Abstract
Salmonella is a major cause of morbidity and mortality in the developing and underdeveloped nations. Being a foodborne disease, Salmonella infection is primarily contracted through the ingestion of contaminated food or water, or due to close contact with infected/carrier individuals. It is an intracellular pathogen, which can survive and replicate in various cells including macrophages, dendritic cells, epithelial cells, and other white blood cells. Once Salmonella crosses the intestinal barrier, it disseminates to various systemic sites by circulation via immune cells. One of the major cell types which are involved in Salmonella infection are host macrophages. They are the niche for intracellular survival and proliferation of Salmonella and a mode of dissemination to distal systemic sites. These cells are very crucial as they mediate the mounting of an appropriate innate and adaptive anti- Salmonella immune response. In this review, we have tried to concise the current knowledge of complex interactions that occur between Salmonella and macrophages.
Keywords: Macrophage, TLR, NLR, ROS, Pyroptosis, Metal starvation, AMP, Exosome
Introduction
Salmonella is a Gram-negative pathogen which causes a variety of diseases ranging from life-threatening systemic infection like typhoid to mild gastroenteritis in humans. Global estimates indicate 21 million typhoid cases and 5 million cases of paratyphoid fever along with 215,000 fatalities each year [1]. There is an estimated 93 million nontyphoidal salmonellosis with 155,000 fatalities every year [2]. These estimates make Salmonella a major cause of morbidity and mortality in developing and underdeveloped nations. Annually, 175–388 cases per 100,000 children and 2,000–7,500 cases per 100,000 HIV-infected adults are approximated in sub-Saharan Africa [3]. In 95% of the cases, nontyphoidal serovars cause gastroenteritis; however, 5% of the cases result in bacteremia and systemic infection called invasive nontyphoidal salmonellosis (iNTS). The incidence of iNTS is prevalent in African countries, particularly among children. 20–25% of iNTS tends to be fatal [4]. Apart from the human host, Salmonella enterica subspecies have been reported to infect other warm-blooded animals of economic importance such as poultry, cattle, etc. The vast diversity showed by these serovars with respect to host range adaptation and virulence strategy makes Salmonella a daunting pathogen.
Salmonella infection is primarily contracted through ingestion of contaminated food or water or due to close contact with infected/carrier individuals. Once ingested, Salmonella crosses the host intestinal barrier by entering through “M cells” (microfold cells) and dendritic cells present in Peyer's patches. Additionally, it can induce bacterial uptake by intestinal epithelial cells via injection of virulence factors into the host cell. These virulence factors are encoded by various horizontally acquired pathogenicity islands present in the Salmonella genome, called the Salmonella pathogenicity islands (SPIs), which are critical for the entry and intracellular survival [5]. After colonization, the bacteria disseminate through the reticuloendothelial system and reside in host macrophages, dendritic cells, polymorphonuclear cells, and hepatic cells. During its systemic phase, the pathogen spreads from the intestine to the mesenteric lymph node (MLN), spleen, liver, gallbladder, and even into bone marrow [6].
Macrophages play an essential role in anti- Salmonella response as it is involved in mediating both innate and adaptive immune responses. Its importance in Salmonella pathology is further emphasized by the fact that the mutants which are incapable of intracellular life in macrophages are in general defective in causing a systemic infection [7]. As a part of innate immune response, macrophages sense the presence of Salmonella-derived pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and NOD-like receptors (NLRs). This subsequently leads to an antibacterial response which comprises reactive oxygen species (ROS), reactive nitrogen species (RNS), acidic environment, metal starvation, and antimicrobial peptides (AMPs). As a part of adaptive immune response, it is essential to mount a Th1 response against Salmonella to avoid a chronic infection. During Salmonella infection, macrophage polarization to M1 or M2 phenotype plays a determinant role in dictating the disease outcome. In this review, we have tried to bring together the intricacies of macrophage- Salmonella interaction.
Intracellular Life of Salmonella in Macrophage
In the host phagocytic cells, Salmonella resides in a modified endosome known as Salmonella-containing vacuole (SCV). The membrane properties of SCV are dynamic and the nascent bacteria containing endosome matures into a modified late endosome. Salmonella avoids SCV lysosome fusion, providing it a safe intracellular niche for bacterial survival and replication [8, 9]. Early SCV contains markers like EEA1 and Rab4 and 5 and transferrin receptors. During the late maturation stage, about 20–40 min after internalization, the late endosomal markers like LAMPs, Rab7, 11, and vATPase replace the early endosome markers [10]. For replication of bacteria within the cell, perinuclear localization of SCV is obligatory. During its intracellular life, SPI-2 effectors are instrumental for both replication and SCV positioning [11]. In case of host epithelial cells, SCVs form filamentous structures called Salmonella-induced filaments (Sifs) [9, 12]. MYD88- and TRIF-mediated TLR signalling is essential for initial SCV acidification [13]. Within an hour of infection, the SCV attains the pH of 4–5. In response to the acidic environment of SCV, the cytoplasm of the bacteria also undergoes acidification. This acidification process is EnvZ sensor kinase and OmpR mediated. EnvZ senses the osmotic stress and in response to it OmpR regulates expression of various pH regulatory genes, including CadC/BA operon and ATP synthase gene of the bacteria [14]. The low pH and low Mg2+ environment of SCV activates the PhoP/Q two-component system. This leads to the upregulation of the Mg2+ transporter (mgtCBR operon), lipopolysaccharide (LPS) modification, and SPI-2 expression [15]. During the process of internalization as well as inside the host macrophage, Salmonella activates signalling of the host PRRs. This process activates various antibacterial responses in macrophages.
