortality or stroke) in randomised controlled trials (RCTs) compared to a rate control strategy (Caldeira 2012; Chatterjee 2013; Sethi 2017).
Chronic treatment with antiarrhythmic drugs can be associated with severe adverse effects, including the potential induction of life‐threatening arrhythmias (a phenomenon called proarrhythmia). Adverse effects could compromise any benefits of maintaining sinus rhythm, or even outweigh them, leading to worse outcomes overall. In fact, the results of some trials show increased mortality associated with the long‐term use of some antiarrhythmics, as in the case with quinidine (Coplen 1990; SPAF 1992), or flecainide (CAST 1991). Finally, it is not known if all antiarrhythmic drugs are equivalent in their effectiveness and safety in the treatment of atrial fibrillation.
Why it is important to do this review
Many trials have studied long‐term treatment with diverse antiarrhythmic drugs for maintaining sinus rhythm, sometimes compared to placebo and sometimes compared to other antiarrhythmic drugs. Attempts to summarise this evidence in systematic reviews of trials or meta‐analyses have been incomplete. They were combined in one narrative review (Golzari 1996); trials using different antiarrhythmics and with very dissimilar lengths of treatment were pooled together (Nichol 2002); and outcomes other than sinus rhythm maintenance were not evaluated (Miller 2000). Consequently, we planned to conduct a more exhaustive systematic review of RCTs studying the long‐term use of antiarrhythmic drugs to maintain sinus rhythm and aimed to determine their effects not only on the recurrence of atrial fibrillation but also on other important clinical outcomes.
After the first publication of this review, another meta‐analysis on the same subject was published by Freemantle and colleagues (Freemantle 2011). The meta‐analysis employed a mixed treatment comparison method, combining the estimates obtained from direct and indirect comparisons in a network of trials. Network meta‐analysis represents an interesting extension of traditional pair‐wise meta‐analyses and can potentially provide a more complete overview of a health set. However, appropriate use of these methods requires strict assumptions and standardisation (Caldwell 2015). Although assumptions underlying classical pair‐wise meta‐analyses are well understood, the conduction of network meta‐analysis still poses multiple challenges that should be carefully considered when using such methods (Cipriani 2013; Tonin 2017).
In any case, after the first publication of this review in 2007 and the publication of the meta‐analysis by Freemantle 2011, several new RCTs have been published. We have systematically searched, assessed and, when found adequate, included any new trial in this domain in the successive updates of this review.
Objectives
To determine the effects of long‐term treatment with antiarrhythmic drugs on death, stroke, drug adverse effects and recurrence of atrial fibrillation in people who have recovered sinus rhythm after having atrial fibrillation.
The primary aim was to assess the effects of any antiarrhythmic drug compared with no antiarrhythmic treatment, that is, no treatment, placebo or treatment for rate control. If several antiarrhythmic drugs appeared to be effective the secondary aim was to compare them.
Methods
Criteria for considering studies for this review
Types of studies
RCTs with concealed allocation of participants to intervention or placebo. We excluded studies that were not randomised or that used an overt allocation method, where future assignments could be anticipated (e.g. by date, by entry number, alternating or rotating). We also excluded cross‐over studies (as the recurrence rate of atrial fibrillation is not uniform over time), cluster‐randomised studies (more prone to selection bias and to local variations in other intervention applied to people with atrial fibrillation) and studies where duration of follow‐up was less than six months.
Types of participants
Adults (aged more than 16 years) who had atrial fibrillation of any type and duration and in whom sinus rhythm had been restored, spontaneously or by any therapeutic intervention.
We excluded people with atrial fibrillation following cardiac surgery and people with any condition causing a life expectancy of less than 12 months.
Types of interventions
To be included, studies must have randomly allocated participants to an intervention group or a control group. The intervention group must have received oral long‐term treatment with any available antiarrhythmic drug, at an appropriate dosing regimen, aimed at preventing new episodes of atrial fibrillation and maintaining sinus rhythm.
For the primary comparison of the review, the control group was no active treatment, this is, any of the following: placebo, no treatment or drugs for rate control (digoxin, calcium channel blockers, beta‐blockers).
For the secondary objective of evaluating differences between antiarrhythmic drugs, the control group could have been any of the other antiarrhythmic drugs that have shown effectiveness compared to no antiarrhythmic treatment.
Both groups, intervention and control, had to be similar with regard to cardiac disease (frequency, type and severity) and type of atrial fibrillation (especially duration). Also, both groups must have been treated similarly apart from the experimental therapy, that is:
the guidelines used to manage initiation, discontinuation, dose and surveillance of anticoagulation had to be the same in both the intervention and control groups;
management and drugs used for hypertension and heart failure had to be similar.
Types of outcome measures
Primary outcomes
All‐cause mortality
Adverse effects: withdrawals from taking the study drug caused by adverse events.
Adverse effects: proarrhythmia, including any of the following: sudden death, any new symptomatic arrhythmia (including symptomatic bradycardia), aggravation of existing arrhythmias (i.e. rapid atrial fibrillation) and new appearance on electrocardiogram (ECG) of QRS or QT widening that led to stopping treatment (Friedman 1998).
Stroke, all types.
Secondary outcomes
Recurrence of atrial fibrillation (number of participants who had a recurrence of atrial fibrillation during follow‐up).
Use of anticoagulation (number of participants started on long‐term treatment with anticoagulants at the end of follow‐up).
Heart failure.
We analysed all outcomes at 12 months. If a trial did not measure outcomes at this exact time point then we used the nearest measure point (e.g. at six, nine or 15 months instead of 12 months).
Search methods for identification of studies
Electronic searches
We updated the searches from 2005 (Appendix 1), 2010 (Appendix 2), and 2014 (Appendix 3) and reran them on 31 January 2019 (Appendix 4).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2019, Issue 1 of 12), MEDLINE (Ovid, 1946 to 28 January 2019) and Embase (Ovid, 1980 to 2019 week 4).
We also searched two clinical trials registers; ClinicalTrials.gov (www.clinicaltrials.gov) (up to 7 February 2019) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) (up to 7 February 2019).
We applied the RCT filter for MEDLINE was the Cochrane sensitivity‐maximising RCT filter, and for Embase, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Searching other resources
In addition, we checked the reference lists of retrieved studies and the reference lists of recent guidelines, meta‐analyses and general reviews on atrial fibrillation.
We applied no language restrictions.
Data collection and analysis
Selection of studies
Any of the authors read the titles (and abstracts where available) and retrieved any publication that seemed to possibly meet the inclusion criteria. Two authors independently read the full texts of the studies that were retrieved and selected the trials that met the criteria for inclusion. We developed and used a predefined form for this task. We compared the selected trials and resolved any discrepancy by discussion and consensus between the authors. We checked the articles that were finally selected for the review to avoid duplication of data. We kept records of the selection process and prepared a PRISMA flowchart (PRISMA 2009).
Data extraction and management
Two authors (from LV, WJ, JB, CLL) extracted data independently using a data collection form specifically developed for this task. When necessary, we contacted the authors of primary studies for additional information. We checked the completed data forms for agreement and resolved any differences by discussion and consensus.
In addition to data relating to the outcomes of the review, we collected information on the following.
Study methods and design (randomisation, allocation concealment and blinding).
Baseline characteristics of participants (age, gender, frequency and type of heart disease, echocardiographic measures, duration and type of atrial fibrillation, as defined in each study and knowing that definitions employed were not always consistent).
Details of treatments (method of cardioversion employed, time interval between conversion to sinus rhythm and initiation of intervention, antiarrhythmic drugs used and dose, treatment used in control group, concomitant treatments (beta‐blockers, angiotensin‐converting enzyme inhibitors, antiplatelets and warfarin)).
Follow‐up duration, participants lost to follow‐up and withdrawals.
Assessment of risk of bias in included studies
Two authors (from LV, EA, WJ, JB, CLL) independently assessed the risk of bias of the selected studies across the following domains recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017): random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other source of bias.
We resolved any differences of opinion by discussion and consensus.
Measures of treatment effect
We determined the risk ratio (RR) with 95% confidence intervals (CI) for all outcomes as they were all dichotomous variables. If evidence of an effect appeared for any outcome and the control group rates of the outcomes were broadly similar, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) to prevent or produce, respectively, one adverse outcome for the specified duration of treatment. We used the pooled RR and the pooled rate from the control groups.
Unit of analysis issues
The review includes no cross‐over trials or cluster randomised trials. For trials with multiple time points, we included only data at one year (or the nearest time point). For trials comparing two antiarrhythmics and placebo or no treatment, we divided the placebo (or no treatment) group into two groups with smaller sample size, to include two different comparisons.
Dealing with missing data
We analysed the data on the basis of intention‐to‐treat. By default, we considered missing participants not to have experienced an event and we used the randomised number of participants as the denominator. Nevertheless, we also carried out the worst‐case scenario intention‐to‐treat‐analysis for all outcomes as a sensitivity analysis.
Assessment of heterogeneity
We tested heterogeneity using the Mantel‐Haenszel Chi2 test and the I2 statistic (Higgins 2011). If we found important heterogeneity, we searched for an explanation based on the differences in clinical characteristics of the included studies. If the studies were clinically very dissimilar, they were not statistically combined.
Assessment of reporting biases
We used funnel plots to test for the presence of publication bias, based on the data for each primary and secondary outcome.
Data synthesis
We pooled data using Review Manager 5 (Review Manager 2014). If there was no heterogeneity, we calculated Mantel‐Haenszel RRs for all outcomes using a fixed‐effect model. If there was heterogeneity between studies, we calculated RRs using a random‐effects model.
We pooled data for all antiarrhythmic drugs and analysed them individually (for each specific drug).
'Summary of findings' table
We created 'Summary of findings' tables using the following outcomes: all‐cause mortality, withdrawals due to adverse effects, proarrhythmia, stroke and recurrence of atrial fibrillation. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT. We prepared a separate 'Summary of findings' table for each drug. We justified all decisions to downgrade the certainty of studies using footnotes and we made comments to aid reader's understanding of the review where necessary.
Two authors (AT, CLL) made GRADE assessments and justified, documented and incorporated their judgements into reporting of results for each outcome.
Subgroup analysis and investigation of heterogeneity
Predefined subgroup analyses were:
paroxysmal atrial fibrillation and persistent atrial fibrillation;
people with heart failure compared to people who had never developed heart failure;
studies where warfarin was mandatory versus studies where warfarin was discretionary; and
people with a structurally normal heart ('lone' atrial fibrillation).
Sensitivity analysis
Sensitivity analyses were performed by selectively pooling:
studies having low risk of bias in the following domains: allocation concealment, blinding and incomplete outcome data; and
studies including more than 200 participants.
In addition, we carried out the worst‐case scenario intention‐to‐treat‐analysis (i.e. considering all missing participants as having events) for all outcomes to test if any potential difference might have arisen due to losses to follow‐up.
Results
Description of studies
Results of the search
We found 6332 references and assessed 205 articles in more detail for the previous publication of this review (Lafuente‐Lafuente 2015). We retrieved, translated, when needed, and assessed articles in Chinese, English, French, German, Italian, Spanish and Swedish. Finally, 59 studies fulfilled the inclusion criteria and had useable data. They comprised 20,981 participants in total.
Compared with the previous publication of this review in 2015, which searched the medical literature until January 2014, we read 2185 additional references (LV, CLL, AT), assessed in detail 22 new articles (LV, EA, CLL, WJ), included one new RCT (Chun 2014), and identified one ongoing study (Park 2017). The new included trial compared dronedarone and propafenone, added 100 more participants and reported only atrial fibrillation recurrence rates, but not mortality or adverse events.
During our process of checking papers for duplicate publications, we became aware that the data from one study we had previously included by the SVA‐4 Investigators (SVA‐4 2008a), was already reported in another included publication (ASAP 2003). Therefore, we removed this study from the analysis, and listed it with the main ASAP 2003 reference in the list of Included studies.
Figure 1 illustrates the selection of articles, following the PRISMA model. Agreement between authors was good for both selecting studies and extracting the data. Details of each included study are shown in the Characteristics of included studies table, and the reasons for exclusion are shown in the Characteristics of excluded studies table.
1.

Selection of studies for inclusion. AF: atrial fibrillation.
Included studies
Participants
Entry criteria differed between studies in several aspects. In some trials, atrial fibrillation was documented in the history but participants were in sinus rhythm at the time of inclusion, while in other trials, participants were in atrial fibrillation and needed to be converted to sinus rhythm (only those converted were included in the review). The duration of atrial fibrillation when persistent, or the time from the last documented episode of atrial fibrillation when paroxysmal, was highly variable (from one month to one year, or no time limit in some studies). Some of the studies required atrial fibrillation to be symptomatic while others did not. Six studies enrolled both people with atrial fibrillation and people with atrial flutter. When available, we used only data from people with atrial fibrillation.
Regarding the type of atrial fibrillation, eight studies included exclusively paroxysmal or recent‐onset atrial fibrillation, 28 studies included only persistent atrial fibrillation (i.e. lasting more than seven days), and the remaining 23 studies included both types. Overall, 48% of the pooled population had persistent or permanent atrial fibrillation.
The mean age of participants varied from 46 to 72 years in the included studies and was 65 years in the pooled population. The proportion of participants having underlying heart disease varied widely, from 29% to 100%, with only one study selectively including people without structural heart disease (FAPIS 1996). The most frequent diseases were coronary artery disease (5% to 50% of participants), hypertension, and valvular abnormalities (less frequent in recent studies). The mean left ventricle ejection fraction was greater than 50% in almost all trials (exceptions being DIAMOND 2001; Kalusche 1994; Nergårdh 2007; Plewan 2001; Vijayalakshmi 2006).
Interventions
Twenty‐nine trials (with 13,443 participants) compared an antiarrhythmic with a control, 12 trials (4536 participants) compared two different antiarrhythmics and a control, and 18 trials (3,002 participants) compared two or more antiarrhythmics with each other. The comparator used in the 41 trials with control groups was a placebo in 32 trials, a beta‐blocker in two trials (DAPHNE 2008; Plewan 2001), digoxin in one trial (Steinbeck 1988), and no treatment in six trials (Flec‐SL 2012; Hillestad 1971; Santas 2012; Sodermark 1975; Van Gelder 1989; Vijayalakshmi 2006).
Drugs included in this review, for which there was at least one well‐designed RCT, were class IA: quinidine, disopyramide; class IC: flecainide, propafenone; class II (beta‐blockers): metoprolol; and class III: amiodarone, dofetilide, dronedarone and sotalol.
Follow‐up
The most frequent length of follow‐up was one year. It was shorter in 17 trials (six to nine months) and longer in six trials (15 to 19 months). Five trials followed participants for two years or more (AFFIRM Substudy 2003; ATHENA 2009; Kochiadakis 2000; Kochiadakis 2004a; Kochiadakis 2004b). We extracted and pooled all outcomes at one year of follow‐up or the nearest time point available. For studies with shorter duration of follow‐up, we used the last observation available. Overall, the mean follow‐up of the pooled population analysed was 10.2 months.
Excluded studies
Main reasons for exclusion of studies were not being controlled or randomised (43 studies), having a follow‐up shorter than six months (16 studies) and including in the control group participants who did not revert to sinus rhythm (10 studies). Additional details on excluded studies are given in the Characteristics of excluded studies table.
Risk of bias in included studies
There was asymmetry in the funnel plot of withdrawals because of adverse effects on treatment with sotalol (Figure 2). It showed fewer small studies on the left side (i.e. there were more small studies showing a trend to more withdrawals on active treatment). However, funnel plots for other outcomes with sotalol were symmetric, so we think the risk of substantial publication bias was low. Funnel plots for the remaining drugs were symmetric.
2.
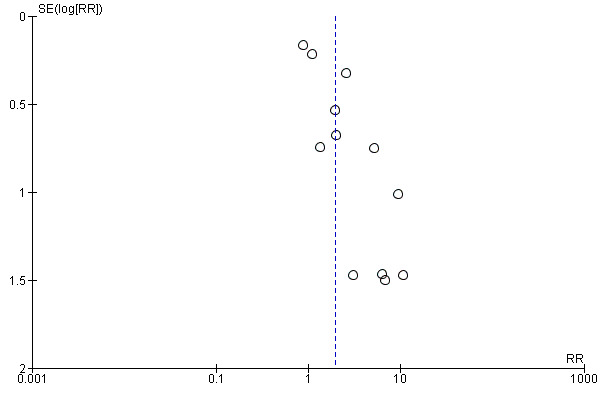
Funnel plot of comparison: 9 Sotalol versus placebo/no treatment, outcome: 9.6 Withdrawals due to adverse effects – main analysis.
The results of the assessment of the risk of bias of included studies across different domains are showed in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were described as RCTs. However, only a minority detailed how the random number sequence was generated (18 studies, 30.5%) or how the allocation of participants was concealed (17 studies, 28.8%). Because of lack of details, the risk of bias on these items was unclear for the remaining studies.
Blinding
The majority of trials comparing an antiarrhythmic versus a control were described as blinded (of 41 trials: 25 were double‐blind and five single‐blind, the remaining 11 were open‐label). In contrast, most trials comparing two or more different antiarrhythmics were open‐label (15 out of 18). However, only 17 of the 25 studies said to be double‐blind adequately reported the method of blinding (and it was adequate in all cases). Nonetheless, we think that the risk of bias associated to this lack of adequate blinding is not very high because: 1. most outcomes assessed in this review were objective ones: recurrence of atrial fibrillation and proarrhythmia were established by ECG records, mortality and stroke are objective outcomes; 2. results from adequately double‐blind studies and open‐label studies were very consistent; 3. well described, adequate blinding was more frequent in studies comparing an active drug with no active treatment, which is the main comparison of the review.
Incomplete outcome data
Most studies adequately reported withdrawals and dropouts. The percentage of participants lost to follow‐up was detailed in 47 of the 59 included trials, was small (5% to 10%) and was well balanced across arms. However, virtually all studies only followed participants until atrial fibrillation recurred or until treatment was stopped for any reason. Therefore, data for some outcomes, such as mortality, were not extensive.
Selective reporting
All studies but three (Chun 2014; DAPHNE 2008; Santas 2012) had data on all‐cause mortality, all but two (ASAP 2003; PITAGORA 2008) on atrial fibrillation recurrence rates, and all but three (AFIB 1997; Chun 2014; Santas 2012) presented data for adverse effects, either withdrawals or proarrhythmia (Table 10). Other outcomes were less frequently reported: in studies with a placebo or no treatment arm, 11 trials reported stroke, to trials reported heart failure and none reported the actual frequency of anticoagulation. All studies reported the outcomes they had prespecified in the way they had prespecified.
1. Number of studies assessing each primary outcome.
| Primary outcomes | n trials reporting (n participants) | n trials NOT reporting (n participants) |
| All‐cause mortality | 39 (17,586) | 3a (393) |
| Cardiovascular mortality | Same as total mortality | Same as total mortality |
| Stroke | 11 (9139) | 30 (8840) |
| Adverse effects (proarrhythmia and withdrawals due to adverse effects) | 39 (16,558) | 3b (1421) |
Out of 41 studies comparing an active drug with a control group receiving no antiarrhythmic (total 17,979 participants).
aChun 2014; DAPHNE 2008; Santas 2012.
bAFIB 1997; Chun 2014; Santas 2012. Others studies did not reported proarrhythmia but reported withdrawals (DAPHNE 2008; Niu 2006; Villani 1992).
Other potential sources of bias
Conflict of interest could exist as almost all the studies included in the review were funded by the company manufacturing the antiarrhythmic drug tested.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. Quinidine compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Quinidine compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: quinidine Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with quinidine | |||||
| All‐cause mortality follow‐up: median 12 months | Study population | RR 2.01 (0.84 to 4.77) | 1646 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 8 per 1000 | 15 per 1000 (6 to 36) | |||||
| Withdrawals due to adverse effects follow‐up: median 12 months | Study population | RR 1.56 (0.87 to 2.78) | 1669 (7 RCTs) | ⊕⊕⊕⊝ Moderatec,d,e | Heterogeneity was high for the main analysis (I2 = 67%), but the test for subgroup differences indicated that the RR was higher in older studies which used a higher dose. | |
| 163 per 1000 | 254 per 1000 (142 to 452) | |||||
| Proarrhythmia follow‐up: median 12 months | Study population | RR 2.05 (0.95 to 4.41) | 1676 (7 RCTs) | ⊕⊕⊕⊕ Highc,f | — | |
| 11 per 1000 | 22 per 1000 (10 to 48) | |||||
| Stroke follow‐up: median 12 months | Study population | RR 0.97 (0.25 to 3.83) | 1107 (4 RCTs) | ⊕⊕⊝⊝ Lowa,g | — | |
| 5 per 1000 | 5 per 1000 (1 to 19) | |||||
| Recurrence of atrial fibrillation follow‐up: median 12 months | Study population | RR 0.83 (0.78 to 0.88) | 1624 (7 RCTs) | ⊕⊕⊕⊕ Highc | — | |
| 80.5 per 100 | 66.8 per 100 (62.8 to 70.8) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: majority of studies were at low or unclear risk of bias for at least one of the key domains (allocation concealment, blinding, incomplete outcome data). bDowngraded one level for imprecision: confidence interval included no effect, the possibility of a beneficial effect and a strong harmful effect. cNot downgraded for study limitations, as the two studies contributing majority of weight were at low risk for key domains (allocation concealment, blinding, incomplete outcome data). dNot downgraded for inconsistency: although heterogeneity was high for the main analysis, this was partially explained by subgroup analysis. eDowngraded one level for imprecision: confidence interval included possibility of no effect or small beneficial effect as well as harmful effect. fNot downgraded for imprecision, although CI just included null. gDowngraded one level for imprecision: confidence interval included both important benefits and harms, and event rate was very low.
Summary of findings 2. Disopyramide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Disopyramide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: disopyramide Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with disopyramide | |||||
| All‐cause mortality follow‐up: mean 12 months | Study population | RR 5.00 (0.25 to 101.37) | 92 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Anticipated absolute effects per 1000 could not be calculated because there were no deaths in the control group. Risks were the data from the RCT. | |
| 0/71 | 5/75 | |||||
| Withdrawals due to adverse effects follow‐up: range 6–12 months | Study population | RR 3.68 (0.95 to 14.24) | 146 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | — | |
| 28 per 1000 | 104 per 1000 (27 to 401) | |||||
| Proarrhythmia | — | — | — | — | — | Not reported |
| Stroke follow‐up: range 6–12 months | Study population | RR 0.31 (0.03 to 2.91) | 146 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 28 per 1000 | 9 per 1000 (1 to 82) | |||||
| Recurrence of atrial fibrillation follow‐up: range 6–12 months | Study population | RR 0.77 (0.59 to 1.01) | 146 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | — | |
| 69.0 per 100 | 53.1 per 100 (40.7 to 69.7) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: both studies had unclear risk of bias for one of the key domains. bDowngraded two levels for imprecision: very small sample size and wide confidence intervals including both important benefits and harms. cDowngraded one level for imprecision: very small sample size.
Summary of findings 3. Propafenone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Propafenone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: propafenone Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with propafenone | |||||
| All‐cause mortality follow‐up: range 6–15 months | Study population | RR 0.19 (0.02 to 1.68) | 212 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Very few data available for this outcome: only 2 deaths reported in 5 included RCTs. | |
| 26 per 1000 | 5 per 1000 (1 to 44) | |||||
| Withdrawals due to adverse effects follow‐up: range 6–15 months | Study population | RR 1.62 (1.07 to 2.46) | 1098 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 61 per 1000 | 99 per 1000 (65 to 150) | |||||
| Proarrhythmia follow‐up: range 6–15 months | Study population | RR 1.32 (0.39 to 4.47) | 381 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 13 per 1000 | 17 per 1000 (5 to 56) | |||||
| Stroke | — | — | — | — | — | Not reported |
| Recurrence of atrial fibrillation follow‐up: range 6–15 months | Study population | RR 0.67 (0.61 to 0.74) | 1098 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 73.0 per 100 | 48.9 per 100 (44.5 to 54.0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations. All studies had unclear or high risk of bias in at least one of the three key domains (allocation concealment, blinding, incomplete outcome data). bDowngraded two levels for imprecision due to small sample size and confidence interval wide enough to include both important benefit and harm.
Summary of findings 4. Flecainide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Flecainide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: flecainide Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with flecainide | |||||
| All‐cause mortality | — | — | — | — | — | Not reported |
| Withdrawals due to adverse effects follow‐up: mean 6 months | Study population | RR 15.41 (0.91 to 260) | 73 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Anticipated absolute effects per 1000 could not be calculated because there were no withdrawals in the control group. Risks were the data from the RCT. | |
| 0/37 | 7/36 | |||||
| Proarrhythmia follow‐up: range 6–12 months | Study population | RR 4.80 (1.30 to 17.7) | 511 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | — | |
| 6 per 1000 | 30 per 1000 (8 to 112) | |||||
| Stroke follow‐up: mean 6 months | Study population | RR 2.04 (0.11 to 39) | 362 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Anticipated absolute effects per 1000 could not be calculated because there were no strokes in the control group. Risks were the data from the RCT. | |
| 0/81 | 3/281 | |||||
| Recurrence of atrial fibrillation follow‐up: range 6–12 months | Study population | RR 0.65 (0.55 to 0.77) | 511 (4 RCTs) | ⊕⊕⊕⊕ Highd | — | |
| 69.8 per 100 | 45.4 per 100 (38.4 to 53.8) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aNot downgraded for study limitations. the only included study was at high risk of bias for blinding (less relevant for this outcome) but low risk for other key domains. bDowngraded two levels for imprecision due to small sample size and wide confidence interval that included both possible harm and no effect. cDowngraded one level for study limitations; all studies were at high or unclear risk of bias in at least one of the key domains. dNot downgraded for study limitations. Majority of weight came from 2 largest studies which were at high risk of bias for blinding (less relevant for this outcome) but low risk for other key domains.
Summary of findings 5. Metoprolol compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Metoprolol compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: metoprolol Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with Metoprolol | |||||
| All‐cause mortality follow‐up: mean 6 months | Study population | RR 2.02 (0.37 to 11.1) | 562 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 4 per 1000 | 7 per 1000 (1 to 39) | |||||
| Withdrawals due to adverse effects follow‐up: mean 6 months | Study population | RR 3.47 (1.48 to 8.1) | 562 (2 RCTs) | ⊕⊕⊕⊕ High | — | |
| 21 per 1000 | 74 per 1000 (31 to 173) | |||||
| Proarrhythmia follow‐up: mean 6 months | Study population | RR 18.14 (2.42 to 135.6) | 562 (2 RCTs) | ⊕⊕⊕⊕ High | Anticipated absolute effects per 1000 could not be calculated because there were no events in the control group. Risks are the data from the RCTs. | |
| 0 / 282 | 17 / 280 | |||||
| Stroke | — | — | — | — | — | Not reported |
| Recurrence of atrial fibrillation follow‐up: mean 6 months | Study population | RR 0.83 (0.68 to 1.02) | 562 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | — | |
| 72.0 per 100 | 59.7 per 100 (49.0 to 73.4) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision. Confidence intervals included both possible harm and possible benefit. bDowngraded one level for inconsistency: high I2 statistic (59%) indicated heterogeneity and this could not be explored in subgroup analysis due to only two studies being included.
Summary of findings 6. Amiodarone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Amiodarone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: amiodarone Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with amiodarone | |||||
| All‐cause mortality follow‐up: range 6–12 months | Study population | RR 1.66 (0.55 to 4.99) | 444 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 26 per 1000 | 43 per 1000 (14 to 129) | |||||
| Withdrawals due to adverse effects follow‐up: range 6–16 months | Study population | RR 6.70 (1.91 to 23.45) | 319 (4 RCTs) | ⊕⊕⊝⊝ Lowb,c | — | |
| 7 per 1000 | 49 per 1000 (14 to 172) | |||||
| Proarrhythmia follow‐up: range 6–16 months | Study population | RR 2.22 (0.71 to 6.96) | 673 (4 RCTs) | ⊕⊕⊕⊝ Moderatea,d | — | |
| 8 per 1000 | 18 per 1000 (6 to 57) | |||||
| Stroke follow‐up: mean 12 months | Study population | RR 1.15 (0.30 to 4.39) | 399 (1 RCT) | ⊕⊕⊝⊝ Lowe | — | |
| 23 per 1000 | 26 per 1000 (7 to 100) | |||||
| Recurrence of atrial fibrillation follow‐up: median 12 months | Study population | RR 0.52 (0.46 to 0.58) | 812 (6 RCTs) | ⊕⊕⊕⊕ Highd | — | |
| 81.2 per 100 | 42.2 per 100 (37.3 to 47.1) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision: confidence interval included both possible benefit and harm. bDowngraded one level for study limitations: majority of weight was from studies with unclear or high risk of bias in key domains. cDowngraded one level for imprecision: small sample size. dNot downgraded for study limitations, as the majority weight was from studies at low risk of bias in all key domains. eDowngraded two levels for imprecision: small sample size and wide confidence interval which included both possible benefit and harm.
Summary of findings 7. Dofetilide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Dofetilide compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: dofetilide Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with dofetilide | |||||
| All‐cause mortality follow‐up: mean 12 months | Study population | RR 0.98 (0.76 to 1.27) | 1183 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 193 per 1000 | 189 per 1000 (146 to 245) | |||||
| Withdrawals due to adverse effects follow‐up: mean 12 months | Study population | RR 1.77 (0.75 to 4.2) | 677 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 34 per 1000 | 61 per 1000 (26 to 144) | |||||
| Proarrhythmia follow‐up: mean 12 months | Study population | RR 5.50 (1.33 to 22.8) | 1183 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 2 per 1000 | 13 per 1000 (3 to 53) | |||||
| Stroke | — | — | — | — | — | Not reported |
| Recurrence of atrial fibrillation follow‐up: mean 12 months | Study population | RR 0.72 (0.61 to 0.85) | 1183 (3 RCTs) | ⊕⊕⊕⊝ Moderatec,d | — | |
| 84.2 per 100 | 60.6 per 100 (51.4 to 71.6) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: majority of studies had unclear risk of selection bias. bDowngraded one level for imprecision: confidence interval included both possible benefit and harm. cNot downgraded for study limitations as 51% of weight came from a study with low risk of bias across all domains (but other two studies had unclear risk of selection bias). dDowngraded one level for heterogeneity due to very high I2 value (79%).
Summary of findings 8. Dronedarone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Dronedarone compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: dronedarone Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with dronedarone | |||||
| All‐cause mortality follow‐up: range 6–12 months | Study population | RR 0.86 (0.68 to 1.09) | 6071 (3 RCTs) | ⊕⊕⊕⊕ High | — | |
| 51 per 1000 | 44 per 1000 (35 to 56) | |||||
| Withdrawals due to adverse effects follow‐up: range 6–12 months | Study population | RR 1.58 (1.34 to 1.85) | 6071 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 77 per 1000 | 122 per 1000 (104 to 143) | |||||
| Proarrhythmia follow‐up: mean 12 months | Study population | RR 1.95 (0.77 to 4.98) | 5872 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | — | |
| 18 per 1000 | 36 per 1000 (14 to 91) | |||||
| Stroke follow‐up: mean 12 months | Study population | RR 0.66 (0.47 to 0.95) | 5872 (2 RCTs) | ⊕⊕⊕⊕ High | — | |
| 27 per 1000 | 18 per 1000 (13 to 25) | |||||
| Recurrence of atrial fibrillation follow‐up: range 6–12 months | Study population | RR 0.85 (0.80 to 0.91) | 1443 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | — | |
| 76.6 per 100 | 65.1 per 100 (61.3 to 69.7) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: 83% of weight came from a study with unclear blinding, which could be relevant to this outcome. bDowngraded one level for inconsistency due to very high I2 statistic of 78%. cDowngraded one level for study limitations: most weight came from a study with unclear allocation concealment.
Summary of findings 9. Sotalol compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation.
| Sotalol compared to placebo or no treatment for maintaining sinus rhythm after cardioversion of atrial fibrillation | ||||||
| Patient or population: adults in sinus rhythm after cardioversion of atrial fibrillation Setting: hospital/community Intervention: sotalol Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with sotalol | |||||
| All‐cause mortality follow‐up: range 6–12 months | Study population | RR 2.23 (1.03 to 4.81) | 1882 (5 RCTs) | ⊕⊕⊕⊕ High | — | |
| 8 per 1000 | 19 per 1000 (9 to 40) | |||||
| Withdrawals due to adverse effects follow‐up: range 6–19 months; median 12 months | Study population | RR 1.95 (1.23 to 3.11) | 2688 (12 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c | Heterogeneity was high for the main analysis (I2 = 56%), but the test for subgroup differences indicated that the RR was higher in older studies with sotalol. | |
| 94 per 1000 | 183 per 1000 (116 to 293) | |||||
| Proarrhythmia follow‐up: median 12 months | Study population | RR 3.55 (2.16 to 5.83) | 2989 (12 RCTs) | ⊕⊕⊕⊝ Moderatea,c | — | |
| 12 per 1000 | 41 per 1000 (25 to 68) | |||||
| Stroke follow‐up: range 6–12 months | Study population | RR 1.47 (0.48 to 4.51) | 1161 (3 RCTs) | ⊕⊕⊕⊝ Moderated | — | |
| 7 per 1000 | 10 per 1000 (3 to 30) | |||||
| Recurrence of atrial fibrillation follow‐up: range 6–19 months; median 12 months | Study population | RR 0.83 (0.80 to 0.87) | 3179 (14 RCTs) | ⊕⊕⊕⊕ Higha,e,f | — | |
| 78.8 per 100 | 65.4 per 100 (63.1 to 68.6) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aNot downgraded for study limitations. Although the majority of studies had unclear or high risk of bias in at least one of the key domains, the majority of the weight was from studies at low risk of bias in key domains. bNot downgraded for inconsistency. I2 statistic was 56% for the main analysis, but this was partially explained by subgroup analysis. cDowngraded one level for publication bias: forest plot appeared to be asymmetrical. dDowngraded one level for imprecision: confidence interval included both possible benefit and harm. eNot downgraded for publication bias: funnel plot appears to be broadly symmetrical. fNot downgraded for inconsistency. I2 statistic was 54% but the forest plot had good overlap in confidence intervals, so a fixed‐effect model was used to maintain the weight of the few larger studies.
We calculated all outcomes at one year of follow‐up or the nearest time point (overall mean follow‐up: 10.2 months).
Imputing missing participants as events (the worst‐case intention‐to‐treat scenario) generally did not modify the results, so we reported the best‐case intention‐to‐treat analysis (missing participants counted as being free of events) as the default; where differences existed, we reported details.
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9.
All‐cause mortality
The all‐cause mortality rate was low (0% to 5.1% at one year). The only exception to this generally low mortality rate was the DIAMOND study (DIAMOND 2001). This trial recruited people with advanced heart failure and had an overall all‐cause mortality of 31% at one year.
The quantity and quality of data on mortality varied markedly between drugs. We found no data on mortality with flecainide and very few data with disopyramide and propafenone.
More data were available for other drugs. We found evidence suggesting an increase in the risk of death with two drugs, quinidine and sotalol. For the remaining drugs studied, available evidence show no apparent effect in mortality.
There was no important heterogeneity between studies for all‐cause mortality for any of the drugs studied.
Drugs with very few or no data on mortality
Disopyramide
Only one study reported all‐cause mortality in people receiving disopyramide compared with placebo or no treatment. It included only 92 participants and had a very wide CIs for mortality that included both possible benefits and harms (RR 5.00, 95% CI 0.25 to 101.37; I2 = 0%; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.
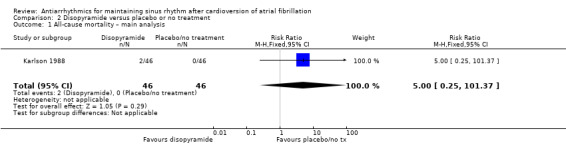
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
Counting missing participants as having died did not change this finding (Analysis 2.2). No other sensitivity analysis could be carried out.
2.2. Analysis.
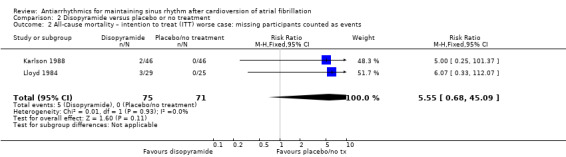
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
Propafenone
Of the five included trials (998 participants), only two studies reported any deaths (one each). The CIs were wide, including both possible benefits and harms, and the results varied markedly between the main analysis (RR 0.19, 95% CI 0.02 to 1.68; studies = 2, participants = 212; I2 = 0%; Analysis 3.1) and the sensitivity analysis which treated missing participants as having died (RR 1.28, 95% CI 0.45 to 3.62; studies = 3, participants = 406; I2 = 19%; Analysis 3.2). Restricting the analysis to the only study at low risk of bias did not differ from the main analysis (Analysis 3.3).
3.1. Analysis.
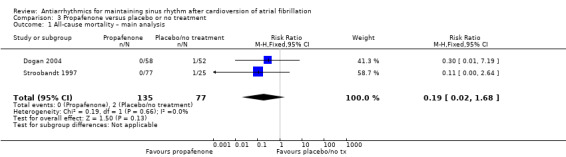
Comparison 3 Propafenone versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
3.2. Analysis.

Comparison 3 Propafenone versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
3.3. Analysis.

