Abstract
Objective
To examine the association between the body mass index (BMI) and the risk of survival, and to evaluate whether tumor characteristics differ by BMI in patients with upper tract urothelial carcinoma (UTUC) managed by surgery.
Methods
A clinical series on 876 patients with localized UTUC following nephroureterectomy with a bladder cuff, with data from Osaka Medical College registry (discovery cohort) and the Nagoya group (validation cohort) was examined. In addition to analyzing the overall survival and cancer-specific survival (CSS), the survival impact adjusted by pathological variables was also assessed by the BMI group.
Results
The percentage of high risk features including positive lymphovascular invasion was doubled in the discovery cohort compared to the validation cohort. The group of BMI ≥ 25 kg/m<sup>2</sup> was associated with improved CSS in the discovery cohort (p = 0.004), and this tendency was verified in the validation cohort (p = 0.006). Nonproportional hazards existed for the group of BMI ≥ 25 kg/m<sup>2</sup> and the BMI 18.5-25 kg/m<sup>2</sup> relative to the group of BMI < 18.5 kg/m<sup>2</sup>, with a change in the CSS hazard. In multivariable Cox models, the BMI group had a superior predictive value compared with other pre-clinical factors both in the discovery cohort (HR = 3.85, p = 0.01; 95%CI: 0.09–0.73) and the validation cohort (HR = 1.56, p = 0.01; 95%CI: 0.45–0.91). When adjusted by lymphovascular invasion, the concordance of the model proposed by the discovery cohort (0.52) challenged in the validation cohort was 0.59.
Conclusions
We found a clinically relevant signature for high risk patients with BMI grouping. Further research is necessary on whether tailoring recommendations for weight and nutrition management to tumor characteristics will improve outcomes.
Key Words: Body mass index, Upper tract urothelial carcinoma, Prognosis
Introduction
Biomarkers help stratify upper tract urothelial carcinoma (UTUC) with aggressive biology and worse outcomes. Most of current prognostic models aim at predicting muscle invasive or non-organ confined disease, after nephroureterectomy and survival outcomes. These tools are still mainly based on molecular markers, imaging, and clinicopathological features, but none were verified on lifestyle factors. Identifying lifestyle factors that can affect the risk of mortality is important for improving the survival experience of pre-operative patients diagnosed with UTUC. Accumulating evidence has shown that body composition may be one such factor. However, risk stratification in UTUC is still suboptimal, especially in the pre-operative setting due to current limitations in staging and grading.
The body mass index (BMI) and body weight have been suggested as affecting the risk of selected cancers [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Potential mediating effects of the BMI have not been formally quantified in a survival analysis setting in urothelial cancer. Hafron et al. [14] investigated the effect of the BMI on survival in patients undergoing radical or partial cystectomy for urothelial bladder cancer. Although, there was no significant association between the BMI and overall or disease specific survival, there was a trend toward better disease specific survival in normal weight patients with organ confined disease. Maurer et al. [15] reviewed 390 consecutive patients who underwent radical cystectomy due to bladder cancer in order to investigate the influence of the BMI on peri- and post-operative morbidity and outcome following radical cystectomy for bladder cancer. They found that the time of cystectomy increased with the degree of patients' obesity, with normal weight being 330 minutes versus overweight being 355 minutes (p = 0.007). In their cohort, between normal weight and overweight patients no significant differences were noted in respect to post-operative TNM stage (pTis-pT2b: 42.6 vs. 48.6%; pT3a-4: 38.2 vs. 27.2%; pN+: 20.2 vs. 24.2%). Notably, mean overall survival times showed no significant differences between normal weight and overweight patients receiving ileal conduits (5-year survival rate: 34.0 vs. 41.1%; p = 0.140) or ileal neobladders (5-year survival rate: 65.1 vs. 70.8%; p = 0.127) [15]. The prognostic impact of the BMI in patients with UTUC is an ongoing debate. Ishioka et al. [16] investigated the association between different BMI groups and UTUC-specific survival using Cox proportional hazards regression models. Multivariate analysis revealed that factors which affected cancer-specific survival (CSS) included pathological T stage (pT2, pT3, and pT4), pathological grade (grade 3), lymph node status (pN1-2), lymphovascular invasion (LVI) (present), and the BMI (BMI ≥ 25 and ≤ 22.5 kg/ m2). Kang et al. [17] investigated the prognostic role of the BMI in patients treated with radical nephroureterectomy for UTUC. They found that the lower quartile BMI group showed significantly increased overall mortality and cancer specific mortality compared to the 25-50% quartiles and upper quartile BMI groups, concluding that pre-operative underweight patients with UTUC had a lower survival rate after radical nephroureterectomy.