TLR and NLR Signalling
TLRs and NLRs are the PRRs responsible for sensing most of the PAMPs. Early detection of Salmonella is dependent on both surface and endosomal TLRs. TLR2 and TLR4 are present on the macrophages and recognize the Salmonella PAMPs. Mice lacking these TLRs exhibit higher extracellular bacterial burden, thus implicating their role in host innate immune responses [16]. Conversely, the absence of TLR signalling in macrophages impairs its acidification, which in turn compromises intracellular replication [13]. Collectively, TLR signalling helps the intracellular replication of bacteria, but inhibits the extracellular growth of the same. As compared to TLR2/4 and TLR4/9 double knockout mice, mice lacking all the three TLRs (i.e., TLR2, 4, and 9) are less susceptible to Salmonella-mediated lethality. Further, complete ablation of TLR (TLR2, 4, 3, 7, 9) signalling results in reduced inflammatory response which in turn enhances susceptibility to Salmonella infection [17].
Apart from the TLRs, NLRs also detect intracellular PAMPs. Salmonella infection activates NLRC4 and NLRP3 signalling, resulting in inflammasome formation [18]. This further leads to activation of caspase-1 and secretion of IL-1β and IL-18 and pyroptosis. NLRC4 recognizes Salmonella flagellin and in order to avoid activation of NLRC4, Salmonella downregulates flagellin production during its intracellular life [19]. The Salmonella PAMP responsible for NLRP3 activation is yet unknown. However, NLRP3 signalling might be linked to ROS production and ROS production is often accompanied by K+ efflux which is a known signal for NLRP3 activation [20]. A deficiency in either of NLRC4 and NLRP3 results in a higher systemic bacterial burden, emphasizing its role in antibacterial response against Salmonella [21]. In contrast, NLRP6 and NLRP12 deletion reduces the bacterial burden and enhances neutrophil and macrophage recruitment to the site of infection [22, 23]. Pathogen sensing by host PRRs induce oxidative burst as an antibacterial response.
ROS and RNS
Recognition of Salmonella PAMPs induce proinflammatory cytokine production by various immune cells. These cytokines further activate macrophages and dendritic cells to produce antibacterial ROS and RNS response. In response to Salmonella infection in macrophages, the initial bactericidal respiratory burst is driven by phagocyte oxidase (phox) also known as NADPH oxidase [24]. This is followed by a long bacteriostatic nitrosative stress driven by inducible nitric oxide synthase (iNOS) [25]. The regulatory effect of cytokines on ROS is proven by the fact that in vivo administration of IFNγ results in increase in ROS, which in turn inhibits systemic spread of Salmonella in rats. The importance of ROS is demonstrated by the fact that mice lacking gp91phox, an essential subunit of NADPH oxidase, succumb to Salmonella infection much faster than wild-type mice. This is also accompanied by a 1,000-fold increase in bacterial burden in the liver of these knockout mice [26]. In addition, patients suffering from chronic granulomatous disease, a condition compromising ROS production, suffer from recurring Salmonella and other bacterial infections [27]. Studies on murine peritoneal macrophages reveal that clearance of up to 99% of intracellular bacteria within the first 6 h of infection is predominantly ROS dependent [28]. However, ROS production reduces after 6 h of infection. Salmonella mutants, which are deficient is ROS and RNS detoxification enzymes such as superoxide dismutase (SodC) [29] and oxidative burst scavenger glutathione, are unable to lead a successful intracellular life in macrophages [30]. Thus, the bacteria are compromised in causing a systemic infection. The SPI-2 is essential for successful evasion of intracellular ROS assault. This is demonstrated by the fact that PhoPQ mutants, responsible for SPI-2 expression in the SCV, are unequipped to handle intra-macrophage ROS [31]. The integrity of the SCV is also crucial to evade ROS. Cytosolic population of Salmonella has been reported to face enhanced ROS levels in both human and murine macrophages [32].
Once the ROS production diminishes, a prolonged iNOS-mediated nitrosative stress follows. iNOS converts L-arginine to nitric oxide (NO) and citrulline, which show a bacteriostatic effect at a later stage of infection [33]. Being a free radicle, NO can react with various intracellular molecules to generate more destructive reactive nitrogen intermediates. The reaction of ROS and NO produces peroxynitrite, which damages DNA and hence results in mutagenesis, further accompanied by damage to proteins as well as lipids. Although, IFN-γ is the main inducer of iNOS [34] even IL-17, IL-22, IL-1, and TNFα can induce its upregulation [33, 35]. Genetically deficient iNOS mice can control the bacterial burden at the initial stage of infection, but are unable to do so at a later stage and show enhanced mortality [36]. This indicates the role of iNOS in controlling Salmonella infection at a later stage. Salmonella also evades RNS by limiting substrate for iNOS. Salmonella infection of murine macrophages results in upregulating of iNOS but simultaneously it enhances arginase II production, which converts L-arginine to ornithine and urea, thus limiting iNOS substrate [37]. PhoPQ plays a crucial role in evasion of nitrosative stress as its deletion results in hyper-susceptibility to NO. Recent reports suggest that PhoPQ-mediated Mg2+ transport is crucial for surviving RNS [38]. The hyper-susceptibility of this mutant to RNS is due to nitrotyrosine formation, DNA damage, and oxidation of iron sulfur cluster. Apart from its antibacterial effect, NO also has immune-modulatory effects as it can suppress T-cell proliferation [38].