Comparison 3 Propafenone versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: low risk of bias studies.
Overall, the evidence for this outcome was very low‐certainty, meaning that we were uncertain of the effect of propafenone on mortality.
Flecainide
None of the four trials studying flecainide (511 participants in total) reported any death from any cause.
Drugs associated with an increase in mortality
Quinidine
Six studies that compared quinidine with placebo or no treatment reported all‐cause mortality. The GRADE rating was low‐certainty for this outcome. The pooled RR suggested that risk of mortality was higher in people receiving quinidine compared with placebo or no treatment, although the CIs also included the possibility of a lower or similar mortality rate (RR 2.01, 95% CI 0.84 to 4.77; studies = 6, participants = 1646; I2 = 0%; Analysis 1.1). This corresponded to 8 deaths per 1000 people in the control group and 15 (95% CI 6 to 36) per 1000 people in the quinidine group.
1.1. Analysis.
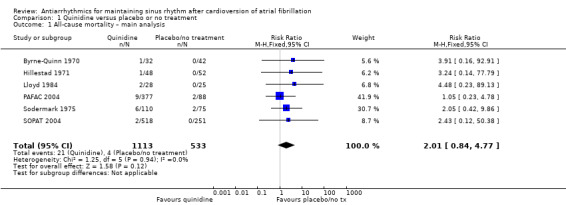
Comparison 1 Quinidine versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
Sensitivity analysis which treated missing participants as having died increased the RR slightly, but was not substantially different to the main analysis (RR 2.12, 95% CI 0.96 to 4.67; studies = 6, participants = 1646; I2 = 0%; Analysis 1.2).
1.2. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 2 All‐cause mortality – sensitivity analysis intention to treat (ITT) worse case: missing participants counted as events.
Conversely, sensitivity analysis of quinidine studies at low risk of bias (Analysis 1.5), or studies with more than 200 participants (Analysis 1.6), left only two studies (PAFAC 2004; SOPAT 2004). There was no evidence of a difference in all‐cause mortality compared with controls (RR 1.29, 95% CI 0.34 to 4.92; studies = 2, participants = 1234; I2 = 0%). These two trials were more recent, employed a lower dose of quinidine (320 mg/day to 480 mg/day) than other studies (800 mg/day to 1800 mg/day) and combined quinidine with verapamil. However, when comparing those two studies against older, higher‐dose studies, the test for subgroup differences did not indicate that the effect differed between those two groups (P = 0.4; Analysis 1.3).
1.5. Analysis.
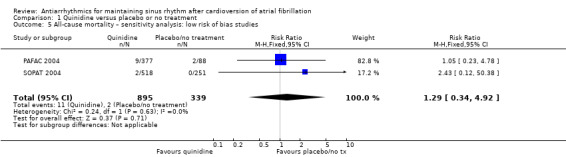
Comparison 1 Quinidine versus placebo or no treatment, Outcome 5 All‐cause mortality – sensitivity analysis: low risk of bias studies.
1.6. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 6 All‐cause mortality – sensitivity analysis: studies > 200 participants.
1.3. Analysis.
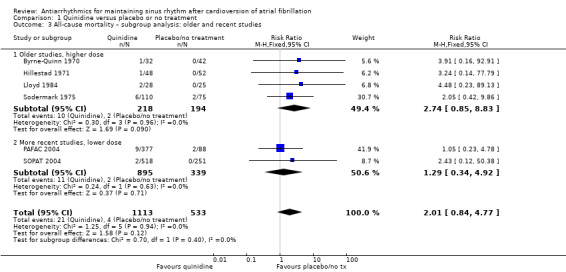
Comparison 1 Quinidine versus placebo or no treatment, Outcome 3 All‐cause mortality – subgroup analysis: older and recent studies.
The other sensitivity analysis did not differ from the main analysis (Analysis 1.4: persistent atrial fibrillation).
1.4. Analysis.
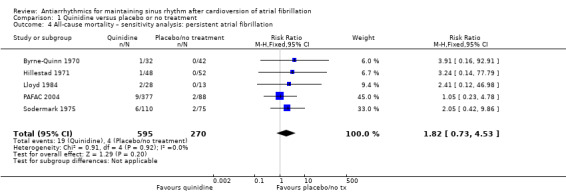
Comparison 1 Quinidine versus placebo or no treatment, Outcome 4 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
Sotalol
High‐certainty evidence from five RCTs indicated that people receiving sotalol had a higher all‐cause mortality rate than those with placebo or no treatment (RR 2.23, 95% CI 1.03 to 4.81; studies = 5, participants = 1882; I2 = 0%; Analysis 9.1; Figure 5). This corresponded to 8 deaths per 1000 people in the control group and 19 (95% CI 9 to 40) deaths per 1000 people in the sotalol group. The NNTH for sotalol was 102 (95% CI 33 to 4167) participants treated for one year to have one additional death, with a wide CI.
9.1. Analysis.
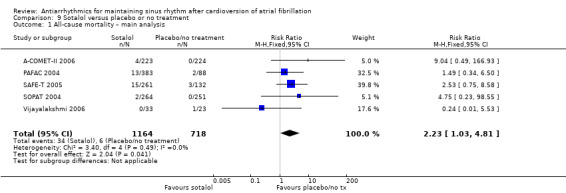
Comparison 9 Sotalol versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
5.

All‐cause mortality with sotalol compared with placebo/no treatment: main analysis.
This association with increased mortality persisted in all sensitivity analyses undertaken, either counting missing participants as deaths (RR 2.02, 95% CI 1.28 to 3.20; studies = 10, participants = 2757; I2 = 0%; Analysis 9.2), restricting to those studies at low risk of bias (RR 2.51, 95% CI 1.06 to 5.98; studies = 3, participants = 1311; I2 = 0%; Analysis 9.4), or which included only persistent atrial fibrillation (RR 2.51, 95% CI 1.06 to 5.98; studies = 3, participants = 1311; I2 = 0%; Analysis 9.3). There was an even larger effect when restricting the analysis to just those studies with at least 200 participants (RR 2.65, 95% CI 1.16 to 6.09; studies = 4, participants = 1826; I2 = 0%; Analysis 9.5).
9.2. Analysis.
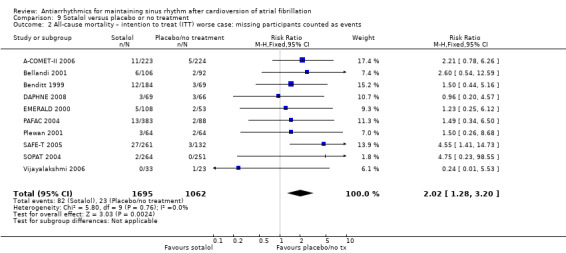
Comparison 9 Sotalol versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
9.4. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 4 All‐cause mortality – sensitivity analysis: low risk of bias studies.
9.3. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
9.5. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 5 All‐cause mortality – sensitivity analysis: studies > 200 participants.
Drugs with no apparent effect on mortality
For the remaining drugs studied, available evidence showed no apparent difference in mortality with respect to placebo or no treatment. However, data for mortality were rarely extensive and the data obtained could have been underpowered to detect mild differences in mortality for several of the drugs studied.
Metoprolol
Moderate‐certainty evidence from two studies comparing metoprolol with placebo or no treatment produced very wide CIs (RR 2.02, 95% CI 0.37 to 11.05; studies = 2, participants = 562; I2 = 47%; Analysis 5.1). Results did not change in any of the sensitivity analyses (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5).
5.1. Analysis.
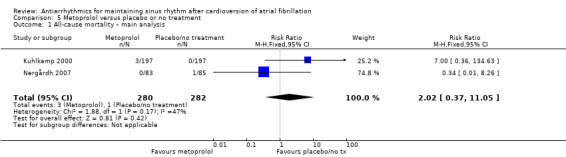
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
5.2. Analysis.
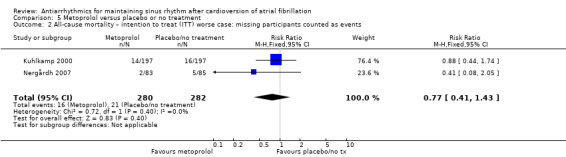
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
5.3. Analysis.
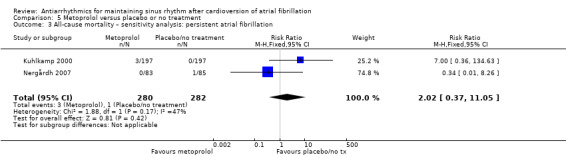
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
5.4. Analysis.

Comparison 5 Metoprolol versus placebo or no treatment, Outcome 4 All‐cause mortality – sensitivity analysis: low risk of bias studies.
5.5. Analysis.
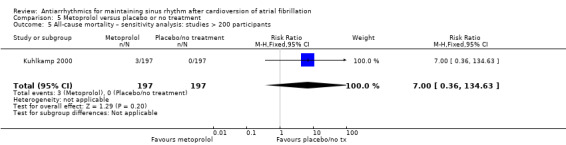
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 5 All‐cause mortality – sensitivity analysis: studies > 200 participants.
Amiodarone
Moderate‐certainty evidence from two studies comparing amiodarone with placebo or no treatment produced wide CIs (RR 1.66, 95% CI 0.55 to 4.99; studies = 2, participants = 444; I2 = 10%; Analysis 6.1). This finding did not change in any of the sensitivity analyses (Analysis 6.2; Analysis 6.3).
6.1. Analysis.
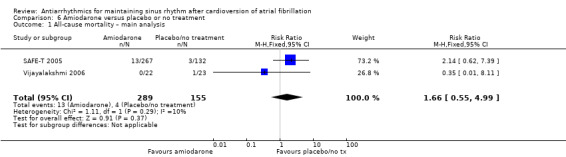
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
6.2. Analysis.
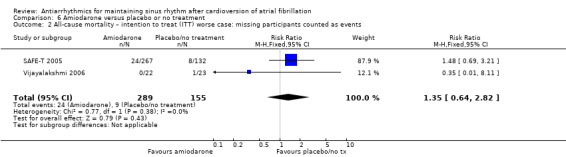
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
6.3. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
Dofetilide
Moderate‐certainty evidence from three RCTs found no evidence of a difference in all‐cause mortality rate between dofetilide and placebo or no treatment groups (RR 0.98, 95% CI 0.76 to 1.27; studies = 3, participants = 1183; I2 = 0%; Analysis 7.1). Sensitivity analyses did not differ substantially from the main analysis (Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5).
7.1. Analysis.
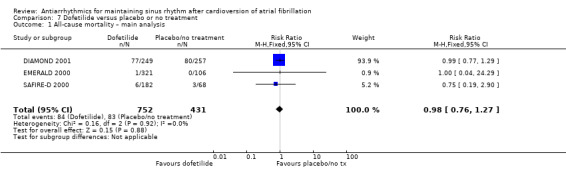
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
7.2. Analysis.
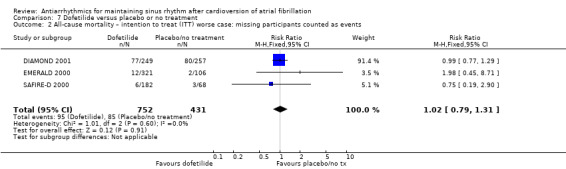
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
7.3. Analysis.
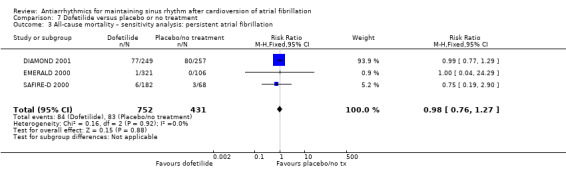
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
7.4. Analysis.
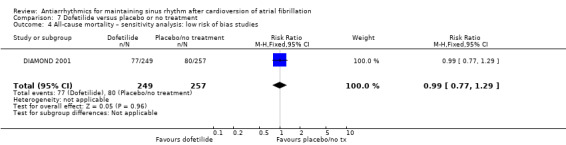
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 4 All‐cause mortality – sensitivity analysis: low risk of bias studies.
7.5. Analysis.
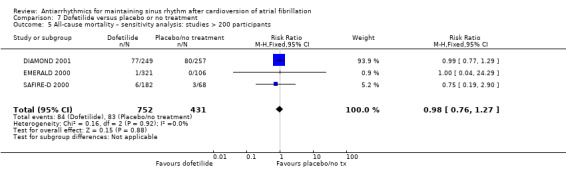
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 5 All‐cause mortality – sensitivity analysis: studies > 200 participants.
Dronedarone
High‐certainty evidence from three RCTs showed no clear difference in all‐cause mortality between dronedarone and placebo or no treatment (RR 0.86, 95% CI 0.68 to 1.09; studies = 3, participants = 6071; I2 = 0%; Analysis 8.1). The ATHENA 2009 study dominated this analysis, with 97% of the weight in the meta‐analysis.
8.1. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 1 All‐cause mortality – main analysis.
There was very little difference between this main result and the different sensitivity analyses (Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5).
8.2. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events.
8.3. Analysis.
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation.
8.4. Analysis.
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 4 All‐cause mortality – sensitivity analysis: low risk of bias studies.
8.5. Analysis.
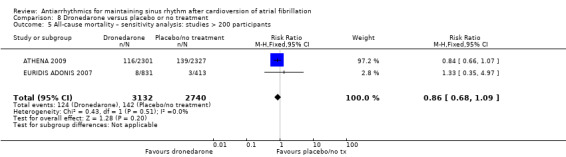
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 5 All‐cause mortality – sensitivity analysis: studies > 200 participants.
Head‐to‐head comparisons
In direct comparisons between antiarrhythmics, there were no differences in mortality (Table 11).
2. Head‐to‐head trials: all‐cause mortality.
| Drug 1 vs drug 2 | Drug 1 | Drug 2 | RR (95% CI) | ||
| Events | Total | Events | Total | ||
| Disopyramide vs other class I drugs | |||||
| Lloyd 1984 | 0 | 29 | 2 | 28 | 0.19 (0.01 to 3.86) |
| PRODIS 1996 | 1 | 31 | 0 | 25 | 2.44 (0.10 to 57.37) |
| Quinidine vs other class I drugs | |||||
| Lloyd 1984 | 2 | 28 | 0 | 29 | 5.17 (0.26 to 103.18) |
| Richiardi 1992 | 0 | 98 | 2 | 102 | 0.21 (0.01 to 4.28) |
| Quinidine vs sotalol | |||||
| Juul‐Moller 1990 | 1 | 85 | 1 | 98 | 1.15 (0.07 to 18.15) |
| Kalusche 1994 | 1 | 41 | 0 | 41 | 3.00 (0.13 to 71.56) |
| PAFAC 2004 | 9 | 377 | 13 | 383 | 0.70 (0.30 to 1.63) |
| SOCESP 1999 | 0 | 63 | 1 | 58 | 0.31 (0.01 to 7.40) |
| SOPAT 2004 | 2 | 518 | 2 | 264 | 0.51 (0.07 to 3.60) |
| Flecainide vs propafenone | |||||
| Aliot 1996 | 0 | 48 | 1 | 49 | 0.34 (0.01 to 8.15) |
| Amiodarone vs class I drugs | |||||
| AFFIRM Substudy 2003 | 10 | 106 | 26 | 116 | 0.42 (0.21 to 0.83) |
| PITAGORA 2008 | 6 | 70 | 2 | 75 | 3.21 (0.67 to 15.40) |
| Amiodarone vs dronedarone | |||||
| DIONYSOS 2010 | 5 | 255 | 2 | 249 | 2.44 (0.48 to 12.47) |
| Amiodarone vs sotalol | |||||
| AFFIRM Substudy 2003 | 15 | 131 | 24 | 125 | 0.60 (0.33 to 1.08) |
| PITAGORA 2008 | 6 | 70 | 0 | 31 | 5.86 (0.34 to 100.89) |
| SAFE‐T 2005 | 13 | 267 | 15 | 261 | 0.85 (0.41 to 1.75) |
| Sotalol vs class I drugs other than quinidine | |||||
| AFFIRM Substudy 2003 | 13 | 88 | 17 | 95 | 0.83 (0.43 to 1.60) |
| Reimold 1993 | 2 | 50 | 0 | 50 | 5.00 (0.25 to 101.58) |
| Sotalol vs dofetilide | |||||
| EMERALD 2000 | 0 | 108 | 1 | 321 | 0.98 (0.04 to 23.99) |
CI: confidence interval; RR: risk ratio.
Withdrawals due to adverse effects
Withdrawals due to adverse effects were more frequent with all studied drugs, compared with placebo or no treatment:
Quinidine
Moderate‐certainty evidence suggested a higher number of withdrawals due to adverse events in the quinidine group than in the placebo or no treatment group, although the CIs included the possibilities of a slightly smaller number of withdrawals and also of no difference between groups (RR 1.56, 95% CI 0.87 to 2.78; studies = 7, participants = 1669; I2 = 67%; Analysis 1.7). This corresponded to 163 withdrawals per 1000 people in the control group and 254 (95% CI 142 to 452) per 1000 people in the quinidine group.
1.7. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 7 Withdrawals due to adverse effects – main analysis.
There was high heterogeneity in the main analysis, which seemed to be related to two more recent studies that employed lower doses of quinidine and combined it with verapamil (PAFAC 2004; SOPAT 2004). A subgroup analysis based on the dose used and age of the studies suggested there was a real difference between these two studies and older studies which employed a higher dose of quinidine (test for subgroup differences, P = 0.009; Analysis 1.8). In older, higher‐dose studies, approximately three times more people withdrew due to adverse effects, compared to placebo or no treatment (RR 3.05, 95% CI 1.29 to 7.22; studies = 5, participants = 435; I2 = 29%). In more‐recent, lower‐dose studies, there was no evidence of a difference in withdrawals (RR 0.88, 95% CI 0.61 to 1.27; studies = 2, participants = 1234; I2 = 51%).
1.8. Analysis.
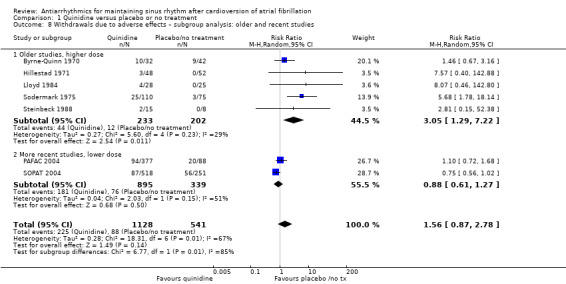
Comparison 1 Quinidine versus placebo or no treatment, Outcome 8 Withdrawals due to adverse effects – subgroup analysis: older and recent studies.
The results of sensitivity analysis varied depending on whether they included mostly older studies, as for the analysis of studies on permanent atrial fibrillation, which showed an increase of withdrawals with quinidine (Analysis 1.9), or whether they included mainly the two more‐recent studies, which showed no difference with controls (Analysis 1.10; Analysis 1.11).
1.9. Analysis.
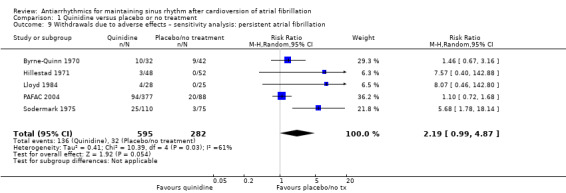
Comparison 1 Quinidine versus placebo or no treatment, Outcome 9 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
1.10. Analysis.
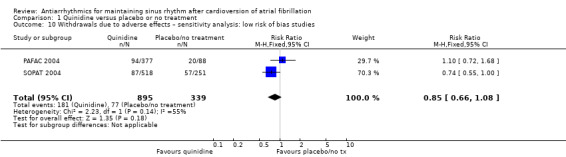
Comparison 1 Quinidine versus placebo or no treatment, Outcome 10 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies.
1.11. Analysis.
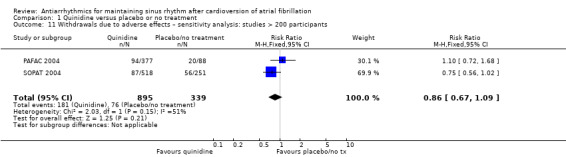
Comparison 1 Quinidine versus placebo or no treatment, Outcome 11 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
Disopyramide
Low‐certainty evidence from two RCTs indicated a more than three‐fold higher risk of withdrawal due to adverse events among people receiving disopyramide compared with placebo or no treatment, although the CIs included the possibility of similar risks of withdrawal due to adverse events (RR 3.68, 95% CI 0.95 to 14.24; studies = 2, participants = 146; I2 = 0%; Analysis 2.3). This corresponded to 28 withdrawals per 1000 people in the control group and 104 (95% CI 27 to 401) per 1000 people in the disopyramide group. The result of the sensitivity analysis was identical to the main analysis (Analysis 2.4). No further sensitivity analyses were possible.
2.3. Analysis.

Comparison 2 Disopyramide versus placebo or no treatment, Outcome 3 Withdrawals due to adverse effects – main analysis.
2.4. Analysis.

Comparison 2 Disopyramide versus placebo or no treatment, Outcome 4 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
Propafenone
Moderate‐certainty evidence indicated a higher risk of withdrawals due to adverse events in people receiving propafenone compared with people receiving placebo or no treatment (RR 1.62, 95% CI 1.07 to 2.46; studies = 5, participants = 1098; I2 = 0%; Analysis 3.4). Corresponding numbers of withdrawals due to adverse events were 61 per 1000 people in the control group and 99 (95% CI 65 to 150) per 1000 people in the propafenone group. The NNTH for propafenone was 26 (95% CI 11 to 234) participants treated for one year to have one additional withdrawal.
3.4. Analysis.
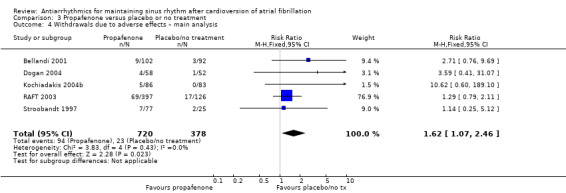
Comparison 3 Propafenone versus placebo or no treatment, Outcome 4 Withdrawals due to adverse effects – main analysis.
Restricting the analysis to the only study with more than 200 participants indicated a lack of evidence for a difference between groups (RR 1.29, 95% CI 0.79 to 2.11; studies = 1, participants = 523; Analysis 3.5).
3.5. Analysis.

Comparison 3 Propafenone versus placebo or no treatment, Outcome 5 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
Flecainide
Only one very small RCT reported withdrawals due to adverse events (RR 15.41, 95% CI 0.91 to 260.19; studies = 1, participants = 73; low‐certainty evidence; Analysis 4.1) (Van Gelder 1989). Seven people receiving flecainide withdrew due to adverse events, compared with none in the control arm. The RR reflected a higher risk of withdrawal due to adverse events when receiving flecainide, but the CIs were wide enough to include no difference between groups and even a small chance of a lower risk, but the very small number of people in this analysis limited the usefulness of this result.
4.1. Analysis.
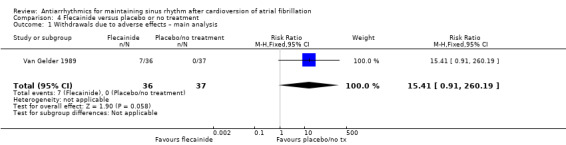
Comparison 4 Flecainide versus placebo or no treatment, Outcome 1 Withdrawals due to adverse effects – main analysis.
Metoprolol
High‐certainty evidence from two RCTs found that the risk of withdrawing due to adverse events was more than three times higher among people receiving metoprolol than people receiving placebo or no treatment (RR 3.47, 95% CI 1.48 to 8.15; studies = 2, participants = 562; I2 = 0%; Analysis 5.6). This represented 21 per 1000 people receiving placebo or no treatment withdrawing due to adverse effects compared with 74 (95% CI 31 to 173) per 1000 people receiving metoprolol. The NNTH was 19 (95% CI 7 to 99) participants treated for one year to have one additional withdrawal.
5.6. Analysis.
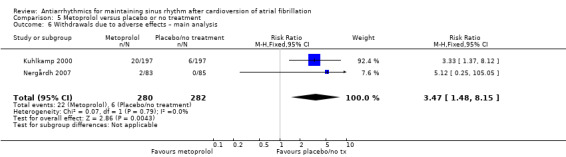
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 6 Withdrawals due to adverse effects – main analysis.
All sensitivity analyses were similar to the main results (Analysis 5.7; Analysis 5.8; Analysis 5.9).
5.7. Analysis.
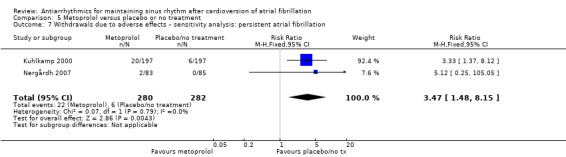
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 7 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
5.8. Analysis.
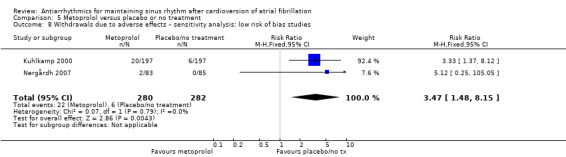
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 8 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies.
5.9. Analysis.
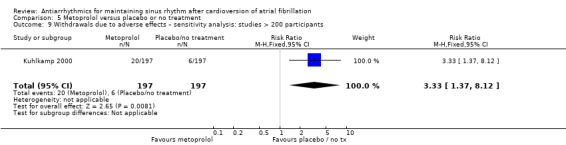
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 9 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
Amiodarone
Pooled analysis of four RCTs found low‐certainty evidence that the risk of withdrawing due to an adverse event was more than six times higher for people receiving amiodarone than for people receiving placebo or no treatment (RR 6.70, 95% CI 1.91 to 23.45; studies = 4, participants = 319; I2 = 0%; Analysis 6.4). This corresponded to seven people out of 1000 people receiving placebo or no treatment withdrawing, compared with 49 (95% CI 14 to 172) per 1000 people receiving amiodarone. The NNTH for amiodarone was 25 (95% CI 6 to 157) participants treated for one year to have one additional withdrawal.
6.4. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 4 Withdrawals due to adverse effects – main analysis.
Sensitivity analysis restricted to the only study at low risk of bias had very wide CIs (RR 4.98, 95% CI 0.65 to 38.29; studies = 1, participants = 99; Analysis 6.5).
6.5. Analysis.
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 5 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies.
Dofetilide
Low‐certainty evidence from two RCTs suggested withdrawals due to adverse effects may have been higher in people receiving dofetilide, but the wide CIs also included the possibility that there was the same risk (or a lower risk) as for people receiving placebo or no treatment (RR 1.77, 95% CI 0.75 to 4.18; studies = 2, participants = 677; I2 = 0%; Analysis 7.6). The risk was 34 per 1000 people in the placebo or no treatment group compared with 61 (95% CI 26 to 144) per 1000 people in the dofetilide group. Sensitivity analyses were identical to the main result (Analysis 7.7; Analysis 7.8).
7.6. Analysis.

Comparison 7 Dofetilide versus placebo or no treatment, Outcome 6 Withdrawals due to adverse effects – main analysis.
7.7. Analysis.
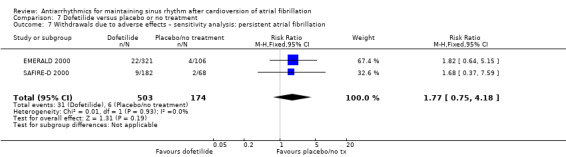
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 7 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
7.8. Analysis.
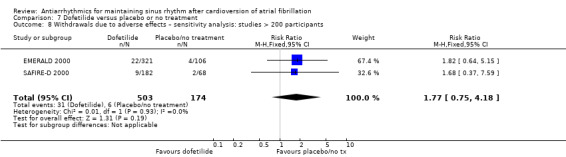
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 8 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
Dronedarone
Three RCTs showed moderate‐certainty evidence of a higher risk of withdrawals due to adverse effects among people receiving dronedarone (RR 1.58, 95% CI 1.34 to 1.85; studies = 3, participants = 6071; I2 = 31%; Analysis 8.6). This corresponded to a risk of 77 withdrawals per 1000 people in the placebo or no treatment group and 122 (95% CI 104 to 143) withdrawals per 1000 people in the dronedarone group. The NNTH was 22 (95% CI 15 to 38) participants treated for one year to have one additional withdrawal.
8.6. Analysis.
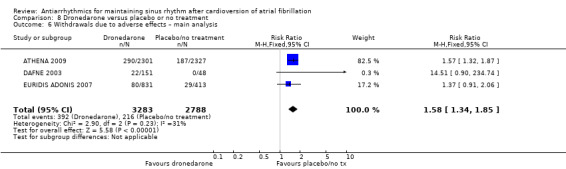
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 6 Withdrawals due to adverse effects – main analysis.
The ATHENA 2009 study had 82.5% of the weight in the main meta‐analysis, so the sensitivity analyses were heavily influenced by this large study. When it was included, they were very similar to the main analysis (Analysis 8.8; Analysis 8.9). The analysis of studies on permanent atrial fibrillation did not include the ATHENA trial and had a very wide CIs (RR 14.51, 95% CI 0.90 to 234.74; Analysis 8.7).
8.8. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 8 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies.
8.9. Analysis.
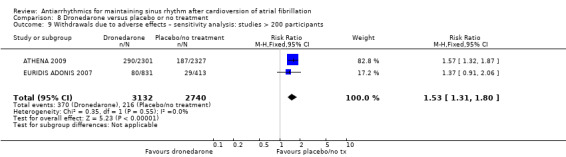
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 9 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
8.7. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 7 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
Sotalol
The risk of withdrawing due to an adverse event was almost twice as high in people receiving sotalol as in people receiving placebo or no treatment (RR 1.95, 95% CI 1.23 to 3.11; studies = 12, participants = 2688; I2 = 56%; Analysis 9.6). The risk was 94 withdrawals per 1000 people in the control group and 183 (95% CI 116 to 293) per 1000 people in the sotalol group. The corresponding NNTH was 11 (95% CI 5 to 46) participants treated for one year to have one additional withdrawal.
9.6. Analysis.
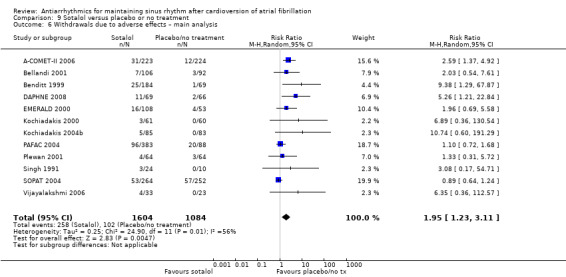
Comparison 9 Sotalol versus placebo or no treatment, Outcome 6 Withdrawals due to adverse effects – main analysis.
Evidence was rated as moderate‐certainty due to suspected publication bias. Although there was an I2 statistic of 56%, we did not downgrade for heterogeneity, because subgroup analysis showed a difference between a subgroup containing the PAFAC 2004 and SOPAT 2004 studies, and a subgroup containing the other studies (P = 0.009 from test for subgroup differences; Analysis 9.7).
9.7. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 7 Withdrawals due to adverse effects – sotalol: heterogeneity study.
Sensitivity analyses all showed an increase in withdrawals on sotalol, giving estimates which were very similar to the main analysis (permanent atrial fibrillation: Analysis 9.8), lower (low risk of bias studies: RR 1.27, 95% CI 1.00 to 1.60; studies = 4, participants = 1686; I2 = 78%; Analysis 9.9), or slightly lower (studies with at least 200 participants: RR 1.81, 95% CI 0.97 to 3.35; studies = 5, participants = 1900; I2 = 79%; Analysis 9.10).
9.8. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 8 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation.
9.9. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 9 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies.
9.10. Analysis.
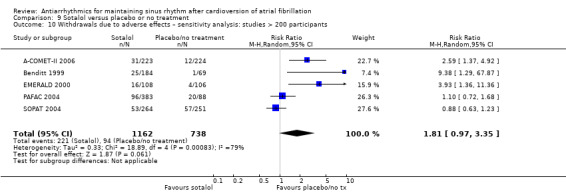
Comparison 9 Sotalol versus placebo or no treatment, Outcome 10 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants.
Head‐to‐head comparisons
In direct comparisons between antiarrhythmics (Table 12), quinidine appeared to cause more withdrawals than flecainide or other class I drugs. Amiodarone seemed to produce fewer withdrawals than class I drugs combined, but showed no difference compared with dronedarone or sotalol. Sotalol caused more withdrawals than dofetilide or beta‐blockers.
3. Head‐to‐head trials: withdrawals due to adverse events.
| Drug 1 vs drug 2 | Drug 1 | Drug 2 | RR (95% CI) | ||
| Events | Total | Events | Total | ||
| Disopyramide vs other class I drugs | |||||
| Lloyd 1984 | 2 | 29 | 4 | 28 | 0.48 (0.10 to 2.43) |
| PRODIS 1996 | 4 | 31 | 8 | 25 | 0.40 (0.14 to 1.19) |
| Quinidine vs flecainide | |||||
| Naccarelli 1996 | 35 | 117 | 22 | 122 | 1.66 (1.04 to 2.65) |
| Steinbeck 1988 | 2 | 15 | 0 | 15 | 5.00 (0.26 to 96.13) |
| Quinidine vs other class I drugs | |||||
| Lloyd 1984 | 4 | 28 | 2 | 29 | 2.07 (0.41 to 10.43) |
| Naccarelli 1996 | 35 | 117 | 22 | 122 | 1.66 (1.04 to 2.65) |
| Richiardi 1992 | 23 | 98 | 10 | 102 | 2.39 (1.20 to 4.77) |
| Steinbeck 1988 | 2 | 15 | 0 | 15 | 5.00 (0.26 to 96.13) |
| Quinidine vs sotalol | |||||
| Hohnloser 1995 | 10 | 25 | 1 | 25 | 10.00 (1.38 to 72.39) |
| Juul‐Moller 1990 | 22 | 85 | 11 | 98 | 2.31 (1.19 to 4.47) |
| Kalusche 1994 | 7 | 41 | 3 | 41 | 2.33 (0.65 to 8.40) |
| PAFAC 2004 | 94 | 377 | 96 | 383 | 0.99 (0.78 to 1.27) |
| SOCESP 1999 | 10 | 63 | 7 | 58 | 1.32 (0.54 to 3.23) |
| SOPAT 2004 | 87 | 518 | 53 | 264 | 0.84 (0.62 to 1.14) |
| Flecainide vs propafenone | |||||
| Aliot 1996 | 2 | 48 | 9 | 49 | 0.23 (0.05 to 1.00) |
| FAPIS 1996 | 10 | 97 | 9 | 103 | 1.18 (0.50 to 2.78) |
| Amiodarone vs class I drugs | |||||
| AFFIRM Substudy 2003 | 20 | 154 | 47 | 121 | 0.33 (0.21 to 0.53) |
| Kochiadakis 2004a | 17 | 72 | 2 | 74 | 8.74 (2.09 to 36.46) |
| PITAGORA 2008 | 5 | 70 | 2 | 31 | 1.11 (0.23 to 5.40) |
| Villani 1992 | 3 | 35 | 10 | 41 | 0.35 (0.10 to 1.18) |
| Vitolo 1981 | 1 | 28 | 1 | 26 | 0.93 (0.06 to 14.09) |
| Amiodarone vs dronedarone | |||||
| DIONYSOS 2010 | 45 | 255 | 32 | 249 | 1.37 (0.90 to 2.09) |
| Amiodarone vs sotalol | |||||
| AFFIRM Substudy 2003 | 20 | 154 | 21 | 135 | 0.83 (0.47 to 1.47) |
| Kochiadakis 2000 | 11 | 65 | 3 | 61 | 3.44 (1.01 to 11.75) |
| Niu 2006 | 5 | 51 | 7 | 51 | 0.71 (0.24 to 2.10) |
| PITAGORA 2008 | 6 | 70 | 0 | 31 | 5.86 (0.34 to 100.89) |
| Vijayalakshmi 2006 | 1 | 22 | 4 | 33 | 0.38 (0.04 to 3.14) |
| Sotalol vs class I drugs other than quinidine | |||||
| AFFIRM Substudy 2003 | 21 | 135 | 47 | 121 | 0.40 (0.25 to 0.63) |
| Kochiadakis 2004b | 5 | 85 | 5 | 86 | 1.01 (0.30 to 3.37) |
| Reimold 1993 | 6 | 50 | 4 | 50 | 1.50 (0.45 to 4.99) |
| Sotalol vs dofetilide | |||||
| EMERALD 2000 | 16 | 108 | 22 | 321 | 2.16 (1.18 to 3.96) |
| Sotalol vs other beta‐blockers | |||||
| DAPHNE 2008 | 11 | 69 | 2 | 66 | 5.26 (1.21 to 22.84) |
| Plewan 2001 | 4 | 64 | 3 | 64 | 1.33 (0.31 to 5.72) |
CI: confidence interval; RR: risk ratio.
Proarrhythmia
Virtually all studied antiarrhythmics showed increased proarrhythmic effects (counting both bradyarrhythmias and tachyarrhythmias attributable to treatment).
Ventricular arrhythmias (torsades, ventricular tachycardia, ventricular fibrillation, widening QRS or QT leading to stopping treatment, sudden death or unexplained syncope) were the most frequent proarrhythmic events reported with dofetilide (100% of all proarrhythmic events), quinidine (94%) and flecainide (69%), while symptomatic bradyarrhythmias (sinus bradycardia leading to stopping treatment; atrio‐ventricular block) were more frequent with metoprolol (94% of all events) and amiodarone (69%). Other drugs demonstrated both types of proarrhythmic events: propafenone (63% ventricular events, 39% bradycardia), sotalol (61% ventricular events, 39% bradycardia) and dronedarone (41% ventricular events, 59% bradycardia).
Quinidine
High‐certainty evidence from seven RCTs showed that the risk of proarrhythmia was twice as high in people the quinidine group compared with people in the placebo or no treatment group, although the CIs did not exclude the possibility of no difference between groups (RR 2.05, 95% CI 0.95 to 4.41; studies = 7, participants = 1676; I2 = 0%; Analysis 1.12). This represented 11 cases per 1000 people in the control group and 22 (95% CI 10 to 48) cases per 1000 people in the quinidine group.
1.12. Analysis.
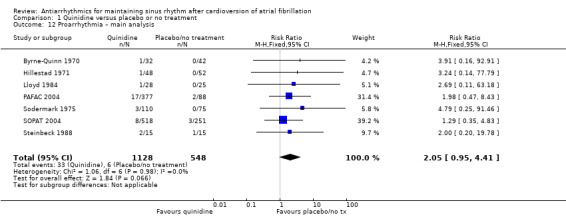
Comparison 1 Quinidine versus placebo or no treatment, Outcome 12 Proarrhythmia – main analysis.
In a way very similar to the analysis of withdrawals due to adverse effects, the results of sensitivity analysis varied depending whether they included mostly older, higher‐dose studies, as the analysis of studies on permanent atrial fibrillation, which showed an increase of proarrhythmia with quinidine (Analysis 1.14); or whether they included mainly the two more recent, lower‐dose studies (PAFAC 2004; SOPAT 2004), which showed no difference compared with controls (Analysis 1.15; Analysis 1.16). However, a subgroup analysis comparing older studies with more‐recent ones found no difference between groups for this outcome (test for difference between subgroups P = 0.41; Analysis 1.3).
1.14. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 14 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
1.15. Analysis.
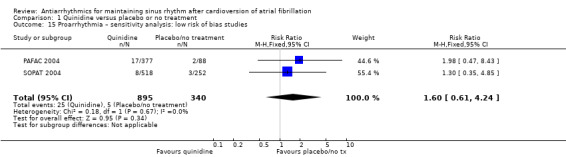
Comparison 1 Quinidine versus placebo or no treatment, Outcome 15 Proarrhythmia – sensitivity analysis: low risk of bias studies.
1.16. Analysis.
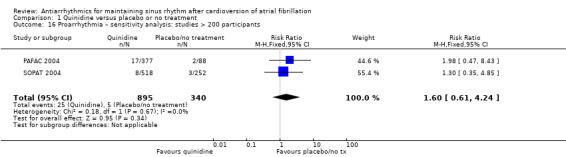
Comparison 1 Quinidine versus placebo or no treatment, Outcome 16 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Disopyramide
We found no disopyramide studies reporting proarrhythmia.
Propafenone
Three RCTs reported proarrhythmia, but the very low‐certainty evidence and wide CIs meant that we were uncertain of the effect of propafenone on this outcome (RR 1.32, 95% CI 0.39 to 4.47; studies = 3, participants = 381; studies = 3; I2 = 8%; Analysis 3.6). Sensitivity analysis restricted to the only study at low risk of bias showed a lack of evidence for a difference between groups (RR 0.49, 95% CI 0.09 to 2.75; studies = 1, participants = 102; Analysis 3.7).
3.6. Analysis.