We hypothesized that being overweight could counteract the success of surgery, and that there is a desirable BMI which affect the patients' survival. To address this hypothesis and arrive at universal findings, we utilized 2 different cohorts in which patients were treated by different medical centers so that we could exclude the influence of surgical success.
Methods
Study Design and Population
Two UTUC databases from the Osaka Medical College registry and Nagoya group were analyzed that included a retrospective clinical series on patients with localized UTUC managed by nephroureterectomy with a bladder cuff [18]. Metastatic disease was excluded by chest X-ray, abdominal ultrasonography, and computed tomography of the abdomen and pelvis. We excluded patients with a mixed histology. Patients with a sufficient comprehensive dataset available for extensive background evaluation were included in the present cohort. For each patient comprehensive clinicopathological data were collected and entered into an institutional review board approved database. Previously, open radical nephroureterectomy with open excision of the distal ureter with a bladder cuff was performed to dissect the kidney with the entire length of the ureter and adjacent segment of the bladder cuff. The hilar and regional lymph nodes adjacent to the ipsilateral great vessel were resected, if possible. All surgical specimens were processed according to institutes' standard pathological procedures. LVI was defined as the unequivocal presence of cancer cells in an endothelium-lined space but not in the underlying muscular walls. After the surgery, the patients follow-up evaluation was comprised of a medical history and physical examination, laboratory tests, urine analysis, urine cytology, abdominal ultrasonography, or chest X-rays. To exclude local or distant metastases, computerized tomography or magnetic resonance imaging was conducted.
Statistical Methods
Outcomes were measured by time to deaths. An event for overall survival (OS) included all deaths within the cohort under investigation, and did not separate those due to the disease of interest from those due to other causes. The period of CSS was defined as the time between the date of surgery and death due to UTUC. CSS and OS, as functions of the BMI (discreet and continuous) were evaluated. Censored survival values represent patients who were alive without any evidence of disease at the last follow-up. Cause of death was defined by the physicians, by a review of a chart corroborated by death incidence. Pearson's chi-square test was performed to examine the relationships of the BMI with pathological parameters. The Kaplan-Meier method was used to estimate the survival rate and the log-rank test was used to compare it among the 3 BMI cohorts. CSS and survival probabilities after surgery were estimated using multivarible Cox regression analysis to address time to cancer-specific deaths after surgery. All reported p values are two-sided and the level of statistical significance was set at p < 0.05. All statistical tests were performed with SPSS Statistics ver24.
Results
The clinical baseline characteristics of the 2 cohorts are shown in Table 1. In the discovery cohort, the age at diagnosis ranged from 23 to 91 years old and 66% were males. In the validation cohort, the age at diagnosis ranged from 22 to 91 years old and 70.1% were males. All patients underwent nephroureterectomy with a bladder cuff. The majority of cases (80 and 79% in each cohort) had unifocal tumors (Table 1). Our discovery cohort was observed to have a higher incidence of advanced pT stage than the validation cohort and tended to be LVI positive more frequently at the time of surgery. In addition, the discovery cohort tended to present with a BMI of < 18.5 kg/m2 compared with patients in the validation cohort (Table 1). All available baseline parameters were well balanced between the 2 cohorts (Table 1).
Table 1.