Apart from ROS and RNS response, macrophages control bacterial replication by limiting the availability of metal ions.
Metal Starvation/Toxicity
Intracellular availability of trace elements is essential for the survival of an intracellular pathogen. One of the innate immune responses against pathogens is metal starvation. Macrophages and neutrophils restrict intracellular bacterial replication by limiting iron and zinc. Additionally, macrophages utilize iron as a co-factor for the generation of ROS and RNS, indicating that the right equilibrium of metal ions is essential for successful pathogen clearance.
Iron is most commonly available in bound form with proteins like transferrin, ferritin, lactoferrin, and lipocalin-2. Transferrin receptor present on the surface of the macrophage binds to plasma ferric ion-bound transferrin which results in receptor-mediated endocytosis. During the endosomal maturation, iron dissociates from transferrin protein due to the acidic pH of the vacuole and empty transferrin receptors are recycled to the cell surface for the next cycle [39].
The importance of iron availability is demonstrated by the fact that individuals suffering from iron overload such as hemochromatosis are more susceptible to Salmonella infection. Mice lacking HEF allele which is associated with hemochromatosis show better survival during Salmonella infection [40, 41]. Furthermore, iron supplementation increases intracellular survival of Salmonella [42]. During Salmonella infection, macrophages increase iron efflux by upregulating an iron export protein ferroprotein-1, thereby limiting the bioavailability of iron to the pathogen [43]. In addition, Salmonella further decreases the intracellular iron pool by enhancing the level of both haem oxygenase, a haem-degrading enzyme, and lipocalin-2, an iron siderophore [39]. All these changes in macrophage iron homeostasis are reported to be IFNγ mediated [44]. Apart from being an iron siderophore, lipocalin-2 also represses IL-10 production and enhances proinflammatory response by upregulation TNFα, IL-6, and NOS2 levels [45]. Macrophage iron-regulatory protein (IRB) 1 and 2 deficiency results in increased mortality in response to Salmonella infection, emphasizing the role of iron homeostasis in disease severity [46]. Live Salmonella also induce the transformation of macrophages to hemophagocytes, these are phagocytes which have engulfed erythrocyte or leukocyte. In these cells, intracellular bacterial survival is greatly enhanced within hemophagocytes, due to a surplus of iron [47, 48].
The importance of divalent transition metal ions like iron and manganese in host defense is further supported by the fact that the presence of natural resistance-associated protein 1 (Nramp1) [49] in mice results in survival of acute infection, whereas those which lack them are susceptible and succumb to the infection. In some individuals, conversion of one glycine residue to asparagine due to a single nucleotide polymorphism results in a nonfunctional Nramp1 protein [50]. This results in uncontrolled replication of Salmonella in the SCV. Nramp1 is expressed in phagocytic cells and is localized to late endosome and lysosome. It is LPS inducible and exports manganese ions from the phagosome in a pH-dependent manner [51]. Further, Mn2+ is reported to inversely regulate NO production and IFNγ response [52].
Zinc is crucial for both the structural aspect and gene expression of various proteins and its sequestration is one of the host defense mechanism against microbial assault. As a response to infection, macrophages upregulate zinc scavengers, metallothioneins 1 and 2 [53]. Their ablation results in a reduction in ROS and RNS levels and an increase in free zinc levels. Collectively, this leads to increased bacterial intracellular survival of Salmonella. The increase in intracellular free zinc level inhibits NFκB and hence proinflammatory responses [54].
Metal ion availability can act as a double-edged sword: on the one hand, it is essential for bacterial survival in trace amounts, and on the other hand, at a higher concentration they can be antibacterial in nature. The best example for this is zinc and copper toxicity. Both human and murine macrophages show increased uptake of copper in response to TLR4 activation. In the intracellular vacuoles, Salmonella is subjected to an increased copper concentration. In murine bone marrow-derived macrophages, treatment with copper chelator enhances intracellular survival of Salmonella [55]. The antibacterial role of copper is further validated by reduced organ load in CueO, a copper oxidase mutant as compared to wild-type Salmonella [56]. Salmonella also express CopA, a copper efflux pump to avoid copper toxicity.
In human monocyte-derived macrophages, although Salmonella induces the formation of zinc-containing vesicles, simultaneously it also upregulates the expression of a zinc efflux pump ZntA [57]. Further, prolonged stimulation of NOD-2 results in accumulation of intracellular zinc which in turn induces autophagy and hence clearance of Salmonella [58]. In addition to starving the intracellular bacteria with the unavailability of metal ions, macrophages also mount an AMP response.