Comparison 3 Propafenone versus placebo or no treatment, Outcome 6 Proarrhythmia – main analysis.
3.7. Analysis.
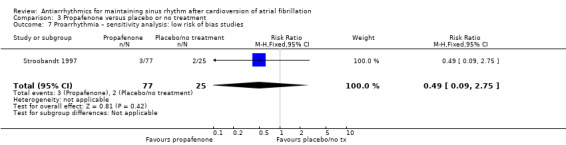
Comparison 3 Propafenone versus placebo or no treatment, Outcome 7 Proarrhythmia – sensitivity analysis: low risk of bias studies.
Flecainide
Risk of proarrhythmia was over four times higher among people receiving flecainide than placebo or no treatment (RR 4.80, 95% CI 1.30 to 17.77; studies = 4, participants = 511; I2 = 0%; moderate‐certainty evidence; Analysis 4.2). This corresponded to a risk of 6 per 1000 among people in the placebo or no treatment group compared with a risk of 30 (95% CI 8 to 112) per 1000 people in the flecainide group. The NNTH for flecainide was 44 (95% CI 10 to 556) participants treated for one year to have one additional proarrhythmic event.
4.2. Analysis.
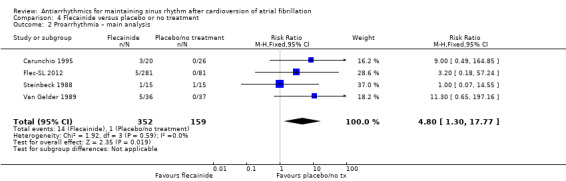
Comparison 4 Flecainide versus placebo or no treatment, Outcome 2 Proarrhythmia – main analysis.
All sensitivity analyses suggested an increased risk of proarrhythmia with flecainide, but their CIs were wider and included the possibility of no difference between groups (Analysis 4.3; Analysis 4.4; Analysis 4.5).
4.3. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 3 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
4.4. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 4 Proarrhythmia – sensitivity analysis: low risk of bias studies.
4.5. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 5 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Metoprolol
High‐certainty evidence showed an important increase of proarrhythmia with metoprolol compared to placebo due mainly to symptomatic bradyarrhythmias (94% of all proarrhythmic events) (RR 18.14, 95% CI 2.42 to 135.66; studies = 2, participants = 562; I2 = 0%; Analysis 5.10). In the pooled population, proarrhythmic events were reported in no participants in the placebo group and in 60 participants per 1000 people in the metoprolol group. The corresponding NNTH was 19 (95% CI 2 to 235) participants treated for one year to have one additional bradyarrhythmia.
5.10. Analysis.
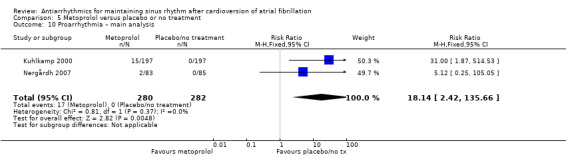
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 10 Proarrhythmia – main analysis.
All sensitivity analyses showed results similar to the main analysis (Analysis 5.11; Analysis 5.12; Analysis 5.13).
5.11. Analysis.
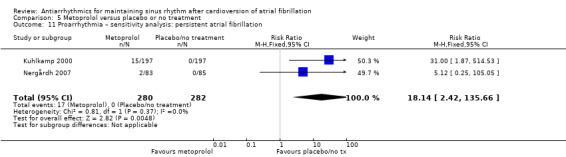
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 11 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
5.12. Analysis.
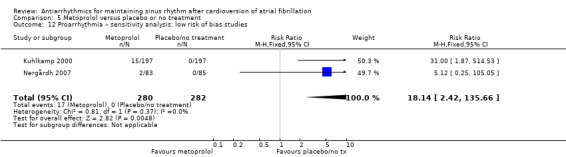
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 12 Proarrhythmia – sensitivity analysis: low risk of bias studies.
5.13. Analysis.
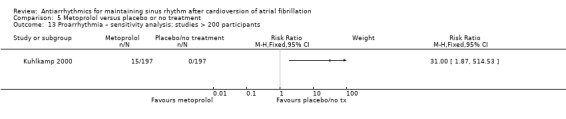
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 13 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Amiodarone
Moderate‐certainty evidence suggested an increase in proarrhythmia with amiodarone compared to placebo or no treatment, but the CIs included the possibility of no difference (or even a reduction) (RR 2.22, 95% CI 0.71 to 6.96; studies = 4, participants = 673; I2 = 0%; Analysis 6.6). This corresponded to a risk of 8 per 1000 per people with placebo or no treatment and 18 (95% CI 6 to 57) per 1000 people with amiodarone. Symptomatic bradyarrhythmias represented 69% of events with amiodarone.
6.6. Analysis.
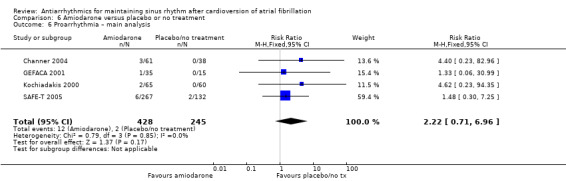
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 6 Proarrhythmia – main analysis.
Sensitivity analyses gave similar results, the only difference was that they pooled fewer studies and the CIs were wider (Analysis 6.7; Analysis 6.8; Analysis 6.9).
6.7. Analysis.
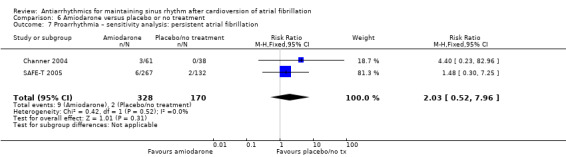
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 7 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
6.8. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 8 Proarrhythmia – sensitivity analysis: low risk of bias studies.
6.9. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 9 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Dofetilide
Moderate‐certainty evidence found a five‐fold increase in proarrhythmic events with dofetilide compared to placebo or no treatment (RR 5.50, 95% CI 1.33 to 22.76; studies = 3, participants = 1183; I2 = 0%; Analysis 7.9). This corresponded to 2 cases per 1000 people in the control group and 13 (95% CI 3 to 53) cases per 1000 people in the dofetilide group. The NNTH for dofetilide was 111 (95% CI 23 to 1515) participants treated for one year to have one additional proarrhythmic event.
7.9. Analysis.
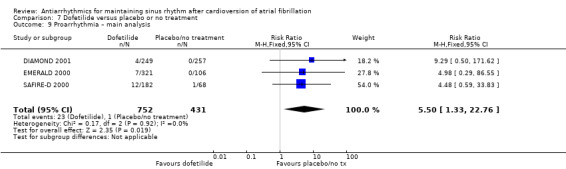
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 9 Proarrhythmia – main analysis.
Sensitivity analyses did not differ from the main analysis (Analysis 7.10; Analysis 7.11; Analysis 7.12).
7.10. Analysis.
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 10 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
7.11. Analysis.
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 11 Proarrhythmia – sensitivity analysis: low risk of bias studies.
7.12. Analysis.

Comparison 7 Dofetilide versus placebo or no treatment, Outcome 12 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Dronedarone
Moderate‐certainty evidence from two RCTs suggested an increase of proarrhythmia with dronedarone compared with placebo, but the CIs included the possibility of no difference or even a benefit on this outcome (RR 1.95, 95% CI 0.77 to 4.98; studies = 2, participants = 5872; I2 = 78%; Analysis 8.6). This represented 18 cases per 1000 people in the placebo group and 36 (95% CI 14 to 91) cases per 1000 people in the dronedarone group.
In sensitivity analysis, there was only one study rated at low risk of bias or including more than 200 participants (ATHENA 2009). This study found an increased risk of proarrhythmia with dronedarone compared to placebo (RR 2.94, 95% CI 2.08 to 4.15, participants = 4628; Analysis 8.11; Analysis 8.12).
8.11. Analysis.
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 11 Proarrhythmia – sensitivity analysis: low risk of bias studies.
8.12. Analysis.
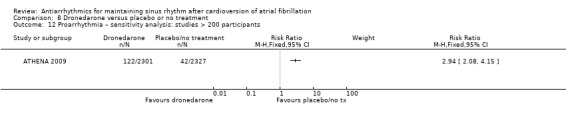
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 12 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Sotalol
Moderate‐certainty evidence showed increased proarrhythmia rates on sotalol compared to placebo or no treatment (RR 3.55, 95% CI 2.16 to 5.83; studies = 12, participants = 2989; I2 = 20%; Analysis 9.11). This corresponded to 12 cases per 1000 people in the control group and 41 (95% CI 25 to 68) cases per 1000 people in the sotalol group. The corresponding NNTH was 33 (95% CI 17 to 72) participants treated for one year to have one additional proarrhythmic event.
9.11. Analysis.
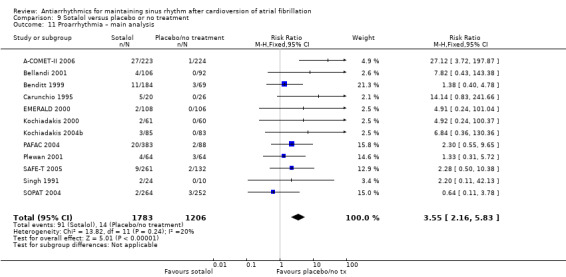
Comparison 9 Sotalol versus placebo or no treatment, Outcome 11 Proarrhythmia – main analysis.
All sensitivity analyses were very similar to the main analysis (Analysis 9.13; Analysis 9.14; Analysis 9.15).
9.13. Analysis.
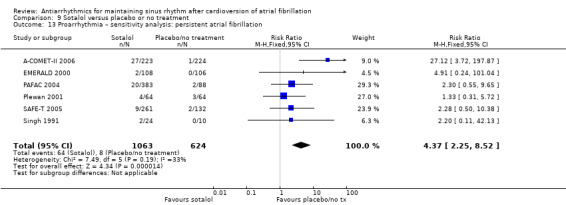
Comparison 9 Sotalol versus placebo or no treatment, Outcome 13 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation.
9.14. Analysis.
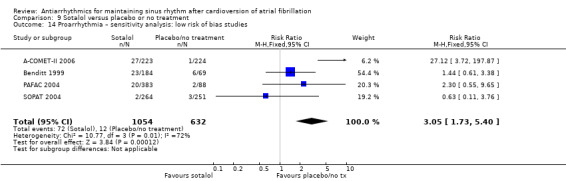
Comparison 9 Sotalol versus placebo or no treatment, Outcome 14 Proarrhythmia – sensitivity analysis: low risk of bias studies.
9.15. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 15 Proarrhythmia – sensitivity analysis: studies > 200 participants.
Head‐to‐head comparisons
In direct comparisons between antiarrhythmics (Table 13), amiodarone seemed to produce fewer proarrhythmic events than class I drugs combined, but showed no clear differences compared with dronedarone or sotalol. There were no other differences between drugs.
4. Head‐to‐head trials: proarrhythmia.
| Drug 1 vs drug 2 | Drug 1 | Drug 2 | RR (95% CI) | ||
| Events | Total | Events | Total | ||
| Disopyramide vs other class I drugs | |||||
| Lloyd 1984 | 0 | 29 | 1 | 28 | 0.32 (0.01 to 7.59) |
| PRODIS 1996 | 1 | 31 | 1 | 25 | 0.81 (0.05 to 12.26) |
| Quinidine vs flecainide | |||||
| Naccarelli 1996 | 10 | 117 | 7 | 122 | 1.49 (0.59 to 3.78) |
| Steinbeck 1988 | 2 | 15 | 1 | 15 | 2.00 (0.20 to 19.78) |
| Quinidine vs other class I drugs | |||||
| Lloyd 1984 | 1 | 28 | 0 | 29 | 3.10 (0.13 to 73.12) |
| Naccarelli 1996 | 10 | 117 | 7 | 122 | 1.49 (0.59 to 3.78) |
| Richiardi 1992 | 2 | 98 | 2 | 102 | 1.04 (0.15 to 7.24) |
| Steinbeck 1988 | 2 | 15 | 1 | 15 | 2.00 (0.20 to 19.78) |
| Quinidine vs sotalol | |||||
| Hohnloser 1995 | 3 | 25 | 1 | 25 | 3.00 (0.33 to 26.92) |
| Juul‐Moller 1990 | 1 | 85 | 1 | 98 | 1.15 (0.07 to 18.15) |
| Kalusche 1994 | 1 | 41 | 2 | 41 | 0.50 (0.05 to 5.30) |
| PAFAC 2004 | 17 | 377 | 20 | 383 | 0.86 (0.46 to 1.62) |
| SOCESP 1999 | 2 | 63 | 3 | 58 | 0.61 (0.11 to 3.54) |
| SOPAT 2004 | 8 | 518 | 2 | 264 | 2.04 (0.44 to 9.53) |
| Flecainide vs propafenone | |||||
| Aliot 1996 | 0 | 48 | 4 | 49 | 0.11 (0.01 to 2.05) |
| FAPIS 1996 | 2 | 97 | 1 | 103 | 2.12 (0.20 to 23.05) |
| Amiodarone vs class I drugs | |||||
| AFFIRM Substudy 2003 | 5 | 154 | 20 | 121 | 0.20 (0.08 to 0.51) |
| Kochiadakis 2004a | 2 | 72 | 2 | 74 | 1.03 (0.15 to 7.10) |
| Vitolo 1981 | 1 | 28 | 1 | 26 | 0.93 (0.06 to 14.09) |
| Amiodarone vs dronedarone | |||||
| DIONYSOS 2010 | 4 | 255 | 2 | 249 | 1.95 (0.36 to 10.57) |
| Amiodarone vs sotalol | |||||
| AFFIRM Substudy 2003 | 5 | 154 | 9 | 135 | 0.49 (0.17 to 1.42) |
| Kochiadakis 2000 | 2 | 65 | 2 | 61 | 0.94 (0.14 to 6.46) |
| SAFE‐T 2005 | 6 | 267 | 9 | 261 | 0.65 (0.24 to 1.81) |
| Sotalol vs class I drugs other than quinidine | |||||
| AFFIRM Substudy 2003 | 9 | 135 | 20 | 121 | 0.40 (0.19 to 0.85) |
| Carunchio 1995 | 5 | 20 | 3 | 20 | 1.67 (0.46 to 6.06) |
| Kochiadakis 2004b | 3 | 85 | 2 | 86 | 1.52 (0.26 to 8.86) |
| Reimold 1993 | 9 | 50 | 6 | 50 | 1.50 (0.58 to 3.90) |
| Sotalol vs dofetilide | |||||
| EMERALD 2000 | 2 | 108 | 7 | 321 | 0.85 (0.18 to 4.03) |
| Sotalol vs other beta‐blockers | |||||
| Plewan 2001 | 4 | 64 | 3 | 64 | 1.33 (0.31 to 5.72) |
CI: confidence interval; RR: risk ratio.
Stroke
There were limited data for stroke. Only 11 of 41 studies with a control group (placebo or no treatment arm) reported stroke outcomes (ATHENA 2009; Benditt 1999; Carunchio 1995; EURIDIS ADONIS 2007; Flec‐SL 2012; Hillestad 1971; Karlson 1988; Lloyd 1984; SAFE‐T 2005; Sodermark 1975; SOPAT 2004), and we were uncertain that reporting of stroke was complete. The reported stroke rate was very low (1% to 2% at one year).
Drugs with no data on stroke
None of the studies of propafenone, metoprolol or dofetilide reported data on stroke.
Drugs with no apparent effect on stroke
Low‐ to very low‐certainty evidence showed no apparent effect on stroke rates, compared to placebo or no treatment, with the following drugs:
quinidine (RR 0.97, 95% CI 0.25 to 3.83; studies = 4, participants = 1107; I2 = 0%; Analysis 1.17);
disopyramide (RR 0.31, 95% CI 0.03 to 2.91; studies = 2, participants = 146; I2 = 0%; Analysis 2.5);
flecainide (RR 2.04, 95% CI 0.11 to 39.00; studies = 1, participants = 362; I2 = 0%; Analysis 4.6);
amiodarone (RR 1.15, 95% CI 0.30 to 4.39; studies = 1, participants = 399; I2 = 0%; Analysis 6.10).
1.17. Analysis.
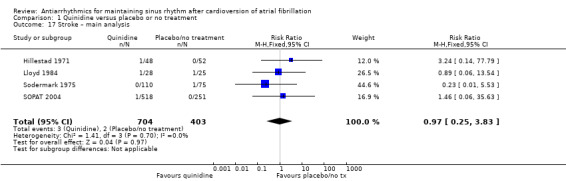
Comparison 1 Quinidine versus placebo or no treatment, Outcome 17 Stroke – main analysis.
2.5. Analysis.
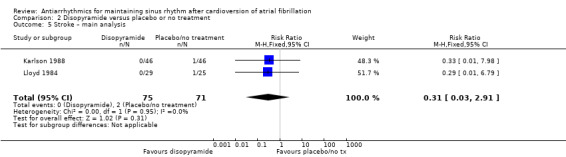
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 5 Stroke – main analysis.
4.6. Analysis.
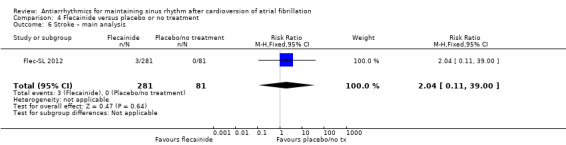
Comparison 4 Flecainide versus placebo or no treatment, Outcome 6 Stroke – main analysis.
6.10. Analysis.
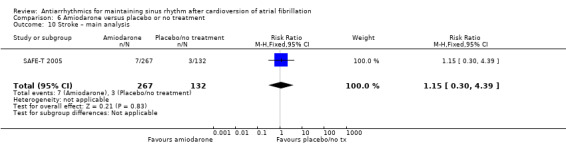
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 10 Stroke – main analysis.
Moderate‐certainty evidence showed no apparent effect on stroke rates compared to placebo or no treatment with sotalol (RR 1.47, 95% CI 0.48 to 4.51; studies = 3, participants = 1161; I2 = 0%; Analysis 9.16).
9.16. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 16 Stroke – main analysis.
The corresponding sensitivity analyses, when these were possible, showed no notable difference with the main analyses.
Drugs with an effect on stroke
Dronedarone
High‐certainty evidence from two RCTs suggested that dronedarone may be associated with reduced risk of stroke (RR 0.66, 95% CI 0.47 to 0.95; studies = 2, participants = 5872; I2 = 0%; Analysis 8.13). This corresponded to a risk of stroke of 27 per 1000 people in the placebo group and 18 per 1000 (13 to 25) people in the dronedarone group. The corresponding NNTB was 109 (95% CI 70 to 741) participants treated for one year to prevent one stroke.
8.13. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 13 Stroke – main analysis.
However, this result was due to one large study, which accounted for 94.6% of the weight in the meta‐analysis (ATHENA 2009). Sensitivity analysis restricted to studies with more than 200 participants included the same two studies so produced identical results (Analysis 8.14).
8.14. Analysis.
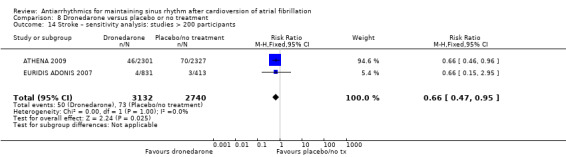
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 14 Stroke – sensitivity analysis: studies > 200 participants.
Recurrence of atrial fibrillation
All antiarrhythmic drugs included in this review, including metoprolol, reduced the risk of recurrence of atrial fibrillation. Recurrence rates of atrial fibrillation at one year were high: 69% to 84% in the control group not receiving antiarrhythmic treatment, reduced to 43% to 67% in participants in the antiarrhythmic group.
Quinidine
High‐certainty evidence showed a reduction in atrial fibrillation recurrences with quinidine (RR 0.83, 95% CI 0.78 to 0.88; studies = 7, participants = 1624; I2 = 0%; Analysis 1.21). Recurrence rates at one year were 80.5% in participants in the placebo or no treatment group and 66.8% (62.8% to 70.8%) in participants in the quinidine group. The NNTB for quinidine was 7 (95% CI 6 to 10) participants treated for one year to avoid one recurrence.
1.21. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 21 Atrial fibrillation recurrence – main analysis.
Results of sensitivity analyses did not differ from the main analysis (Analysis 1.22; Analysis 1.23; Analysis 1.24).
1.22. Analysis.

Comparison 1 Quinidine versus placebo or no treatment, Outcome 22 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
1.23. Analysis.
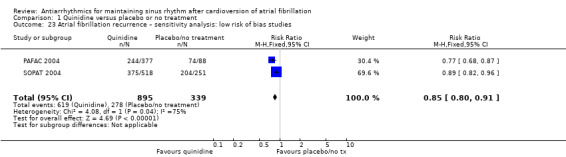
Comparison 1 Quinidine versus placebo or no treatment, Outcome 23 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
1.24. Analysis.
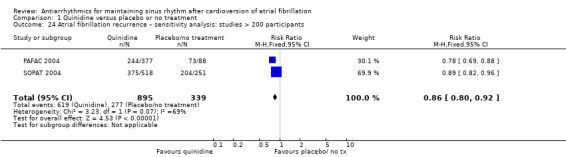
Comparison 1 Quinidine versus placebo or no treatment, Outcome 24 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Disopyramide
Evidence for disopyramide was low‐certainty because it consisted of two small RCTs with unclear risk of bias. It suggested disopyramide reduced recurrences of atrial fibrillation (RR 0.77, 95% CI 0.59 to 1.01; studies = 2, participants = 146; I2 = 0%; Analysis 2.7). This corresponded to a recurrence rate, at six months to one year, of 69.0% in the control group and 53.1% (95% CI 40.7% to 69.7%) in the disopyramide group. Both studies included only people with permanent atrial fibrillation (Analysis 2.8), and no other sensitivity analysis was possible.
2.7. Analysis.
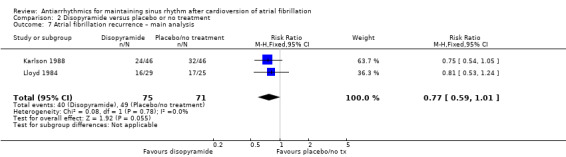
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 7 Atrial fibrillation recurrence – main analysis.
2.8. Analysis.
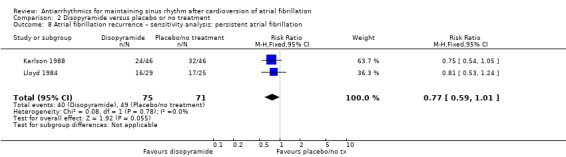
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 8 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
Propafenone
Moderate‐certainty evidence from five RCTs indicated that propafenone reduced atrial fibrillation recurrences by about a third (RR 0.67, 95% CI 0.61 to 0.74; studies = 5, participants = 1098; I2 = 0%; Analysis 3.8). Recurrence rate was 73.0% in the control group and 48.9% (44.5% to 54.0%) in the propafenone group. The corresponding NNTB was 4 (95% CI 3 to 5) participants treated for one year to avoid one recurrence.
3.8. Analysis.
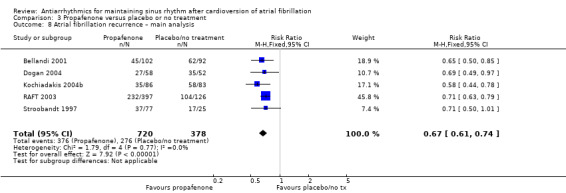
Comparison 3 Propafenone versus placebo or no treatment, Outcome 8 Atrial fibrillation recurrence – main analysis.
Results from sensitivity analyses were very similar (Analysis 3.9; Analysis 3.10).
3.9. Analysis.
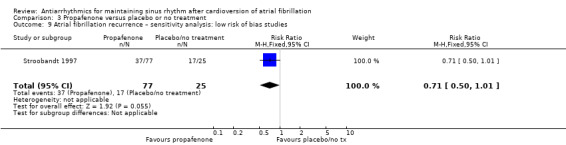
Comparison 3 Propafenone versus placebo or no treatment, Outcome 9 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
3.10. Analysis.

Comparison 3 Propafenone versus placebo or no treatment, Outcome 10 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Flecainide
High‐certainty evidence showed that flecainide reduced atrial fibrillation recurrences by about a third (RR 0.65, 95% CI 0.55 to 0.77; studies = 4, participants = 511; I2 = 29%; Analysis 4.10). That corresponded to a recurrence rate of 69.8% in people not treated or receiving placebo and 45.4% (38.4% to 53.8%) in people receiving flecainide. The NNTB for flecainide was 4 (95% CI 3 to 6) participants treated for one year to avoid one recurrence.
4.10. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 10 Atrial fibrillation recurrence – main analysis.
Results from sensitivity analyses did not differ substantially (Analysis 4.11; Analysis 4.12; Analysis 4.13).
4.11. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 11 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
4.12. Analysis.
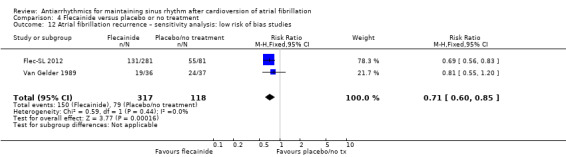
Comparison 4 Flecainide versus placebo or no treatment, Outcome 12 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
4.13. Analysis.

Comparison 4 Flecainide versus placebo or no treatment, Outcome 13 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Metoprolol
Moderate‐certainty evidence from two RCTs suggested that metoprolol reduced recurrences of atrial fibrillation, compared with placebo, but the CI included the possibility of no difference (RR 0.83, 95% CI 0.68 to 1.02; studies = 2, participants = 562; I2 = 59%; Analysis 5.14). The corresponding recurrence rates were 72.0% in people receiving placebo and 59.7% (49.0% to 73.4%) in people receiving metoprolol. All sensitivity analyses included the same two trials so obtained identical results (Analysis 5.12; Analysis 5.15), except the analysis restricted to studies including more than 200 participants, which included only one study and showed no difference between metoprolol and placebo (Analysis 4.13).
5.14. Analysis.

Comparison 5 Metoprolol versus placebo or no treatment, Outcome 14 Atrial fibrillation recurrence – main analysis.
5.15. Analysis.
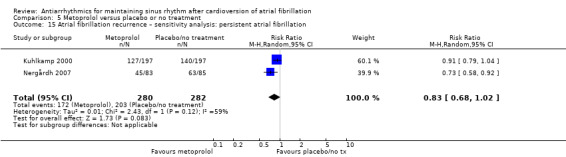
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 15 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
Amiodarone
High‐certainty evidence showed a reduction of atrial fibrillation recurrences with amiodarone of about a half, compared to placebo or no treatment (RR 0.52, 95% CI 0.46 to 0.58; studies = 6, participants = 812; I2 = 33%; Analysis 6.13). This corresponded to a recurrence rate of 81.2% in people not receiving active treatment and 42.2% (95% CI 37.3% to 47.1%) in people receiving amiodarone. The NNTB for amiodarone was 3 (95% CI 2 to 4) participants treated for one year to avoid one recurrence.
6.13. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 13 Atrial fibrillation recurrence – main analysis.
All sensitivity analyses obtained very similar results (Analysis 6.14; Analysis 6.15; Analysis 6.16).
6.14. Analysis.
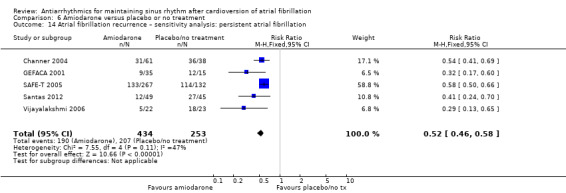
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 14 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
6.15. Analysis.
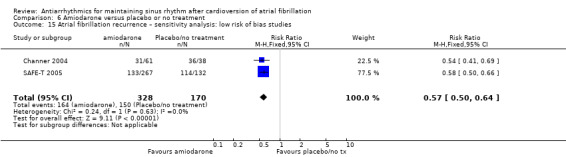
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 15 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
6.16. Analysis.

Comparison 6 Amiodarone versus placebo or no treatment, Outcome 16 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Dofetilide
Moderate‐certainty evidence indicated that dofetilide reduced recurrences of atrial fibrillation, compared to placebo, by about a quarter (RR 0.72, 95% CI 0.61 to 0.85; studies = 3, participants = 1183; I2 = 79%; Analysis 7.13). Recurrence rates were 84.2% in people receiving placebo and 60.6% (95% CI 51.4% to 71.6%) in people receiving dofetilide. The corresponding NNTB was 4 (95% CI 3 to 8) participants treated for one year to avoid one recurrence.
7.13. Analysis.
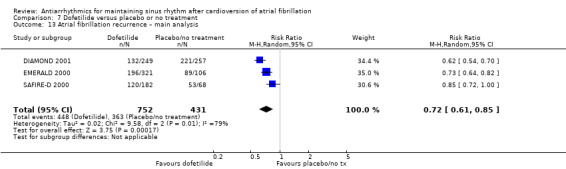
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 13 Atrial fibrillation recurrence – main analysis.
There was substantial heterogeneity between studies on dofetilide for this outcome (I2 = 79%, P = 0.008). All studies showed the same direction of effect (i.e. a reduction of atrial fibrillation recurrences) and the heterogeneity was probably caused by differences in the characteristics of recruited participants.
Sensitivity analyses did not differ from the main analysis (Analysis 7.14; Analysis 7.15; Analysis 7.16).
7.14. Analysis.
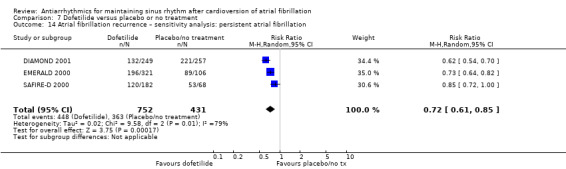
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 14 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
7.15. Analysis.

Comparison 7 Dofetilide versus placebo or no treatment, Outcome 15 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
7.16. Analysis.
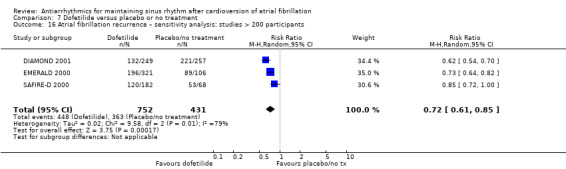
Comparison 7 Dofetilide versus placebo or no treatment, Outcome 16 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Dronedarone
Moderate‐certainty evidence from two RCTs showed a reduction of recurrences of atrial fibrillation with dronedarone of about 15% (RR 0.85, 95% CI 0.80 to 0.91; studies = 2, participants = 1443; I2 = 0%; Analysis 8.15). This corresponded to a recurrence rate of 76.6% in people treated with placebo and 65.1% (95% CI 61.3% to 69.7%) in people treated with dronedarone. The NNTB for dronedarone was 9 (95% CI 7 to 15) participants treated for one year to avoid one recurrence.
8.15. Analysis.
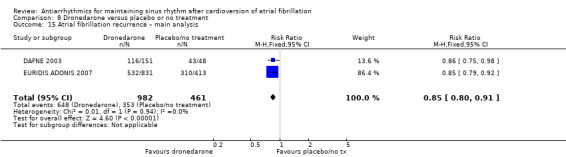
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 15 Atrial fibrillation recurrence – main analysis.
Results from sensitivity analyses were quasi‐identical (Analysis 8.16; Analysis 8.17).
8.16. Analysis.
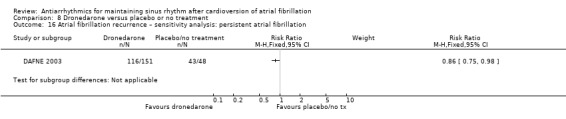
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 16 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
8.17. Analysis.