Patients background characteristics
| Discovery cohort (n = 188) | Validation cohort (n= 688) | |
|---|---|---|
| Gender, n (%) | 124 (66.0%) | 482 (70.1%) |
| Male | 64 (34.0%) | 200 (29.1%) |
| Female | 6 (0.90%) | |
| Unknown | ||
| Age (mean, median, min-max) | 69, 71, 23–91 | 69, 69, 22–91 |
| Bladder tumor at diagnosis, n (%) | ||
| None | 127 (67.6%) | 504 (73.3%) |
| Yes | 49 (26.1%) | 172 (25.0%) |
| Unknown | 12 (6.4%) | 12 (1.7%) |
| Cytology, n (%) | ||
| Negative | 3 (1.6%) | 156 (22.7%) |
| Positive | 20 (10.6%) | 185 (26.9%) |
| Suspicious | 45 (23.9%) | 271 (39.4%) |
| Unknown | 120 (63.8%) | 76 (11.0%) |
| Tumor focality, n (%) | ||
| Unifocal | 150 (79.8%) | 541 (78.6%) |
| Multifocal | 38 (20.2%) | 147 (21.4%) |
| Grade, n (%) | ||
| Low | 67 (35.6%) | 77 (15.2%) |
| High | 88 (46.8%) | 430 (84.8%) |
| Unknown | 33 (17.6%) | |
| LVI, n (%) | ||
| No | 81(43.1%) | 562 (81.7%) |
| Yes | 75 (39.9%) | 126 (18.3%) |
| Unknown | 32 (17.0%) | |
| pT classification, n (%) | ||
| pT0/pTls/pTa | 26 (13.8%) | 134 (19.5%) |
| pT1 | 36 (19.1%) | 120 (17.4%) |
| pT2 | 15 (8.0%) | 117 (17.0%) |
| pT3 | 67 (35.6%) | 282 (41.0%) |
| pT4 | 11 (5.9%) | 31 (4.5%) |
| Unknown | 33 (17.6%) | 4 (0.6%) |
| BMI grouped, n (%) | ||
| < 18.5 | 51 (27.1%) | 76 (11.0%) |
| 18.5–24.9 | 107 (56.9%) | 466 (67.7%) |
| 25 or larger | 30 (16.0%) | 146 (21.2%) |
Table 2 shows the correlation of the grouped BMI with all clinical and histopathological parameters in the 2 cohorts. The extent of the BMI did not statistically significantly correlate with gender, and tumor focality in the discovery cohort (Table 2). We found a statistically significant association of bladder tumor at diagnosis (p = 0.01), cytology (p = 0.04), grade (p < 0.01), LVI (p < 0.01), and pT classification (p < 0.01) with the extent of the BMI in the discovery cohort. Among patients in the BMI ≥ 25 kg/m2 group, LVI was significantly less common. There was a statistically significant association of the extent of the BMI with the pT stage, although there was no clear linear correlation between these parameters. In the validation cohort, we found a statistically significant association of the extent of the BMI with cytology and a casual relation with LVI. A large BMI was associated with negative urine cytology and the altitude of positive urine cytology in the BMI ≥ 25 kg/m2 group was smaller than the BMI of the 18.5–24.9 kg/m2 group (Table 2). LVI in the BMI ≥ 25 kg/m2 group was more likely to show negative results than the other 2 groups with a smaller BMI (Table 2).
Table 2.
Patients background characteristics grouped by BMI category in 2 cohorts
| Discovery cohort (n = 188) by BMI category |
Validation cohort (n = 688) by BMI category |
|||||||
|---|---|---|---|---|---|---|---|---|
| < 18.5 | 18.5–24.9 | 25 or larger | p | < 18.5 | 18.5–24.9 | 25 or larger | p | |
| Gender | 0.13 | 0.28 | ||||||
| Male | 28 (54.9%) | 76 (71.0%) | 20 (66.7%) | 46 (60.5%) | 324 (69.5%) | 112 (76.7%) | ||
| Female | 23 (45.1%) | 31 (29.0%) | 10 (33.3%) | 29 (38.2%) | 140 (30.0%) | 31 (21.2%) | ||
| Unknown | 1 (1.3%) | 2 (0.4%) | 3 (2.1%) | |||||
| Age (mean, median, min-max) | 60, 69, 10–83 | 68,70, 10–91 | 69, 71,42–84 | 67,71, 10–88 | 68,69, 10–94 | 67,68, 36–90 | ||
| Bladder tumor at diagnosis | 0.01 | 0.91 | ||||||
| None | 30 (58.8%) | 71 (66.4%) | 26 (86.7%) | 55 (72.4%) | 345 (74.0%) | 104 (71.2%) | ||
| Yes | 13 (25.5%) | 32 (29.9%) | 4 (13.3%) | 20 (26.3%) | 112 (24.0%) | 40 (27.4%) | ||
| Unknown | 8 (15.7%) | 4 (3.7%) | 0 (0.0%) | 1 (1.3%) | 9 (1.9%) | 2 (1.4%) | ||