AMP Response
AMPs are generally 10- to 50-amino-acid-long cationic peptides which show direct bactericidal effects. As the name suggests, it contains positively charged basic amino acids along with hydrophobic amino acid residues that can interact with the LPS and bacterial membrane resulting in pore formation. Cathelicidins and defensins are the two major classes of cationic AMPs. Most of the AMP studies regarding Salmonella have been carried out in epithelial cells as they are the major producers of AMPs in vertebrates. The status of macrophage-derived AMPs during Salmonella infection remains largely unexplored. However, the existing limited literature suggests a role of cathelicidin in macrophages [59]. In macrophages, cathelicidin resides in the lysosome. It is composed of a C-terminal antimicrobial domain which is masked by a conserved N-terminal domain. Removal of the N-terminal domain by a cellular serine protease is crucial for its antimicrobial activity. Humans express cathelicidin hCAP-18 gene, whereas mouse expresses cathelicidin-related antimicrobial peptide (CRAMP). Salmonella infection of macrophages results in the upregulation of CRAMP [60]. It has been reported to show an antibacterial effect both in vitro and in vivo. Salmonella-mediated upregulation of CRAMP is both ROS and serine protease activity dependent. Salmonella infection of antioxidant pretreated macrophages were unable to show upregulation of CRAMP, confirming that the role of ROS is cathelicidin function. PhoP mutants are more sensitive to CRAMP which might be due to the absence of PhoP-mediated LPS modification [61]. In case of human macrophages, hCAP-18 production is TLR dependent. Stimulation of TLR2, 4, and 9 results in the upregulation of hCAP expression in alveolar macrophages. Furthermore, TLR regulation of hCAP-18 is vitamin D dependent [60, 62]. In case of human monocyte-derived macrophages, Salmonella does not induce cathelicidin hCAP-18 [61]. The effects of other macrophage-derived AMPs in anti- Salmonella response requires further investigation. Another area which remains hugely unexplored is the miRNA regulation in macrophages upon Salmonella infection.
miRNAs in Salmonella Infection
miRNAs belong to the class of noncoding RNAs of about 18–22 nucleotides in length. They play major roles in regulating the expression of protein-coding genes by either controlling at the transcriptional level or by repression of translation [63]. Since the host-pathogen interaction is a complex network, many of the host gene expression levels are, or should be, altered by the pathogen to tend to its own needs. Thus miRNA regulation also has a role to play during Salmonella infection of macrophages (Table 1).
Table 1.
The miRNAs altered in macrophages upon Salmonella infection
| miRNA | Modulation by Salmonella infection | Molecules/pathways altered | Authors [Ref.], year |
|---|---|---|---|
| let-7a | Downregulation | IL-6 and IL-10/ pro- and anti-inflammatory responses, respectively | Das et al. [64], 2016 |
| miR-155 | Downregulation | Inflammatory mediators, cytokines | Das et al. [64], 2016 |
| miR-146a | Upregulation | IRAK4 and TRAF6/important molecules of TLR signalling | Heale et al. [72], 2010 |
| miR-125b | Downregulation | Alteration in TNFα levels | Heale et al. [72], 2010 |
Salmonella is known to modulate the levels of certain miRNAs in vitro in both macrophages and epithelial cells. In murine macrophage-like RAW 264.7 cells, miRNAs like miR-21, miR-146, and miR-155 were found to be upregulated [64]. Interestingly, None of these miRNAs have known roles associated with invasion or intracellular replication of Salmonella. Another miRNA, let-7a, was found to be downregulated in both RAW 264.7 cells and HeLa cells. This downregulation was found to be a response to the TLR4 stimulation by the LPS. Target genes of this let-7a miRNA are IL-6 and IL-10 whose repression is relieved upon its downregulation. These cytokines mediate the pro- and anti-inflammatory responses, respectively [65, 66].
The mammalian adenosine deaminases, ADAR1 and ADAR2, have been found to deaminate the adenosines in dsRNAs leading to the A to I conversions. ADAR1 is an ubiquitously expressed protein [67] ADAR2 has high levels of expression in the brain [68]. ADAR1 knockout mice die at the embryogenesis stages [69], whereas ADAR2 knockout mice die by postnatal day 20 with epileptic seizures [70]. Defective editing by these enzymes has been implicated in several diseases like amyotrophic lateral sclerosis, cancers, and metabolic disorders like type 2 diabetes mellitus [71]. These A to I conversions in miRNAs lead to the reduction of the mature miRNA levels. This is either due to the failure of the processing enzymes to bind to the pri-miRNAs after editing, or due to the inability of the miRNA to bind to their targets as a result of the loss of complementarity after editing. It has been found that the levels of ADAR1 increase dramatically upon Salmonella infection. LPS stimulation of macrophages is found to alter the levels of miR-146a, miR-155, and miR125b [72]. miR-146a has roles in regulating the levels of IRAK1 and TRAF6; two important members of TLR signalling. miR-146a also regulates the levels of TNFα which is one of the major cytokines produced via TLR signalling that mediates proinflammatory responses.
Exosomes
Many intracellular pathogens induce exosome secretion which has immunomodulatory properties [73]. Exosomes are small vesicles secreted by various cells of the dimension of 50–100 nm. The role of exosomes during Salmonella infection is largely unexplored. Salmonella stimulates exosome secretion in infected THP-1 macrophages [74]. These exosomes are CD63+ and CD9+ positive. They contain LPS and can activate a TLR4-dependent pathway in naive macrophages. They can induce TNFα production in naive macrophages in a Myd-88-dependent manner. In addition to LPS, Salmonella-induced exosomes have also been reported to contain OUT deubiquitinase1 which does not contain any secretory motif. The secretion of exosomes is mediated via multivesicular bodies [74, 75]. Furthermore, Salmonella Typhi infection of both epithelial cells and macrophages also results in the secretion of outer membrane vesicles containing cytolethal distending toxins (CDTs) [76]. This process requires the microenvironment of SCV or its mimic. Infected cells release CDT-containing exosomes via actin and microtubule-dependent anterograde transport. Internalization of these OMVs by bystander cells requires active endocytosis and retrograde transport, ultimately resulting in DNA damage. Salmonella infection also results in various types of host cell death [77].
Cell Death
One of the many strategies employed by various pathogens to manipulate host is via host cell death. Salmonella causes host cell death by both apoptosis, pyroptosis, necroptosis, and autophagy.