Comparison 8 Dronedarone versus placebo or no treatment, Outcome 17 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Sotalol
High‐certainty evidence found a reduction of atrial fibrillation recurrences of about a fifth with sotalol compared with placebo or no treatment (RR 0.83, 95% CI 0.80 to 0.87; studies = 14, participants = 3179; I2 = 54%; Analysis 9.20). The corresponding recurrence rates were 78.8% in participants not receiving an antiarrhythmic and 65.4% (95% CI 63.1% to 68.6%) in participants receiving sotalol. The NNTB for sotalol was 7 (95% CI 6 to 10) participants treated for one year to avoid one recurrence.
9.20. Analysis.
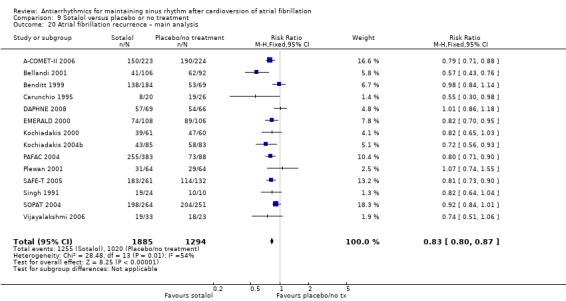
Comparison 9 Sotalol versus placebo or no treatment, Outcome 20 Atrial fibrillation recurrence – main analysis.
There were no substantial difference with the main analysis in any of the sensitivity analyses (Analysis 9.21; Analysis 9.22; Analysis 9.23).
9.21. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 21 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation.
9.22. Analysis.
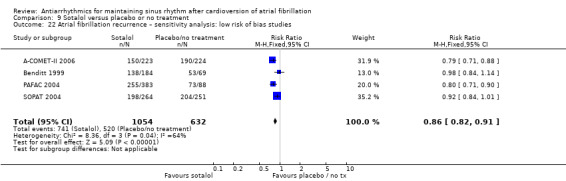
Comparison 9 Sotalol versus placebo or no treatment, Outcome 22 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
9.23. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 23 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Head‐to‐head comparisons
In direct comparisons between antiarrhythmics (Table 14), amiodarone appeared to reduce the recurrence of atrial fibrillation more than the combined class I drugs, more than dronedarone and more than sotalol. There were no other differences in head‐to‐head comparisons between antiarrhythmics.
5. Head‐to‐head trials: recurrence of atrial fibrillation.
| Drug 1 vs drug 2 | Drug 1 | Drug 2 | RR (95% CI) | ||
| Events | Total | Events | Total | ||
| Disopyramide vs other class I drugs | |||||
| Lloyd 1984 | 16 | 29 | 16 | 28 | 0.97 (0.61 to 1.53) |
| PRODIS 1996 | 10 | 31 | 11 | 25 | 0.73 (0.37 to 1.44) |
| Quinidine vs flecainide | |||||
| Naccarelli 1996 | 93 | 117 | 93 | 122 | 1.04 (0.91 to 1.19) |
| Steinbeck 1988 | 10 | 15 | 6 | 15 | 1.67 (0.81 to 3.41) |
| Quinidine vs other class I drugs | |||||
| Lloyd 1984 | 16 | 28 | 16 | 29 | 1.04 (0.65 to 1.64) |
| Naccarelli 1996 | 93 | 117 | 93 | 122 | 1.04 (0.91 to 1.19) |
| Richiardi 1992 | 57 | 98 | 53 | 102 | 1.12 (0.87 to 1.44) |
| Steinbeck 1988 | 10 | 15 | 6 | 15 | 1.67 (0.81 to 3.41) |
| Quinidine vs sotalol | |||||
| Hohnloser 1995 | 7 | 25 | 12 | 25 | 0.58 (0.28 to 1.23) |
| Juul‐Moller 1990 | 49 | 85 | 50 | 98 | 1.13 (0.87 to 1.47) |
| Kalusche 1994 | 15 | 41 | 21 | 41 | 0.71 (0.43 to 1.18) |
| PAFAC 2004 | 244 | 377 | 255 | 383 | 0.97 (0.88 to 1.08) |
| SOCESP 1999 | 25 | 63 | 20 | 58 | 1.15 (0.72 to 1.84) |
| SOPAT 2004 | 375 | 518 | 198 | 264 | 0.97 (0.88 to 1.05) |
| Flecainide vs propafenone | |||||
| Aliot 1996 | 19 | 48 | 26 | 49 | 0.75 (0.48 to 1.16) |
| FAPIS 1996 | 30 | 97 | 30 | 103 | 1.06 (0.70 to 1.62) |
| Amiodarone vs class I drugs | |||||
| AFFIRM Substudy 2003 | 60 | 106 | 99 | 116 | 0.66 (0.55 to 0.80) |
| Kochiadakis 2004a | 20 | 72 | 32 | 74 | 0.64 (0.41 to 1.01) |
| PITAGORA 2008 | 42 | 70 | 54 | 75 | 0.83 (0.66 to 1.06) |
| Villani 1992 | 14 | 35 | 30 | 41 | 0.55 (0.35 to 0.85) |
| Vitolo 1981 | 6 | 28 | 14 | 26 | 0.40 (0.18 to 0.88) |
| Amiodarone vs dronedarone | |||||
| DIONYSOS 2010 | 116 | 255 | 163 | 249 | 0.69 (0.59 to 0.82) |
| Amiodarone vs sotalol | |||||
| AFFIRM Substudy 2003 | 58 | 131 | 81 | 125 | 0.68 (0.54 to 0.86) |
| Kochiadakis 2000 | 27 | 65 | 39 | 61 | 0.65 (0.46 to 0.92) |
| Niu 2006 | 24 | 51 | 36 | 51 | 0.67 (0.47 to 0.94) |
| PITAGORA 2008 | 42 | 70 | 24 | 31 | 0.78 (0.59 to 1.01) |
| SAFE‐T 2005 | 133 | 267 | 183 | 261 | 0.71 (0.62 to 0.82) |
| Vijayalakshmi 2006 | 3 | 11 | 10 | 17 | 0.46 (0.16 to 1.32) |
| Dronedarone vs propafenone | |||||
| Chun 2014 | 36 | 50 | 37 | 50 | 0.97 (0.77 to 1.24) |
| Sotalol vs class I drugs other than quinidine | |||||
| AFFIRM Substudy 2003 | 67 | 88 | 81 | 95 | 0.89 (0.77 to 1.03) |
| Carunchio 1995 | 8 | 20 | 6 | 20 | 1.33 (0.57 to 3.14) |
| Kochiadakis 2004b | 43 | 85 | 35 | 86 | 1.24 (0.89 to 1.73) |
| Reimold 1993 | 32 | 50 | 35 | 50 | 0.91 (0.69 to 1.20) |
| Sotalol vs dofetilide | |||||
| EMERALD 2000 | 74 | 108 | 196 | 321 | 1.12 (0.96 to 1.31) |
| Sotalol vs beta‐blockers | |||||
| DAPHNE 2008 | 57 | 69 | 54 | 66 | 1.01 (0.86 to 1.18) |
| Plewan 2001 | 31 | 64 | 29 | 64 | 1.07 (0.74 to 1.55) |
CI: confidence interval; RR: risk ratio.
Other outcomes
Chronic anticoagulation with warfarin was mandatory (i.e. every participant received anticoagulation therapy throughout the whole follow‐up period) in only three studies (Channer 2004; Hillestad 1971; Van Gelder 1989). In the rest of the studies, the decision on anticoagulation use was left to the judgement of the attending physician. Unfortunately, no trial reported the actual frequency of anticoagulation in the different treatment groups during follow‐up.
Seven trials reported some data on the incidence of heart failure, which was low (ATHENA 2009; DIONYSOS 2010; FAPIS 1996; Hohnloser 1995; Kuhlkamp 2000; PRODIS 1996; Reimold 1993). There were no differences in those trials between participants receiving antiarrhythmics and participants receiving placebo or no treatment.
Subgroup analysis
Twenty‐three of the studies with a control group (placebo or no treatment) included only people with persistent atrial fibrillation. The mean duration of atrial fibrillation in those studies varied greatly, from three to 36 months. Only four studies exclusively included people with paroxysmal atrial fibrillation. The remaining studies included people with both paroxysmal and persistent atrial fibrillation; none reported outcomes separately by type of atrial fibrillation.
It was not possible to compare subgroups of people with permanent and paroxysmal atrial fibrillation for any given antiarrhythmic drug. Therefore, we analysed people with permanent atrial fibrillation separately, for the outcomes and drugs that was possible, but as a sensitivity analysis.
Other planned subgroup analyses (people with heart failure, studies where warfarin was mandatory versus those where it was discretionary, people with a structurally normal heart) were not possible as separate data for each group of participants were seldom available. A more detailed analysis by left ventricular function or by the New York Heart Association (NYHA) class was not possible either, for the same reason.
Discussion
In the third update of this systematic review, we found and included just one new RCT which added little additional information (100 participants, reported only atrial fibrillation recurrence rates). We excluded a previously included study as we become aware its data were already reported in another included study. Additionally, we restructured the analysis of the review to treat each drug separately, in order to present all analyses and results in a clearer way. In the end, some of the results regarding specific antiarrhythmics and conclusions of the review have changed.
Summary of main results
The primary aim of this review was to determine if long‐term treatment with antiarrhythmics carried any clinical benefit to participants in addition to maintenance of sinus rhythm. Consequently, we focused on all‐cause mortality, stroke and potential adverse effects of treatment as the main outcomes.
Concerning all‐cause mortality, we found that no antiarrhythmic drug produced a benefit on mortality and that some antiarrhythmics, sotalol and very probably quinidine, were actually associated with an increase in all‐cause mortality. Results for sotalol were particularly strong and the certainty of evidence was high: included studies had a low risk of bias for this outcome; results were consistent in all sensitivity analyses, replicating the results of the main analysis and indicating a clear association with increased mortality. The mortality rate in the pooled population was low, 0.8% in control participants (placebo or no treatment), but it was doubled in participants receiving sotalol. The mean NNTH was estimated at 102 participants treated for one year to have one additional death.
The results suggesting an increase in mortality also with quinidine were less solid. The CIs included the possibility of no difference and when the analysis was restricted to more recent, larger and higher‐certainty studies, two studies remained that showed no increase in all‐cause mortality in the active treatment groups (PAFAC 2004; SOPAT 2004). A possible explanation is that both studies used a lower dose of quinidine than earlier trials and that quinidine was combined with verapamil, which has been shown to reduce some of the proarrhythmic effects of quinidine, such as accelerated atrio‐ventricular conduction. Finally, the proportion of participants having structural heart disease was lower in the PAFAC 2004 and SOPAT 2004 studies than in earlier trials. Therefore, the certainty of the evidence pointing to increased all‐cause mortality with quinidine was low.
It is important to note that our data do not allow us to exclude a small increase in mortality with other antiarrhythmics, similar to those observed with quinidine and sotalol. Pooled data for other drugs included fewer studies and participants than for quinidine or sotalol and could be underpowered to detect effects that are of small size. In particular, we found very few data on mortality with flecainide. This is concerning because this drug has been shown to induce an excess of mortality in some trials (CAST 1991), and it showed a high risk of proarrhythmia in our review, similar to that of sotalol. The combined flecainide data had only a fifth of the participants included for sotalol and, despite the fact that several of the included studies stated that they analysed mortality, there were no deaths in any treatment group. Thus, we are very unsure about what the effect of long‐term treatment with flecainide on mortality might be. Similarly, the combined data for amiodarone for this outcome included four times fewer participants than with sotalol, so our power to detect small increases in mortality was very limited. Amiodarone has a well‐known high toxicity profile, it showed in our analysis one of the highest risk of withdrawing treatment due to adverse effects (RR 6.70, 95% CI 1.91 to 23.45) and was associated, in other meta‐analyses employing different methods, to a possible increase in mortality (Freemantle 2011; Piccini 2009) (see below: Agreements and disagreements with other studies or reviews).
With respect to adverse effects, virtually all the antiarrhythmics showed more withdrawals from treatment due to adverse effects and were associated with increased proarrhythmic events, compared with participants receiving placebo or no treatment. It is important to remember that we employed an extended definition of proarrhythmia that included severe, symptomatic bradycardia and AV blocks. Metoprolol was associated with an increase in proarrhythmia, precisely because of an increased incidence of severe bradycardias. Of all antiarrhythmics, quinidine at higher doses and sotalol appeared to be the drugs with more withdrawals because of adverse events both compared to controls and to other antiarrhythmics. Withdrawal rates with quinidine were as high as 25% in the pooled population analysed. Amiodarone, even if it compared favourably with class I drugs combined, had a very high RR (6.70) for increasing withdrawals compared to placebo. Moreover, these were the results at one‐year follow‐up, and the adverse effects of amiodarone are known to increase in frequency over time (Harris 1983; Lafuente‐Lafuente 2009).
Regarding other outcomes, our results showed that all the antiarrhythmic drugs studied reduced the recurrence of atrial fibrillation. However, the effectiveness of antiarrhythmics was limited: they reduced recurrences by 20% to 50% compared to controls, which meant that atrial fibrillation still recurred in many participants (43% to 67%) treated with antiarrhythmics at one year. Amiodarone seemed to be the most effective drug in preventing recurrences as it had the lowest RR and in head‐to‐head comparisons it was better than combined class I drugs, dronedarone or sotalol. In spite of this, atrial fibrillation recurred at one year in 43% of participants treated with amiodarone.
Above all, we did not find evidence of any clinical benefit derived from this reduction of recurrences of atrial fibrillation. The results on mortality showed no benefit with any drug, rather the contrary, as we have already discussed. Fewer data existed on stroke or heart failure, but what data we found showed no difference between participants receiving active antiarrhythmic treatment and those not receiving it. The only exception was a single study in which the stroke rate was lower in the dronedarone arm than in the placebo arm (ATHENA 2009). This finding was not confirmed by other studies of dronedarone. This lack of observable clinical benefit from the reduction of atrial fibrillation recurrences could have several explanations: 1. any potential benefit obtained with antiarrhythmics might be erased by the associated toxicity and increased proarrhythmic events; 2. clinical evolution and prognosis might be determined in many participants mostly by their underlying heart disease, rather than by atrial fibrillation itself.
An interesting result of this review was that metoprolol, a beta‐blocker, also showed a reduction in atrial fibrillation recurrence, based on the pooled data from two high‐certainty RCTs (Kuhlkamp 2000; Nergårdh 2007). Besides, there was no difference in preventing recurrences between beta‐blockers and sotalol in two other trials (DAPHNE 2008 comparing sotalol against metoprolol or atenolol, and Plewan 2001 against bisoprolol). The effect of beta‐blockers in reducing the recurrence of atrial fibrillation could be due to their ability to suppress atrial extrasystoles, known to be a frequent precipitant of paroxysmal atrial fibrillation (Haïssaguerre 1998). Beta‐blocker effects might also relate to antihypertensive and anti‐ischaemic actions or to their effect in reducing cardiac remodelling associated with coronary artery disease or heart failure. Like most of the active drugs we studied, metoprolol was associated with increased withdrawals due to adverse effects and increased cases of severe, symptomatic bradycardia.
Overall completeness and applicability of evidence
Most of the included trials reported data on all‐cause mortality, recurrence of atrial fibrillation and main adverse drug events. We also intended to analyse other clinically relevant outcomes such as the frequency of systemic embolism and use of long‐term anticoagulation, or the influence of heart failure and structural heart disease in the response to treatment. Unfortunately data on those outcomes were sparse, if reported at all. In the few trials where they were reported, the frequencies of stroke and heart failure were very low, perhaps because the populations that were included were low risk. The frequency of use of anticoagulants during follow‐up was not reported in any study.
Similarly, we wanted to analyse the influence of structural heart disease on effectiveness, especially with respect to left ventricular ejection fraction and left atrial size, and the influence of duration of atrial fibrillation before cardioversion. These are factors well known to influence the risk of recurrence of atrial fibrillation. Unfortunately this analysis was not possible as separate data were not available for those participants subgroups.
This lack of data for some clinical outcomes was the main limitation of our review. Another limitation could be that in many studies participants were followed up until atrial fibrillation recurred, and not thereafter, hence additional events between that point and the complete one year of follow‐up might have been missed. Also, the populations included in most studies were at low risk of events, the mean age of included participants was 64 years old and most of them had a normal left ventricular ejection fraction. We do not know if our results can be extrapolated to other patient populations, especially older people and those with a reduced left ventricular ejection fraction.
Finally, it is important to remember that maintaining sinus rhythm using long‐term antiarrhythmic drugs is only one possible step in the more general 'rhythm control' strategy, and antiarrhythmic drugs should be put within the perspective of the global strategy chosen for the patient (AHA/ACC/HRS 2014; NICE 2014). Other therapies have proven useful to prevent or reduce recurrence of atrial fibrillation in selected patients, especially catheter ablation (APAF 2006; Oral 2006; Terasawa 2009); and antiarrhythmics have been occasionally used for terminating recurrences (Alboni 2004). However, the effects of these therapies on the important clinical endpoints of all‐cause mortality, stroke and incidence of heart failure are still not well known. A different Cochrane Review has studied the effectiveness of catheter ablation for paroxysmal and persistent atrial fibrillation (Chen 2012).
Quality of the evidence
Two areas of concern regarding the risk of bias of included studies were present: 1. a lack of details, in about 70% of studies, on the procedures followed for randomisation and for concealing the allocation of participants; and 2. a lack of double‐blinding in approximately 60% of studies. The lack of details on the randomisation and concealing procedures can probably be explained, at least partly, by the fact that many of the studies were conducted in the 1980s and 1990s, when the standards for reporting research methods were less developed. Also, it was very difficult to obtain additional data from authors for studies so old. The lack of blinding particularly concerned studies comparing two antiarrhythmics and much less so those studies comparing an antiarrhythmic with no active treatment. Nevertheless, these concerns did not allow us to consider the evidence as 'high certainty'.
In addition to the risk of bias of included studies, another problem was that few data were available for some outcomes, causing imprecision, as analysis produced wide CIs including both the possibility of significant benefit and harm. This problem was more frequent with older drugs (e.g. quinidine, disopyramide, propafenone and flecainide) than newer ones (e.g. metoprolol, dronedarone and sotalol) and affected particularly mortality and, above all, stroke, outcomes that had a low frequency in the studied population. There was occasional inconsistency between studies for some outcomes (e.g. the effect on withdrawals with quinidine and sotalol), but was rare.
However, despite those potential limitations, there were two characteristics that increased our confidence in the results of the review. 1. Consistency of results: for each analysis, there were always several studies available and results were very consistent across individual studies, despite their differences in blinding or in the description of the allocation procedures. 2. Objective outcomes: with the only exception of withdrawals because of adverse effects, the outcomes analysed were measured objectively (ECG records) or were objective outcomes (stroke, mortality), which reduced the risk of bias associated to the lack of blinding.
In the end, we judged the available evidence for most analysed outcomes (all‐cause mortality, withdrawals due to adverse effects, proarrhythmia and recurrence of atrial fibrillation) as moderate certainty.
Potential biases in the review process
There was asymmetry in the funnel plot of one isolated outcome with sotalol (withdrawals because of adverse effects) but not for the other outcomes or with other drugs. Thus, we think the risk of substantial publication bias was low.
There were very few disagreements between authors regarding the inclusion and exclusion of candidate studies. There were also few disagreements regarding the data extracted from included studies. Disagreements were easily resolved by discussion and consensus in all cases.
Conflicts of interest could exist as most studies included in the review were funded by the company manufacturing the antiarrhythmic drug tested.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis by Coplen 1990 found that quinidine increased all‐cause mortality. A meta‐analysis by Nichol 2002 found no difference in all‐cause mortality with any antiarrhythmic, but most of the trials that they pooled had very short follow‐up periods.
A more recent network meta‐analysis, using a mixed treatment comparison method (where the estimates obtained from direct and indirect comparisons are combined in a network of trials), also found an increase in all‐cause mortality associated with sotalol (Freemantle 2011). This meta‐analysis, as well as a different meta‐analysis that compared amiodarone and dronedarone (Piccini 2009), raised the possibility of an increase in mortality associated with amiodarone treatment compared with placebo. However, this result appeared in exploratory analysis (restricted to inclusion of larger studies) and not in the main analysis. Freemantle 2011 did not study quinidine in the meta‐analysis.
Another systematic review, published in 2013, employed different methods to ours (RCTs with follow‐up of three months or more, different statistical methods) but found very similar results: antiarrhythmic drugs reduced atrial fibrillation recurrences but increased withdrawals due to adverse effects, serious adverse effects and proarrhythmia (Sullivan 2013). This study also found, compared to placebo, an increased mortality in participants receiving sotalol and a trend to increased mortality with amiodarone. It did not study quinidine.
Two meta‐analysis, conducted by separate teams but using the same methods, focused on dronedarone and included people with atrial fibrillation but also with heart failure (Chatterjee 2012; De Vecchis 2019). Both found a trend to increased all‐cause and cardiovascular mortality with dronedarone, compared to placebo, in this population.
Authors' conclusions
Implications for practice.
There is high‐certainty evidence of increased all‐cause mortality associated with sotalol treatment, when used for maintaining sinus rhythm in people who had atrial fibrillation. This evidence may have implications for clinical practice, and careful consideration of prescribing for this population would be prudent.
We found low‐certainty evidence suggesting that quinidine may be associated with increased mortality, as well as moderate‐certainty evidence of a marked increase in withdrawals due to adverse events and high‐certainty evidence of increased proarrhythmic events. Therefore, the evidence from this review may have implications for prescribing this drug for maintaining sinus rhythm in people who had atrial fibrillation.
Flecainide has been shown to induce an excess of mortality in some trials in other heart conditions (CAST 1991). Very few data on mortality were available for this drug when employed for maintaining sinus rhythm, making any reliable estimation of mortality in people with atrial fibrillation impossible. However, we found moderate‐certainty evidence of an important increase in proarrhythmic events with flecainide, which would imply a degree of caution may be necessary in using this drug for this population.
Overall, chronic treatment with antiarrhythmics drugs may not be the most appropriate first‐line treatment for people with atrial fibrillation given 1. the concerns regarding increased mortality with several drugs; 2. the modest effectiveness of antiarrhythmic drugs for preventing recurrences of atrial fibrillation; 3. the evidence of increased adverse events with all drugs studied; 4. the evidence of increased proarrhythmic events with most drugs studied, and 5. the absence of evidence of any benefit obtained with these drugs on clinical endpoints. Other treatments, or strategies, with fewer associated adverse events, or higher effectiveness, could be considered before using antiarrhythmics, such as no treatment at all, rate control strategy (Caldeira 2012; Chatterjee 2013), pulmonary vein catheter ablation (CASTLE‐AF 2018; Khan 2014), or, in selected people with paroxysmal atrial fibrillation, episodic, very short‐term use of antiarrhythmics (in hospital or as needed approach) (Alboni 2004; Saborido 2010).
Implications for research.
Adequate evidence exists for some outcomes (withdrawals, proarrhythmia and atrial fibrillation recurrences) for all drugs included in this review. There is good evidence regarding mortality for several antiarrhythmics, but there is an important lack of data on mortality for some drugs, particularly flecainide and propafenone, and limited data for other drugs, such as amiodarone, which does not exclude the possibility of small increases in mortality with them.
Available evidence is limited by the lack of systematic assessment in many studies of important clinical outcomes: stroke, heart failure and functional measures (exercise capacity, quality of life). Trials studying antiarrhythmic drugs should measure their effects on these outcomes in addition to prevention of arrhythmia recurrences. Pending questions include the effects of antiarrhythmics on these clinical outcomes, and their effects in specific subgroups of patients, specifically people with heart failure or reduced left ventricular ejection fraction, and older people.
Finally, new drugs or other procedures that are more effective in preventing atrial fibrillation recurrence or are associated with fewer adverse effects, or both, would be desirable.
What's new
| Date | Event | Description |
|---|---|---|
| 14 June 2019 | New citation required and conclusions have changed | One new trial included. One previously included study excluded because of double publication. Analysis reorganised into nine individual drug comparisons. Conclusions changed. |
| 12 June 2019 | New search has been performed | Searches rerun to January 2019. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 21 April 2015 | Amended | Minor corrections in Figures 6 and 7 |
| 25 July 2014 | New search has been performed | Searches rerun to January 2014. Three new trials were included, studying flecainide, amiodarone and sotalol. Conclusions of the review did not change |
| 25 July 2014 | New citation required but conclusions have not changed | The inclusion of three new trials did not change the conclusions of this review |
| 15 March 2011 | New citation required and conclusions have changed | Searches were re‐run for this update to February 2010. Eleven new publications were included. This new trials studied several drugs (amiodarone, azimilide, dofetilide, dronedarone, metoprolol and sotalol) and added 8212 more patients. Some of the conclusions have changed in light of this new evidence: a) Beta‐blockers (metoprolol) showed a significant effect in preventing AF recurrence; b) In addition to class IA drugs, sotalol was also associated with increased all‐cause mortality. |
| 25 February 2011 | New search has been performed | Eleven new studies added and results changed |
| 8 September 2008 | Amended | Converted to new review format. |
| 23 June 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank all the trial authors who answered our request for additional data; Prof Charles Caulin for his most valuable suggestions; Dr Barbara Stadler for translating articles from German, Lone Gale for helping with articles in Swedish, Dr Qian Zhang for translating from Chinese; Dr Miguel Angel Longas‐Tejero, Prof Stéphane Mouly and Prof Jean‐François Bergmann for their help in previous versions of this review; and all the staff of Cochrane Heart Group for their most important help and support.
Appendices
Appendix 1. Search strategies 2005
CENTRAL
1 ATRIAL FIBRILLATION 2 (atrial near fibrillat*) 3 (auricular* near fibrillat*) 4 (atrium near fibrillat*) 5 (atrial next arrhythmi*) 6 (#1 or #2 or #3 or #4 or #5) 7 ANTI‐ARRHYTHMIA AGENTS 8 antiarrhythmi* 9 anti‐arrhythmi* 10 (anti next arrhythmi*) 11 procainamide 12 disopyramide 13 quinidine 14 mexiletine 15 flecainide 16 propafenone 17 bisoprolol 18 esmolol 19 amiodarone 20 dofetilide 21 sotalol 22 azimilide 23 ibutilide 24 cibenzoline 25 moricizine 26 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17) 27 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26) 28 (#26 or #27) 29 (#6 and #28)
MEDLINE in PubMed
("Atrial Fibrillation" (mh) OR ((atrial OR atrium OR auricular) AND fibrillat*)) AND ("Anti‐Arrhythmia Agents" (mh) OR antiarrhythmi* (tw) OR anti‐arrhythmi* (tw) OR procainamide (tw) OR disopyramide (tw) OR quinidine (tw) OR mexiletine (tw) OR flecainide (tw) propafenone (tw) OR bisoprolol (tw) OR esmolol (tw) OR amiodarone (tw) OR dofetilide (tw) OR sotalol (tw) OR ibutilide (tw) OR azimilide (tw) OR moricizine (tw) OR cibenzoline (tw)) AND ("randomized controlled trial" (pt) OR "controlled clinical trial" (pt) OR "randomized controlled trials" (mh) OR "random allocation" (mh) OR "double‐blind method" (mh) OR "single‐blind method" (mh) OR "clinical trial" (pt) OR "clinical trials" (mh) OR ("clinical trial" (tw)) OR ((singl* (tw) OR doubl* (tw) OR trebl* (tw) OR tripl* (tw)) AND (mask* (tw) OR blind* (tw))) OR ( placebos (mh) OR placebo* (tw) OR random* (tw) OR "research design" (mh:noexp) OR "comparative study" (mh) OR "evaluation studies" (mh) OR "follow‐up studies" (mh) OR "prospective studies" (mh) OR control* (tw) OR prospectiv* (tw) OR volunteer* (tw)) NOT (animal (mh) NOT human (mh))) Notes: The strategy to locate randomized controlled trials is the Cochrane highly sensitive search strategy (all phases), as contained in the Cochrane Reviewer's Handbook (ref: CR Handbook 2003). The "related articles" feature of PubMed MEDLINE was also used.
Search strategy for EMBASE.com
# 1 (atrial OR 'atrium'/exp OR auricular) AND fibrillat* # 2 'anti‐arrhythmic' OR antiarrhythmi* OR 'procainamide'/exp OR 'disopyramide'/exp OR 'quinidine'/exp OR 'mexiletine'/exp OR 'flecainide'/exp OR 'propafenone'/exp OR 'bisoprolol'/exp OR 'esmolol'/exp OR 'amiodarone'/exp OR 'dofetilide'/exp OR 'sotalol'/exp OR 'ibutilide'/exp OR 'azimilide'/exp OR 'dronedarone'/exp OR 'moricizine'/exp OR 'cibenzoline'/exp # 3 'randomized controlled trial'/exp OR 'controlled clinical trial'/exp OR 'randomized controlled trials'/exp OR 'random allocation'/exp OR 'double‐blind method'/exp OR 'single‐blind method'/exp OR 'clinical trial'/exp OR 'clinical trials'/exp OR ((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR ('placebos'/exp OR placebo* OR random* OR 'comparative study'/exp OR 'evaluation studies'/exp OR 'follow‐up studies'/exp OR 'prospective studies'/exp OR control* OR prospectiv* OR volunteer*) # 4 #1 AND #2 AND #3 Note: The "related articles" feature was also used.
Appendix 2. Search strategies 2010
CENTRAL in the Cochrane Library
#1 MeSH descriptor Atrial Fibrillation this term only #2 (atrial in All Text near/3 fibrillat* in All Text) #3 (auricular* in All Text near/3 fibrillat* in All Text) #4 (atrium in All Text near/3 fibrillat* in All Text) #5 atrial next arrhythmi* in All Text #6 (#1 or #2 or #3 or #4 or #5) #7 MeSH descriptor Anti‐Arrhythmia Agents explode all trees #8 antiarrhythmi* in All Text #9 anti‐arrhythmi* in All Text #10 dronedarone in All Text #11 amiodarone in All Text #12 bisoprolol in All Text #13 disopyramide in All Text #14 dofetilide in All Text #15 azimilide in All Text #16 ibutilide in All Text #17 flecainide in All Text #18 propafenone in All Text #19 quinidine in All Text #20 cibenzoline in All Text #21 moricizine in All Text #22 mexiletine in All Text #23 procainamide in All Text #24 sotalol in All Text #25 esmolol in All Text #26 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #27 (#17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) #28 (#26 or #27) #29 (#6 and #28)
MEDLINE Ovid
1 Atrial Fibrillation/ 2 atrial fibrillation.tw. 3 atrium fibrillation.tw. 4 auricular fibrillation.tw. 5 or/1‐4 6 exp Anti‐Arrhythmia Agents/ 7 antiarrhythmi$.tw. 8 anti‐arrhythmi$.tw. 9 dronedarone.tw. 10 amiodarone.tw. 11 bisoprolol.tw. 12 disopyramide.tw. 13 dofetilide.tw. 14 azimilide.tw. 15 ibutilide.tw. 16 flecainide.tw. 17 propafenone.tw. 18 quinidine.tw. 19 cibenzoline.tw. 20 moricizine.tw. 21 mexiletine.tw. 22 procainamide.tw. 23 sotalol.tw. 24 esmolol.tw. 25 or/6‐24 26 5 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 Randomized controlled trials/ 30 random allocation/ 31 double blind method/ 32 single‐blind method/ 33 or/27‐32 34 exp animal/ not humans/ 35 33 not 34 36 clinical trial.pt. 37 exp Clinical Trials as Topic/ 38 (clin$ adj25 trial$).ti,ab. 39 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 40 placebos/ 41 placebo$.ti,ab. 42 random$.ti,ab. 43 research design/ 44 or/36‐43 45 44 not 34 46 35 or 45 47 26 and 46 48 limit 47 to yr="2005 ‐ 2010"
Embase Ovid to 2010 Week 6
1 heart atrium fibrillation/ 2 atrial fibrillation.tw. 3 atrium fibrillation.tw. 4 auricular fibrillation.tw. 5 or/1‐4 6 exp antiarrhythmic agent/ 7 antiarrhythmi$.tw. 8 anti‐arrhythmi$.tw. 9 dronedarone.tw. 10 amiodarone.tw. 11 bisoprolol.tw. 12 disopyramide.tw. 13 dofetilide.tw. 14 azimilide.tw. 15 ibutilide.tw. 16 flecainide.tw. 17 propafenone.tw. 18 quinidine.tw. 19 cibenzoline.tw. 20 moricizine.tw. 21 mexiletine.tw. 22 procainamide.tw. 23 sotalol.tw. 24 esmolol.tw. 25 or/6‐24 26 5 and 25 27 random$.tw. 28 factorial$.tw. 29 (crossover$ or cross‐over$).tw. 30 placebo$.tw. 31 (doubl$ adj blind$).tw. 32 (singl$ adj blind$).tw. 33 assign$.tw. 34 allocat$.tw. 35 volunteer$.tw. 36 Crossover Procedure/ 37 Double‐blind Procedure/ 38 Randomized Controlled Trial/ 39 Single‐blind Procedure/ 40 or/27‐39 41 (animal/ or nonhuman/) not human/ 42 40 not 41 43 26 and 42 44 limit 43 to yr="2005 ‐ 2010"
Appendix 3. Search strategies 2014
Note: the RCT filter for MEDLINE was updated. The RCT filter for MEDLINE is now the Cochrane sensitivity‐maximising RCT filter, and for Embase, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions have been applied (Lefebvre 2011).
CENTRAL
#1 MeSH descriptor Atrial Fibrillation this term only #2 (atrial in All Text near/3 fibrillat* in All Text) #3 (auricular* in All Text near/3 fibrillat* in All Text) #4 (atrium in All Text near/3 fibrillat* in All Text) #5 atrial next arrhythmi* in All Text #6 (#1 or #2 or #3 or #4 or #5) #7 MeSH descriptor Anti‐Arrhythmia Agents explode all trees #8 antiarrhythmi* in All Text #9 anti‐arrhythmi* in All Text #10 dronedarone in All Text #11 amiodarone in All Text #12 bisoprolol in All; Text #13 disopyramide in All Text #14 dofetilide in All Text #15 azimilide in All Text #16 ibutilide in All Text #17 flecainide in All Text #18 propafenone in All Text #19 quinidine in All Text #20 cibenzoline in All Text #21 moricizine in All Text #22 mexiletine in All Text #23 procainamide in All Text #24 sotalol in All Text #25 esmolol in All Text #26 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #27 (#17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25) #28 (#26 or #27) #29 (#6 and #28)
MEDLINE Ovid (up to October 2013)
1 Atrial Fibrillation/ 2 atrial fibrillation.tw. 3 atrium fibrillation.tw. 4 auricular fibrillation.tw. 5 or/1‐4 6 exp Anti‐Arrhythmia Agents/ 7 antiarrhythmi$.tw. 8 anti‐arrhythmi$.tw. 9 dronedarone.tw. 10 amiodarone.tw. 11 bisoprolol.tw. 12 disopyramide.tw. 13 dofetilide.tw. 14 azimilide.tw. 15 ibutilide.tw. 16 flecainide.tw. 17 propafenone.tw. 18 quinidine.tw. 19 cibenzoline.tw. 20 moricizine.tw. 21 mexiletine.tw. 22 procainamide.tw. 23 sotalol.tw. 24 esmolol.tw. 25 or/6‐24 26 5 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomized.ab. 30 placebo.ab. 31 drug therapy.fs. 32 randomly.ab. 33 trial.ab. 34 groups.ab. 35 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36 exp animals/ not humans.sh. 37 35 not 36 38 26 and 37(6063) 39 (201002* or 201003* or 201004* or 201005* or 201006* or 201007* or 201008* or 201009* or 201010* or 201011* or 201012* or 2011* or 2012* or 2013*).ed. 40 38 and 39
MEDLINE PubMed (October 2013 to January 2014)
("Atrial Fibrillation" (mh) OR ((atrial OR atrium OR auricular) AND fibrillat*)) AND ("Anti‐Arrhythmia Agents" (mh) OR antiarrhythmi* (tw) OR anti‐arrhythmi* (tw) OR procainamide (tw) OR disopyramide (tw) OR quinidine (tw) OR mexiletine (tw) OR flecainide (tw) propafenone (tw) OR bisoprolol (tw) OR esmolol (tw) OR amiodarone (tw) OR dofetilide (tw) OR sotalol (tw) OR ibutilide (tw) OR azimilide (tw) OR moricizine (tw) OR cibenzoline (tw)) AND ("randomized controlled trial" (pt) OR "controlled clinical trial" (pt) OR randomized (tiab) OR placebo (tiab) OR "drug therapy" (sh) OR randomly (tiab) OR trial (tiab) OR groups (tiab)) NOT (animal (mh) NOT human (mh)))
Embase Ovid (up to October 2013)
1 exp heart atrium fibrillation/ 2 atrial fibrillation.tw. 3 atrium fibrillation.tw. 4 auricular fibrillation.tw. 5 or/1‐4 6 exp antiarrhythmic agent/ 7 antiarrhythmi$.tw. 8 anti‐arrhythmi$.tw. 9 dronedarone.tw. 10 amiodarone.tw. 11 bisoprolol.tw. 12 disopyramide.tw. 13 dofetilide.tw. 14 azimilide.tw. 15 ibutilide.tw. 16 flecainide.tw. 17 propafenone.tw. 18 quinidine.tw. 19 cibenzoline.tw. 20 moricizine.tw. 21 mexiletine.tw. 22 procainamide.tw. 23 sotalol.tw. 24 esmolol.tw. 25 or/6‐24 26 5 and 25 27 random$.tw. 28 factorial$.tw. 29 crossover$.tw. 30 cross over$.tw. 31 cross‐over$.tw. 32 placebo$.tw. 33 (doubl$ adj blind$).tw. 34 (singl$ adj blind$).tw. 35 assign$.tw. 36 allocat$.tw. 37 volunteer$.tw. 38 crossover procedure/ 39 double blind procedure/ 40 randomized controlled trial/ 41 single blind procedure/ 42 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 43 (animal/ or nonhuman/) not human/ 44 42 not 43 45 26 and 44 46 (2010* or 2011* or 2012* or 2013*).em. 47 (2010* or 2011* or 2012* or 2013*).dd. 48 46 or 47 49 45 and 48
EMBASE.com (October 2013 to January 2014)
("Atrial Fibrillation" (mh) OR ((atrial OR atrium OR auricular) AND fibrillat*)) # 1 (atrial OR 'atrium'/exp OR auricular) AND fibrillat* # 2 'anti‐arrhythmic' OR antiarrhythmi* OR 'procainamide'/exp OR 'disopyramide'/exp OR 'quinidine'/exp OR 'mexiletine'/exp OR 'flecainide'/exp OR 'propafenone'/exp OR 'bisoprolol'/exp OR 'esmolol'/exp OR 'amiodarone'/exp OR 'dofetilide'/exp OR 'sotalol'/exp OR 'ibutilide'/exp OR 'azimilide'/exp OR 'dronedarone'/exp OR 'moricizine'/exp OR 'cibenzoline'/exp # 3 'randomized controlled trial'/exp OR 'randomized controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR randomized OR 'placebo'/exp OR placebo OR 'drug therapy'/exp OR 'drug therapy' OR randomly OR trial OR groups NOT ('animal'/exp OR animal NOT ('human'/exp OR human)) # 4 #1 AND #2 AND #3
Appendix 4. Search strategies 2019
CENTRAL
#1 MeSH descriptor: [Atrial Fibrillation] this term only
#2 atrial near/3 fibrillat*
#3 auricular* near/3 fibrillat*
#4 atrium near/3 fibrillat*
#5 atrial next arrhythmi*
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Anti‐Arrhythmia Agents] explode all trees
#8 antiarrhythmi*
#9 anti‐arrhythmi*
#10 dronedarone
#11 amiodarone
#12 bisoprolol
#13 disopyramide
#14 dofetilide
#15 azimilide
#16 ibutilide
#17 flecainide
#18 propafenone
#19 quinidine
#20 cibenzoline
#21 moricizine
#22 mexiletine
#23 procainamide
#24 sotalol
#25 esmolol
#26 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#27 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25
#28 #26 or #27
#29 #6 and #28 Publication Year from 2014 to 2019
MEDLINE Ovid
1 Atrial Fibrillation/
2 atrial fibrillat*.tw.
3 atrium fibrillat*.tw.
4 auricular fibrillat*.tw.
5 or/1‐4
6 exp Anti‐Arrhythmia Agents/
7 antiarrhythmi$.tw.
8 anti‐arrhythmi$.tw.
9 dronedarone.tw.
10 amiodarone.tw.
11 bisoprolol.tw.
12 disopyramide.tw.
13 dofetilide.tw.
14 azimilide.tw.
15 ibutilide.tw.
16 flecainide.tw.
17 propafenone.tw.
18 quinidine.tw.
19 cibenzoline.tw.
20 moricizine.tw.
21 mexiletine.tw.
22 procainamide.tw.
23 sotalol.tw.
24 esmolol.tw.
25 or/6‐24
26 5 and 25
27 randomized controlled trial.pt.
28 controlled clinical trial.pt.
29 randomized.ab.
30 placebo.ab.
31 drug therapy.fs.
32 randomly.ab.
33 trial.ab.
34 groups.ab.
35 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36 exp animals/ not humans.sh.
37 35 not 36
38 26 and 37
39 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).ed.
40 38 and 39
41 38 not (1* or 2*).ed.
42 40 or 41
Embase Ovid
1 exp heart atrium fibrillation/
2 atrial fibrillation.tw.
3 atrium fibrillation.tw.
4 auricular fibrillation.tw.
5 or/1‐4
6 exp antiarrhythmic agent/
7 antiarrhythmi$.tw.
8 anti‐arrhythmi$.tw.
9 dronedarone.tw.
10 amiodarone.tw.
11 bisoprolol.tw.
12 disopyramide.tw.
13 dofetilide.tw.
14 azimilide.tw.
15 ibutilide.tw.
16 flecainide.tw.
17 propafenone.tw.
18 quinidine.tw.
19 cibenzoline.tw.
20 moricizine.tw.
21 mexiletine.tw.
22 procainamide.tw.
23 sotalol.tw.
24 esmolol.tw.
25 or/6‐24
26 5 and 25
27 random$.tw.
28 factorial$.tw.
29 crossover$.tw.
30 cross over$.tw.
31 cross‐over$.tw.
32 placebo$.tw.
33 (doubl$ adj blind$).tw.
34 (singl$ adj blind$).tw.
35 assign$.tw.
36 allocat$.tw.
37 volunteer$.tw.
38 crossover procedure/
39 double blind procedure/
40 randomized controlled trial/
41 single blind procedure/
42 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41
43 (animal/ or nonhuman/) not human/
44 42 not 43
45 26 and 44
46 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).em.
47 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dd.
48 46 or 47
49 45 and 48
ClinicalTrials.gov Condition or disease: Atrial Fibrillation OR atrial fibrillat* OR atrium fibrillat* OR auricular fibrillat* Other terms: antiarrhythmi* OR anti‐arrhythmi* OR dronedarone OR amiodarone OR bisoprolol OR disopyramide OR dofetilide OR azimilide OR ibutilide OR flecainide OR propafenone OR quinidine OR cibenzoline OR moricizine OR mexiletine OR procainamide OR sotalol Filters: Recruiting: All ; Country: All WHO ICTRP Advanced Search:
title: atrial fibrillation Condition: Atrial Fibrillation OR atrial fibrillat* OR atrium fibrillat* OR auricular fibrillat* Intervention: antiarrhythmi* OR anti‐arrhythmi* OR dronedarone OR amiodarone OR bisoprolol OR disopyramide OR dofetilide OR azimilide OR ibutilide OR flecainide OR propafenone OR quinidine OR cibenzoline OR moricizine OR mexiletine OR procainamide OR sotalol Filters: Recruiting: All ; Country: All ; Phases: All
Data and analyses
Comparison 1. Quinidine versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality – main analysis | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.84, 4.77] |
| 2 All‐cause mortality – sensitivity analysis intention to treat (ITT) worse case: missing participants counted as events | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.96, 4.67] |
| 3 All‐cause mortality – subgroup analysis: older and recent studies | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.84, 4.77] |
| 3.1 Older studies, higher dose | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.85, 8.83] |
| 3.2 More recent studies, lower dose | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
| 4 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation | 5 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.73, 4.53] |
| 5 All‐cause mortality – sensitivity analysis: low risk of bias studies | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
| 6 All‐cause mortality – sensitivity analysis: studies > 200 participants | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
| 7 Withdrawals due to adverse effects – main analysis | 7 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.87, 2.78] |
| 8 Withdrawals due to adverse effects – subgroup analysis: older and recent studies | 7 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.87, 2.78] |
| 8.1 Older studies, higher dose | 5 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.29, 7.22] |
| 8.2 More recent studies, lower dose | 2 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.27] |
| 9 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.99, 4.87] |
| 10 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 11 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.09] |
| 12 Proarrhythmia – main analysis | 7 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.95, 4.41] |
| 13 Proarrhythmia – subgroup analysis: older and recent studies | 7 | 1677 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.96, 4.42] |
| 13.1 Older studies, higher dose | 5 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.87, 11.32] |
| 13.2 More recent studies, lower dose | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
| 14 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation | 5 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.93, 7.53] |
| 15 Proarrhythmia – sensitivity analysis: low risk of bias studies | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
| 16 Proarrhythmia – sensitivity analysis: studies > 200 participants | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
| 17 Stroke – main analysis | 4 | 1107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.25, 3.83] |
| 18 Stroke – sensitivity analysis: persistent atrial fibrillation | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.01] |
| 19 Stroke – sensitivity analysis: low risk of bias studies | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20 Stroke – sensitivity analysis: studies > 200 participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21 Atrial fibrillation recurrence – main analysis | 7 | 1624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.78, 0.88] |
| 22 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation | 5 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.70, 0.85] |
| 23 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.80, 0.91] |
| 24 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.92] |
1.13. Analysis.
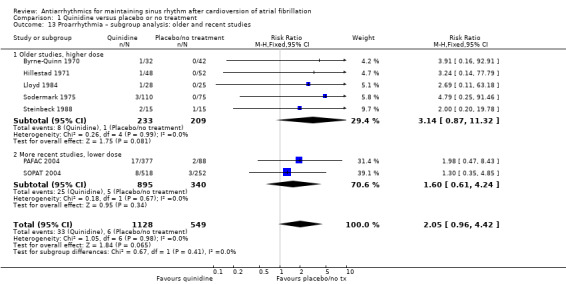
Comparison 1 Quinidine versus placebo or no treatment, Outcome 13 Proarrhythmia – subgroup analysis: older and recent studies.
1.18. Analysis.
Comparison 1 Quinidine versus placebo or no treatment, Outcome 18 Stroke – sensitivity analysis: persistent atrial fibrillation.
1.19. Analysis.
Comparison 1 Quinidine versus placebo or no treatment, Outcome 19 Stroke – sensitivity analysis: low risk of bias studies.
1.20. Analysis.
Comparison 1 Quinidine versus placebo or no treatment, Outcome 20 Stroke – sensitivity analysis: studies > 200 participants.
Comparison 2. Disopyramide versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality – main analysis | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.37] |
| 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [0.68, 45.09] |
| 3 Withdrawals due to adverse effects – main analysis | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [0.95, 14.24] |
| 4 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [0.95, 14.24] |
| 5 Stroke – main analysis | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.91] |
| 6 Stroke – subgroup analysis: persistent atrial fibrillation | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.91] |
| 7 Atrial fibrillation recurrence – main analysis | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.01] |
| 8 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.01] |
2.6. Analysis.
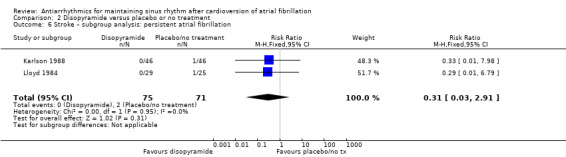
Comparison 2 Disopyramide versus placebo or no treatment, Outcome 6 Stroke – subgroup analysis: persistent atrial fibrillation.
Comparison 3. Propafenone versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality – main analysis | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.68] |
| 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.45, 3.62] |
| 3 All‐cause mortality – sensitivity analysis: low risk of bias studies | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.64] |
| 4 Withdrawals due to adverse effects – main analysis | 5 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.07, 2.46] |
| 5 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants | 1 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.79, 2.11] |
| 6 Proarrhythmia – main analysis | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.39, 4.47] |
| 7 Proarrhythmia – sensitivity analysis: low risk of bias studies | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.75] |
| 8 Atrial fibrillation recurrence – main analysis | 5 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.61, 0.74] |
| 9 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.50, 1.01] |
| 10 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants | 1 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.63, 0.79] |
Comparison 4. Flecainide versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals due to adverse effects – main analysis | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.41 [0.91, 260.19] |
| 2 Proarrhythmia – main analysis | 4 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.80 [1.30, 17.77] |
| 3 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.35 [0.91, 44.22] |
| 4 Proarrhythmia – sensitivity analysis: low risk of bias studies | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.35 [0.91, 44.22] |
| 5 Proarrhythmia – sensitivity analysis: studies > 200 participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Stroke – main analysis | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.11, 39.00] |
| 7 Stroke – subgroup analysis: persistent atrial fibrillation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Stroke – sensitivity analysis: low risk of bias studies | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Stroke – sensitivity analysis: studies > 200 participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Atrial fibrillation recurrence – main analysis | 4 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.55, 0.77] |
| 11 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.60, 0.85] |
| 12 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.60, 0.85] |
| 13 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
4.7. Analysis.
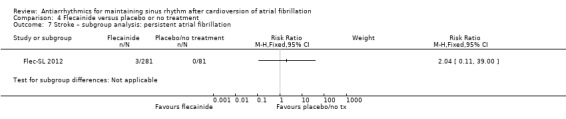
Comparison 4 Flecainide versus placebo or no treatment, Outcome 7 Stroke – subgroup analysis: persistent atrial fibrillation.
4.8. Analysis.
Comparison 4 Flecainide versus placebo or no treatment, Outcome 8 Stroke – sensitivity analysis: low risk of bias studies.
4.9. Analysis.
Comparison 4 Flecainide versus placebo or no treatment, Outcome 9 Stroke – sensitivity analysis: studies > 200 participants.
Comparison 5. Metoprolol versus placebo or no treatment.
5.16. Analysis.