| Cytology | 0.04 | 0.03 | ||||||
| Negative | 0 (0.0%) | 3 (2.8%) | 0 (0.0%) | 13 (17.1%) | 114 (24.5%) | 29 (19.9%) | ||
| Positive | 8 (15.7%) | 9 (8.4%) | 3 (10.0%) | 27 (35.5%) | 125(26.8%) | 33 (22.6%) | ||
| Suspicious | 15 (29.4%) | 29 (27.1%) | 1 (3.3%) | 27 (35.5%) | 170 (36.5%) | 74 (50.7%) | ||
| Unknown | 28 (54.9%) | 66 (61.7%) | 26 (86.7%) | 9 (11.8%) | 57 (12.2%) | 10 (6.8%) | ||
| Tumor focality | 0.84 | 0.22 | ||||||
| Unifocal | 41 (80.4%) | 84 (78.5%) | 25 (83.3%) | 58 (76.3%) | 375 (80.5%) | 108 (74.0%) | ||
| Multifocal | 10 (19.6%) | 23 (21.5%) | 5 (16.7%) | 18 (23.7%) | 91 (19.5%) | 38 (26.0%) | ||
| Grade | < 0.01 | 0.64 | ||||||
| Low | 8 (15.7%) | 48 (44.8%) | 11 (36.7%) | 5 (6.6%) | 51 (10.9%) | 21 (14.4%) | ||
| High | 25 (49.0%) | 45 (42.1%) | 18 (60.0%) | 52 (68.4%) | 289 (62.0%) | 89 (61.0%) | ||
| Unknown | 18 (35.3%) | 14 (13.1%) | 1 (3.3%) | 19 (25.0%) | 126 (27.0%) | 36 (24.7%) | ||
| LVI | < 0.01 | 0.09 | ||||||
| No | 10 (19.6%) | 52 (48.6%) | 19 (63.3%) | 63 (82.9%) | 371 (79.6%) | 128 (87.7%) | ||
| Yes | 23 (45.1%) | 42 (39.3%) | 10 (33.3%) | 13 (17.1%) | 95 (20.4%) | 18 (12.3%) | ||
| Unknown | 18 (35.3%) | 13 (12.1%) | 1 (3.3%) | 0 (0.0%) | 0(0.0%) | 0 (0.0%) | ||
| pT classification | < 0.01 | 0.80 | ||||||
| pTO/pTis/pTa | 2 (3.9%) | 17 (15.9%) | 7 (23.3%) | 16 (21.1%) | 94 (20.2%) | 24 (16.4%) | ||
| pT1 | 6 (11.8%) | 27 (25.2%) | 3 (10.0%) | 10 (13.2%) | 83 (17.8%) | 27 (18.5%) | ||
| pT2 | 4 (7.8%) | 6 (5.6%) | 5 (16.7%) | 15 (19.7%) | 71 (15.2%) | 31 (21.2%) | ||
| pT3 | 15 (29.4%) | 39 (36.4%) | 13 (43.3%) | 30 (39.5%) | 195 (41.8%) | 57 (39.0%) | ||
| pT4 | 6 (11.8%) | 4 (3.7%) | 1 (3.3%) | 5 (6.6%) | 20 (4.3%) | 6 (4.1%) | ||
| Unknown | 18 (35.3%) | 14 (13.1%) | 1 (3.3%) | 0 (0.0%) | 3 (0.6%) | 1 (0.7%) | ||
In these 2 cohorts, the 5-year survival rates were 93 and 97%, respectively. Cox regression models were developed to evaluate the effect of the BMI on CSS and OS for UTUC (Tables 3, 4). This regression model was done to estimate the adjusted HRs for OS and CSS and their 95%CI, with adjustment for gender, age, bladder tumor at diagnosis, urine cytology, tumor focality, and the BMI. In the discovery cohort, a higher BMI was the single factor associated with a better CSS and OS (p = 0.01, OR = 3.85, 95%CI = 0.09–0.73; Table 3) and (p = 0.01, OR = 4.17, 95%CI = 0.09–0.68; Table 4), respectively. In the validation cohort, age (p < 0.01, OR = 1.03, 95%CI = 1.01–1.06; Table 3), bladder tumor at diagnosis (p = 0.04, OR = 1.51, 95%CI = 1.00–2.29; Table 3), tumor focality (p = 0.01, OR = 3.85, 95%CI = 1.02–2.36; Table 3), and a higher BMI (p = 0.01, OR = 1.56, 95%CI = 0.45–0.91; Table 3) predicted a better CSS. These factors, except for tumor focality, remained as significant predictive factors for OS (Table 4).
Table 3.
COX multivariate analysis for 10-year CSS
| Discovery cohort (n = 188) by BMI category |
Validation cohort (n = 688) by BMI category |
|||||||
|---|---|---|---|---|---|---|---|---|
| p | OR | 95%CIlower | 95%CIupper | p | OR | 95% CI lower | 95% CI upper | |
| Gender | 0.88 | 1.08 | 0.33 | 2.60 | 0.94 | 1.02 | 0.70 | 1.48 |
| Age | 0.55 | 1.02 | 0.96 | 1.08 | < 0.01 | 1.03 | 1.01 | 1.06 |
| BT at diagnosis | 0.80 | 1.19 | 0.30 | 4.69 | 0.05 | 1.51 | 1.00 | 2.29 |
| Cytology | 0.16 | 1.03 | 0.99 | 1.07 | 0.96 | 1.00 | 0.86 | 1.17 |
| Tumor focality | 0.55 | 1.35 | 0.50 | 3.67 | 0.04 | 1.55 | 1.02 | 2.36 |
| BMI grouped | 0.01 | 3.85 | 0.09 | 0.73 | 0.01 | 1.56 | 0.45 | 0.91 |
Table 4.