Pyroptosis is an inflammatory cell death. It is a caspase-1-dependent programmed cell death which results in the lysis of the infected cell. It is characterized by the secretion of proinflammatory cytokines IL-1β, IL-18, nuclease-mediated cleavage of DNA in the nucleus and membrane lysis, and LDH release [78]. Salmonella stimulates pyroptosis in infected macrophages in a SPI-1-dependent manner. Translocation of flagellin to the cytosol by the type three secretion system (T3SS) results in activation of cytosolic NLRC4-mediated activation of inflammasome which ultimately results in caspase-1-mediated cell death. NLRP3-mediated activation of inflammasome also results in pyroptosis of Salmonella-infected macrophages. Inflammasome formation, Ipaf and adaptor protein ASC interaction is crucial for caspase-1 activation [79]. Further, SPI-1 mutants cannot secrete flagellin and hence cannot stimulate pyroptosis in macrophages. Caspase-1 activation results in IL-1β, IL-18, and LDH release [80]. IL-1β and IL-18 secretion affect the recruitment of T cells and NK cells. Being a well-known pyrogen, IL-1β also affects the extent of fever during salmonellosis. Flagellin-mediated cell death is the most prominent in the early phase of infection but not during the systemic phase. However, prolonged Salmonella infection also results in a caspase-1-dependent delayed cell death in a SPI-2-dependent manner. SPI-2 effector SpvB is crucial for this phenotype. Caspase-1-deficient mice are 1,000-fold more susceptible to Salmonella infection and display a higher organ burden in Peyer's patches, MLN, and spleen when the infection is through the oral route [81]. Peritoneal infection of these mice does not exhibit any difference when compared to wild type, highlighting its role in intestinal invasion and dissemination. Release of proinflammatory cytokines by macrophages undergoing pyroptosis can recruit more immune cells which can be infected by the bacteria and result in dissemination. Deficiency of caspase-1 in resistant mouse strain (Nramp1 positive) also enhances lethality to Salmonella [82]. Among other signalling cascades, Raf-1 kinase is also known to mediate inflammatory signals upon LPS stimulation in macrophages. Raf-1-deficient macrophages are hypersensitive towards pathogen-induced caspase-1 activation and cell death [78].
Infected macrophages also undergo SPI-2-dependent apoptosis. SpvB, a SPI-2 cytotoxin, depolymerizes actin cytoskeleton in human macrophages and causes apoptosis. This process requires TLR4 signalling and bacterial LPS. Salmonella-mediated activation of PKR kinase results in phosphorylation of eIF2α which in turn inhibits protein synthesis. This ultimately leads to delayed apoptosis of infected cells. NFκB and MAPK activity negatively regulates Salmonella-mediated apoptosis of macrophages [83]. Salmonella effector AvrA shows MAPKK acetyl transferase activity which results in inhibition of JNK and NFκB. PhoP has also been reported to play a role in Salmonella-mediated cell death of human macrophages. This kind of apoptosis of macrophages is induced by only invasive Salmonella strains irrespective of their intracellular replication ability. During Salmonella-mediated apoptosis of macrophages, infected cells show membrane blebbing, DNA fragmentation, and activation of effector caspase-3 [78, 80].
Apart from apoptosis and pyroptosis, Salmonella infection has been reported to induce necroptosis in macrophages. Salmonella infection of macrophages stimulates type I interferon signalling which in turn activates RIP1 and 3 kinases resulting in necroptosis of infected cells. Mice deficient in IFN receptor or RIP3 show prolonged survival as compared to wild-type mice. This is attributed to the absence of macrophage necroptosis [84].
Salmonella has also been reported to cause autophagy-mediated cell death in macrophages deficient in caspase-1. This process is SipB dependent. SipB disrupts host mitochondria in infected cells which results in autophagy induction and cell death [85].
Metabolism
In human and murine macrophages, glycolysis is the major source of energy and glucose is the main carbon source. The tri-carboxylic acid (TCA) cycle is partially required for amino acid synthesis; however, complete TCA cycle is not essential for intracellular life in macrophages.
In contrast to epithelial cells, Salmonella present in the macrophages does not require a functional electron transport chain and ATP synthase. Instead, it depends on substrate level phosphorylation for ATP generation rather than proton gradient [86]. Salmonella has been reported to use host amino acids for its survival. For example, Salmonella upregulates arginine uptake by the host macrophage by increasing the cationic amino acid transporter mCAT expression [87]. Localization of mCAT to SCV results in transport of arginine from cytosol to the vacuole. From the vacuolar pool, bacteria uptake arginine in an ArgT-dependent manner [87]. Salmonella also requires a functional purine biosynthesis pathway for successful survival inside macrophages. Macrophage ROS response results in DNA damage in the bacteria and to repair this damage it is crucial to have functional purine biosynthesis. This pathway also regulates Sif formation and SPI-2 T3SS formation [86].
Macrophage Polarization during Salmonella Infection
The initial inflammatory response of the host, upon Salmonella infection may prime the differentiation of the macrophages into two major types, the classically activated macrophages (CAMs or M1 type) or the alternatively activated macrophages (AAMs or M2 type).