Comparison 5 Metoprolol versus placebo or no treatment, Outcome 16 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies.
5.17. Analysis.
Comparison 5 Metoprolol versus placebo or no treatment, Outcome 17 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants.
Comparison 6. Amiodarone versus placebo or no treatment.
6.11. Analysis.
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 11 Stroke – sensitivity analysis: persistent atrial fibrillation.
6.12. Analysis.
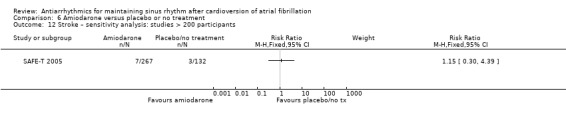
Comparison 6 Amiodarone versus placebo or no treatment, Outcome 12 Stroke – sensitivity analysis: studies > 200 participants.
Comparison 7. Dofetilide versus placebo or no treatment.
Comparison 8. Dronedarone versus placebo or no treatment.
8.10. Analysis.
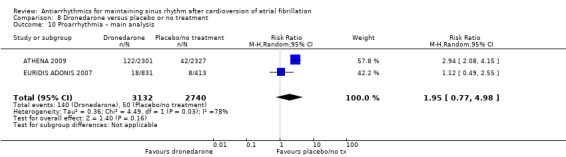
Comparison 8 Dronedarone versus placebo or no treatment, Outcome 10 Proarrhythmia – main analysis.
Comparison 9. Sotalol versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality – main analysis | 5 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.03, 4.81] |
| 2 All‐cause mortality – intention to treat (ITT) worse case: missing participants counted as events | 10 | 2757 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.28, 3.20] |
| 3 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation | 3 | 1311 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.06, 5.98] |
| 4 All‐cause mortality – sensitivity analysis: low risk of bias studies | 3 | 1311 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.06, 5.98] |
| 5 All‐cause mortality – sensitivity analysis: studies > 200 participants | 4 | 1826 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.16, 6.09] |
| 6 Withdrawals due to adverse effects – main analysis | 12 | 2688 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.23, 3.11] |
| 7 Withdrawals due to adverse effects – sotalol: heterogeneity study | 12 | 2688 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.23, 3.11] |
| 7.1 PAFAC and SOPAT trials | 2 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.25] |
| 7.2 Rest of studies | 10 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.81, 4.22] |
| 8 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation | 6 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.28, 2.41] |
| 9 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies | 4 | 1686 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.82, 2.81] |
| 10 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants | 5 | 1900 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.97, 3.35] |
| 11 Proarrhythmia – main analysis | 12 | 2989 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.16, 5.83] |
| 12 Proarrhythmia – sotalol: heterogeneity study | 11 | 2826 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [2.07, 5.67] |
| 12.1 PAFAC and SOPAT trials | 2 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.51, 4.37] |
| 12.2 Rest of studies | 9 | 1840 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.32 [2.40, 7.76] |
| 13 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation | 6 | 1687 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.37 [2.25, 8.52] |
| 14 Proarrhythmia – sensitivity analysis: low risk of bias studies | 4 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.73, 5.40] |
| 15 Proarrhythmia – sensitivity analysis: studies > 200 participants | 6 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [1.77, 5.06] |
| 16 Stroke – main analysis | 3 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.48, 4.51] |
| 17 Stroke – sensitivity analysis: persistent atrial fibrillation | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.36, 5.00] |
| 18 Stroke – sensitivity analysis: low risk of bias studies | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.20, 16.71] |
| 19 Stroke – sensitivity analysis: studies > 200 participants | 3 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.48, 4.51] |
| 20 Atrial fibrillation recurrence – main analysis | 14 | 3179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.80, 0.87] |
| 21 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation | 7 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.77, 0.86] |
| 22 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies | 4 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.82, 0.91] |
| 23 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants | 6 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.81, 0.89] |
9.12. Analysis.
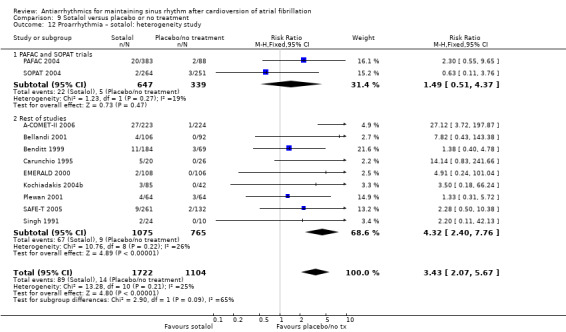
Comparison 9 Sotalol versus placebo or no treatment, Outcome 12 Proarrhythmia – sotalol: heterogeneity study.
9.17. Analysis.