COX multivariate analysis for 10-year OS
| Discovery cohort (n = 188) |
Validation cohort (n = 688) by BMI category |
|||||||
|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI lower | 95%CIupper | p | OR | 95% CI lower | 95% CI upper | |
| Gender | 0.95 | 1.03 | 0.34 | 2.73 | 0.42 | 1.11 | 0.86 | 1.45 |
| Age | 0.59 | 1.02 | 0.96 | 1.08 | < 0.01 | 1.04 | 1.02 | 1.05 |
| BT at diagnosis | 0.97 | 1.03 | 0.27 | 3.89 | 0.01 | 1.58 | 1.14 | 2.19 |
| Cytology | 0.19 | 1.52 | 0.82 | 2.83 | 0.49 | 1.04 | 0.85 | 1.08 |
| Tumor focality | 0.43 | 1.48 | 0.56 | 3.95 | 0.20 | 1.25 | 0.89 | 1.77 |
| BMI grouped | 0.01 | 4.17 | 0.09 | 0.68 | 0.03 | 1.37 | 0.55 | 0.96 |
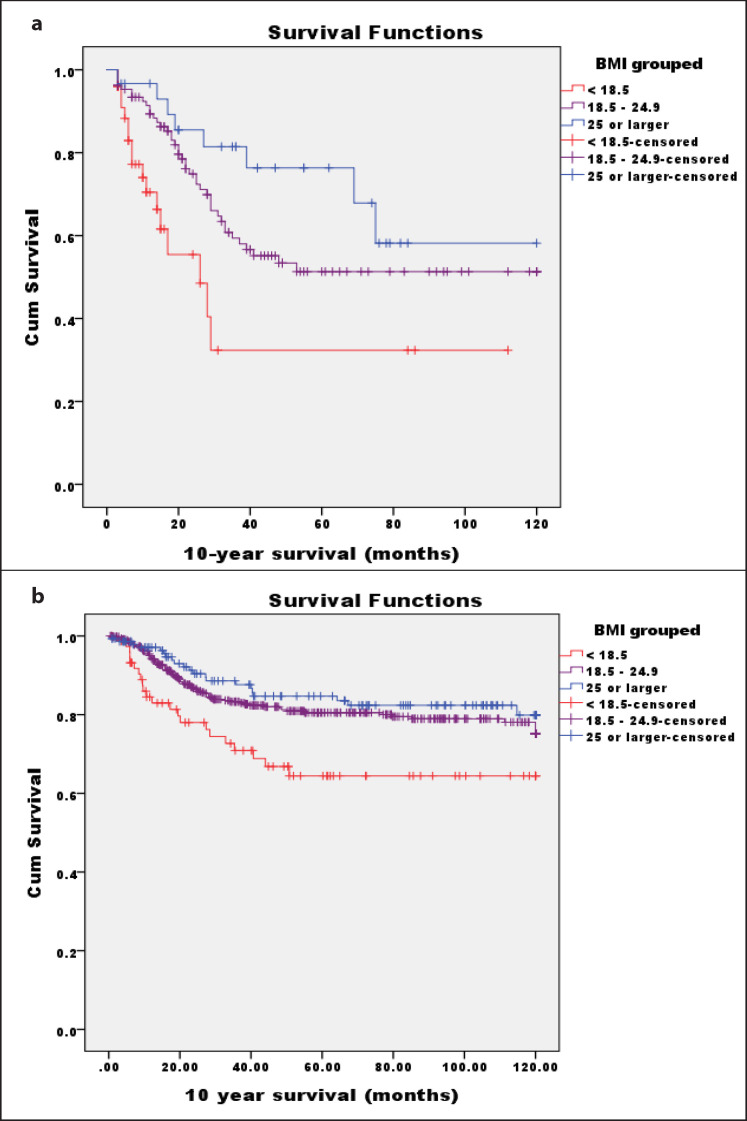
As is shown in Figure 1, a BMI ≥ 25 kg/m2 had higher survival probability in the discovery cohort and validation cohort. The estimated mean survival time in the discovery cohort and validation cohort patient group was 71.6 months (range 63.0–80.3 months) and 99.0 months (range 95.6–102.3 months), respectively. A significant interaction of the BMI with survival was obtained. In the discovery cohort, the logrank test demonstrated a strong trend for an increase in risk for deaths in patients with a smaller BMI (logrank p = 0.004: BMI of 18.5–24.9 kg/ m2 vs. BMI < 18.5 kg/m2, and logrank p = 0.004: BMI ≥ 25 kg/m2 vs. BMI < 18.5 kg/m2) (Fig. 1a). The validation cohort confirmed this trend in determining the prognosis of UTUC patients (Fig. 1b). In a chart review of the whole cohort, we encountered 75 complications among 627 patient who had described intra-operative complications (supplementary table). The amount of bleeding and operation time were more relevant than operation-related complications for a higher BMI in laparoscopic nephroureterectomy (supplementary table).
