Abstract
Minamata disease (MD) is a form of intoxication involving the central nervous system and is caused by ingesting seafood from methylmercury-contaminated areas in Japan. In MD, cerebellar ataxia is a cardinal feature observed in approximately 80% of MD patients. Although cerebellar transcranial magnetic stimulation (TMS) has recently been used for treating cerebellar ataxia, the optimal stimulation conditions remain unclear. Here, we report the first case of cerebellar ataxia in an MD patient that was significantly improved after high-frequency cerebellar TMS. To determine the optimal stimulation conditions, we examined the excitability of the primary motor cortex (M1) using resting-state functional magnetic resonance imaging (rs-fMRI). rs-fMRI revealed M1 hyperconnectivity, which was indicative of activation of the dentato-thalamo-cortical (DTC) pathway. Thus, high-frequency cerebellar TMS was applied to inhibit the DTC pathway. Improvement of cerebellar ataxia was only observed after real TMS, not sham stimulation. As this effect was consistent with inhibition of hyperconnectivity of M1, the effectiveness of high-frequency cerebellar TMS for cerebellar ataxia was thought to be caused by inhibition of the DTC pathway. Therefore, we suggest that the evaluation of M1 excitability using rs-fMRI can be effective for determining the optimal TMS stimulation conditions for cerebellar ataxia.
Keywords: Minamata disease, Cerebellar ataxia, Transcranial magnetic stimulation, Brain network, Resting-state functional magnetic resonance imaging
Introduction
Minamata disease (MD) is a form of intoxication involving the central nervous system and is caused by ingesting seafood from methylmercury-contaminated areas in Japan [1]. Patients typically present with marked distal sensory disturbances, visual field constriction, ataxia, auditory disturbances, and tremors. In MD, cerebellar ataxia is a cardinal feature observed in approximately 80% of MD patients [2]. Cerebellar transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have recently been used as valuable therapeutic modalities for treating cerebellar ataxia [3, 4]. We report the first case of cerebellar ataxia in an MD patient that was significantly improved after high-frequency cerebellar TMS.
Case Report
The patient was an 83-year-old man, who had noticed numbness of the upper limbs approximately 40 years previously, followed by numbness of the lower limbs several months later. At 48 years of age, he was diagnosed with MD based on his residence history and neurological findings [5]. Thereafter, unsteadiness of gait appeared and gradually progressed. Neurological examination revealed a glove-and-stocking type of sensory disturbance, moderate truncal ataxia, intention tremor, and decreased deep tendon reflexes of the lower limbs. Severe cerebellar atrophy was identified on brain magnetic resonance imaging (MRI).
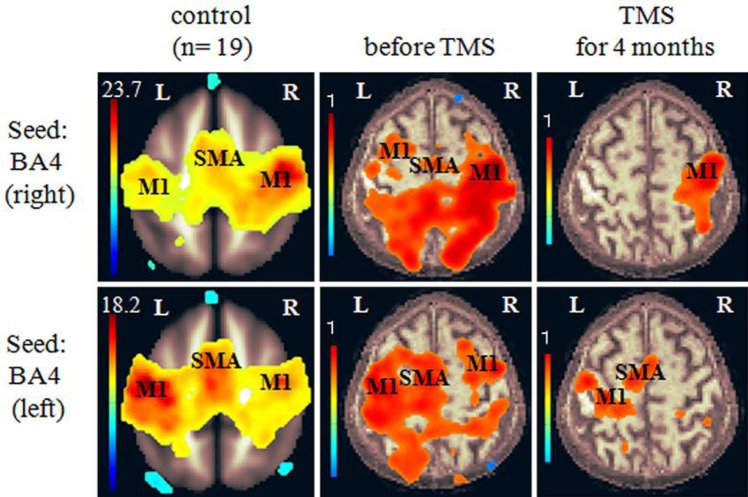
The cerebellum regulates the excitability of the primary motor cortex (M1) via the dentato-thalamo-cortical (DTC) pathway and is involved in cerebellar ataxia [3, 4]. As resting-state functional MRI (rs-fMRI) can be used to identify alterations in functional connectivity in many neurological and psychiatric diseases [6], we examined the functional connectivity of Brodmann area 4 (equivalent to M1) using rs-fMRI. rs-fMRI data were acquired with a 3T Discovery MR750 scanner (GE Healthcare, Milwaukee, WI, USA). The connectivity was compared to that in 19 healthy control subjects (9 females and 10 males, average age 75.7 ± 5.2 years) as described previously [7]. The Ethics Committee of the National Institute for Minamata Disease approved this study, and all subjects provided informed consent to participate. Although we could not statistically analyze the differences between the MD patient and the control group due to there being only 1 case of MD, rs-fMRI revealed M1 hyperconnectivity in the patient compared to control subjects prior to TMS (Fig. 1). This finding was indicative of long-lasting activation of the DTC pathway.