The polarization into M1 or M2 phenotypes is majorly dictated by the cytokines the macrophages encounter [88]. Cytokines like IFNγ or stimulation of TLRs like TLR4 by LPS primes the macrophage to develop into an M1 phenotype which then secretes proinflammatory cytokines and helps in the activation of Th1 arm of the adaptive immune system. This activation of macrophages into CAMs is very tightly regulated as they can also induce tissue damage. Stimulation by cytokines like IL-4 results in the differentiation of macrophages into M2 phenotype, which secrete anti-inflammatory cytokines. These macrophages have critical roles in resolving the inflammation and wound healing. They have reduced microbicidal activity and aid the Th2 arm of the adaptive immune system.
Studies have found that Salmonella prefer inhabiting the AAM during the establishment of chronic infections [89]. There have been rigorous studies performed to elucidate the reason for which the pathogen tends to “choose” one phenotype of the macrophage over another. This can happen because either the bacteria can survive or replicate better in these phagocytes or the bacteria actively guide the polarization to be shifted towards M2 phenotype. The latter seems to be the case with Salmonella.
The nuclear peroxisome proliferator-activated receptors, PPARγ and PPARδ, via their signal transduction are pivotal in dictating the gene regulation patterns of the AAMs [90]. Drugs that target the activity of these PPARs have been known to be effective in a variety of diseases including diabetes, cardiac diseases, and also inflammatory bowel disease [91]. Infection of cultured macrophages with Salmonella induces the expression of genes encoding PPARγ and PPARδ [89]. The same study showed that modulation of PPARγ levels in AAMs directly correlated with the ability of Salmonella to replicate inside these macrophages, whereas they did not show any effect on the proliferation of bacteria such as Mycobacterium, Francisella, and Listeria. This suggests that the pathogen plays an active role in driving the macrophage polarization into the AAM or M2 phenotype.
Protein kinase C (PKC) isotypes belong to the family of serine/threonine protein kinases and play a vital role through signal transduction in the regulation of a variety of cellular functions. One of the isotypes, PKCθ, is known to be predominantly expressed in T cells. Recent reports suggest a role for PKCθ in cholesterol metabolism in human macrophages [92]. Other isotypes like PKCα, PKCβ, PKCδ, and PKCζ have been shown to have critical roles in antimicrobial immune responses of the macrophages. In a recent study, it was shown that in mice lacking PKCθ, the disease progression and lethality of Salmonella Typhimurium infection was exacerbated [93]. It was found that the mRNA levels of PKCθ were strongly upregulated in CAMs but not in AAMs. The results were similar both in murine macrophages and human monocytes. It is suggested that PKCθ might have a subset selective role as signalling intermediate of proinflammatory macrophages. This is speculated to be happening by IL-10 repression which affects the microbicidal activity of the macrophages and also by the regulation or influencing the transcription of certain miRNA clusters [94]. This might be another reason for the pathogen to not choose CAMs.
Since AAMs have reduced microbicidal activity, Salmonella choosing them as a better vehicle than the CAMs whose responses are more robust seems logical. However, direct evidence of this being the reason has not yet been established. It was elegantly suggested by Roop II et al. [95] that this bias could be not because of the reduced microbicidal activity, but something more fundamental. The shift in the cellular metabolism is one of the consequences of macrophage polarization. CAMs rely on glycolysis for their energy and end up consuming a lot of glucose, whereas AAMs degrade fatty acids for acquiring energy. Since glucose is more readily available in the AAMs, as beautifully suggested, the bacteria might have found a “sweet spot” in the AAMs.
Conclusion
Significant advances have been made in understanding the Salmonella pathogenesis in both human and murine models. In this review, we have tried to summarize the complex interaction between Salmonella and macrophage (Fig. 1). Among all cells types that serve as a niche for Salmonella, epithelial cells and macrophages are most studied. However, large gaps still remain to be filled in our understanding of the pathogenesis. Specially, the role of AMPs, metal availability, and Salmonella metabolism in macrophage with respect to acute versus chronic infection require further attention. We are just beginning to understand the role of exosomes, microRNA, and tissue resident macrophages in Salmonella pathogenesis and they might play a crucial role in determining the disease outcome. Further studies are needed to explore these unchartered territories to have a better understanding of the disease. This can help design a new therapeutic strategy against Salmonella infection.
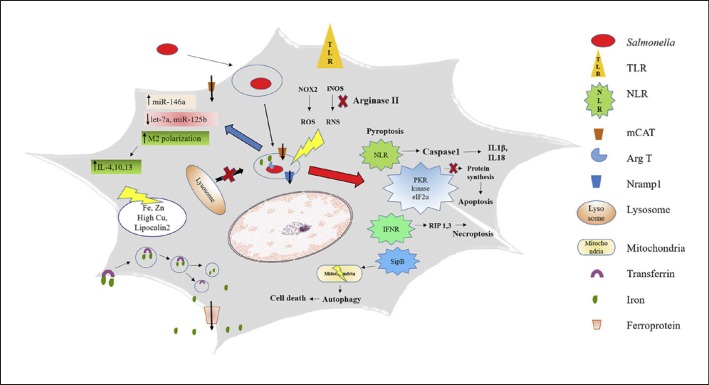
Fig. 1.