Fig. 1.
Time to deaths by age group. Association of the BMI with 10-year cancer-specific survival.a Kaplan-Meier survival curves are presented among discovery cohorts (n = 188) stratified by individual BMI categories; b Kaplan-Meier survival curves among validation cohorts (n = 688) stratified by individual BMI categories.
We further evaluated the impact of the BMI on CSS with the adjustment of conventional pathological parameters (Table 5). In the discovery cohort, the BMI adjusted by the pT stage showed a borderline significant correlation with CSS. The survival impact of the BMI was validated after adjusting for LVI, pT stage, and grade. The BMI was a significant indicator for LVI (p = 0.01, OR = 1.41, 95%CI = 0.54–0.92), pT stage (p = 0.01, OR = 1.43, 95%CI = 0.54–0.91), and grade (p = 0.01, OR = 1.49, 95%CI = 0.51–0.90) in the validation set.
Table 5.
Risk prediction of grouped BMI for cancer-specific survival adjusted by LVI, pT stage, and grade
| LVI |
pT |
Grade |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95%CIlower | 95% CI upper | p | OR | 95% CI lower | 95% CI upper | p | OR | 95% CI lower | 95% CI upper | |
| Discovery cohort | 0.13 | 1.37 | 0.48 | 1.1 | 0.06 | 1.51 | 0.43 | 1.02 | 0.11 | 1.39 | 0.48 | 1.08 |
| Validation cohort | 0.01 | 1.41 | 0.54 | 0.92 | 0.01 | 1.43 | 0.54 | 0.91 | 0.01 | 1.49 | 0.51 | 0.90 |
Discussion
Despite a growing understanding of the biology underlying UTUC, management of this disease remains difficult due to the lack of validated biomarkers and the limitations of current predictive and prognostic tools [19, 20, 21]. Further efforts and collaborations are necessary to allow their integration in daily practice.
In our study, survival analysis was employed to assess a discovery cohort and establish associations with clinico-pathological parameters. We showed a differential clinico-pathological background based on the BMI category in patients with UTUC in separate discovery and validation cohorts. This is clinically valuable because the BMI is easily obtained via a totally noninvasive procedure. Except for BMI, age, bladder tumor at diagnosis, and tumor focality were not associated with CSS and OS in the discovery cohort as determined by COX multivariate analysis, while all of these were predictive for deaths in the validation cohort. UTUC can be stratified into 3 subtypes bases on the BMI. However it is widely accepted that UTUC can be classified into subtypes using a classic pathological staging system, to represent subtypes with different patient prognoses. The present study found that an increasing BMI was associated with a favorable outcome for UTUC independent of other factors.