Comparison 9 Sotalol versus placebo or no treatment, Outcome 17 Stroke – sensitivity analysis: persistent atrial fibrillation.
9.18. Analysis.
Comparison 9 Sotalol versus placebo or no treatment, Outcome 18 Stroke – sensitivity analysis: low risk of bias studies.
9.19. Analysis.
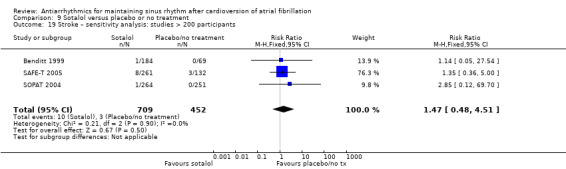
Comparison 9 Sotalol versus placebo or no treatment, Outcome 19 Stroke – sensitivity analysis: studies > 200 participants.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
A‐COMET‐I 2006.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Symptomatic AF in the previous 6 months. Type: recent onset 28%, persistent 72% (mean duration: NS). n = 446 Men: 78% Age (mean): 65 (SD 10) years Structural heart disease: 70%. LAD: NS. LVEF: NS |
|
| Interventions | Azimilide 250 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. "Identical cellulose film‐coated tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts well described. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were reported. Events were classified by an event committee whose members were blinded to treatment. |
| Other bias | Low risk | No other bias apparent. |
A‐COMET‐II 2006.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Symptomatic AF, persistent for > 48 hours, < 6 months' duration. n = 658 Men: 66% Age (mean): 62 (SD 9) years Structural heart disease: 73%. LAD: enlarged in 72%. LVEF: reduced (< 40%) in 10% of participants |
|
| Interventions | Azimilide 125 mg/day vs sotalol 320 mg/day vs placebo Method of AF cardioversion: 6% pharmacological, 94% electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1:1 ratio according to a randomisation code generated before the start of the study. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Quote: "A dummy technique was used to provide the same looking and number of pills to all subjects". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals from study well described and intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Low risk | All prespecified study outcomes of interest and all main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
A‐STAR 2006.
| Methods | RCT Placebo‐controlled, single or double‐blind? Loss to follow‐up reported: no |
|
| Participants | Symptomatic AF in the previous 6 months Type: paroxysmal or recent onset 95%, persistent 5% (mean duration: NS). n = 431 Men: 62% Age (mean): 62 (SD 10) years Structural heart disease: 69%. LAD: NS. LVEF: NS |
|
| Interventions | Azimilide 125 mg/day vs placebo Method of AF cardioversion: 100% spontaneous or pharmacological Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Adverse effects Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Procedure of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebo‐controlled study, but it was not presented as single or double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Few details given on the 61 participants who withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest and main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
AFFIRM Substudy 2003.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | AF likely to be recurrent and to cause illness or death. Type: paroxysmal or recent onset 29%, persistent 71% (mean duration: NS). n = 410 Men: 63% Age (mean): 69 (SD 8) years Structural heart disease: 85%. LAD: enlarged in 71%. LVEF: 55% |
|
| Interventions | Amiodarone 200 mg/day vs class I drugs vs sotalol 240 mg/day Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 3.8 years: Mortality At 12 months: Proarrhythmia Adverse effects AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | Telephone call to the distant, centralised, Clinical Trial Center, after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few participants withdrew and were lost to follow‐up, all well reported. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
AFIB 1997.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Previous AF documented in the last 2 years. Type: NS. n = 1227 Men: 62% Age (mean): 63 (SD 13) years Structural heart disease: 67%. LAD: NS. LVEF: NS |
|
| Interventions | Bidisomide various doses (400 mg/day, 800 mg/day, 1200 mg/day) vs placebo Method of AF cardioversion: pharmacological 70%, electrical 30% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Presented as randomised but method employed not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described as double‐blind or single‐blind, even if it is said that "Medications were packaged identically". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts of participants were well described, and the proportions were not excessive. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. Basic main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Aliot 1996.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal AF documented any time before (70% in last 1 year). n = 97 Men: 53% Age (mean): 63 (SD 12) years Structural heart disease: 45%. LAD: NS. LVEF: NS |
|
| Interventions | Flecainide 100–200 mg/day vs propafenone 600 mg/day Method of AF cardioversion: pharmacological Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial but randomisation procedure not described. |
| Allocation concealment (selection bias) | Unclear risk | Methods to conceal allocation not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and discontinuations from the study were well reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes were well reported. Adverse events and deaths were reviewed by an external safety panel. |
| Other bias | Low risk | No other bias apparent. |
ASAP 2003.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Previous AF documented in the last 2 years. Type: NS. n = 1380 (4 substudies) Men: 66% Age (mean): 63 (SD 13) years Structural heart disease: 73%. LAD: NS. LVEF: NS |
|
| Interventions | Azimilide various doses (35–125 mg/day) vs placebo Method of AF cardioversion: pharmacological 65%, electrical 35% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects Time to AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Presented as randomised, no details given about the procedure used. |
| Allocation concealment (selection bias) | Unclear risk | How allocation was concealed not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as "double‐blind", but the methods employed were not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts from the study were well described and were unlikely to influence overall outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest and main outcomes expected were adequately reported. |
| Other bias | Unclear risk | Combination of 4 similar RCTs (SVA‐1, SVA‐2, SVA‐3 and SVA‐4) assessing various doses of azimilide, pooling subsamples of people with AF. |
ATHENA 2009.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Non‐permanent AF with high risk of recurrence Type: all types, % NS. n = 4628 Men: 53% Age (mean): 72 (SD 9) years Structural heart disease: 57%. LAD: NS. LVEF: reduced (< 45%) in 12% |
|
| Interventions | Dronedarone 800 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary, 60% participants in both groups |
|
| Outcomes | At 22 months: Mortality Proarrhythmia Adverse effects Stroke Hospitalisations due to cardiovascular events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Presented as randomised, no detail given about the procedure used. |
| Allocation concealment (selection bias) | Low risk | Central allocation of participants after randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" trial, but no details given on the procedure followed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts well described and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All expected and prespecified outcomes of interest were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Bellandi 2001.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal recurrent AF (47%), or persistent AF (53%, mean duration: NS). n = 194 Men: 56% Age (mean): 52 (range 20–75) years Structural heart disease: 72%. LAD: 42 mm. LVEF: 55% |
|
| Interventions | Propafenone 900 mg/day vs sotalol 240 mg/day vs placebo Method of AF cardioversion: pharmacological 89%, electrical 11% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Presented as a double‐blind trial, but methods employed were not detailed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost to follow‐up, balanced, well reported. |
| Selective reporting (reporting bias) | Low risk | All main outcomes of interest, prespecified and expected, were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Benditt 1999.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | AF or AFl documented in the last 3 months. Type: paroxysmal or recent onset 77%, persistent 23% (mean duration: NS). n = 253 Men: 64% Age (mean): 62 (range 24–86) years Structural heart disease: 57%. LAD: NS (enlarged in 28%). LVEF: NS |
|
| Interventions | Sotalol various doses (80 mg/day, 120 mg/day, 160 mg/day) vs placebo Method of AF cardioversion: NS Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned according to a computer‐generated random code. |
| Allocation concealment (selection bias) | Low risk | Allocation by a central office. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study, procedures described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts from the study were well reported. |
| Selective reporting (reporting bias) | Low risk | All main outcomes prespecified and expected were well reported. |
| Other bias | Low risk | No other bias apparent. |
Byrne‐Quinn 1970.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (mean duration: 12 months). n = 74 Men: 53% Age (mean): 54 (range 30–70) years Structural heart disease: 80%. LAD: NS. LVEF: NS |
|
| Interventions | Quinidine 1.2 g/day vs placebo Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on the method employed to generate the random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Participants "were randomly allocated to two groups". No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical tablets. Participants and investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High proportion of participants withdrawn, unbalanced between groups. |
| Selective reporting (reporting bias) | Low risk | All study's prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Carunchio 1995.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Recurrent AF (type: NS) with > 3 episodes in previous 1 year. NS. n = 66 Men: 50% Age (mean): 48 (range 30–69) years Structural heart disease: 65%. LAD: 36 mm. LVEF: NS, all > 40% |
|
| Interventions | Flecainide 200 mg/day vs sotalol 240 mg/day vs placebo Method of AF cardioversion: pharmacological 67%, electrical 33% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly allocated", but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | The procedure to conceal allocations was not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All study's prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Channer 2004.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Persistent AF (mean duration: 6 months). n = 99 Men: 78% Age (mean): 67 (SD 10) years Structural heart disease: NS. LAD: 44 mm. LVEF: 58% |
|
| Interventions | Amiodarone 200 mg/day vs placebo Method of AF cardioversion: pharmacological 20%, electrical 80% Warfarin mandatory |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐established random number sequence. |
| Allocation concealment (selection bias) | Low risk | An independent pharmacist assigned treatment according to the random sequence. Investigators were "blinded to treatment allocation". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study using matching placebo. Quote: "(Patients) investigators, and physicians involved … were blinded to treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few withdrawals and lost from follow‐up, well reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest, prespecified and expected, were well reported. |
| Other bias | Low risk | No other bias apparent. |
Chun 2014.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (mean duration: NS, in 38% of participants it was > 1 year). n = 100 Men: 81% Age (mean): 59 (SD 10) years Structural heart disease: NS. Mean LAD: 44 mm. Mean LVEF: 58% |
|
| Interventions | Dronedarone 800 mg/day vs propafenone 450 mg/day Method of AF cardioversion: electrical Warfarin (or direct oral anticoagulants) discretionary, but 94% of participants were taking anticoagulants. |
|
| Outcomes | At 6 months: AF recurrence |
|
| Notes | We tried to contact authors of this study to request additional details on methods employed and outcomes analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few withdrawals happened, balanced and well described. |
| Selective reporting (reporting bias) | High risk | The only prespecified outcome was AF recurrence, and it was adequately reported. However, other important clinical outcomes, such as adverse events and deaths, would be expected in this type of study. |
| Other bias | Low risk | No other bias apparent. |
DAFNE 2003.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Persistent AF (mean duration: 3 months). n = 199 Men: 70% Age (mean): 63 years Structural heart disease: NS. LAD: 45 mm. LVEF: 55% |
|
| Interventions | Dronedarone various doses (800 mg/day, 1200 mg/day, 1600 mg/day) vs placebo Method of AF cardioversion: pharmacological 15%, electrical 85% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not detailed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as a double‐blind trial, but no details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals because adverse events were reported, but other dropouts or lost to follow‐up were not well detailed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and main outcomes of interest were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
DAPHNE 2008.
| Methods | RCT Single‐blind Loss to follow‐up reported: yes |
|
| Participants | Bradycardia‐tachycardia sinus node disease with history of several episodes of AF or AFl and needing a pacemaker AF type: 100% paroxysmal. n = 135 Men: 49.6% Age (mean): 73 (SD 7) years Structural heart disease: 71%. LAD: 43 mm. LVEF: 56% |
|
| Interventions | Sotalol 167 mg/day (mean) vs beta‐blockers (atenolol or metoprolol) Method of AF cardioversion: 100% spontaneous Warfarin discretionary |
|
| Outcomes | At 19 months: Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost to follow‐up, were well balanced and well reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest were reported. |
| Other bias | Unclear risk | Restrictive inclusion criteria: only people with the bradycardia–tachycardia form of sinus node disease requiring pacemaker implantation were included. |
DIAMOND 2001.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (mean duration: NS) in people with heart failure or recent myocardial infarction and reduced LVEF. n = 506 Men: 77% Age (mean): 72 (range 36–92) years Structural heart disease: 100%. LAD: NS. LVEF: NS, all < 35% |
|
| Interventions | Dofetilide 500 µg/day vs placebo Method of AF cardioversion: spontaneous or pharmacological 63%, electrical 37% Warfarin discretionary |
|
| Outcomes | At 12 and 24 months: Mortality Proarrhythmia Heart failure AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation by a central office after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study using matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified and all expected outcomes of interest were well reported. Members of an events committee reviewed available data on a blinded basis and classified deaths. |
| Other bias | Unclear risk | Substudy from the 2 DIAMOND RCTs which were not stratified by rhythm. |
DIONYSOS 2010.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Documented AF for > 72 hours Type: 5% paroxysmal, 22% recent onset, 63% persistent (mean duration: 1.5 months). n = 504 Men: 71% Age (mean): 64 (SD 10) years Structural heart disease: 29%. LAD: NS. LVEF: NS |
|
| Interventions | Amiodarone 200 mg/day vs dronedarone 800 mg/day Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin required |
|
| Outcomes | At 12 months: Mortality Adverse effects Proarrhythmia AF recurrence Heart failure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method employed not described. |
| Allocation concealment (selection bias) | Low risk | Central allocation after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" study, but no details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts from the study were well reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Dogan 2004.
| Methods | RCT Single‐blind Loss to follow‐up reported: yes |
|
| Participants | AF of duration 3 hours to 3 months: recent onset 71%, persistent 29% (mean duration: 0.5 months). n = 110 Men: 45% Age (mean): 61 (SD 12) years Structural heart disease: 79%. LAD: 44 mm. LVEF: 64% |
|
| Interventions | Propafenone 450 mg/day vs placebo Method of AF cardioversion: spontaneous 42%, pharmacological 31%, electrical 27% Warfarin discretionary |
|
| Outcomes | At 15 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported to be "randomized", but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts of participants were well described. The proportions of missing outcomes was not enough to have an impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
EMERALD 2000.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (1 week to 1 year, mean duration < 6 months). n = 535 Men: 70% Age (mean): 64 years Structural heart disease: NS. LAD: NS. LVEF: NS |
|
| Interventions | Dofetilide 250 µg/day, 500 µg/day or 1000 µg/day (3 different groups) vs sotalol 160 mg/day vs placebo Method of AF cardioversion: 10% pharmacological, 90% electrical Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Adverse effects Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the method employed. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Presented as "double‐blind", but no detail given about how blinding was obtained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts were well detailed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are adequately reported. |
| Other bias | Low risk | No other bias apparent. |
EURIDIS ADONIS 2007.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | AF or AFl documented in the previous 3 months. Proportions of paroxysmal and persistent AF not reported. n = 1244 Men: 69% Age (mean): 63 (SD 11) years Structural heart disease: 41%. LAD: 42.5 mm. LVEF: 58% |
|
| Interventions | Dronedarone 800 mg/day vs placebo Method of AF cardioversion: any (frequencies of use not reported) Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stochastic randomisation procedure with balancing for prognostic factors. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial using matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost from follow‐up balanced between groups and well described. |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest were adequately reported. |
| Other bias | Unclear risk | All data‐management and data analyses were performed by the sponsor. |
FAPIS 1996.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal recurrent AF with > 2 episodes in the last 4 months. n = 200 Men: 54% Age (mean): 57 (SD 10) years Structural heart disease: 0%. LAD: 35 mm. LVEF: 61% |
|
| Interventions | Flecainide 200 mg/day vs propafenone 520 mg/day Method of AF cardioversion: pharmacological Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization schedule". |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts and lost to follow‐up well described and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Flec‐SL 2012.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF with indication for cardioversion (mean duration: 20 months). n = 362 Men: 66% Age (mean): 64 (SD 10) years Structural heart disease: NS LAD: 46 mm. LVEF: NS |
|
| Interventions | Flecainide 200–300 mg/day vs no treatment Method of AF cardioversion: pharmacological 20%, electrical 80% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Stroke, embolism AF recurrence |
|
| Notes | A third group of participants randomised to flecainide for only 3 months was not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Low risk | Allocation by a distant central office. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts of participants well described. Missing outcome data balanced across intervention groups. |
| Selective reporting (reporting bias) | Low risk | All of the study's prespecified outcomes were reported in the prespecified way. |
| Other bias | Low risk | No other bias apparent. |
GEFACA 2001.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Persistent AF lasting > 2 months (mean duration: 36 months). n = 50 Men: 73% Age (mean): 62 (SD 7) years Structural heart disease: 94% LAD: 48 mm. LVEF: 60% |
|
| Interventions | Amiodarone 200 mg/day vs placebo Method of AF cardioversion: pharmacological 32%, electrical 68% Warfarin discretionary |
|
| Outcomes | At 16 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind", but no details given on the procedure employed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participant seems to have been lost to follow‐up, but this was not clearly stated. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Hillestad 1971.
| Methods | RCT Open‐label Loss to follow‐up reported: no |
|
| Participants | Persistent AF lasting 1 month to 2 years (mean duration: NS). n = 100 Men: 46% Age (mean): 54 (range 22 to 77) years Structural heart disease: 92%. LAD: NS. LVEF: NS |
|
| Interventions | Quinidine 0.8–1.2 g/day vs no treatment Method of AF cardioversion: electrical Warfarin mandatory |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly allocated", but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear that all loss to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Hohnloser 1995.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF between 2 days and 6 months (mean duration: 1.5 months). n = 50 Men: 36% Age (mean): 62 (SD 11) years Structural heart disease: 86%. LAD: 50 mm. LVEF: 51% |
|
| Interventions | Quinidine 1 g/day vs sotalol 240–320 mg/day Method of AF cardioversion: pharmacological 40%, electrical 60% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not explained. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes were well reported. |
| Other bias | Low risk | No other bias apparent. |
Juul‐Moller 1990.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF between 2 months and 1 year (mean duration: 5 months). n = 183 Men: 81% Age (mean): 59 (SD 9) years Structural heart disease: NS. LAD: 42 mm. LVEF: NS |
|
| Interventions | Quinidine 1.2 g/day vs sotalol 160–320 mg/day Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly allocated", but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐labelled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Kalusche 1994.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | AF lasting from 2 weeks to 2 years. Type: paroxysmal 32%, persistent 68% (mean duration: NS). n = 82 Men: 68% Age (mean): 61 (SD 5) years Structural heart disease: 68%. LAD: 45 mm. LVEF: 30% |
|
| Interventions | Quinidine 1 g/day vs sotalol 240–400 mg/day Method of AF cardioversion: pharmacological 47%, electrical 53% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and lost to follow‐up were well detailed. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Karlson 1988.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF between 6 weeks and 1 year (mean duration: 5 months). n = 92 Men: 71% Age (mean): 60 (range 31–72) years Structural heart disease: 60%. LAD: NS. LVEF: NS |
|
| Interventions | Disopyramide 500 mg/day vs placebo Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random" allocation of participants, but method not described. |
| Allocation concealment (selection bias) | Low risk | Sealed codes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as double‐blind, but method employed was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost to follow‐up, all losses well reported. |
| Selective reporting (reporting bias) | Low risk | All study prespecified outcomes of interest were reported. All main outcomes expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Kochiadakis 2000.
| Methods | RCT Single‐blind Loss to follow‐up reported: no |
|
| Participants | Any documented symptomatic previous or persistent AF. Type: paroxysmal or recent onset 64%, persistent 34% (mean duration: 10 months). n = 186 Men: 52% Age (mean): 63 (SD 9) years Structural heart disease: 35%. LAD: 44 mm. LVEF: 53% |
|
| Interventions | Amiodarone 200 mg/day vs sotalol 320 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 and 24 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up not clearly described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified way. All expected outcomes of interest were reported. |
| Other bias | Low risk | No other bias apparent. |
Kochiadakis 2004a.
| Methods | RCT Single‐blind Loss to follow‐up reported: no |
|
| Participants | Any documented symptomatic previous or persistent AF. Type: paroxysmal or recent onset 63%, persistent 37% (mean duration: 8 months). n = 146 Men: 49% Age (mean): 63 (SD 9) years Structural heart disease: 38%. LAD: 43 mm. LVEF: 53% |
|
| Interventions | Amiodarone 200 mg/day vs propafenone 450 mg/day Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 and 24 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up are not clearly described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified way. All expected outcomes of interest were reported. |
| Other bias | Low risk | No other bias apparent. |
Kochiadakis 2004b.
| Methods | RCT Single‐blind Loss to follow‐up reported: no |
|
| Participants | Any documented symptomatic previous or persistent AF. Type: paroxysmal or recent onset 59%, persistent 41% (mean duration: 8 months). n = 254 Men: 50% Age (mean): 63 (SD 10) years Structural heart disease: 41%. LAD: 44 mm. LVEF: 53% |
|
| Interventions | Propafenone 450 mg/day vs sotalol 300 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 and 24 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up are not clearly described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified way. All expected outcomes of interest were reported. |
| Other bias | Low risk | No other bias apparent. |
Kuhlkamp 2000.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF lasting 2 days to 1 year (mean duration: 3 months). n = 394 Men: 70% Age (mean): 60 (range 24–86) years Structural heart disease: 36%. LAD: 42 mm. LVEF: 64% |
|
| Interventions | Metoprolol 100 mg/day vs placebo Method of AF cardioversion: pharmacological 18%, electrical 82% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Central allocation after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Quote: "The placebo tablets were identical in size, weight, colour, and taste to the metoprolol CR/XL [controlled‐release/extended release] tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost to follow‐up were balanced between groups and well described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Lloyd 1984.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF lasting 1 month to 3 years (mean duration: NS). n = 82 Men: 38 Age (mean): 46 (range 15–79) years Structural heart disease: 94%. LAD: NS. LVEF: NS |
|
| Interventions | Disopyramide 450 mg/day vs quinidine 1.4 g/day vs placebo Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "assigned after randomization", but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Both drugs tested and placebo were "identical in appearance". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost to follow‐up and losses were well reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the study and all outcomes of interest expected were reported. |
| Other bias | Low risk | No other bias apparent. |
Naccarelli 1996.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Any documented symptomatic AF. Type: paroxysmal 74%, persistent 26% (mean duration: 36 months). n = 239 Men: 38 Age (mean): 58 years Structural heart disease: 83%. LAD: NS. LVEF: NS |
|
| Interventions | Flecainide 200–300 mg/day vs quinidine 1–1.5 g/day Method of AF cardioversion: pharmacological Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts were well described. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Nergårdh 2007.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF of < 1 year (mean duration: 5 months). n = 168 Men: 71% Age (mean): 67 (SD 11) years Structural heart disease: NS. LAD: 45 mm. LVEF: 49% |
|
| Interventions | Metoprolol 170 mg/day (mean) vs placebo Method of AF cardioversion: 100% electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Adverse effects Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. The placebo tablets were identical in size, weight, colour and taste to the metoprolol tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost to follow‐up were balanced between group and well described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Niu 2006.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Any type of AF. Type: 41% paroxysmal, 59% persistent (mean duration: NS). n = 102 Men: 56% Age (mean): 56 (SD 11) years Structural heart disease: NS (coronary artery disease 33%, hypertension 25%). LAD: NS. LVEF: NS |
|
| Interventions | Amiodarone 200 mg/day vs sotalol 40–80 mg/day Method of AF cardioversion: pharmacological Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomized", but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts were well described and were not enough to have a clinically relevant impact on the effect estimate. |
| Selective reporting (reporting bias) | Low risk | Main prespecified and expected outcomes were reported. |
| Other bias | Low risk | No other bias apparent. |
Okishige 2000.
| Methods | RCT Single‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF lasting > 6 months (mean duration: 22 months). n = 62 Men: 92% Age (mean): 51 (SD 17) years Structural heart disease: 61%. LAD: 41 mm. LVEF: 61% |
|
| Interventions | Pilsicainide 150 mg/day vs placebo Method of AF cardioversion: pharmacological 21%, electrical 79% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not detailed. |
| Allocation concealment (selection bias) | Unclear risk | Method to conceal participant allocation not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants received either the active treatment or "matching placebo", but it was not report if attending doctors were blind to the treatment administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were few withdrawals and dropouts, which were reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified way. |
| Other bias | Low risk | No other bias apparent. |
PAFAC 2004.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF lasting > 7 days (mean duration: 15 months). n = 848 Men: 66% Age (mean): 63 (SD 9) years Structural heart disease: NS. LAD: 45 mm. LVEF: 60% |
|
| Interventions | Quinidine 0.480 g/day (+ verapamil) vs sotalol 320 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was created by an independent organisation which was not involved in the conduct of the study". |
| Allocation concealment (selection bias) | Low risk | Quote: "Each investigator received a set of sealed random code envelopes for the patients scheduled for enrolment at his/her site". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study of 2 active drugs and placebo using a double‐dummy technique. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost to follow‐up were few, well balanced between groups and adequately described. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
PITAGORA 2008.
| Methods | RCT Single‐blind Loss to follow‐up reported: yes |
|
| Participants | Recurrent symptomatic AF in people with sinus node disease and an indication for pacemaker. Excluded people with underlying coronary disease or reduced LVEF Type of AF: 53% paroxysmal, 47% persistent (mean duration: NS). n = 176 Men: 81% Age (mean): 72 (SD 8) years Structural heart disease: NS%. LAD: 47 mm. LVEF: 56% |
|
| Interventions | Amiodarone 190 mg/day vs class IC (flecainide 170 mg/day or propafenone 530 mg/day) vs sotalol 140 mg/day Method of AF cardioversion: NS Warfarin discretionary |
|
| Outcomes | At 21 months: Mortality Adverse effects Proarrhythmia Stroke |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study, no details given on the procedure followed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few participants were lost to follow‐up. Withdrawals were well described. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Plewan 2001.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (mean duration: 9 months). n = 128 Men: 62% Age (mean): 59 (SD 10) years Structural heart disease: 72%. LAD: 48 mm. LVEF: 41% |
|
| Interventions | Sotalol 160 mg/day vs bisoprolol 5 mg/day Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 8 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method to conceal allocation of participants not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study was not reported to be blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants were lost to follow‐up or withdrew, well balanced between groups and adequately reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
PRODIS 1996.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF (mean duration: 5 months). n = 56 Men: 68% Age (mean): 60 (SD 11) years Structural heart disease: 65%. LAD: 46 mm. LVEF: NS |
|
| Interventions | Disopyramide 750 mg/day vs propafenone 900 mg/day Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomly assigned", but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy employed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the study and all outcomes of interest expected were reported. |
| Other bias | Low risk | No other bias apparent. |
RAFT 2003.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Previous symptomatic AF documented in the last year. Type: NS. n = 523 Men: 59% Age (mean): 63 (range 22–89) years Structural heart disease: 48%. LAD: NS. LVEF: NS |
|
| Interventions | Propafenone at various doses (450 mg/day, 650 mg/day and 850 mg/day) vs placebo Method of AF cardioversion: pharmacological 79%, electrical 21% Warfarin discretionary |
|
| Outcomes | At 9 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not detailed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Quote: "Identical capsules containing either placebo or propafenone". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts were adequately described and seemed balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Reimold 1993.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Any symptomatic AF or AFl. Type: paroxysmal 47%, persistent 53% (mean duration: 36 months). n = 100 Men: 64% Age (mean): 61 (SD 12) years Structural heart disease: 81%. LAD: 46 mm. LVEF: 59% |
|
| Interventions | Propafenone 675 mg/day vs sotalol 320 mg/day Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratification scheme using a permuted blocks design generated before initiation of the trial. |
| Allocation concealment (selection bias) | Low risk | Drug assignment provided in sealed envelopes by a research pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described as a blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up were not clearly detailed but seemed to be few. Analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and expected outcomes were well reported. |
| Other bias | Low risk | No other bias apparent. |
Richiardi 1992.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal AF having > 3 episodes in the last 3 months. n = 200 Men: 54% Age (mean): 57 (range 29–75) years Structural heart disease: 48%. LAD: 45 mm. LVEF: NS |
|
| Interventions | Propafenone 900 mg/day vs quinidine 1 g/day Method of AF cardioversion: pharmacological 88%, electrical 12% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. Not described as blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost at follow‐up well described, balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes of interest and expected outcomes were reported. |
| Other bias | Low risk | No other bias apparent. |
SAFE‐T 2005.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF lasting 3 days to 1 year (mean duration: NS). n = 655 Men: 99% Age (mean): 67 (SD 9) years Structural heart disease: 33%. LAD: 48 mm. LVEF: 51% Type of AF: persistent, mean duration: NS |
|
| Interventions | Amiodarone 300 mg/day vs sotalol 320 mg/day vs placebo Method of AF cardioversion: pharmacological 20%, electrical 80% |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted‐block randomisation, with stratification. |
| Allocation concealment (selection bias) | Low risk | Both the investigators and participants were unaware of the study‐group assignments. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study, matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost to follow‐up were well balanced and described. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
SAFIRE‐D 2000.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF or AFl lasting 2 weeks to 6 months (mean duration: NS). n = 250 Men: 84% Age (mean): 67 (range 30–88) years Structural heart disease: 67%. LAD: NS. LVEF: NS |
|
| Interventions | Dofetilide various doses (250 µg/day, 500 µg/day and 1000 µg/day) vs placebo Method of AF cardioversion: pharmacological 15%, electrical 85% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as "double blind", but details on the procedure employed were not given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and dropouts were well detailed, not many and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes of interest were well described. |
| Other bias | Low risk | No other bias apparent. |
Santas 2012.
| Methods | RCT Open‐label Loss to follow‐up reported: no |
|
| Participants | First episode of persistent AF submitted for cardioversion (mean duration: NS). n = 94 Men: NS Age (mean): NS Structural heart disease: NS. LAD: NS. LVEF: NS |
|
| Interventions | Amiodarone (dose: NS) vs no antiarrhythmic Method of AF cardioversion: pharmacological 19%, electrical 81% Warfarin: NS |
|
| Outcomes | At 15 months: AF recurrence |
|
| Notes | Only data from a congress poster presentation available. Authors have been contacted. All participants received ibesartan. The study compared ibesartan alone with ibesartan + amiodarone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study stated that was randomised but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedures given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals and dropouts of participants after randomisation were not described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Unclear risk | Insufficient information to permit judgement. Only data from a congress abstract were available. Contacted authors for further details, but this has been unsuccessful. |
Singh 1991.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Persistent AF or AFl lasting 2 weeks to 1 year (mean duration: 3 months). n = 34 Men: 71% Age (mean): 60 (SD 14) years Structural heart disease: NS. LAD: 44 mm. LVEF: NS |
|
| Interventions | Sotalol 80–320 mg/day vs placebo Method of AF cardioversion: pharmacological 17%, electrical 83% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method employed to generate the random sequence not detailed. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not explained. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated repeatedly that treatment or placebo was given in double‐blind conditions, but no other details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts were adequately described and well balanced. |
| Selective reporting (reporting bias) | Low risk | All prespecified and expected outcomes were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
SMART 2002.
| Methods | RCT Double‐blind Loss to follow‐up reported: no |
|
| Participants | Symptomatic paroxysmal AF having > 1 episode monthly (59%) or persistent AF lasting < 1 month (41%). n = 94 Men: 72% Age (mean): 60 (SD 12) years Structural heart disease: NS. LAD: NS. LVEF: NS |
|
| Interventions | Aprindine 40 mg/day vs placebo Method of AF cardioversion: pharmacological 50%, electrical 50% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Central allocation, after inclusion, using a randomisation list common to all centres. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Matching placebo capsules and tablets identical in size, weight, colour and taste. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals well described but unclear whether additional participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified manner. |
| Other bias | Low risk | No other bias apparent. |
SOCESP 1999.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | AF lasting < 6 months. Type: recent onset 61%, persistent 39% (mean duration: NS). n = 121 Men: 59% Age (mean): 54 (SD 13) years Structural heart disease: 54%. LAD: 39 mm. LVEF: 68% |
|
| Interventions | Quinidine 700 mg/day vs sotalol 240 mg/day Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not detailed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were well detailed but unclear whether there were other participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Sodermark 1975.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF or AFl lasting < 3 years (mean duration: 3–6 months). n = 185 Men: 78% Age (mean): 58 (range 24–78) years Structural heart disease: 94%. LAD: NS. LVEF: NS |
|
| Interventions | Quinidine 1.2–1.8 g/day vs no treatment Method of AF cardioversion: pharmacological 49%, electrical 51% Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly allocated" but methods of randomisation were not described. |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. Control group received no antiarrhythmic treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost at follow‐up were few and well described. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes of interest and expected outcomes were reported. |
| Other bias | Low risk | No other bias apparent. |
SOPAT 2004.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal AF documented in the last 1 month (mean duration: NS). n = 1033 Men: 63% Age (mean): 60 (SD 11) years Structural heart disease: NS. LAD: 39 mm. LVEF: 61% |
|
| Interventions | Quinidine 320 mg/day or 480 mg/day (+ verapamil) vs sotalol 320 mg/day vs placebo Method of AF cardioversion: both pharmacological and electrical, % NS Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Stroke Proarrhythmia Adverse effects AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Low risk | Allocation by a central office, after inclusion. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Quote: "Placebo in double‐dummy technique". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost to follow‐up, adequately described, well balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and expected outcomes of interest were well reported. |
| Other bias | Low risk | No other bias apparent. |
Steinbeck 1988.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Paroxysmal symptomatic AF of any duration (mean duration: 6 years). n = 45 Men: 58% Age (mean): 59 years Structural heart disease: 73%. LAD: NS. LVEF: NS |
|
| Interventions | Quinidine 1 g/day (+ digoxin) vs flecainide 200–300 mg/day (+ digoxin) vs digoxin alone Method of AF cardioversion: pharmacological Warfarin discretionary |
|
| Outcomes | At 12 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned" but the procedure was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method to conceal allocation not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All expected main outcomes and prespecified outcomes were adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Stroobandt 1997.
| Methods | RCT Double‐blind Loss to follow‐up reported: yes |
|
| Participants | Recent onset AF (46%) or persistent AF lasting > 2 weeks (54%, mean duration: NS). n = 102 Men: 73% Age (mean): 62 (range 27–84) years Structural heart disease: 71%. LAD: 39 mm. LVEF: NS |
|
| Interventions | Propafenone 450 mg/day vs placebo Method of AF cardioversion: pharmacological 34%, electrical 66% Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Procedure to conceal allocations not well described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Matching placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts from the study were adequately reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest were reported in the prespecified way. |
| Other bias | Low risk | No other bias apparent. |
Van Gelder 1989.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Any persistent AF or AFl (mean duration: 12 months). n = 73 Men: 55% Age (mean): 60 (SD 11) years Structural heart disease: 82%. LAD: 44 mm. LVEF: NS |
|
| Interventions | Flecainide 200–300 mg/day vs no treatment Method of AF cardioversion: electrical Warfarin mandatory? |
|
| Outcomes | At 6 months: Mortality Adverse effects Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed blinded by drawing from a box, prepared before the start of the study, containing 90 lots. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. Control group received no treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and lost at follow‐up were well balanced and well described. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes of interest and all expected outcomes were reported. |
| Other bias | Low risk | No other bias apparent. |
Vijayalakshmi 2006.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Persistent AF in whom cardioversion was planned (mean duration: 7 months). n = 78 Men: 71% Age (mean): 64 (SD 9) years Structural heart disease: NS LAD: 43 mm. LVEF: 43% |
|
| Interventions | Amiodarone 200 mg/day vs sotalol 160–320 mg/day vs no treatment Method of AF cardioversion: pharmacological 22%, electrical 78% Warfarin required for 6 weeks, discretionary afterwards |
|
| Outcomes | At 6 months: Mortality Adverse effects Proarrhythmia AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedures given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and dropouts of participants were well described. Missing outcome data were balanced across intervention groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the prespecified way. All main outcomes expected in this type of study were reported. |
| Other bias | Low risk | No other bias apparent. |
Villani 1992.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Symptomatic recent‐onset AF lasting > 1 hour, being at least the second episode. n = 76 Men: 49% Age (mean): 65 (range 37–85) years Structural heart disease: 86%. LAD: 38 mm. LVEF: NS |
|
| Interventions | Amiodarone 200 mg/day vs disopyramide 500 mg/day Method of AF cardioversion: pharmacological 74%, electrical 26% Warfarin discretionary |
|
| Outcomes | At 14 months: Mortality Adverse effects AF recurrence Symptomatic recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that participants were randomised, but method employed not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and reasons for withdrawals well described. No participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest and all expected outcomes adequately reported. |
| Other bias | Low risk | No other bias apparent. |
Vitolo 1981.
| Methods | RCT Open‐label Loss to follow‐up reported: yes |
|
| Participants | Any persistent AF (mean duration: NS). n = 54 Men: 37% Age (mean): 53 (SD 11) years Structural heart disease: 100%. LAD: NS. LVEF: NS |
|