Summary of the Salmonella-macrophage interactions. TLR stimulation by the Salmonella PAMPs trigger the burst of ROS and RNS . Salmonella tries to avoid the ill effects of RNS by inducing the simultaneous enhancement in the production of arginase II, which limits the substrate for iNOS activity. Arginase is also recruited into the SCVs. Salmonella actively avoids the SCV fusion with the lysosomes. Macrophages try to establish the balance in the level of metal ions in order to limit the comforts extended to the pathogen. Salmonella effectors trigger various kinds of cell death, like (a) caspase-1-mediated pyroptosis, (b) activation of PKR kinase which leads to the phosphorylation of eIF2α, hampering protein synthesis, leading to apoptosis, (c) activation of IFNR, leading to necroptosis, and (d) SipB mediating the autophagy. Salmonella infection also leads to the alteration of the levels of certain miRNAs. Salmonella prefers the alternatively activated macrophages over the classically activated macrophages for its intracellular life.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Bhutta ZA, Threlfall J. Addressing the global disease burden of typhoid fever. JAMA. 2009;302:898–899. doi: 10.1001/jama.2009.1259. [DOI] [PubMed] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM; International Collaboration on Enteric Disease ‘Burden of Illness Studies’ The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 3.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MI, Katrinak C, Freeman A, Franco-Paredes C. Enteric Fever and Invasive Nontyphoidal Salmonellosis - 9th International Conference on Typhoid and Invasive NTS Disease, Bali, Indonesia, April 30–May 3, 2015. Emerg Infect Dis. 2016:22. doi: 10.3201/eid2204.151463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilyas B, Tsai CN, Coombes BK. Evolution of Salmonella-host cell interactions through a dynamic bacterial genome. Frontiers Cell Infect Microbiol. 2017;7:428. doi: 10.3389/fcimb.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 7.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meresse S, Unsworth KE, Habermann A, Griffiths G, Fang F, Martinez-Lorenzo MJ, Waterman SR, Gorvel JP, Holden DW. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 10.Gorvel JP, Meresse S. Maturation steps of the Salmonella-containing vacuole. Microbes Infect. 2001;3:1299–1303. doi: 10.1016/s1286-4579(01)01490-3. [DOI] [PubMed] [Google Scholar]
- 11.Steele-Mortimer O, Brumell JH, Knodler LA, Meresse S, Lopez A, Finlay BB. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol. 2002;4:43–54. doi: 10.1046/j.1462-5822.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem. 2005;280:24634–24641. doi: 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
- 13.Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, Jones MB, Dracheva T, Peterson SN, Monack DM, Barton GM. TLR signaling is required for Salmonella typhimurium virulence. Cell. 2011;144:675–688. doi: 10.1016/j.cell.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty S, Mizusaki H, Kenney LJ. A FRET-based DNA biosensor tracks OmpR-dependent acidification of Salmonella during macrophage infection. PLoS Biol. 2015;13:e1002116. doi: 10.1371/journal.pbio.1002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 17.Sivick KE, Arpaia N, Reiner GL, Lee BL, Russell BR, Barton GM. Toll-like receptor-deficient mice reveal how innate immune signaling influences Salmonella virulence strategies. Cell Host Microbe. 2014;15:203–213. doi: 10.1016/j.chom.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai MA, Quarles EK, Lopez-Yglesias AH, Zhao X, Hajjar AM, Smith KD. Innate immune detection of flagellin positively and negatively regulates Salmonella infection. PLoS One. 2013;8:e72047. doi: 10.1371/journal.pone.0072047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster N, Hulme SD, Barrow PA. Induction of antimicrobial pathways during early-phase immune response to Salmonella spp. in murine macrophages: gamma interferon (IFN-gamma) and upregulation of IFN-gamma receptor alpha expression are required for NADPH phagocytic oxidase gp91-stimulated oxidative burst and control of virulent Salmonella spp. Infect Immun. 2003;71:4733–4741. doi: 10.1128/IAI.71.8.4733-4741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez-Torres A, Fang FC. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect. 2001;3:1313–1320. doi: 10.1016/s1286-4579(01)01492-7. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Ari J, Wolach O, Gavrieli R, Wolach B. Infections associated with chronic granulomatous disease: linking genetics to phenotypic expression. Expert Rev Anti Infect Ther. 2012;10:881–894. doi: 10.1586/eri.12.77. [DOI] [PubMed] [Google Scholar]
- 28.Mehta A, Singh S, Ganguly NK. Role of reactive oxygen species in Salmonella typhimurium-induced enterocyte damage. Scand J Gastroenterol. 1998;33:406–414. doi: 10.1080/00365529850171044. [DOI] [PubMed] [Google Scholar]
- 29.Uzzau S, Bossi L, Figueroa-Bossi N. Differential accumulation of Salmonella[Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: correlation with their relative contribution to pathogenicity. Mol Microbiol. 2002;46:147–156. doi: 10.1046/j.1365-2958.2002.03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Song M, Husain M, Jones-Carson J, Liu L, Henard CA, Vazquez-Torres A. Low-molecular-weight thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis. Mol Microbiol. 2013;87:609–622. doi: 10.1111/mmi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Torres A, Fang FC. Oxygen-dependent anti- Salmonella activity of macrophages. Trends Microbiol. 2001;9:29–33. doi: 10.1016/s0966-842x(00)01897-7. [DOI] [PubMed] [Google Scholar]