Despite significant heterogeneity being found in analyzing the relation of body weight with UTUC mortality, it provided strong evidence that a higher BMI is beneficial. We were the first to describe the prognostic impact of UTUC in an Asian cohort [18]. Two years later, Ishioka et al. [16] investigated the impact of the BMI with different categories on UTUC using a Japanese database. HRs for CSS of the BMI ≥ 25 kg/m2 and the BMI < 22.5 kg/m2 group were 1.62 and 1.68, respectively, concluding that both higher and lower BMI affect the prognosis of UTUC treated with radical nephroureterectomy [16]. In our study, the HR for CSS and OS of the BMI were 3.85 in the discovery cohort/1.56 in the validation cohort, and 4.17 in the discovery cohort/1.37 in the validation cohort, respectively. One year later, Kim et al. [22] used another cut-off to assess the relationship between the BMI and survival. There were no significant differences in the urothelial recurrence-free survival rates according to the BMI classification (p = 0.488) [22]. In the univariate analysis, the BMI ≥ 25 kg/m2 was a significant predictor of better CSS (HR 1.89; 95% CI 0.33–0.84, p = 0.007) than normal weight, although they did not observe significant associations in the multivariable analysis [22]. Recently, Fukushima et al. [23] investigated the prognostic significance of sarcopenia, which was defined in combination with skeletal muscle by computed tomography and the BMI, in 81 UTUC patients. In locally advanced or node positive patients, those with sarcopenia showed significantly worse CSS and OS than those without (5-year CSS rate: 55 vs. 100%, p = 0.014; 5-year OS rate: 40 vs. 86%, p = 0.007), while no prognostic difference was observed between patients with and without sarcopenia in those with organ-confined disease [23]. A few studies that investigated the association between BMI and the risk of death from UTUC were found and a worse prognostic outcome seems to be associated with a higher BMI in a Western cohort and a lower BMI in an Asian cohort [16, 18, 24]. The reason for this discrepancy is simple. The baseline patients' BMI is totally different in Asian countries than in Western countries. Ehdaie et al. [24] showed a median patient BMI of 27.9 kg/m2 (IQR: 6.7) from 520 Western patients, while in the present cohort, the median BMI was 22 kg/m2 (IQR: 2.0). In our total cohort, only 17 patients in 876 patients (1.9%) showed a BMI > 30 kg/m2, the cut-off for obesity. Again, we do not mean that being obese renders a good prognosis, because in our validation cohort for patients with a BMI ≥ 30 kg/ m2, the mean estimated CSS was 65.5 months which was shorter than a BMI of 18.5–24.9 kg/m2 and a BMI ≥ 25 kg/m2, and the overall cohort (data not shown).
Keeping an adequate BMI might benefit the improvement of survival among UTUC patients. Future clinical trials will include the association of confounding factors, such as lifestyle, nutrition, and smoking history. Where possible, the impact of the BMI change on the death of patients with UTUC should be evaluated.
Conclusions
The results presented here provide a summary of the status of UTUC patients undergoing nephroureterectomy with a bladder cuff from two different cohorts. The BMI may be a more important determining factor of patient survival risk status after treatment with surgery than formerly assumed. The present study is descriptive in nature and not predictive. Although, we believe this is a good resource to characterize these patients.
Clinical Practice Points
- Current information on the prognostic importance of the BMI for patients with UTUC is based on a variety of equivocal reports.
- Because of inconsistent and uncertain research results as well as racial and ethnic differences, we sought to evaluate the prognostic significance of the BMI in two different Japanese UTUC cohorts (discovery cohort and validation cohort) managed by surgery.
- The study aimed to analyze the association between the BMI at time of diagnosis, UTUC histopathological features (tumor focality, T stage, nuclear grade, lymphatic/vascular invasion), incidence of concomitant bladder cancer, and positive urine cytology to evaluate the impact of the BMI on CSS by multivariate analysis.
- A BMI ≥ 25 kg/m2 had higher survival probability in the discovery cohort and validation cohort.
Supplementary Material
Supplementary data
References
- 1.Smits A, Lopes A, Bekkers R, Galaal K. Body mass index and the quality of life of endometrial cancer survivors - a systematic review and meta-analysis. Gynecol Oncol. 2015;137:180–187. doi: 10.1016/j.ygyno.2015.01.540. [DOI] [PubMed] [Google Scholar]
- 2.Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, Goldberg RM, Degramont A, O'Connell MJ, Sargent DJ. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119:1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J, Halazun K, Dove L, Brown RS, Jr, Neugut AI, Kato T, Remotti H, Coppleson YJ, Emond JC. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539–543. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepesi B, Gold KA, Correa AM, Heymach JV, Vaporciyan AA, Roszik J, Dmitrovsky E, Liu X. The influence of body mass index on overall survival following surgical resection of non-small cell lung cancer. J Thorac Oncol. 2017;12:1280–1287. doi: 10.1016/j.jtho.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ademuyiwa FO, Groman A, O'Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117:4132–4140. doi: 10.1002/cncr.26019. [DOI] [PubMed] [Google Scholar]