Fig. 1.
Seed-to-voxel-based connectivity of Brodmann area 4, which is equivalent to the primary motor cortex (M1), before and after transcranial magnetic stimulation (TMS). Compared to that in the controls (left column), M1 hyperconnectivity was observed in the patient prior to TMS (middle column). The hyperconnectivity of M1 was reduced after sequential therapy for 4 months (right column). The color bars indicate connectivity strength. SMA, supplementary motor area.
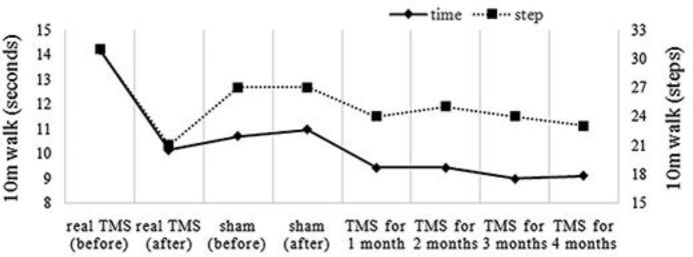
As activation of the DTC pathway was assumed in this case, we selected high-frequency cerebellar TMS to inhibit the DTC pathway. The resting motor threshold (RMT) for the tibialis anterior muscle was measured before TMS using a Cool D-B80 butterfly coil powered by a MagPro ×100 stimulator (MagVenture, Lucernemarken, Denmark). The sites for cerebellar stimulation were amenable to the method described by Shiga et al. [8]: (1) coil centered 4 cm lateral to the right of the inion (3 trains); (2) coil centered on the inion (4 trains); and (3) coil centered 4 cm lateral to the left of the inion (3 trains). Each train consisted of 5 Hz stimulation at 90% intensity of the RMT for 10 s with a 50-s inter-train interval. For sham stimulation, we reduced the stimulation intensity to 18% of the RMT. Real and sham TMS was performed twice weekly, and 1 week of therapy equated to 1 round. A round of sham stimulation was performed after the real TMS round. TMS was continued twice weekly to maintain therapeutic effects. To estimate truncal ataxia, we measured the time and number of steps required to walk 10 m. The estimation of intention tremor was performed using a voluntary movement analysis system (Human Technology Laboratory, Kumamoto, Japan) [7, 9].
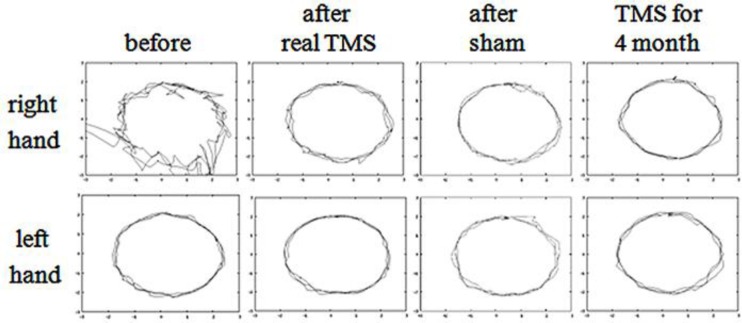
The patient noted reduced gait instability after 1 round of real TMS but experienced gait instability and fell once after sham stimulation. Reductions in the time and number of steps required to walk 10 m were observed only after real TMS (Fig. 2). He also continued to note the reduced intention tremor after 1 round of real TMS. A reduced gap between the pen and target was observed not only after real TMS but also after sham stimulation (Fig. 3), which could be explained by a carry-over effect of TMS because sham stimulation was performed after real TMS. These effects continued after sequential therapy for 4 months (Fig. 2, Fig. 3). These results were consistent with inhibition of M1 hyperconnectivity (Fig. 1).
Fig. 2.
The time and number of steps required to walk 10 m. Reductions in the time and number of steps required were observed after real TMS, but not after sham stimulation. This effect continued after sequential therapy for 4 months.
Fig. 3.
Figure tracing before and after real TMS or sham stimulation over the cerebellum. A decrease in the gap between the pen and target was sustained after real TMS. This effect continued after sequential therapy for 4 months.
Discussion
For the first time, we applied TMS to treat cerebellar ataxia in a chronic MD patient. We found that high-frequency cerebellar TMS improved cerebellar ataxia, in conjunction with inhibition of the activated DTC pathway.
The possibility of TMS therapy to target network deficits has received much attention [10]. As broad areas of the cerebral cortex and cerebellum are typically disturbed in MD [11], brain network impairment may underlie the pathophysiology of MD and could thus provide a therapeutic target in MD.