| Interventions | Amiodarone 400 mg/day vs quinidine 1.2 g/day Method of AF cardioversion: electrical Warfarin discretionary |
|
| Outcomes | At 6 months: Mortality Proarrhythmia Adverse effects AF recurrence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant was loss to follow‐up in this small study. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest and all expected outcomes were reported. |
| Other bias | Low risk | No other bias apparent. |
AF: atrial fibrillation; AFl: atrial flutter; LAD: left atrium diameter; LVEF: left ventricle ejection fraction; n: number of participants included in the study; NS: not stated; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aberg 1969 | Non‐controlled study: all participants were initially treated with quinidine for 1 year, then allocated to procainamide alone or procainamide + quinidine and followed for only 3 months. |
| Adamyan 2015 | Inadequate comparison: did not compare individual antiarrhythmic drugs, but combinations of 2 drugs (amiodarone + ivabradine vs amiodarone + bisoprolol). |
| AF‐CHF 2002 | Rate vs rhythm control comparison. Participants in control group (rate control) were in persistent AF not reverted to sinus rhythm. Use of long‐term oral anticoagulants was significantly different between rate and rhythm control groups. |
| AFFIRM 2002 | Rate vs rhythm control comparison. People in persistent AF at inclusion, not reverted to sinus rhythm. Multiple different antiarrhythmics used in intervention group (rhythm control), not analysed separately. Warfarin mandatory in control group (rate control) but discretionary in antiarrhythmics group and actual use was very different. |
| Anderson 1994 | Cross‐over study. Follow‐up < 6 months (4 months). |
| Andromeda 2008 | People with heart failure were randomised to dronedarone or placebo. About 25% of participants had AF but it was not possible to obtain separate data for those participants. Mean follow‐up was only 2 months as the trial was terminated early because of increased deaths in the dronedarone group. |
| Antman 1990 | Non‐controlled trial. |
| Aros 1978 | Inadequate comparison: quinidine vs quinidine + amiodarone. Probably not truly randomised. All participants underwent cardiac surgery. |
| Babuty 1999 | Comparison of drugs not relevant: flecainide vs cibenzoline, but the effectiveness of cibenzoline was not known. Included people with atrial tachyarrhythmias of various types, not only AF. |
| Beck 1978 | Acute pharmacological conversion of AF only, no long‐term therapy with antiarrhythmics. |
| Berns 1987 | Non‐controlled trial. |
| Blevins 1987 | Non‐controlled trial. |
| Blomstrom 1984 | Non‐controlled trial. |
| Boissel 1981 | Follow‐up < 6 months (3 months). Some participants followed for 1 year but they had not been randomised. |
| Brodsky 1987 | Non‐controlled trial. |
| CHF‐STAF 1998 | Recruited people with heart failure, only 15% had AF, not reverted to sinus rhythm, not analysed separately. |
| Chun 1995 | Non‐controlled trial. |
| Clementy 1992 | Non‐controlled trial. |
| Connolly 1989 | Cross‐over study. Follow‐up < 6 months (4 months). |
| CTAF 2000 | Initially included, but useable data could not be extracted: amiodarone compared against the sequential use of propafenone and sotalol, and separate data on each drug were not available. |
| Cuan‐Perez 1971 | Non‐randomised, retrospective study. |
| Darkner 2014 | Short‐term treatment: only 8 weeks of antiarrhythmic drug (amiodarone). |
| Di Biase 2016 | Inadequate comparison: compared an antiarrhythmic (amiodarone) with catheter ablation, not against placebo or no treatment. |
| Enriquez 2014 | Only antiarrhythmic drugs for acute cardioversion studied. |
| ERAFT 2002 | Follow‐up < 6 months (3 months). |
| Faivre 1970 | Non‐randomised trial, retrospective control series. |
| Farkowski 2012 | Only antiarrhythmics for acute cardioversion studied. |
| Fernández 1998 | Acute pharmacological conversion of AF only, no long‐term therapy with antiarrhythmics. |
| Feyrer 2014 | Non‐randomised study. |
| Fragakis 2012 | Very short‐term study (24 hours follow‐up) on the efficacy for converting recent onset AF. All groups received amiodarone. |
| Frances 1985 | Comparison of drugs not relevant: quinidine vs cibenzoline, but the effectiveness of cibenzoline was not known. |
| Galperin 2014 | All randomised participants received amiodarone; treatment for 3 months was compared with treatment for 18 months. A control group existed but included only 9 participants and they were not randomly allocated. |
| Gold 1986 | Non‐controlled trial. |
| Gosselink 1992 | Non‐controlled trial. |
| Graboys 1983 | Non‐controlled trial. |
| Gramley 2011 | Data unusable: compared a group receiving dronedarone with other group receiving flecainide or amiodarone. Separate data for amiodarone and flecainide not available. Only global outcomes (all groups pooled) were available at 6 months. |
| Grigoryan 2011 | Non‐randomised study with only 16 weeks' follow‐up. Compared ivabradine vs placebo. |
| Gu 2012 | 1 antiarrhythmic drug (amiodarone or propafenone) vs a combination of both, but separate data for amiodarone and propafenone were not available. |
| GUSTO 2002 | Randomised trial but allocation to antiarrhythmics was not randomised. Multiple different antiarrhythmics used, mainly for acute cardioversion, only 19% of participants received long‐term treatment with an antiarrhythmic. |
| Hammill 1988 | Non‐controlled trial. |
| Hartel 1974 | Follow‐up < 6 months (3 months). |
| Hopson 1996 | Non‐controlled trial. |
| Horowitz 1985 | Non‐controlled trial. |
| HOT‐CAFE 2004 | Rate vs rhythm control comparison. Participants in control group in persistent AF not reverted to sinus rhythm. Various antiarrhythmics used sequentially in intervention group (rhythm control), not analysed separately. Warfarin mandatory in control group (rate control) but discretionary in antiarrhythmics group. |
| Härtel 1970 | Quasi‐randomised: allocation by year of birth. Follow‐up < 6 months (3 months). |
| Ishiguro 2008 | Non‐controlled trial: all participants received bisoprolol. |
| J‐BAF 2009 | Follow‐up < 6 months (3 months only). The main endpoint of the study was the rate of cardioversion achieved rather than the maintaining of sinus rhythm. Rates of participants reverted to sinus rhythm were largely different between study groups. |
| J‐RHYTHM 2009 | Rate vs rhythm control comparison. Participants in control group (rate control) in persistent AF not reverted to sinus rhythm. Multiple different antiarrhythmics used in intervention group (rhythm control), not analysed separately. |
| Jong 2006 | Inadequate comparison: 2 different doses of amiodarone were studied, without any control (placebo or a different drug) group. |
| Kanoupakis 2004 | Follow‐up < 6 months (4 weeks). |
| Kennelly 1977 | Non‐randomised trial. Comparison of drugs not relevant: quinidine vs lidoflazine, but the effectiveness of lidoflazine was not known. Stopped prematurely due to mortality excess with lidoflazine. |
| Kerr 1988 | Non‐controlled trial. |
| Khitri 2012 | Follow‐up < 6 months (3 months only). Compared celivarone (drug related to amiodarone) with amiodarone and placebo. |
| Komatsu 2006 | Comparison of drug not relevant: cibenzoline vs pilsicainide, but the effectiveness of both drugs in AF was unknown (no studies comparing them with placebo or no treatment). |
| Kosior 2001 | Non‐controlled trial. |
| Kosior 2009 | Very short‐term study (24 hours' follow‐up only) comparing quinidine vs propafenone for the conversion of paroxysmal AF. |
| Kyles 1991 | Non‐controlled trial. |
| Lardoux 1996 | Comparison of drugs not relevant: propafenone vs cibenzoline, but the effectiveness of cibenzoline was not known. Included people with atrial tachyarrhythmias of various types, not only AF. |
| Lau 1992 | Cross‐over study. |
| Levi 1973 | Acute pharmacological conversion of AF only, no long‐term therapy with antiarrhythmics. |
| Li 2004 | Non‐randomised, retrospective study. |
| Lodziński 2014 | Short‐term treatment: only 2 months of antiarrhythmic treatment (amiodarone or sotalol). |
| Löbe 2013 | Non‐controlled, non‐randomised trial. All participants received dronedarone. |
| Manios 2003 | Follow‐up < 6 months (6 weeks). |
| Martin 1986 | Not truly randomised. Unknown if AF was reverted in all participants. |
| Mary‐Rabine 1990 | Non‐controlled trial. |
| Massacci 1991 | Cross‐over study. |
| Maĭkov 2015 | Protocol. Only antiarrhythmics for acute cardioversion were studied. |
| Meng 2015 | Inadequate comparison: compared sotalol vs Wenxin Kel, a Chinese herbal medicine. Furthermore, essential data on participants' characteristics at inclusion and methods employed not available. |
| Mizutani 1995 | Non‐controlled trial for long‐term use of antiarrhythmics after conversion. |
| Mont 2014 | Inadequate comparison: compared several antiarrhythmics with catheter ablation, not against placebo or no treatment. No separate data for each antiarrhythmic drug employed was provided. Furthermore, unclear if all participants in the antiarrhythmic group underwent cardioversion to sinus rhythm. |
| Nedostup 1990 | Non‐randomised, retrospective study. |
| Opolski 1997 | Non‐controlled trial. |
| Park 2014 | Short‐term treatment: only 3‐months' treatment with antiarrhythmic drugs. |
| PEPS 2002 | Non‐controlled trial. |
| PIAF 2000 | Rate vs rhythm control comparison. Participants in control group in persistent AF not reverted to sinus rhythm. |
| Pietersen 1991 | Follow‐up < 6 months (3 months). |
| Piot 1998 | Comparison of drugs not relevant: disopyramide vs cibenzoline, but the effectiveness of cibenzoline was not known. |
| Porterfield 1989 | Non‐controlled trial. |
| PSVT 1995 | Cross‐over study. Follow‐up < 6 months (3 months). |
| Qin 2016 | Non‐randomised trial. Retrospective cohort analysis. |
| RACE 2002 | Rate vs rhythm control comparison. Participants in control group in persistent AF not reverted to sinus rhythm. Various antiarrhythmics used sequentially in intervention group (rhythm control), not analysed separately. Warfarin mandatory in control group (rate control) but discretionary in antiarrhythmics group. |
| Rakhmanova 2014 | Inadequate comparison: non‐commercialised drug (allapinine) compared to quinidine. Lack of essential data: unable to translate from Russian and unable to contact the authors. |
| Rasmussen 1981 | Cross‐over study. Follow‐up < 6 months (3 months). |
| Resnekov 1971 | Non‐controlled trial. |
| STAF 2003 | Rate vs rhythm control comparison. People in persistent AF at inclusion, not reverted to sinus rhythm. Multiple different antiarrhythmics used in intervention group (rhythm control), not analysed separately. |
| Steeds 1999 | Cross‐over study. Follow‐up < 6 months (2 months). |
| Tonet 1986 | Cross‐over study. |
| Torp‐Pedersen 2011 | Follow‐up < 6 months (3 months). Multicentre randomised controlled trial comparing several doses of vernakalant with placebo. |
| Touboul 1995 | Comparison of drugs not relevant: quinidine vs cibenzoline, but the effectiveness of cibenzoline was not known. |
| Van Wijk 1989 | Cross‐over study. Follow‐up < 6 months (3 months). |
| VEPARAF 2003 | Follow‐up < 6 months (3 months). |
| Wanless 1997 | Follow‐up < 6 months (4–8 weeks). |
| Zehender 1992 | Follow‐up < 6 months (3 months). Some participants followed longer but all received quinidine, and there was no control group. |
| Zeriouh 2014 | Non‐randomised, non‐comparative study. |
AF: atrial fibrillation.
Characteristics of ongoing studies [ordered by study ID]
Park 2017.
| Trial name or title | |
| Methods | Randomised controlled trial Open label |
| Participants | Adults with a first episode of paroxysmal atrial fibrillation |
| Interventions | Flecainide (100 mg twice daily) vs no treatment |
| Outcomes | At 12 months: Atrial fibrillation recurrence Others? |
| Starting date | Unknown |
| Contact information | Dr Eak Kyun Shin, Gachon University, Gil Medical Center, Incheon, Republic of Korea |
| Notes |
Differences between protocol and review
Some of the original planned outcomes and planned subgroup analyses could not be performed because the data needed were not recorded or not reported in the original studies. Some planned outcomes were thus modified.
All‐cause mortality and cardiovascular mortality were virtually identical in all studies, so we chose to report only all‐cause mortality.
We finally analysed only stroke instead of the originally planned "embolic complications (stroke and peripheral embolism combined)" as data for peripheral embolism was lacking.
Heart failure was added as a secondary outcome, because it is an important outcome in these patients.
Other modifications were included in the successive updates with respect to the original protocol:
Assessment of the risk of bias of included studies was expanded to comply with the latest Cochrane MECIR methodological requirements;
We decided to report risk ratios instead of Peto odds ratios, as originally done, because risk ratios are more interpretable by clinicians and non‐statisticians.
Initially, we analysed data not only by each individual drug but also grouped by pharmacological class, following the classification of Vaughan Williams (Vaughan Williams 1984). However, individual antiarrhythmics are very different from one another even inside the same class and it is unclear what would be the clinical implications of grouping them by classes. Consequently, after discussion, we decided to analyse data only by individual drugs.
We decide to drop out several drugs that have never been marketed for this indication (never proven to be effective): aprindine (class IB), bidisomide (class IB) and azimilide (class III).
Contributions of authors
CL‐L, JB: prepared and designed the review,
CL‐L: searched for primary studies and contacted authors of primary studies when needed.
LV, AT, WJ: screened search results, and retrieved papers.
LV, EA, WJ, JB, CL‐L: assessed papers for inclusion and risk of bias.
LV, EA, WJ, CL‐L: extracted data.
CL‐L, AT: performed analysis and interpreted data.
LV, WJ: interpreted data and reviewed the manuscript.
CL‐L, AT, JB: wrote the review.
CL‐L, AT: assessed the GRADE domains.
Sources of support
Internal sources
Unité de Recherches Thérapeutiques, Hôpital Lariboisière, Paris, France.
Assistance Publique – Hôpitaux de Paris, France.
Sorbonne Université, Paris, France.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
LV: none.
EA: none.
AT: none.
WJ: none.
JB: none.
CL‐L has received consultant fees (less than EUR 5000 total) from Sanofi‐Aventis, in 2009 and 2010, for helping to conduct a study (a mixed treatment comparison meta‐analysis) on several antiarrhythmic drugs for the management of atrial fibrillation. Sanofi‐Aventis is the manufacturer of amiodarone and dronedarone, two of the antiarrhythmics studied in this review. He also received personal fees from Bayer Healthcare and BMS, outside this work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
A‐COMET‐I 2006 {published data only}
- A‐COMET‐I Investigators, Pritchett EL, Kowey P, Connolly S, Page RL, Kerr C, et al. Antiarrhythmic efficacy of azimilide in patients with atrial fibrillation. Maintenance of sinus rhythm after conversion to sinus rhythm. American Heart Journal 2006;151(5):1043‐9. [PUBMED: PMID: 16644334] [DOI] [PubMed] [Google Scholar]
A‐COMET‐II 2006 {published data only}
- A‐COMET‐II Investigators, Lombardi F, Borggrefe M, Ruzyllo W, Lüderitz B. Azimilide vs. placebo and sotalol for persistent atrial fibrillation: the A‐COMET‐II (Azimilide‐CardiOversion MaintEnance Trial‐II) trial. European Heart Journal 2006;27(18):2224‐31. [PUBMED: PMID: 16935870] [DOI] [PubMed] [Google Scholar]
AFFIRM Substudy 2003 {published data only}
- AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. Journal of the American College of Cardiology 2003;42(1):20‐9. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
AFIB 1997 {published data only}
- The Atrial Fibrillation Investigation with Bidisomide (AFIB) Investigators. Treatment of atrial fibrillation and paroxysmal supraventricular tachycardia with bidisomide. Circulation 1997;96(8):2625‐32. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
Aliot 1996 {published data only}
- Aliot E, Denjoy I, The Flecainide AF French Study Group. Comparison of the safety and efficacy of flecainide versus propafenone in hospital out‐patients with symptomatic paroxysmal atrial fibrillation/flutter. American Journal of Cardiology 1996;77(3):66A‐71A. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
ASAP 2003 {published data only}
- Connolly SJ, Schnell DJ, Page RL, Wilkinson WE, Marcello SR, Pritchett EL. Dose‐response relations of azimilide in the management of symptomatic, recurrent, atrial fibrillation. American Journal of Cardiology 2001;88(9):974‐9. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Schnell DJ, Page RL, Wilkinson WE, Marcello SR, Pritchett EL, Azimilide Supraventricular Arrhythmia Program Investigators. Symptoms at the time of arrhythmia recurrence in patients receiving azimilide for control of atrial fibrillation or flutter: results from randomized trials. American Heart Journal 2003;146(3):489‐93. [MEDLINE: ; ISSN: 0002‐8703] [DOI] [PubMed] [Google Scholar]
- Page RL, Connolly SJ, Wilkinson WE, Marcello SR, Schnell DJ, Pritchett EL, Azimilide Supraventricular Arrhythmia Program (ASAP) Investigators. Antiarrhythmic effects of azimilide in paroxysmal supraventricular tachycardia: efficacy and dose‐response. American Heart Journal 2002;143(4):643‐9. [MEDLINE: ; ISSN 0002‐8703] [DOI] [PubMed] [Google Scholar]
- Page RL, Tilsch TW, Connolly SJ, Schnell DJ, Marcello SR, Wilkinson WE, et al. Azimilide Supraventricular Arrhythmia Program (ASAP) Investigators. Asymptomatic or "silent" atrial fibrillation: frequency in untreated patients and patients receiving azimilide. Circulation 2003;107(8):1141‐5. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
- Pritchett EL, Page RL, Connolly SJ, Marcello SR, Schnell DJ, Wilkinson WE. Antiarrhythmic effects of azimilide in atrial fibrillation: efficacy and dose‐response. Azimilide Supraventricular Arrhythmia Program 3 (SVA‐3) Investigators. Journal of the American College of Cardiology 2000;36(3):794‐802. [MEDLINE: ; ISSN 0735‐1097] [DOI] [PubMed] [Google Scholar]
- SVA‐4 Investigators, Page RL, Pritchett EL, Connolly S, Wilkinson WE. Azimilide for the treatment of atrial fibrillation, atrial flutter, and paroxysmal supraventricular tachycardia: results of a randomized trial and insights on the concordance of symptoms and recurrent arrhythmias. Journal of Cardiovascular Electrophysiology 2008;19(2):172‐7. [PUBMED: PMID: 17916138] [DOI] [PubMed] [Google Scholar]
A‐STAR 2006 {published data only}
- A‐STAR Investigators, Kerr CR, Connolly SJ, Kowey P, Page RL, Pritchett EL, et al. Efficacy of azimilide for the maintenance of sinus rhythm in patients with paroxysmal atrial fibrillation in the presence and absence of structural heart disease. American Journal of Cardiology 2006;98(2):215‐8. [PUBMED: PMID: 16828595] [DOI] [PubMed] [Google Scholar]
ATHENA 2009 {published and unpublished data}
- ATHENA Investigators, Hohnloser SH, Crijns HJ, Eickels M, Gaudin C, Page RL, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. New England Journal of Medicine 2009;360(2):668‐78. [PUBMED: PMID: 19213680] [DOI] [PubMed] [Google Scholar]
- ATHENA Investigators, Connolly SJ, Crijns HJ, Torp‐Pedersen C, Eickels M, Gaudin C, et al. Analysis of stroke in ATHENA: a placebo‐controlled, double‐blind, parallel‐arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death in patients with atrial fibrillation/atrial flutter. Circulation 2009;120(13):1174‐80. [PUBMED: PMID: 19752319] [DOI] [PubMed] [Google Scholar]
Bellandi 2001 {published data only}
- Bellandi F, Dabizzi RP, Niccoli L, Cantini F. Propafenone and sotalol in the prevention of paroxysmal atrial fibrillation – long‐term safety and efficacy study [Propafenon und sotalol bei der pravention von paroxysmalem vorhofflimmern. Langzeitstudie zur beurteilung von sicherheit und wirksamkeit]. Munchener Medizinische Wochenschrift 1996;138(12):39‐46. [MEDLINE: ; EMBASE 1996098252; ISSN: 0027‐2973] [Google Scholar]
- Bellandi F, Dabizzi RP, Niccoli L, Cantini F. Propafenone and sotalol in the prevention of paroxysmal atrial fibrillation: long‐term safety and efficacy study. Current Therapeutic Research, Clinical and Experimental 1995;56(11):1154‐68. [MEDLINE: ; EMBASE 1996000327; ISSN: 0011‐393X] [Google Scholar]
- Bellandi F, Dabizzi RP, Niccoli L, Cantini F, Palchetti R. Propafenone and sotalol: long‐term efficacy and tolerability in the prevention of paroxysmal atrial fibrillation. A placebo‐controlled double‐blind study [Propafenone e sotalolo: efficacia e tollerabilita a lungo termine nella prevenzione della fibrillazione atriale parossistica. Studio in doppio cieco controllato con placebo]. Giornale Italiano di Cardiologia 1996;26(4):379‐90. [MEDLINE: ; ISSN 0046‐5968] [PubMed] [Google Scholar]
- Bellandi F, Leoncini M, Maioli M, Gallopin M, Dabizzi RP. Comparing agents for prevention of atrial fibrillation recurrence. Cardiology Review 2002;19(9):18‐21. [EMBASE 2002284566; ISSN 1061‐5377] [Google Scholar]
- Bellandi F, Simonetti I, Leoncini M, Frascarelli F, Giovannini T, Maioli M, et al. Long‐term efficacy and safety of propafenone and sotalol for the maintenance of sinus rhythm after conversion of recurrent symptomatic atrial fibrillation. American Journal of Cardiology 2001;88(6):640‐5. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Benditt 1999 {published data only (unpublished sought but not used)}
- Benditt DG, Williams JH, Jin J, Deering TF, Zucker R, Browne K, et al. Maintenance of sinus rhythm with oral d,l‐sotalol therapy in patients with symptomatic atrial fibrillation and/or atrial flutter. D,L‐Sotalol Atrial Fibrillation/Flutter Study Group. American Journal of Cardiology 1999;84(3):270‐7. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Byrne‐Quinn 1970 {published data only}
- Byrne‐Quinn E, Wing AJ. Maintenance of sinus rhythm after DC reversion of atrial fibrillation. A double‐blind controlled trial of long‐acting quinidine bisulphate. British Heart Journal 1970;32(3):370‐6. [MEDLINE: ; ISSN 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carunchio 1995 {published data only}
- Carunchio A, Fera MS, Mazza A, Burattini M, Greco G, Galati A, et al. A comparison between flecainide and sotalol in the prevention of recurrences of paroxysmal atrial fibrillation [Confronto tra flecainide e sotalolo nella profilassi delle recidive di fibrillazione atriale parossistica]. Giornale Italiano di Cardiologia 1995;25(1):51‐68. [MEDLINE: ; ISSN 0046‐5968] [PubMed] [Google Scholar]
Channer 2004 {published and unpublished data}
- Channer KS, Birchall A, Steeds RP, Walters SJ, Yeo WW, West JN, et al. A randomized placebo‐controlled trial of pre‐treatment and short‐ or long‐term maintenance therapy with amiodarone supporting DC cardioversion for persistent atrial fibrillation. European Heart Journal 2004;25(2):144‐50. [MEDLINE: ; ISSN 0195‐668X] [DOI] [PubMed] [Google Scholar]
Chun 2014 {published data only}
- Chun KJ, Byeon K, Im SI, Park KM, Park SJ, Kim JS, et al. Efficacy of dronedarone versus propafenone in the maintenance of sinus rhythm in patients with atrial fibrillation after electrical cardioversion. Clinical Therapeutics 2014;36(9):1169‐75. [DOI: 10.1016/j.clinthera.2014.07.013; PUBMED: 25134972] [DOI] [PubMed] [Google Scholar]
DAFNE 2003 {published data only}
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose‐ranging study. European Heart Journal 2003;24(16):1481‐7. [MEDLINE: ; ISSN 0195‐668X] [DOI] [PubMed] [Google Scholar]
DAPHNE 2008 {published data only}
- DAPHNE Study Investigators, Capucci A, Botto G, Molon G, Spampinato A, Favale S, et al. The Drug And Pace Health cliNical Evaluation (DAPHNE) study: a randomized trial comparing sotalol versus beta‐blockers to treat symptomatic atrial fibrillation in patients with brady‐tachycardia syndrome implanted with an antitachycardia pacemaker. American Heart Journal 2008;156(2):373.e1‐8. [PUBMED: PMID: 18657671] [DOI] [PubMed] [Google Scholar]
DIAMOND 2001 {published data only}
- Moller M, Torp‐Pedersen CT, Kober L. Dofetilide in patients with congestive heart failure and left ventricular dysfunction: safety aspects and effect on atrial fibrillation. The Danish Investigators of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group. Congestive Heart Failure 2001;7(3):146‐50. [MEDLINE: ; ISSN 1527‐5299] [DOI] [PubMed] [Google Scholar]
- Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp‐Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation‐flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (DIAMOND) substudy. Circulation 2001;104(3):292‐6. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
- Torp‐Pedersen C, Moller M, Bloch‐Thomsen PE, Kober L, Sandoe E, Egstrup K, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. New England Journal of Medicine 1999;341(12):857‐65. [MEDLINE: ; ISSN 0028‐4793] [DOI] [PubMed] [Google Scholar]
- Torp‐Pedersen CT, Moller M, Bloch‐Thomsen PE, Kober L, Sandoe E, Egstrup K, et al. Dofetilide to patients with heart failure and left ventricular dysfunction [Dofetilid til patienter med hjerteinsufficiens og darligt fungerende venstre ventrikel]. Ugeskrift for Laeger 2000;10(44):5948‐53. [MEDLINE: ; ISSN: 0041‐5782] [PubMed] [Google Scholar]
DIONYSOS 2010 {published data only (unpublished sought but not used)}
- Heuzey JY, Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short‐term, randomized, double‐blind, parallel‐group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. Journal of Cardiovascular Electrophysiology 2010;21(6):597‐605. [PUBMED: PMID: 20384650 ] [DOI] [PubMed] [Google Scholar]
Dogan 2004 {published data only}
- Dogan A, Ergene O, Nazli C, Kinay O, Altinbas A, Ucarci Y, et al. Efficacy of propafenone for maintaining sinus rhythm in patients with recent onset or persistent atrial fibrillation after conversion: a randomized, placebo‐controlled study. Acta Cardiologica 2004;59(3):255‐61. [MEDLINE: ; ISSN: 0001‐5385] [DOI] [PubMed] [Google Scholar]
EMERALD 2000 {published and unpublished data}
- Cambell TJ, Greenbaum RA, Channer KS, Dalrymple HW, Kingma JH, Santini M, et al. Mortality in patients with atrial fibrillation – 1 year follow up of EMERALD (European and Australian multicenter evaluative research on atrial fibrillation dofetilide). Journal of the American College of Cardiology 2000;35(2 Suppl A):154A‐5A. [Google Scholar]
- Dalrymple HW, Cambell TJ, Channer KS, Greenbaum R, Kingma JH, Santini M, et al. Maintenance of sinus rhythm by dofetilide improves quality of life. The EMERALD (European and Australian multicenter evaluative research on atrial fibrillation dofetilide) study. Circulation 1998;98(Suppl 13‐14):66. [Google Scholar]
- Greenbaum R, Campbell TJ, Channer KS, Dalrymple HW, Kingma JH, Santini M, et al. Conversion of atrial fibrillation and maintenance of sinus rhythm by dofetilide. The EMERALD (European and Australian multicenter evaluative research on atrial fibrillation dofetilide) study. Circulation 1998;98(Suppl I‐633):3326. [Google Scholar]
- US Food, Drug Administration. Drug Approval Package: Tikosyn (Dofetilide). Medical Review: Parts 1 and 2 (Study number 345). www.accessdata.fda.gov/drugsatfda_docs/nda/99/20‐931_Tikosyn.cfm (accessed 10 August 2010).
EURIDIS ADONIS 2007 {published and unpublished data}
- Brookes L. Dronedarone on Trial: EURIDIS and ADONIS, 2004. www.medscape.com/viewarticle/489226 (accessed January 2007).
- EURIDIS and ADONIS Investigators, Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. New England Journal of Medicine 2007;357(10):987‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hohnloser SH. EURIDIS and ADONIS: maintenance of sinus rhythm with dronedarone in patients with atrial fibrillation or flutter. European Society of Cardiology Congress; 2004 Aug 28 – Sep 1; Munich, Germany. Munich, Germany, 2004. [MEDLINE: ; none]
FAPIS 1996 {published data only}
- Chimienti M, Cullen MT Jr, Casadei G. Safety of flecainide versus propafenone for the long‐term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. European Heart Journal 1995;16(12):1943‐51. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
- Chimienti M, Cullen MT Jr, Casadei G. Safety of long‐term flecainide and propafenone in the management of patients with symptomatic paroxysmal atrial fibrillation: report from the Flecainide and Propafenone Italian Study Investigators. American Journal of Cardiology 1996;77(3):60A‐75A. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Flec‐SL 2012 {published data only}
- Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U, et al. Short‐term versus long‐term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec‐SL): a prospective, randomised, open‐label, blinded endpoint assessment trial. Lancet 2012;380(9838):238‐46. [PUBMED: 22713626] [DOI] [PubMed] [Google Scholar]
GEFACA 2001 {published data only}
- Galperin J, Elizari MV, Chiale PA, Molina RT, Ledesma R, Scapin AO, et al. Efficacy of amiodarone for the termination of chronic atrial fibrillation and maintenance of normal sinus rhythm: a prospective, multicenter, randomized, controlled, double blind trial. Journal of Cardiovascular Pharmacology and Therapeutics 2001;6(4):341‐50. [MEDLINE: ; ISSN 1074‐2484] [DOI] [PubMed] [Google Scholar]
Hillestad 1971 {published data only}
- Hillestad L, Bjerkelund C, Dale J, Maltau J, Storstein O. Quinidine in maintenance of sinus rhythm after electroconversion of chronic atrial fibrillation. A controlled clinical study. British Heart Journal 1971;33(4):518‐21. [MEDLINE: ; ISSN 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hohnloser 1995 {published data only}
- Hohnloser SH, Loo A, Baedeker F. Efficacy and proarrhythmic hazards of pharmacologic cardioversion of atrial fibrillation: prospective comparison of sotalol versus quinidine. Journal of the American College of Cardiology 1995;26(4):852‐8. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
Juul‐Moller 1990 {published data only}
- Juul‐Moller S, Edvardsson N, Rehnqvist‐Ahlberg N. Sotalol versus quinidine for the maintenance of sinus rhythm after direct current conversion of atrial fibrillation. Circulation 1990;82(6):1932‐9. [MEDLINE: ; ISSN: 0009‐7322] [DOI] [PubMed] [Google Scholar]
Kalusche 1994 {published data only}
- Kalusche D, Stockinger J, Betz P, Roskamm H. Sotalol and quinidine/verapamil (Cordichin) in chronic atrial fibrillation – conversion and 12‐month follow‐up – a randomized comparison [Sotalol und Chinidin/Verapamil (Cordichin) bei chronischem Vorhoflimmern – Konversion und 12‐Monats‐Follow‐up – ein randomisierter Vergleich]. Zeitschrift fur Kardiologie 1994;83 Suppl 5:109‐16. [MEDLINE: ; ISSN: 0300‐5860] [PubMed] [Google Scholar]
Karlson 1988 {published data only}
- Karlson BW, Torstensson I, Abjorn C, Jansson SO, Peterson LE. Disopyramide in the maintenance of sinus rhythm after electroconversion of atrial fibrillation. A placebo‐controlled one‐year follow‐up study. European Heart Journal 1988;9(3):284‐90. [MEDLINE: ; ISSN 0195‐668X] [DOI] [PubMed] [Google Scholar]
- Karlson BW, Torstensson I, Abjorn C, Kallryd A, Jonsson J, Jansson SO, et al. Preventive disopyramide after electroconversion of atrial fibrillation – a good alternative [Disopyramid som profylax efter elregularisering av formaksflimmer – ett bra alternativ]. Lakartidningen 1991;88(24):2242‐5. [MEDLINE: ; ISSN: 0023‐7205] [PubMed] [Google Scholar]
Kochiadakis 2000 {published data only}
- Kochiadakis GE, Igoumenidis NE, Marketou ME, Kaleboubas MD, Simantirakis EN, Vardas PE. Low dose amiodarone and sotalol in the treatment of recurrent, symptomatic atrial fibrillation: a comparative, placebo controlled study. Heart 2000;84(3):251‐7. [MEDLINE: ; ISSN 1355‐6037] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochiadakis GE, Igoumenidis NE, Marketou ME, Solomou MC, Kanoupakis EM, Vardas PE. Low‐dose amiodarone versus sotalol for suppression of recurrent symptomatic atrial fibrillation. American Journal of Cardiology 1998;81(8):995‐8. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
- Kochiadakis GE, Marketou ME, Igoumenidis NE, Chrysostomakis SI, Mavrakis HE, Kaleboubas MD, et al. Amiodarone, sotalol, or propafenone in atrial fibrillation: which is preferred to maintain normal sinus rhythm?. Pacing and Clinical Electrophysiology 2000;23(11 Pt 2):1883‐7. [MEDLINE: ; ISSN 0147‐8389] [DOI] [PubMed] [Google Scholar]
Kochiadakis 2004a {published data only}
- Kochiadakis GE, Igoumenidis NE, Hamilos MI, Tzerakis PG, Klapsinos NC, Zacharis EA, et al. Long‐term maintenance of normal sinus rhythm in patients with current symptomatic atrial fibrillation: amiodarone vs propafenone, both in low doses. Chest 2004;125(2):377‐83. [MEDLINE: ; ISSN: 0012‐3692] [DOI] [PubMed] [Google Scholar]
Kochiadakis 2004b {published data only}
- Kochiadakis GE, Igoumenidis NE, Hamilos ME, Tzerakis PG, Klapsinos NC, Chlouverakis GI, et al. Sotalol versus propafenone for long‐term maintenance of normal sinus rhythm in patients with recurrent symptomatic atrial fibrillation. American Journal of Cardiology 2004;94(12):1563‐6. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Kuhlkamp 2000 {published data only}
- Kuhlkamp V, Schirdewan A, Stangl K, Homberg M, Ploch M, Beck OA. Use of metoprolol CR/XL to maintain sinus rhythm after conversion from persistent atrial fibrillation: a randomized, double‐blind, placebo‐controlled study. Journal of the American College of Cardiology 2000;36(1):139‐46. [MEDLINE: ; ISSN 0735‐1097] [DOI] [PubMed] [Google Scholar]
Lloyd 1984 {published data only}
- Lloyd EA, Gersh BJ, Forman R. The efficacy of quinidine and disopyramide in the maintenance of sinus rhythm after electroconversion from atrial fibrillation. A double‐blind study comparing quinidine, disopyramide and placebo. South African Medical Journal 1984;65(10):367‐9. [MEDLINE: ; ISSN: 0038‐2469] [PubMed] [Google Scholar]
Naccarelli 1996 {published data only}
- Naccarelli GV, Dorian P, Hohnloser SH, Coumel P. Prospective comparison of flecainide versus quinidine for the treatment of paroxysmal atrial fibrillation/flutter. American Journal of Cardiology 1996;77(3):53A‐9A. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Nergårdh 2007 {published data only}
- Nergårdh AK, Rosenqvist M, Nordlander R, Frick M. Maintenance of sinus rhythm with metoprolol CR initiated before cardioversion and repeated cardioversion of atrial fibrillation: a randomized double‐blind placebo‐controlled study. European Heart Journal 2007;28(11):1351‐7. [PUBMED: PMID: 17329409] [DOI] [PubMed] [Google Scholar]
Niu 2006 {published data only}
- Niu F, Huang CX, Jiang H, Yang B, Guo WL, Chen YX, et al. Effects of amiodarone versus sotalol in treatment of atrial fibrillation: a random controlled clinical study. Zhonghua Yi Xue Za Zhi 2006;86(2):121‐3. [PUBMED: PMID: 16620720] [PubMed] [Google Scholar]
Okishige 2000 {published data only}
- Okishige K, Nishizaki M, Azegami K, Igawa M, Yamawaki N, Aonuma K. Pilsicainide for conversion and maintenance of sinus rhythm in chronic atrial fibrillation: a placebo‐controlled, multicenter study. American Heart Journal 2000;140(3):e13. [MEDLINE: ; ISSN 0002‐8703] [DOI] [PubMed] [Google Scholar]
PAFAC 2004 {published data only}
- Fetsch T, Bauer P, Engberding R, Koch HP, Lukl J, Meinertz T, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. European Heart Journal 2004;25(16):1385‐94. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
- Fetsch T, Burschel G, Breithardt G, Engberding R, Koch HP, Lukl J, et al. Medicamentous prevention after electric cardioversion of chronic atrial fibrillation. Goals and design of the PAFAC Study [Die medikamentöse Prophylaxe nach elektronischer Kardioversion von chronischem Vorhofflimmern. Ziele und Design der PAFAC‐Studie]. Zeitschrift fur Kardiologie 1999;88(3):195‐207. [MEDLINE: ; ISSN 0300‐5860] [DOI] [PubMed] [Google Scholar]
PITAGORA 2008 {published data only}
- PITAGORA Study Investigators, Gulizia M, Mangiameli S, Orazi S, Chiarandà G, Piccione G, et al. A randomized comparison of amiodarone and class IC antiarrhythmic drugs to treat atrial fibrillation in patients paced for sinus node disease: the PITAGORA trial. American Heart Journal 2008;155(1):107.e1. [PUBMED: PMID: 18082498] [DOI] [PubMed] [Google Scholar]
Plewan 2001 {published data only}
- Plewan A, Lehmann G, Ndrepepa G, Schreieck J, Alt EU, Schomig A, et al. Maintenance of sinus rhythm after electrical cardioversion of persistent atrial fibrillation; sotalol vs bisoprolol. European Heart Journal 2001;22(16):1504‐10. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
PRODIS 1996 {published data only}
- Crijns HJ, Gosselink AT, Lie KI. Propafenone versus disopyramide for maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation: a randomized, double‐blind study. Cardiovascular Drugs and Therapy 1996;10(2):145‐52. [MEDLINE: ; ISSN: 0920‐3206] [DOI] [PubMed] [Google Scholar]
RAFT 2003 {published data only}
- Pritchett EL, Page RL, Carlson M, Undesser K, Fava G. Efficacy and safety of sustained‐release propafenone (propafenone SR) for patients with atrial fibrillation. American Journal of Cardiology 2003;92(8):941‐6. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Reimold 1993 {published data only}
- Reimold SC, Cantillon CO, Friedman PL, Antman EM. Propafenone versus sotalol for suppression of recurrent symptomatic atrial fibrillation. American Journal of Cardiology 1993;71(7):558‐63. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Richiardi 1992 {published data only}
- Richiardi E, Gaita F, Greco C, Gaschino G, Comba Costa G, Rosettani E, et al. Propafenone versus hydroquinidine in long‐term pharmacological prophylaxis of atrial fibrillation [Propafenone versus idrochinidina nella profilassi farmacologica a lungo termine della fibrillazione atriale]. Cardiologia 1992;37(2):123‐7. [MEDLINE: ; ISSN: 0393‐1978] [PubMed] [Google Scholar]
SAFE‐T 2005 {published data only}
- Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, et al. Amiodarone versus sotalol for atrial fibrillation. New England Journal of Medicine 2005;352(18):1861‐72. [MEDLINE: ; ISSN: 0028‐4793] [DOI] [PubMed] [Google Scholar]
- Singh SN, Singh BN, Reda DJ, Fye CL, Ezekowitz MD, Fletcher RD, et al. Comparison of sotalol versus amiodarone in maintaining stability of sinus rhythm in patients with atrial fibrillation (Sotalol‐Amiodarone Fibrillation Efficacy Trial [Safe‐T]). American Journal of Cardiology 2003;92(4):468‐72. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
SAFIRE‐D 2000 {published data only}
- Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE‐D) study. Circulation 2000;102(19):2385‐90. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
Santas 2012 {published data only (unpublished sought but not used)}
- Santas E, Dominguez E, Martinez‐Brotons A, Ruiz‐Granell R, Ruiz V, Ferrero A, et al. Early withdrawal of amiodarone after cardioversion in atrial fibrillation patients treated with irbesartan. A prospective randomized study. European Heart Journal 2012;33 Suppl 1:380. [DOI: 10.1093/eurheartj/ehs282; PUBMED: 23805432]23805432 [DOI] [Google Scholar]
Singh 1991 {published data only}
- Singh S, Saini RK, DiMarco J, Kluger J, Gold R, Chen YW. Efficacy and safety of sotalol in digitalized patients with chronic atrial fibrillation. The Sotalol Study Group. American Journal of Cardiology 1991;68(11):1227‐30. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
SMART 2002 {published data only}
- Atarashi H, Inoue H, Fukunami M, Sugi K, Hamada C, Origasa H. Double‐blind placebo‐controlled trial of aprindine and digoxin for the prevention of symptomatic atrial fibrillation. Circulation Journal 2002;66(6):553‐6. [MEDLINE: ; ISSN 1346‐9843] [DOI] [PubMed] [Google Scholar]
SOCESP 1999 {published data only}
- Veloso HH, Paola AA. Analysis of atrial fibrillation recurrence during therapy with sotalol or quinidine. Researchers of +SOCESP [Analise da recorrencia de fibrilacao atrial durante terapia com sotalol ou quinidina. Investigadores da SOCESP]. Arquivos Brasileiros de Cardiologia 1998;70(1):43‐9. [MEDLINE: ; ISSN: 0066‐782X] [DOI] [PubMed] [Google Scholar]
- Paola AA, Veloso HH. Efficacy and safety of sotalol versus quinidine for the maintenance of sinus rhythm after conversion of atrial fibrillation. SOCESP Investigators. American Journal of Cardiology 1999;84(9):1033‐7. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Sodermark 1975 {published data only}
- Sodermark T, Jonsson B, Olsson A, Oro L, Wallin H, Edhag O, et al. Effect of quinidine on maintaining sinus rhythm after conversion of atrial fibrillation or flutter. A multicentre study from Stockholm. British Heart Journal 1975;37(5):486‐92. [MEDLINE: ; ISSN 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
SOPAT 2004 {published data only}
- Patten M, Koch HP, Sonntag F, Luderitz B, Meinertz T. Medicamentous prevention of symptomatic paroxysmal atrial fibrillation/flutter onset. Goals and design of the SOPAT study. Executive committee of the investigators representing trial physicians [Die medikamentose Anfallsprophylaxe bei symptomatischem paroxysmalem Vorhofflimmern/‐flattern. Ziele und Design der SOPAT‐Studie. Investigators in Vertretung der Prufarzte]. Zeitschrift fur Kardiologie 1999;88(3):185‐94. [MEDLINE: ; ISSN: 0300‐5860] [DOI] [PubMed] [Google Scholar]
- Patten M, Maas R, Bauer P, Luderitz B, Sonntag F, Dluzniewski M, et al. Suppression of paroxysmal atrial tachyarrhythmias – results of the SOPAT trial. European Heart Journal 2004;25(16):1395‐404. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
Steinbeck 1988 {published data only}
- Steinbeck G, Doliwa R, Bach P. Therapy of paroxysmal atrial fibrillation. Cardiac glycosides alone or combined with anti‐arrhythmia agents? [Therapie des paroxysmalen Vorhofflimmerns. Herzglykoside allein oder in Kombination mit Antiarrhythmika?]. Deutsche Medizinische Wochenschrift 1988;113(48):1867‐71. [MEDLINE: ; ISSN: 0012‐0472] [DOI] [PubMed] [Google Scholar]
Stroobandt 1997 {published data only}
- Stroobandt R, Stiels B, Hoebrechts R. Propafenone for conversion and prophylaxis of atrial fibrillation. Propafenone Atrial Fibrillation Trial Investigators. American Journal of Cardiology 1997;79(4):418‐23. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Van Gelder 1989 {published data only}
- Gelder IC, Crijns HJ, Gilst WH, Wijk LM, Hamer HP, Lie KI. Efficacy and safety of flecainide acetate in the maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation or atrial flutter. American Journal of Cardiology 1989;64(19):1317‐21. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
- Gelder IC, Crijns HJ, Gilst WH, Wijk LM, Hamer HP, Lie KI. Efficacy of flecainide to maintain sinus rhythm after cardioversion of chronic atrial fibrillation and flutter. Circulation 1988;78(4 Pt 2):III626. [MEDLINE: ; ISSN: 0009‐7322] [Google Scholar]
Vijayalakshmi 2006 {published data only}
- Vijayalakshmi K, Whittaker VJ, Sutton A, Campbell P, Wright RA, Hall JA, et al. A randomized trial of prophylactic antiarrhythmic agents (amiodarone and sotalol) in patients with atrial fibrillation for whom direct current cardioversion is planned. American Heart Journal 2006;151(4):863.e1‐6. [PUBMED: 16569550] [DOI] [PubMed] [Google Scholar]
Villani 1992 {published data only}
- Villani R, Zoletti F, Veniani M, Locati F, Nava S. A comparison between amiodarone and disopyramide in a delayed‐release formulation in the prevention of recurrences of symptomatic atrial fibrillation [Confronto fra amiodarone e disopiramide in formulazione retard nella prevenzione delle recidive di fibrillazione atriale sintomatica]. La Clinica Terapeutica 1992;140(1 Pt 2):35‐9. [MEDLINE: ; ISSN: 0009‐9074] [PubMed] [Google Scholar]
Vitolo 1981 {published data only}
- Vitolo E, Tronci M, Larovere MT, Rumolo R, Morabito A. Amiodarone versus quinidine in the prophylaxis of atrial fibrillation. Acta Cardiologica 1981;36(6):431‐44. [MEDLINE: ; ISSN: 0001‐5385] [PubMed] [Google Scholar]
References to studies excluded from this review
Aberg 1969 {published data only}
- Aberg H. Procaine amide as a prophylactic drug against atrial fibrillation. A controlled study [Prokainamid som profylax mot formaksflimmer. En kontrollerad studie]. Nordisk Medicin 1969;82(33):1011‐3. [MEDLINE: ; ISSN: 0029‐1420] [PubMed] [Google Scholar]
- Aberg H, Cullhed I. Procaine amide quinidine as prophylactic drugs against relapse of atrial fibrillation [Prokainamid och kinidin som profylaktikum mot recidiv av formaksflimmer]. Nordisk Medicin 1968;79(24):781‐2. [MEDLINE: ; ISSN 0029‐1420] [PubMed] [Google Scholar]
Adamyan 2015 {published data only}
- Adamyan KG, Tunyan LG, Chilingaryan AL. Comparative efficacy of amiodarone with ivabradin combination or amiodarone with bisoprolol combination in the prevention of atrial fibrillation recurrence in patients with left ventricular diastolic dysfunction. Rational Pharmacotherapy in Cardiology 2015;11(5):483‐8. [DOI: 10.20996/1819-6446-2015-11-5-483-488] [DOI] [Google Scholar]
AF‐CHF 2002 {published data only}
- Atrial Fibrillation and Congestive Heart Failure (AF‐CHF) investigators. Rationale and design of a study assessing treatment strategies of atrial fibrillation in patients with heart failure: the Atrial Fibrillation and Congestive Heart Failure (AF‐CHF) trial. American Heart Journal 2002;144(4):597‐607. [PUBMED: PMID: 12360154] [PubMed] [Google Scholar]
- Atrial Fibrillation and Congestive Heart Failure (AF‐CHF) investigators. Rationale and design of a study assessing treatment strategies of atrial fibrillation in patients with heart failure: the Atrial Fibrillation and Congestive Heart Failure (AF‐CHF) trial. American Heart Journal 2002;144(4):597‐607. [MEDLINE: ; ISSN 0002‐8703, EMBASE 2002364426] [PubMed] [Google Scholar]
AFFIRM 2002 {published data only}
- AFFIRM Investigators, Chung MK, Shemanski L, Sherman DG, Greene HL, Hogan DB, Kellen JC, et al. Functional status in rate‐ versus rhythm‐control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) Functional Status Substudy. Journal of the American College of Cardiology 2005;46(10):1891‐9. [PUBMED: PMID: 16286177] [DOI] [PubMed] [Google Scholar]
- AFFIRM Investigators, Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109(12):1509‐13. [PUBMED: PMID: 15007003] [DOI] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. New England Journal of Medicine 2002;347(23):1825‐33. [MEDLINE: ; ISSN 0028‐4793] [DOI] [PubMed] [Google Scholar]
Anderson 1994 {published data only}
- Anderson JL. Long‐term safety and efficacy of flecainide in the treatment of supraventricular tachyarrhythmias: the United States experience. The Flecainide Supraventricular Tachyarrhythmia Investigators. American Journal of Cardiology 1992;70(5):11A‐7A. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
- Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, Bhandari AK, et al. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double‐blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation 1989;80(6):1557‐70. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
- Anderson JL, Platt ML, Guarnieri T, Fox TL, Maser MJ, Pritchett EL. Flecainide acetate for paroxysmal supraventricular tachyarrhythmias. The Flecainide Supraventricular Tachycardia Study Group. American Journal of Cardiology 1994;74(6):578‐84. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Andromeda 2008 {published data only}
- Dronedarone Study Group, Køber L, Torp‐Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. New England Journal of Medicine 2008;358(25):2678‐87. [PUBMED: 18565860] [DOI] [PubMed] [Google Scholar]
Antman 1990 {published data only}