- 6.Bachir BG, Aprikian AG, Izawa JI, Chin JL, Fradet Y, Fairey A, Estey E, Jacobsen N, Rendon R, Cagiannos I, Lacombe L, Lattouf JB, Kapoor A, Matsumoto E, Saad F, Bell D, Black PC, So AI, Drachenberg D, Kassouf W. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: results from a Canadian multicenter collaboration. Urol Oncol. 2014;32:441–448. doi: 10.1016/j.urolonc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM, Holmberg SB, Lindtner J, Erien D, Collins J, Snyder R, Thürlimann B, Fey MF, Mendiola C, Werner ID, Simoncini E, Crivellari D, Gelber RD, Goldhirsch A. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 9.D'Aiuto M, Chirico A, De Riggi MA, Frasci G, De Laurentiis M, Di Bonito M, Vici P, Pizzuti L, Sergi D, Maugeri-Saccà M, Barba M, Giordano A. Body mass index and treatment outcomes following neoadjuvant therapy in women aged 45 y or younger: evidence from a historic cohort. Cancer Biol Ther. 2016;17:470–476. doi: 10.1080/15384047.2016.1156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, Kohno H, Nagasue N. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 11.Donat SM, Salzhauer EW, Mitra N, Yanke BV, Snyder ME, Russo P. Impact of body mass index on survival of patients with surgically treated renal cell carcinoma. J Urol. 2006;175:46–52. doi: 10.1016/S0022-5347(05)00054-6. [DOI] [PubMed] [Google Scholar]
- 12.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanning CV, Staerkel GA, Sneige N, Thomsen S, Myhre MJ, Von Eschenbach AC. Spindling artifact of urothelial cells in post-laser treatment urinary cytology. Diagn Cytopathol. 1993;9:279–281. doi: 10.1002/dc.2840090307. [DOI] [PubMed] [Google Scholar]
- 14.Hafron J, Mitra N, Dalbagni G, Bochner B, Herr H, Donat SM. Does body mass index affect survival of patients undergoing radical or partial cystectomy for bladder cancer? J Urol. 2005;173:1513–1517. doi: 10.1097/01.ju.0000154352.54965.14. [DOI] [PubMed] [Google Scholar]
- 15.Maurer T, Maurer J, Retz M, Paul R, Zantl N, Gschwend JE, Treiber U. Influence of body mass index on operability, morbidity and disease outcome following radical cystectomy. Urol Int. 2009;82:432–439. doi: 10.1159/000218533. [DOI] [PubMed] [Google Scholar]
- 16.Ishioka J, Masuda H, Kijima T, Tatokoro M, Yoshida S, Yokoyama M, Matsuoka Y, Numao N, Koga F, Saito K, Fujii Y, Sakai Y, Arisawa C, Okuno T, Nagahama K, Kamata S, Yonese J, Kageyama Y, Noro A, Morimoto S, Tsujii T, Kitahara S, Gotoh S, Kihara K. Bimodal pattern of the impact of body mass index on cancer-specific survival of upper urinary tract urothelial carcinoma patients. Anticancer Res. 2014;34:5683–5688. [PubMed] [Google Scholar]
- 17.Kang HW, Jung HD, Ha YS, Kim TH, Kwon TG, Byun SS, Yun SJ, Kim WJ, Choi YD. Preoperative underweight patients with upper tract urothelial carcinoma survive less after radical nephroureterectomy. J Korean Med Sci. 2015;30:1483–1489. doi: 10.3346/jkms.2015.30.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inamoto T, Komura K, Watsuji T, Azuma H. Specific body mass index cut-off value in relation to survival of patients with upper urinary tract urothelial carcinomas. Int J Clin Oncol. 2012;17:256–262. doi: 10.1007/s10147-011-0284-5. [DOI] [PubMed] [Google Scholar]
- 19.Rink M, Ehdaie B, Cha EK, Green DA, Karakiewicz PI, Babjuk M, Margulis V, Raman JD, Svatek RS, Fajkovic H, Lee RK, Novara G, Hansen J, Daneshmand S, Lotan Y, Kassouf W, Fritsche HM, Pycha A, Fisch M, Scherr DS, Shariat SF. Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62:677–684. doi: 10.1016/j.eururo.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Szarvas T, Modos O, Horvath A, Nyirady P. Why are upper tract urothelial carcinoma two different diseases? Transl Androl Urol. 2016;5:636–647. doi: 10.21037/tau.2016.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simhan J, Smaldone MC, Egleston BL, Canter D, Sterious SN, Corcoran AT, Ginzburg S, Uzzo RG, Kutikov A. Nephron-sparing management vs radical nephroureterectomy for low- or moderate-grade, low-stage upper tract urothelial carcinoma. BJU Int. 2014;114:216–220. doi: 10.1111/bju.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Can body mass index predict survival outcomes in patients treated with radical nephroureterectomy for upper-tract urothelial carcinoma? Int Urol Nephrol. 2015;47:1311–1320. doi: 10.1007/s11255-015-1039-4. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic significance of sarcopenia in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Cancer Med. 2016;5:2213–2220. doi: 10.1002/cam4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehdaie B, Chromecki TF, Lee RK, Lotan Y, Margulis V, Karakiewicz PI, Novara G, Raman JD, Ng C, Lowrance WT, Scherr DS, Shariat SF. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol. 2011;186:66–72. doi: 10.1016/j.juro.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data