As the DTC pathway activity is controlled by inhibition from Purkinje cells (PCs), TMS and tDCS have recently been applied to treat cerebellar ataxia [3, 4]. Rodríguez-Labrada et al. [3] proposed that low-frequency cerebellar TMS exerted its therapeutic effect in hereditary cerebellar ataxia via suppression of the PCs (activation of the DTC pathway), resulting in disinhibition of the contralateral M1. However, van Dun et al. [4] proposed that high-frequency cerebellar TMS or anodal tDCS exerted its therapeutic effect in cerebellar ataxia by activating the PCs (inhibition of the DTC pathway), resulting in inhibition of the contralateral M1. Therefore, the optimal stimulation conditions in cerebellar ataxia remain unclear. To determine the optimal stimulation conditions in this case, we examined the excitability of M1 using rs-fMRI. As the cerebellar granule cells activating the PCs are disturbed in MD [1, 2], it is conceivable that the DTC pathway may be activated in MD. rs-fMRI in this case revealed M1 hyperconnectivity, suggesting activation of the DTC pathway. Therefore, we applied high-frequency cerebellar TMS to inhibit the DTC pathway by activating the PCs. As the improvement of cerebellar ataxia was consistent with the inhibition of M1 hyperconnectivity, high-frequency cerebellar TMS was considered to be effective in improving cerebellar ataxia via inhibition of the DTC pathway. These findings indicate that the evaluation of M1 excitability using rs-fMRI can be effective for determining the optimal TMS stimulation conditions for cerebellar ataxia. Additional cases will be needed to clarify the pathogenesis of cerebellar ataxia and to establish the utility of TMS therapy in MD.
Conclusion
To our knowledge, this is the first report of the activated DTC pathway in MD and of the efficacy of high-frequency cerebellar TMS for treating cerebellar ataxia in a chronic MD patient. The evaluation of M1 excitability using rs-fMRI may be helpful for determining the optimal stimulation conditions of TMS therapy for cerebellar ataxia.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The patient and control subjects have given their written informed consent. The study protocol was approved by the Ethics Committee of the National Institute for Minamata Disease.
Disclosure Statement
The authors declare that there is no conflict of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Masaaki Nakamura designed the study, interpreted the results, and wrote the manuscript. Masafumi Bekki, Youko Miura, Mina Itatani, and Liu Xiao Jie acquired the data. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank the patient and the control subjects for participating in this study. We thank Ms. N. Tabata and Ms. T. Yamashita for their assistance in carrying out TMS and rs-fMRI analysis. We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003 Oct;349((18)):1731–7. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 2.Tokuomi H. Study Group of Minamata Disease, editors. Minamata disease. Kumamoto: Shuhan; 1968. Clinical investigations on Minamata disease: A. Minamata disease in human adult; pp. p. 37–72. [Google Scholar]
- 3.Rodríguez-Labrada R, Velázquez-Pérez L, Ziemann U. Transcranial magnetic stimulation in hereditary ataxias: diagnostic utility, pathophysiological insight and treatment. Clin Neurophysiol. 2018 Aug;129((8)):1688–98. doi: 10.1016/j.clinph.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 4.van Dun K, Mitoma H, Manto M. Cerebellar cortex as a therapeutic target for neurostimulation. Cerebellum. 2018 Dec;17((6)):777–87. doi: 10.1007/s12311-018-0976-8. [DOI] [PubMed] [Google Scholar]
- 5.Director General of Environmental Health Department . Diagnostic guidelines for Minamata disease. In: Tsubaki T, Takahashi H, editors. Recent advances in Minamata disease studies. Tokyo: Kodansha; 1986. pp. pp. 207–210. [Google Scholar]
- 6.Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014 Jul;272((1)):29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Etoh S, Sakamoto T, Nakamura T, Jie LX, Miura Y, et al. Potential therapeutic effect of repetitive transcranial magnetic stimulation for tremor in Minamata disease: A case report. J Neurol Sci. 2018 May;388:47–9. doi: 10.1016/j.jns.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Shiga Y, Tsuda T, Itoyama Y, Shimizu H, Miyazawa KI, Jin K, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. 2002 Jan;72((1)):124–6. doi: 10.1136/jnnp.72.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005 Jan;128((Pt 1)):104–15. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- 10.Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014 Sep;34((36)):12049–56. doi: 10.1523/JNEUROSCI.1776-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eto K. Minamata disease. Neuropathology. 2000 Sep;20((s1 Suppl)):S14–9. doi: 10.1046/j.1440-1789.2000.00295.x. [DOI] [PubMed] [Google Scholar]