- Antman EM, Beamer AD, Cantillon C, McGowan N, Friedman PL. Therapy of refractory symptomatic atrial fibrillation and atrial flutter: a staged care approach with new antiarrhythmic drugs. Journal of the American College of Cardiology 1990;15(3):698‐707. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
- Antman EM, Beamer AD, Cantillon C, McGowan N, Goldman L, Friedman PL. Long‐term oral propafenone therapy for suppression of refractory symptomatic atrial fibrillation and atrial flutter. Journal of the American College of Cardiology 1988;12(4):1005‐11. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
Aros 1978 {published data only}
- Aros F, Valles V, Alegria E, Loma‐Osorio A, Malpartida F. Maintaining sinus rhythm with quinidine and amiodarone after electric cardioversion [Mantenimiento del ritmo sinusal con quinidina y amiodarona tras cardioversion electrica]. Revista Espanola de Cardiologia 1978;31(1 Pt 2):185‐91. [MEDLINE: ; ISSN: 0300‐8932] [PubMed] [Google Scholar]
Babuty 1999 {published data only}
- Babuty D, D'Hautefeuille B, Scheck F, Mycinsky C, Pruvost P, Peraudeau P. Cibenzoline versus flecainide in the prevention of paroxysmal atrial arrhythmias: a double‐blind randomized study. Journal of Clinical Pharmacology 1995;35(5):471‐7. [MEDLINE: ; ISSN: 0091‐2700] [DOI] [PubMed] [Google Scholar]
- Babuty D, Maison‐Blanche P, Fauchier L, Brembilla‐Perrot B, Medvedowsky JL, Bine‐Scheck F. Double‐blind comparison of cibenzoline versus flecainide in the prevention of recurrence of atrial tachyarrhythmias in 139 patients. Annals of Noninvasive Electrocardiology 1999;4(1):53‐9. [MEDLINE: ; EMBASE 1999048462; ISSN: 1082‐720X] [Google Scholar]
- Maison‐Blanche P, Brembilla‐Perrot B, Fauchier JP, Babuty D, Garnier LF, Rouesnel P, et al. Comparative study of cibenzoline and flecainide by oral route for preventing recurrence of paroxysmal atrial tachyarrhythmias [Étude comparative de la cibenzoline et du flecaïnide administrés par voie orale dans la prévention de récidive des tachycardies auriculaires]. Annales de Cardiologie et d Angeiologie 1997;46(2):109‐16. [MEDLINE: ; ISSN: 0003‐3928] [PubMed] [Google Scholar]
Beck 1978 {published data only}
- Beck OA, Lehmann HU, Hochrein H. Propafenone and lidoflazine in chronic atrial fibrillation and flutter (author's translation) [Propafenon und Lidoflazin bei chronischem Vorhofflimmern und ‐flattern. Vergleichende Untersuchungen]. Deutsche Medizinische Wochenschrift 1978;103(26):1068‐72. [MEDLINE: ; ISSN: 0012‐0472] [DOI] [PubMed] [Google Scholar]
Berns 1987 {published data only}
- Berns E, Rinkenberger RL, Jeang MK, Dougherty AH, Jenkins M, Naccarelli GV. Efficacy and safety of flecainide acetate for atrial tachycardia or fibrillation. American Journal of Cardiology 1987;59(15):1337‐41. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Blevins 1987 {published data only}
- Blevins RD, Kerin NZ, Benaderet D, Frumin H, Faitel K, Jarandilla R, et al. Amiodarone in the management of refractory atrial fibrillation. Archives of Internal Medicine 1987;147(8):1401‐4. [MEDLINE: ; ISSN: 0003‐9926] [PubMed] [Google Scholar]
Blomstrom 1984 {published data only}
- Blomstrom P, Edvardsson N, Olsson SB. Amiodarone in atrial fibrillation. Acta Medica Scandinavica 1984;216(5):517‐24. [MEDLINE: ; ISSN: 0001‐6101] [DOI] [PubMed] [Google Scholar]
Boissel 1981 {published data only}
- Boissel JP, Wolf E, Gillet J, Soubrane A, Cavallaro A, Mazoyer G, et al. Controlled trial of a long‐acting quinidine for maintenance of sinus rhythm after conversion of sustained atrial fibrillation. European Heart Journal 1981;2(1):49‐55. [MEDLINE: ; ISSN 0195‐668X] [DOI] [PubMed] [Google Scholar]
Brodsky 1987 {published data only}
- Brodsky MA, Allen BJ, Walker CJ 3rd, Casey TP, Luckett CR, Henry WL. Amiodarone for maintenance of sinus rhythm after conversion of atrial fibrillation in the setting of a dilated left atrium. American Journal of Cardiology 1987;60(7):572‐5. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
CHF‐STAF 1998 {published data only}
- Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the Veterans Affairs Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF‐STAT). Circulation 1998;98(23):2574‐9. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
Chun 1995 {published data only}
- Chun SH, Sager PT, Stevenson WG, Nademanee K, Middlekauff HR, Singh BN. Long‐term efficacy of amiodarone for the maintenance of normal sinus rhythm in patients with refractory atrial fibrillation or flutter. American Journal of Cardiology 1995;76(1):47‐50. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Clementy 1992 {published data only}
- Clementy J, Dulhoste MN, Laiter C, Denjoy I, Dos Santos P. Flecainide acetate in the prevention of paroxysmal atrial fibrillation: a nine‐month follow‐up of more than 500 patients. American Journal of Cardiology 1992;70(5):44A‐9A. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Connolly 1989 {published data only}
- Connolly SJ, Hoffert DL. Usefulness of propafenone for recurrent paroxysmal atrial fibrillation. American Journal of Cardiology 1989;63(12):817‐9. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
CTAF 2000 {published data only}
- Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. American Heart Journal 2002;143(6):984‐90. [MEDLINE: ; ISSN: 0002‐8703] [DOI] [PubMed] [Google Scholar]
- Lumer GB, Roy D, Talajic M, Couturier A, Lambert J, Frasure‐Smith N, et al. Amiodarone reduces procedures and costs related to atrial fibrillation in a controlled clinical trial. European Heart Journal 2002;23(13):1050‐6. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. New England Journal of Medicine 2000;342(13):913‐20. [MEDLINE: ; ISSN: 0028‐4793] [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Thibault B, Dubuc M, Nattel S, Eisenberg MJ, et al. Pilot study and protocol of the Canadian Trial of Atrial Fibrillation (CTAF). American Journal of Cardiology 1997;80(4):464‐8. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Cuan‐Perez 1971 {published data only}
- Cuan‐Perez M, Ortiz A. Comparative study of quinidine, propranolol and diphenylhydantoin for preventing recurrence in post‐cardioversion auricular fibrillation [Estudio comparativo entre quinidina, propranolol y difenil‐hidantoinato para prevenir la recaida en fibrilacion auricular post‐cardioversion]. Archivos del Instituto de Cardiologia de Mexico 1971;41(3):278‐84. [MEDLINE: ; ISSN 0020‐3785] [PubMed] [Google Scholar]
Darkner 2014 {published data only}
- Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, et al. Recurrence of arrhythmia following short‐term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double‐blind, randomized, placebo‐controlled study (AMIO‐CAT trial). European Heart Journal 2014;35(47):3356‐64. [DOI: 10.1093/eurheartj/ehu354] [DOI] [PubMed] [Google Scholar]
Di Biase 2016 {published data only}
- Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133(17):1637‐44. [DOI: 10.1161/CIRCULATIONAHA.115.019406] [DOI] [PubMed] [Google Scholar]
Enriquez 2014 {published data only}
- Enriquez A, Conde D, Hopman W, Mondragon I, Chiale PA, Luna AB, et al. Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovascular Therapeutics 2014;32(2):52‐6. [DOI: 10.1111/1755-5922.12063] [DOI] [PubMed] [Google Scholar]
ERAFT 2002 {published data only}
- Meinertz T, Lip GY, Lombardi F, Sadowski ZP, Kalsch B, Camez A, et al. Efficacy and safety of propafenone sustained release in the prophylaxis of symptomatic paroxysmal atrial fibrillation. The European Rythmol/Rytmonorm Atrial Fibrillation Trial (ERAFT) Study. American Journal of Cardiology 2002;90(12):1300‐6. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Faivre 1970 {published data only}
- Faivre G, Polu JM. Disopyramide in the preparation for electric shock in atrial fibrillation and in the maintenance of subsequent sinus rhythm [La disopiramide nella preparazione allo shock elettrico della fibrillazione atriale e nel mantenimento dell'ulteriore ritmo sinusale]. Minerva Medica 1970;61(71 Suppl):3745‐7. [MEDLINE: ; ISSN 0026‐4806] [PubMed] [Google Scholar]
Farkowski 2012 {published data only}
- Farkowski MM, Maciag A, Dabrowski R, Pytkowski M, Kowalik I, Szwed H. Clinical efficacy of antazoline in rapid cardioversion of paroxysmal atrial fibrillation – a protocol of a single center, randomized, double‐blind, placebo‐controlled study (the AnPAF Study). Trials 2012;13:162. [DOI: 10.1186/1745-6215-13-162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández 1998 {published data only}
- Fernández JM, Martínez‐Marcos FJ, Ortega A, García JL, Camacho C, Santos JM, et al. Comparative study of propafenone, amiodarone and flecainide in the treatment of paroxystic atrial fibrillation [Estudio comparativo de propafenona, amiodarona y flecainida en el tratamiento de la fibrilación auricular paroxística]. Revista Espanola de Cardiologia 1998;51 Suppl 5:84. [MEDLINE: ; ISSN: 0300‐8932] [Google Scholar]
Feyrer 2014 {published data only}
- Feyrer R, Ballazhi F, Seitz T, Weyand M, Harig F. Impact of medical treatment on long‐term results after surgical ablation of atrial fibrillation in cardiac surgical patients. Annals of Thoracic and Cardiovascular Surgery 2014;20(3):207‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fragakis 2012 {published data only}
- Fragakis N, Koskinas KC, Katritsis DG, Pagourelias ED, Zografos T, Geleris P. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent‐onset atrial fibrillation. American Journal of Cardiology 2012;110(5):673‐7. [DOI: 10.1016/j.amjcard.2012.04.044; PUBMED: 22621799] [DOI] [PubMed] [Google Scholar]
- Koskinas KC, Fragakis N, Pagourelias ED, Rossios K, Abramidou A, Sotiriou P, et al. Ranolazine added to amiodarone vs. amiodarone alone for the conversion of recent‐onset atrial fibrillation. European Heart Journal 2012;33 Suppl 1:383. [DOI: 10.1093/eurheartj/ehs282; PUBMED: 23805432]23805432 [DOI] [Google Scholar]
Frances 1985 {published data only}
- Frances Y, Luccioni R, Delaage M, Donnarel G, Medvedowsky JL, Quiret JC. Long‐term prevention of the recurrence of auricular fibrillation using cibenzoline. Multicenter study apropos of 89 case reports [Prevention au long cours des recidives de fibrillation auriculaire par la cibenzoline. Etude multicentrique a propos de 89 observations]. Archives des Maladies du Coeur et des Vaisseaux 1985;78(Spec No):99‐103. [MEDLINE: ; ISSN: 0003‐9683] [PubMed] [Google Scholar]
Galperin 2014 {published data only}
- Galperin J, Elizari MV, Bonato R, Ledesma R, Vazquez Blanco M, Lago M, et al. Short‐term amiodarone therapy after reversion of persistent atrial fibrillation reduces recurrences at 18 months. Cardiology Journal 2014;21(4):397‐404. [DOI: 10.5603/CJ.a2013.0152; PUBMED: 24293165] [DOI] [PubMed] [Google Scholar]
Gold 1986 {published data only}
- Gold RL, Haffajee CI, Charos G, Sloan K, Baker S, Alpert JS. Amiodarone for refractory atrial fibrillation. American Journal of Cardiology 1986;57(1):124‐7. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Gosselink 1992 {published data only}
- Gosselink AT, Crijns HJ, Gelder IC, Hillige H, Wiesfeld AC, Lie KI. Low‐dose amiodarone for maintenance of sinus rhythm after cardioversion of atrial fibrillation or flutter. JAMA 1992;267(24):3289‐93. [MEDLINE: ; ISSN 0098‐7484] [PubMed] [Google Scholar]
Graboys 1983 {published data only}
- Graboys TB, Podrid PJ, Lown B. Efficacy of amiodarone for refractory supraventricular tachyarrhythmias. American Heart Journal 1983;106(4 Pt 2):870‐6. [MEDLINE: ; ISSN: 0002‐8703] [DOI] [PubMed] [Google Scholar]
Gramley 2011 {published data only}
- Gramley F, Koellensperger E, Munzel T, Kettering KC. Dronedarone therapy after catheter ablation of atrial fibrillation: a new hybrid therapy option. Europace 2011;13 Suppl 3:NP. [DOI: ] [Google Scholar]
Grigoryan 2011 {published data only}
- Grigoryan S, Hazarapetyan LGC. Procoralan as a preventive treatment for paroxysmal atrial fibrillation. European Heart Journal 2011;32 Suppl 1:624. [DOI: 10.1093/eurheartj/ehr323] [DOI] [Google Scholar]
Gu 2012 {published data only}
- Gu J, Liu X, Tan H, Zhou L, Gu J, Jiang W, et al. Extensive antiarrhythmic drugs after catheter ablation of persistent atrial fibrillation. Acta Cardiologica 2012;67(4):407‐14. [PUBMED: 22997994] [DOI] [PubMed] [Google Scholar]
GUSTO 2002 {published data only}
- Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. Management and outcome of patients with atrial fibrillation during acute myocardial infarction: the GUSTO‐III experience. Global use of strategies to open occluded coronary arteries. Heart 2002;88(4):357‐62. [MEDLINE: ; ISSN 1355‐6037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammill 1988 {published data only}
- Hammill SC, Wood DL, Gersh BJ, Osborn MJ, Holmes DR Jr. Propafenone for paroxysmal atrial fibrillation. American Journal of Cardiology 1988;61(6):473‐4. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Härtel 1970 {published data only}
- Hartel G, Louhija A, Konttinen A, Halonen PI. Value of quinidine in maintenance of sinus rhythm after electric conversion of atrial fibrillation. British Heart Journal 1970;32(1):57‐60. [MEDLINE: ; ISSN 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel G, Louhija A, Halonen PI. Investigations on the value of quinidine therapy after electrical rhythmization of atrial fibrillation [Untersuchungen über den Wert der Chinidinbehandlung nach elektrischer Rhythmisierung von Vorhofflimmern]. Die Medizinische Welt 1969;20(25):1464. [MEDLINE: ; CENTRAL: CN‐00247601] [Google Scholar]
- Härtel G, Louhija A, Halonen PI. The value of quinidine therapy in order to maintain sinus rhythm after electrical rhythmization of atrial fibrillation [Der Wert der Chinidinbehandlung für die Erhaltung des Sinusrhythmus nach elektrischer Rhythmisierung von Vorhofflimmern]. Klinische Wochenschrift 1969;47(17):942. [MEDLINE: ; CENTRAL: CN‐00247602; ISSN: 0023‐2173] [Google Scholar]
Hartel 1974 {published data only}
- Hartel G, Louhija A, Konttinen A. Disopyramide in the prevention of recurrence of atrial fibrillation after electroconversion. Clinical Pharmacology and Therapeutics 1974;15(6):551‐5. [MEDLINE: ; ISSN 0009‐9236] [DOI] [PubMed] [Google Scholar]
Hopson 1996 {published data only}
- Hopson JR, Buxton AE, Rinkenberger RL, Nademanee K, Heilman JM, Kienzle MG. Safety and utility of flecainide acetate in the routine care of patients with supraventricular tachyarrhythmias: results of a multicenter trial. The Flecainide Supraventricular Tachycardia Study Group. American Journal of Cardiology 1996;77(3):72A‐82A. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Horowitz 1985 {published data only}
- Horowitz LN, Spielman SR, Greenspan AM, Mintz GS, Morganroth J, Brown R, et al. Use of amiodarone in the treatment of persistent and paroxysmal atrial fibrillation resistant to quinidine therapy. Journal of the American College of Cardiology 1985;6(6):1402‐7. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
HOT‐CAFE 2004 {published data only}
- Opolski G, Torbicki A, Kosior D, Stolarz P, Dawidowska R, Zawadzka M, et al. Should sinus rhythm be restored in patients with chronic atrial fibrillation? Preliminary results from the Polish "Hot Cafe" study [Czy przywracac rytm zatokowy u chorych z przewleklym migotaniem przedsionkow? Wstepne wyniki polskiego badania "Hot Cafe"]. Polskie Archiwum Medycyny Wewnetrznej 1999;101(5):413‐8. [MEDLINE: ; ISSN: 0032‐3772] [PubMed] [Google Scholar]
- Opolski G, Torbicki A, Kosior D, Szulc M, Zawadzka M, Pierscinska M, et al. Rhythm control versus rate control in patients with persistent atrial fibrillation. Results of the HOT CAFE Polish Study. Kardiologia Polska 2003;59(7):1‐16. [MEDLINE: ; ISSN 0022‐9032] [PubMed] [Google Scholar]
- Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska‐Kaplon B, Kolodziej P, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest 2004;126(2):476‐86. [MEDLINE: ; ISSN: 0012‐3692] [DOI] [PubMed] [Google Scholar]
Ishiguro 2008 {published data only}
- Ishiguro H, Ikeda T, Abe A, Tsukada T, Mera H, Nakamura K, et al. Antiarrhythmic effect of bisoprolol, a highly selective beta1‐blocker, in patients with paroxysmal atrial fibrillation. International Heart Journal 2008;49(3):281‐93. [PUBMED: 18612186] [DOI] [PubMed] [Google Scholar]
J‐BAF 2009 {published data only}
- J‐BAF Investigators, Yamashita T, Ogawa S, Sato T, Aizawa Y, Atarashi H, Fujiki A, et al. Dose‐response effects of bepridil in patients with persistent atrial fibrillation monitored with transtelephonic electrocardiograms: a multicenter, randomized, placebo‐controlled, double‐blind study (J‐BAF Study). Circulation Journal 2009;73(6):1020‐7. [PUBMED: PMID: 19359813] [DOI] [PubMed] [Google Scholar]
Jong 2006 {published data only}
- Jong GP, Chang MH, Chang TC, Chou P, Fu CY, Tien LY, et al. Long‐term efficacy and safety of very‐low‐dose amiodarone treatment for the maintenance of sinus rhythm in patients with chronic atrial fibrillation after successful direct‐current cardioversion. Chinese Medical Journal (English) 2006;119(24):2030‐5. [PUBMED: PMID: 17199952] [PubMed] [Google Scholar]
J‐RHYTHM 2009 {published data only}
- J‐RHYTHM Investigators, Ogawa S, Yamashita T, Yamazaki T, Aizawa Y, Atarashi H, Inoue H, et al. Optimal treatment strategy for patients with paroxysmal atrial fibrillation: J‐RHYTHM Study. Circulation Journal 2009;73(2):242‐8. [PUBMED: PMID: 19060419] [DOI] [PubMed] [Google Scholar]
Kanoupakis 2004 {published data only}
- Kanoupakis EM, Manios EG, Mavrakis HE, Tzerakis PG, Mouloudi HK, Vardas PE. Comparative effects of carvedilol and amiodarone on conversion and recurrence rates of persistent atrial fibrillation. American Journal of Cardiology 2004;94(5):659‐62. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Kennelly 1977 {published data only}
- Kennelly BM. Comparison of lidoflazine and quinidine in prophylactic treatment of arrhythmias. British Heart Journal 1977;39(5):540‐6. [MEDLINE: ; CENTRAL: CN‐00345795; ISSN: 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 1988 {published data only}
- Kerr CR, Klein GJ, Axelson JE, Cooper JC. Propafenone for prevention of recurrent atrial fibrillation. American Journal of Cardiology 1988;61(11):914‐6. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Khitri 2012 {published data only}
- Khitri AR, Aliot EM, Capucci A, Connolly SJ, Crijns H, Hohnloser SH, et al. Celivarone for maintenance of sinus rhythm and conversion of atrial fibrillation/flutter. Journal of Cardiovascular Electrophysiology 2012;23(5):462‐72. [DOI: 10.1111/j.1540-8167.2011.02234.x; PUBMED: 22171925] [DOI] [PubMed] [Google Scholar]
Komatsu 2006 {published data only}
- Komatsu T, Sato Y, Tachibana H, Nakamura M, Horiuchi D, Okumura K. Randomized crossover study of the long‐term effects of pilsicainide and cibenzoline in preventing recurrence of symptomatic paroxysmal atrial fibrillation: influence of the duration of arrhythmia before therapy. Circulation Journal 2006;70(6):667‐72. [PUBMED: 16723785] [DOI] [PubMed] [Google Scholar]
Kosior 2001 {published data only}
- Kosior D, Karpinski G, Wretowski D, Stolarz P, Stawicki S, Rabczenko D, et al. Sequential prophylactic antiarrhythmic therapy for maintenance of sinus rhythm after cardioversion of persistent atrial fibrillation – one year follow‐up. Kardiologia Polska 2002;56(4):361‐7. [MEDLINE: ; EMBASE: 2002162110; ISSN: 0022‐9032] [Google Scholar]
- Kosior D, Opolski G, Torbicki A. Efficacy of sequential antiarrhythmic treatment in sinus rhythm maintenance after successful electrocardioversion in patients with chronic non‐valvular atrial fibrillation. Medical Science Monitor 2001;7(1):68‐73. [MEDLINE: ; ISSN 1234‐1010] [PubMed] [Google Scholar]
- Kosior D, Szulc M, Zawadzka M, PierScinska M, Stawicki S, Rabczenko D, et al. Role of amiodarone in sinus rhythm maintenance after successful cardioversion in patients with chronic non‐valvular atrial fibrillation [Rola amiodaronu w utrzymaniu rytmu zatokowego po skutecznej kardiowersji elektrycznej u chorych z przetrwalym migotaniem]. Polskie Archiwum Medycyny Wewnetrznej 2002;108(6):1151‐60. [MEDLINE: ; ISSN 0032‐3772] [PubMed] [Google Scholar]
Kosior 2009 {published data only}
- Kosior DA, Kochanowski J, Scisło P, Piatkowski R, Postuła M, Rabczenko D, Opolski G. Efficacy and tolerability of oral propafenone versus quinidine in the treatment of recent onset atrial fibrillation: a randomized, prospective study. Cardiology Journal 2009;16(6):521‐7. [PUBMED: 19950088] [PubMed] [Google Scholar]
Kyles 1991 {published data only}
- Kyles AE, Murdock CJ, Yeung‐Lai‐Wah JA, Vorderbrugge S, Kerr CR. Long term efficacy of propafenone for prevention of atrial fibrillation. Canadian Journal of Cardiology 1991;7(9):407‐9. [MEDLINE: ; ISSN: 0828‐282X] [PubMed] [Google Scholar]
Lardoux 1996 {published data only}
- Lardoux H, Maison Blanche P, Marchand X, Canler A, Rouesnel P, Bleinc D, et al. Cibenzoline versus propafenone by the oral route for preventing recurrence of atrial arrhythmia: multicenter, randomized, double‐blind study [Cibenzoline versus propafénone par voie orale dans la prévention de la récidive des arythmies auriculaires: étude multicentrique randomisée realisée en double aveugle]. Annales de Cardiologie et d Angeiologie 1996;45(8):469‐79. [MEDLINE: ; ISSN: 0003‐3928] [PubMed] [Google Scholar]
Lau 1992 {published data only}
- Lau CP, Leung WH, Wong CK. A randomized double‐blind crossover study comparing the efficacy and tolerability of flecainide and quinidine in the control of patients with symptomatic paroxysmal atrial fibrillation. American Heart Journal 1992;124(3):645‐50. [MEDLINE: ; ISSN: 0002‐8703] [DOI] [PubMed] [Google Scholar]
Levi 1973 {published data only}
- Levi G, Proto C, Rovetta A. Double‐blind evaluation of practolol and quinidine in the treatment of chronic atrial fibrillation. Cardiology 1973;58(6):364‐8. [MEDLINE: ; ISSN 0008‐6312] [DOI] [PubMed] [Google Scholar]
- Quadri A, Levi GP, Proto C. Treatment of chronic atrial fibrillation with practolol‐quinidine [Trattamento della fibrillazione atriale cronica con practololo‐chinidina]. Minerva Cardioangiologica 1973;21(10):668‐71. [MEDLINE: ; ISSN 0026‐4725] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li H, Riedel R, Oldemeyer JB, Rovang K, Hee T. Comparison of recurrence rates after direct‐current cardioversion for new‐onset atrial fibrillation in patients receiving versus those not receiving rhythm‐control drug therapy. American Heart Journal 2004;93(1):45‐8. [MEDLINE: ; ISSN 0002‐9149] [DOI] [PubMed] [Google Scholar]
Löbe 2013 {published data only}
- Löbe S, Salmáš J, John S, Kornej J, Husser D, Hindricks G, et al. Usefulness of dronedarone in patients with atrial arrhythmias. American Journal of Cardiology 2013;111(9):1311‐4. [DOI: 10.1016/j.amjcard.2012.12.057; PUBMED: 23465099] [DOI] [PubMed] [Google Scholar]
Lodziński 2014 {published data only}
- Lodziński P, Kiliszek M, Koźluk E, Piątkowska A, Balsam P, Kochanowski J, et al. Does a blanking period after pulmonary vein isolation impact long‐term results? Results after 55 months of follow‐up. Cardiology Journal 2014;21(4):384‐91. [DOI: 10.5603/CJ.a2013.0144] [DOI] [PubMed] [Google Scholar]
Maĭkov 2015 {published data only}
- Maĭkov EB, Luricheva IuA, Mironov NIu, Sokolov SF, Golitsyn SP, Rozenshtraukh LV, et al. Refralon (niferidil) is a new class III antiarrhythmic agent for pharmacological cardioversion for persistent atrial fibrillation and atrial flutter. Terapevticheskii Arkhiv 2015;87(1):38‐48. [DOI: 10.17116/terarkh201587138-48] [DOI] [PubMed] [Google Scholar]
Manios 2003 {published data only}
- Manios EG, Mavrakis HE, Kanoupakis EM, Kallergis EM, Dermitzaki DN, Kambouraki DC, et al. Effects of amiodarone and diltiazem on persistent atrial fibrillation conversion and recurrence rates: a randomized controlled study. Cardiovascular Drugs and Therapy 2003;17(1):31‐9. [MEDLINE: ; ISSN: 0920‐3206] [DOI] [PubMed] [Google Scholar]
Martin 1986 {published data only}
- Martin A, Benbow LJ, Leach C, Bailey RJ. Comparison of amiodarone and disopyramide in the control of paroxysmal atrial fibrillation and atrial flutter. British Journal of Clinical Practice. Supplement 1986;44:52‐60. [MEDLINE: ; ISSN: 0260‐8767] [PubMed] [Google Scholar]
Mary‐Rabine 1990 {published data only}
- Mary‐Rabine L, Kulbertus HE. Clinical efficacy of flecainide acetate in atrial fibrillation. Cardiology 1990;77(6):443‐9. [MEDLINE: ; ISSN: 0008‐6312] [DOI] [PubMed] [Google Scholar]
Massacci 1991 {published data only}
- Massacci E, Morellini C, De PG, Chioffi M, Longobardi M, Cellino F, et al. Flecainide versus amiodarone in preventing paroxysmal idiopathic atrial fibrillation. New Trends in Arrhythmias 1991;7(4):693‐8. [MEDLINE: ; EMBASE 1992241383] [Google Scholar]
Meng 2015 {published data only}
- Meng Z, Tan J, He Q, Zhu M, Li X, Zhang J, et al. Wenxin Keli versus sotalol for paroxysmal atrial fibrillation caused by hyperthyroidism: a prospective, open label, and randomized study. Evidence‐Based Complementary and Alternative Medicine 2015;2015:101904. [DOI: 10.1155/2015/101904] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizutani 1995 {published data only}
- Mizutani N, Oki Y, Wakida Y, Iwa T, Haga M, Mizutani K, et al. Pilsicainide in the acute conversion and the prevention of paroxysmal atrial fibrillation. Japanese Pharmacology & Therapeutics 1995;23(7):113‐20. [MEDLINE: ; EMBASE 1995251738] [Google Scholar]
Mont 2014 {published data only}
- Mont L, Bisbal F, Hernández‐Madrid A, Pérez‐Castellano N, Viñolas X, Arenal A, et al. SARA investigators. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). European Heart Journal 2014;35(8):501‐7. [DOI: 10.1093/eurheartj/eht457] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nedostup 1990 {published data only}
- Nedostup AV, Alekseevskaia MA, Novikov ID, Maevskaia IV. A comparison of the efficacy of quinidine and kordaron as agents to stabilize the recovered sinus rhythm in patients with the permanent form of atrial fibrillation [Sravnenie effektivnosti khinidina i kordarona kak sredstv stabilizatsii vosstanovlennogo sinusovogo ritma u bol'nykh s postoianno formo mertsatel'no aritmii]. Terapevticheskii Arkhiv 1990;62(9):47‐51. [MEDLINE: ; ISSN: 0040‐3660] [PubMed] [Google Scholar]
Opolski 1997 {published data only}
- Opolski G, Stanislawska J, Gorecki A, Swiecicka G, Torbicki A, Kraska T. Amiodarone in restoration and maintenance of sinus rhythm in patients with chronic atrial fibrillation after unsuccessful direct‐current cardioversion. Clinical Cardiology 1997;20(4):337‐40. [MEDLINE: ; ISSN: 0160‐9289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park L, Park S, Ko L, Choi L, Park K. Effect of pilsicainide versus other class IC anti‐arrhythmic drugs after catheter ablation of paroxysmal atrial fibrillation; prospective randomized study. 35th Annual Scientific Sessions of the Heart Rhythm Society; 2014 May 7‐10; San Francisco (CA). [clinicaltrials.gov/ct2/show/NCT01775891]
PEPS 2002 {published data only}
- Simon T, Mary‐Krause M, Funck‐Brentano C, Davy JM, Weingrod M, Jaillon P. Efficacy and tolerance of propafenone after correction of atrial fibrillation: PEPS pharmaco‐epidemiologic study [Efficacité et tolérance de la propafénone après régularisation de la fibrillation auriculaire: étude pharmaco‐épidémiologique PEPS]. Archives des Maladies du Coeur et des Vaisseaux 2002;95(6):567‐72. [MEDLINE: ; ISSN 0003‐9683] [PubMed] [Google Scholar]
PIAF 2000 {published data only}
- Gronefeld GC, Lilienthal J, Kuck KH, Hohnloser SH. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. European Heart Journal 2003;24(15):1430‐6. [MEDLINE: ; ISSN: 0195‐668X] [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation – Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet 2000;356(9244):1789‐94. [MEDLINE: ; ISSN 0140‐6736] [DOI] [PubMed] [Google Scholar]
Pietersen 1991 {published data only}
- Pietersen AH, Hellemann H. Usefulness of flecainide for prevention of paroxysmal atrial fibrillation and flutter. Danish‐Norwegian Flecainide Multicenter Study Group. American Journal of Cardiology 1991;67(8):713‐7. [MEDLINE: ; EMBASE 1991186337; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Piot 1998 {published data only}
- Piot O, Flammang D, Dambrine P, Cheikel J, Jouannon C, Graux P, et al. A randomized double‐blind trial comparing cibenzoline and disopyramide in the prevention of recurrences of atrial tachyarrhythmia [Etude randomisee en double aveugle comparant la cibenzoline et le disopyramide dans la prevention de recidives de tachyarythmies atriales]. Archives des Maladies du Coeur et des Vaisseaux 1998;91(12):1481‐6. [MEDLINE: ; ISSN: 0003‐9683] [PubMed] [Google Scholar]
Porterfield 1989 {published data only}
- Porterfield JG, Porterfield LM. Therapeutic efficacy and safety of oral propafenone for atrial fibrillation. American Journal of Cardiology 1989;63(1):114‐6. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
PSVT 1995 {published data only}
- Cobbe SM, Rae AP, Poloniecki JD, Chong E, Balnave K, Moriarty A, et al. A randomized, placebo‐controlled trial of propafenone in the prophylaxis of paroxysmal supraventricular tachycardia and paroxysmal atrial fibrillation. UK Propafenone PSVT Study Group. Circulation 1995;92(9):2550‐7. [MEDLINE: ; ISSN 0009‐7322] [PubMed] [Google Scholar]
Qin 2016 {published data only}
- Qin D, Leef G, Bilal Alam M, Rattan R, Bilal Munir M, Patel D, et al. Comparative effectiveness of antiarrhythmic drugs for rhythm control of atrial fibrillation. Journal of Cardiology 2016;67(5):471‐6. [PUBMED: 26233885] [DOI] [PubMed] [Google Scholar]
RACE 2002 {published data only}
- Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. New England Journal of Medicine 2002;347(23):1834‐40. [MEDLINE: ; ISSN 0028‐4793] [DOI] [PubMed] [Google Scholar]
Rakhmanova 2014 {published data only (unpublished sought but not used)}
- Rakhmanova MM. Efficacy and safety of allapinine and quinidine bisulphate in the treatment of patients with persistent atrial fibrillation after cardioversion. Kardiologiia 2014;54(9):33‐8. [PUBMED: 25702400] [DOI] [PubMed] [Google Scholar]
Rasmussen 1981 {published data only}
- Rasmussen K, Wang H, Fausa D. Comparative efficiency of quinidine and verapamil in the maintenance of sinus rhythm after DC conversion of atrial fibrillation. A controlled clinical trial. Acta Medica Scandinavica. Supplementum 1981;645:23‐8. [MEDLINE: ; ISSN 0365‐463X] [DOI] [PubMed] [Google Scholar]
Resnekov 1971 {published data only}
- Resnekov L, Gibson D, Waich S, Muir J, McDonald L. Sustained‐release quinidine (Kinidin Durules) in maintaining sinus rhythm after electroversion of atrial dysrhythmias. British Heart Journal 1971;33(2):220‐5. [MEDLINE: ; ISSN 0007‐0769] [DOI] [PMC free article] [PubMed] [Google Scholar]
STAF 2003 {published data only}
- Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, et al. Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. Journal of the American College of Cardiology 2003;41(10):1690‐6. [MEDLINE: ; ISSN 0735‐1097] [DOI] [PubMed] [Google Scholar]
Steeds 1999 {published data only}
- Steeds RP, Birchall AS, Smith M, Channer KS. An open label, randomised, crossover study comparing sotalol and atenolol in the treatment of symptomatic paroxysmal atrial fibrillation. Heart 1999;82(2):170‐5. [MEDLINE: ; ISSN 1355‐6037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonet 1986 {published data only}
- Tonet JL, Bernardeau C, Lechat P, Frank R, Touzet I, Fontaine G, et al. Comparison between the efficacy of amiodarone and quinidine in the treatment of atrial cardiac arrhythmias. British Journal of Clinical Practice. Supplement 1986;44:42‐8. [MEDLINE: ; ISSN: 0262‐8767] [PubMed] [Google Scholar]
Torp‐Pedersen 2011 {published data only}
- Torp‐Pedersen C, Raev DH, Dickinson G, Butterfield NN, Mangal B, Beatch GN. A randomized, placebo‐controlled study of vernakalant (oral) for the prevention of atrial fibrillation recurrence after cardioversion. Circulation. Arrhythmia and Electrophysiology 2011;4(5):637‐43. [DOI: 10.1161/CIRCEP.111.962340; PUBMED: 21841207] [DOI] [PubMed] [Google Scholar]
Touboul 1995 {published data only}
- Touboul P, Brembilla‐Perrot B, Scheck F, Gabriel A, Lardoux H, Marchand X. Comparative effects of cibenzoline and hydroquinidine in the prevention of auricular fibrillation. A randomized double‐blind study [Effets comparés de la cibenzoline et de l'hydroquinidine dans la prévention de la fibrillation auriculaire. Une étude randomisée en double aveugle]. Annales de Cardiologie et d Angeiologie 1995;44(9):525‐31. [MEDLINE: ; ISSN: 0003‐3928] [PubMed] [Google Scholar]
Van Wijk 1989 {published data only}
- Wijk LM, Heijer P, Crijns HJ, Gilst WH, Lie KI. Flecainide versus quinidine in the prevention of paroxysms of atrial fibrillation. Journal of Cardiovascular Pharmacology 1989;13(1):32‐6. [MEDLINE: ; ISSN: 0160‐2446] [DOI] [PubMed] [Google Scholar]
VEPARAF 2003 {published data only}
- Simone A, Pasquale M, Matteis C, Canciello M, Manzo M, Sabino L, et al. VErapamil plus antiarrhythmic drugs reduce atrial fibrillation recurrences after an electrical cardioversion (VEPARAF Study). European Heart Journal 2003;25(15):1425‐9. [MEDLINE: ; 0195‐668X] [DOI] [PubMed] [Google Scholar]
Wanless 1997 {published data only}
- Wanless RS, Anderson K, Joy M, Joseph SP. Multicenter comparative study of the efficacy and safety of sotalol in the prophylactic treatment of patients with paroxysmal supraventricular tachyarrhythmias. American Heart Journal 1997;133(4):441‐6. [MEDLINE: ; ISSN: 0002‐8703] [DOI] [PubMed] [Google Scholar]
Zehender 1992 {published data only}
- Zehender M, Hohnloser S, Muller B, Meinertz T, Just H. Effects of amiodarone versus quinidine and verapamil in patients with chronic atrial fibrillation: results of a comparative study and a 2‐year follow‐up. Journal of the American College of Cardiology 1992;19(5):1054‐9. [MEDLINE: ; ISSN: 0735‐1097] [DOI] [PubMed] [Google Scholar]
- Zehender M, Meinertz T, Just H. Amiodarone and verapamil/chinidin in the treatment of patients with chronic atrial fibrillation [Amiodaron und Verapamil/Chinidin in der Behandlung von Patienten mit chronischem Vorhofflimmern]. Zeitschrift fur Kardiologie 1994;83 Suppl 5:101‐8. [MEDLINE: ; EMBASE 1994379000; ISSN: 0300‐5860] [PubMed] [Google Scholar]
Zeriouh 2014 {published data only}
- Zeriouh M, Heider A, Rahmanian PB, Choi YH, Sabashnikov A, Scherner M, et al. A novel treatment strategy of new onset atrial fibrillation after cardiac surgery: an observational prospective study. Journal of Cardiothoracic Surgery 2014;9:83. [DOI: 10.1186/1749-8090-9-83] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Park 2017 {published data only}
- Park YM, Cha MS, Kang WC, Han SH, Koh KK, Ahn T, et al. Efficacy of short‐ and long‐term antiarrhythmic use (flecainide) in maintaining sinus rhythm in patients with first developed paroxysmal atrial fibrillation; prospective‐randomized study, interim analysis. Heart Rhythm 2017;14(5 Suppl 1):S304. [Google Scholar]
- Park YM, Cha MS, Kang WC, Han SH, Koh KK, Ahn T, et al. Efficacy of short‐and long‐term anti‐arrhythmic use in maintaining sinus rhythm in patients with first developed paroxysmal atrial fibrillation; prospective‐randomized study, interim analysis. Europace 2017;19(Suppl 3):iii46. [Google Scholar]
Additional references
AHA/ACC/HRS 2014
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal American College Cardiology 2014;64(21):e1‐76. [DOI: 10.1016/j.jacc.2014.03.022; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alboni 2004
- Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, et al. Outpatient treatment of recent‐onset atrial fibrillation with the "pill‐in‐the‐pocket" approach. New England Journal of Medicine 2004;351(23):2384‐91. [MEDLINE: ; ISSN: 0028‐4793] [DOI] [PubMed] [Google Scholar]
Anter 2009
- Anter E, Callans DJ, Wyse DG. Pharmacological and electrical conversion of atrial fibrillation to sinus rhythm is worth the effort. Circulation 2009;120(14):1436‐43. [PUBMED: 19805660] [DOI] [PubMed] [Google Scholar]
APAF 2006
- Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. Journal of the American College of Cardiology 2006;48(11):2340‐7. [DOI: 10.1016/j.jacc.2006.08.037; PUBMED: 17161267] [DOI] [PubMed] [Google Scholar]
Ayala 2003
- Ayala C, Wattigney WA, Croft JB, Hyduk A, Mensah GA, Davis H. Atrial fibrillation as a contributing cause of death and Medicare hospitalization – United States, 1999. MMWR. Morbidity and Mortality Weekly Report 2003;52(7):128‐31. [PMID: 12617537] [PubMed] [Google Scholar]
Benjamin 1998
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98(10):946‐52. [MEDLINE: ; ISSN 0009‐7322] [DOI] [PubMed] [Google Scholar]
Caldeira 2012
- Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta‐analysis of randomized controlled trials. Archives of Cardiovascular Diseases 2012;105(4):226‐38. [DOI: 10.1016/j.acvd.2011.11.005; PUBMED: 22633297] [DOI] [PubMed] [Google Scholar]
Caldwell 2015
- Caldwell DM, Dias S, Welton NJ. Extending treatment networks in health technology assessment: how far should we go?. Value Health 2015;18(5):673‐81. [DOI: 10.1016/j.jval.2015.03.1792] [DOI] [PMC free article] [PubMed] [Google Scholar]
CAST 1991
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. New England Journal of Medicine 1991;324:781‐8. [PMID: 1900101] [DOI] [PubMed] [Google Scholar]
CASTLE‐AF 2018
- Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. New England Journal of Medicine 2018;378(5):417‐27. [DOI: 10.1056/NEJMoa1707855; PUBMED: 29385358] [DOI] [PubMed] [Google Scholar]
Chatterjee 2012
- Chatterjee S, Ghosh J, Lichstein E, Aikat S, Mukherjee D. Meta‐analysis of cardiovascular outcomes with dronedarone in patients with atrial fibrillation or heart failure. American Journal of Cardiology 2012;110(4):607‐13. [DOI: 10.1016/j.amjcard.2012.04.034; PUBMED: 22608952] [DOI] [PubMed] [Google Scholar]
Chatterjee 2013
- Chatterjee S, Sardar P, Lichstein E, Mukherjee D, Aikat S. Pharmacologic rate versus rhythm‐control strategies in atrial fibrillation: an updated comprehensive review and meta‐analysis. Pacing and Clinical Electrophysiology: PACE 2013;36(1):122‐33. [DOI: 10.1111/j.1540-8159.2012.03513.x; PUBMED: 22978656] [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen HS, Wen JM, Wu SN, Liu JP. Catheter ablation for paroxysmal and persistent atrial fibrillation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD007101.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chugh 2014
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129(8):837‐47. [DOI: 10.1161/CIRCULATIONAHA.113.005119; PUBMED: 24345399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013;159(2):130‐137. [DOI: 10.7326/0003-4819-159-2-201307160-00008] [DOI] [PubMed] [Google Scholar]
Connolly 2000
- Connolly SJ. Appropriate outcome measures in trials evaluating treatment of atrial fibrillation. American Heart Journal 2000;139:752‐60. [PMID: 10783204] [DOI] [PubMed] [Google Scholar]
Coplen 1990
- Coplen SE, Antman EM, Berlin JA, Hewitt P, Chalmers TC. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta‐analysis of randomized control trials. Circulation 1990;82:1106‐16. [PMID: 2144796] [DOI] [PubMed] [Google Scholar]
De Vecchis 2019
- Vecchis R, Ariano C. Effects of dronedarone on all‐cause mortality and on cardiovascular events in patients treated for atrial fibrillation: a meta‐analysis of RCTs. Minerva Cardioangiol 2019;67(2):163‐71. [DOI: 10.23736/S0026-4725.18.04719-9; PUBMED: 30260141] [DOI] [PubMed] [Google Scholar]
ESC 2016
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC Scientific Document Group. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal 2016;37(38):2893‐962. [DOI: 10.1093/eurheartj/ehw210; PUBMED: 27567408 ] [DOI] [PubMed] [Google Scholar]
Flaker 1995
- Flaker GC, Fletcher KA, Rothbart RM, Halperin JL, Hart RG: Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. American Journal of Cardiology 1995;76:355‐8. [PMID: 7639159] [DOI] [PubMed] [Google Scholar]
Freemantle 2011
- Freemantle N, Lafuente‐Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide and propafenone, for the management of atrial fibrillation. Europace 2011;13(3):329‐45. [PUBMED: 21227948] [DOI] [PubMed] [Google Scholar]
Frick 2001
- Frick M, Frykman V, Jensen‐Urstad M, Ostergren J, Rosenqvist M. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clinical Cardiology 2001;24:238‐44. [PMID: 11288971] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 1998
- Friedman PL, Stevenson WG. Proarrhythmia. American Journal of Cardiology 1998;82(8A):50N‐8N. [MEDLINE: ; ISSN: 0002‐9149] [DOI] [PubMed] [Google Scholar]
Geleris 2001
- Geleris P, Stavrati A, Afthonidis D, Kirpizidis H, Boudoulas H. Spontaneous conversion to sinus rhythm of recent (within 24 hours) atrial fibrillation. Journal of Cardiology 2001;37:103‐7. [PMID: 11255692] [PubMed] [Google Scholar]
Go 2001
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370‐5. [PMID: 11343485] [DOI] [PubMed] [Google Scholar]
Golzari 1996
- Golzari H, Cebul RD, Bahler RC. Atrial fibrillation: restoration and maintenance of sinus rhythm and indications for anticoagulation therapy. Annals of Internal Medicine 1996;125:311‐23. [PMID: 8678396] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed December 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Harris 1983
- Harris L, McKenna WJ, Roxland E, Holt DW, Storey GC, Krikler DM. Side effects of long‐term amiodarone therapy. Circulation 1983;67(1):45‐51. [MEDLINE: ; ISSN: 0009‐7322] [DOI] [PubMed] [Google Scholar]
Haïssaguerre 1998
- Haïssaguerre S, Bordier P, Jaïs P, Shah DC, Hocini M, Raherison C, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine 1998;339(10):659‐66. [PUBMED: 9725923] [DOI] [PubMed] [Google Scholar]
Heeringa 2006
- Heeringa J, Kuip DA, Hofman A, Kors JA, Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. European Heart Journal 2006;27(8):949‐53. [PUBMED: 16527828] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook. Cochrane.
Khan 2014
- Khan AR, Khan S, Sheikh MA, Khuder S, Grubb B, Moukarbel GV. Catheter ablation and antiarrhythmic drug therapy as first‐ or second‐line therapy in the management of atrial fibrillation: systematic review and meta‐analysis. Circulation. Arrhythmia and Electrophysiology 2014;7(5):853‐60. [DOI: 10.1161/CIRCEP.114.001853; PMID: 25110162] [DOI] [PubMed] [Google Scholar]
Knuiman 2014
- Knuiman M, Briffa T, Divitini M, Chew D, Eikelboom J, McQuillan B, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. European Journal of Epidemiology 2014;29(3):181‐90. [DOI: 10.1007/s10654-013-9875-y; PUBMED: 24389686] [DOI] [PubMed] [Google Scholar]
Krahn 1995
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. American Journal of Medicine 1995;98:476‐84. [PMID: 7733127] [DOI] [PubMed] [Google Scholar]
Lafuente‐Lafuente 2009
- Lafuente‐Lafuente C, Alvarez JC, Leenhardt A, Mouly S, Extramiana F, Caulin C, et al. Amiodarone concentrations in plasma and fat tissue during chronic treatment and related toxicity. British Journal of Clinical Pharmacology 2009;67(5):511‐9. [PUBMED: 19552745] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Miller 2000
- Miller MR, McNamara RL, Segal JB, Kim N, Robinson KA, Goodman SN, et al. Efficacy of agents for pharmacologic conversion of atrial fibrillation and subsequent maintenance of sinus rhythm: a meta‐analysis of clinical trials. Journal of Family Practice 2000;49:1033‐46. [PMID: 11093570] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. (Clinical Guideline 180), 2014. guidance.nice.org.uk/CG180 (accessed 17 June 2014).
Nichol 2002
- Nichol G, McAlister F, Pham B, Laupacis A, Shea B, Green M, et al. Meta‐analysis of randomised controlled trials of the effectiveness of antiarrhythmic agents at promoting sinus rhythm in patients with atrial fibrillation. Heart 2002;87:535‐43. [PMID: 12010934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oral 2006
- Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary‐vein ablation for chronic atrial fibrillation. New England Journal of Medicine 2006;354(9):934‐41. [MEDLINE: ; ISSN: 0028‐4793] [DOI] [PubMed] [Google Scholar]
Piccini 2009
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM, Kong DF. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. Journal of the American College of Cardiology 2009;54(12):1089‐95. [DOI: 10.1016/j.jacc.2009.04.085] [DOI] [PubMed] [Google Scholar]
PRISMA 2009
- PRISMA Group, Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruigomez 2002
- Ruigomez A, Johansson S, Wallander MA, Rodriguez LA. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. Journal of Clinical Epidemiology 2002;55:358‐63. [PMID: 11927203] [DOI] [PubMed] [Google Scholar]
Saborido 2010
- Saborido CM, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D. Systematic review and cost‐effectiveness evaluation of 'pill‐in‐the‐pocket' strategy for paroxysmal atrial fibrillation compared to episodic in‐hospital treatment or continuous antiarrhythmic drug therapy. Health Technology Assessment 2010;14(31):iii‐iv, 1‐75. [DOI: 10.3310/hta14310; PUBMED: 20569652] [DOI] [PubMed] [Google Scholar]
Sethi 2017
- Sethi NJ, Feinberg J, Nielsen EE, Safi S, Gluud C, Jakobsen JC. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: a systematic review with meta‐analysis and Trial Sequential Analysis. PloS One 2017;12(10):e0186856. [DOI: 10.1371/journal.pone.0186856; PUBMED: 29073191] [DOI] [PMC free article] [PubMed] [Google Scholar]
SPAF 1992
- Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Journal of the American College of Cardiology 1992;20:527‐32. [PMID: 1512329] [DOI] [PubMed] [Google Scholar]
Stewart 2002
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. American Journal of Medicine 2002;113:359‐64. [PMID: 12401529] [DOI] [PubMed] [Google Scholar]
Sullivan 2013
- Sullivan SD, Orme ME, Morais E, Mitchell SA. Interventions for the treatment of atrial fibrillation: a systematic literature review and meta‐analysis. International Journal of Cardiology 2013;165(2):229‐36. [DOI: 10.1016/j.ijcard.2012.03.070; PUBMED: 22469557] [DOI] [PubMed] [Google Scholar]
Terasawa 2009
- Terasawa T, Balk EM, Chung M, Garlitski AC, Alsheikh‐Ali AA, Lau J, et al. Systematic review: comparative effectiveness of radiofrequency catheter ablation for atrial fibrillation. Annals of Internal Medicine 2009;15(3):191‐202. [PUBMED: 19581635] [DOI] [PubMed] [Google Scholar]
Tonin 2017
- Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta‐analysis: a technique to gather evidence from direct and indirect comparisons. Pharmacy Practice 2017;15(1):943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Gelder 1996
- Gelder IC, Crijns HJ, Tieleman RG, Brugemann J, Kam PJ, Gosselink AT, et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Archives of Internal Medicine 1996;156:2585‐92. [PMID: 8951302] [DOI] [PubMed] [Google Scholar]
Vaughan Williams 1984
- Vaughan Williams EM. A classification of antiarrhythmic actions reassessed after a decade of new drugs. Journal of Clinical Pharmacology 1984;24(4):129‐47. [MEDLINE: ; ISSN: 0091‐2700] [DOI] [PubMed] [Google Scholar]
Wattigney 2003
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 2003;108:711‐6. [PMID: 12885749] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lafuente‐Lafuente 2006
- Lafuente‐Lafuente C, Mouly S, Longás‐Tejero MA, Mahé I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomised controlled trials. Archives of Internal Medicine 2006;166(7):719‐28. [MEDLINE: ; ISSN: 0003‐9926] [DOI] [PubMed] [Google Scholar]
Lafuente‐Lafuente 2012
- Lafuente‐Lafuente C, Longas‐Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD005049.pub3] [DOI] [PubMed] [Google Scholar]
Lafuente‐Lafuente 2015
- Lafuente‐Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD005049.pub4] [DOI] [PubMed] [Google Scholar]