- 32.van der Heijden J, Bosman ES, Reynolds LA, Finlay BB. Direct measurement of oxidative and nitrosative stress dynamics in Salmonella inside macrophages. Proc Natl Acad Sci USA. 2015;112:560–565. doi: 10.1073/pnas.1414569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, Miyamoto Y, Tamura F, Maeda H. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002;70:3130–3142. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441–C450. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- 35.Cherayil BJ, Antos D. Inducible nitric oxide synthase and Salmonella infection. Microbes Infect. 2001;3:771–776. doi: 10.1016/s1286-4579(01)01428-9. [DOI] [PubMed] [Google Scholar]
- 36.Gogoi M, Ravikumar V, Dixit NM, Chakravortty D. Salmonella escapes antigen presentation through K63 ubiquitination mediated endosomal proteolysis of MHC II via modulation of endosomal acidification in dendritic cells. Pathog Disease. 2018;76:ftx125. doi: 10.1093/femspd/ftx125. [DOI] [PubMed] [Google Scholar]
- 37.Lahiri A, Das P, Chakravortty D. Arginase modulates Salmonella induced nitric oxide production in RAW264.7 macrophages and is required for Salmonella pathogenesis in mice model of infection. Microbes Infect. 2008;10:1166–1174. doi: 10.1016/j.micinf.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Bourret TJ, Liu L, Shaw JA, Husain M, Vazquez-Torres A. Magnesium homeostasis protects Salmonella against nitrooxidative stress. Sci Rep. 2017;7:15083. doi: 10.1038/s41598-017-15445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 40.Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, Fang FC, Weiss G. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–3651. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 42.Kortman GA, Boleij A, Swinkels DW, Tjalsma H. Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One. 2012;7:e29968. doi: 10.1371/journal.pone.0029968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown DE, Nick HJ, McCoy MW, Moreland SM, Stepanek AM, Benik R, O'Connell KE, Pilonieta MC, Nagy TA, Detweiler CS. Increased ferroportin-1 expression and rapid splenic iron loss occur with anemia caused by Salmonella enterica serovar Typhimurium infection in mice. Infect Immun. 2015;83:2290–2299. doi: 10.1128/IAI.02863-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 45.Nairz M, Schroll A, Haschka D, Dichtl S, Sonnweber T, Theurl I, Theurl M, Lindner E, Demetz E, Asshoff M, Bellmann-Weiler R, Muller R, Gerner RR, Moschen AR, Baumgartner N, Moser PL, Talasz H, Tilg H, Fang FC, Weiss G. Lipocalin-2 ensures host defense against Salmonella typhimurium by controlling macrophage iron homeostasis and immune response. Eur J Immunol. 2015;45:3073–3086. doi: 10.1002/eji.201545569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nairz M, Ferring-Appel D, Casarrubea D, Sonnweber T, Viatte L, Schroll A, Haschka D, Fang FC, Hentze MW, Weiss G, Galy B. Iron regulatory proteins mediate host resistance to Salmonella infection. Cell Host Microbe. 2015;18:254–261. doi: 10.1016/j.chom.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilonieta MC, Moreland SM, English CN, Detweiler CS. Salmonella enterica infection stimulates macrophages to hemophagocytose. MBio. 2014;5:e02211. doi: 10.1128/mBio.02211-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagy TA, Moreland SM, Detweiler CS. Salmonella acquires ferrous iron from haemophagocytic macrophages. Mol Microbiol. 2014;93:1314–1326. doi: 10.1111/mmi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canonne-Hergaux F, Gruenheid S, Govoni G, Gros P. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc Assoc Am Phys. 1999;111:283–289. doi: 10.1046/j.1525-1381.1999.99236.x. [DOI] [PubMed] [Google Scholar]
- 50.Salinas-Delgado Y, Galaviz-Hernandez C, Toral RG, Avila Rejon CA, Reyes-Lopez MA, Martinez AR, Martinez-Aguilar G, Sosa-Macias M. The D543N polymorphism of the SLC11A1/NRAMP1 gene is associated with treatment failure in male patients with pulmonary tuberculosis. Drug Metab Pers Ther. 2015;30:211–214. doi: 10.1515/dmpt-2015-0019. [DOI] [PubMed] [Google Scholar]
- 51.Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CJ, Ou YC, Lin SY, Liao SL, Chen SY, Chen JH. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem Int. 2006;49:62–71. doi: 10.1016/j.neuint.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Manuel Y, Thomas Y, Pellegrini O. Metallothionein and tissue damage. IARC Sci Publ. 1992:231–237. [PubMed] [Google Scholar]
- 54.Wu A, Tymoszuk P, Haschka D, Heeke S, Dichtl S, Petzer V, Seifert M, Hilbe R, Sopper S, Talasz H, Bumann D, Lass-Florl C, Theurl I, Zhang K, Weiss G. Salmonella utilizes zinc to subvert anti-microbial host defense of macrophages via modulation of NF-kappaB signaling. Infect Immun. 2017 doi: 10.1128/IAI.00418-17. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J. 2012;444:51–57. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- 56.Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun. 2010;78:2312–2319. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapetanovic R, Bokil NJ, Achard ME, Ong CL, Peters KM, Stocks CJ, Phan MD, Monteleone M, Schroder K, Irvine KM, Saunders BM, Walker MJ, Stacey KJ, McEwan AG, Schembri MA, Sweet MJ. Salmonella employs multiple mechanisms to subvert the TLR- inducible zinc-mediated antimicrobial response of human macrophages. FASEB J. 2016;30:1901–1912. doi: 10.1096/fj.201500061. [DOI] [PubMed] [Google Scholar]
- 58.Lahiri A, Abraham C. Activation of pattern recognition receptors up-regulates metallothioneins, thereby increasing intracellular accumulation of zinc, autophagy, and bacterial clearance by macrophages. Gastroenterology. 2014;147:835–846. doi: 10.1053/j.gastro.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agier J, Efenberger M, Brzezinska-Blaszczyk E. Cathelicidin impact on inflammatory cells. Cent Eur J Immunol. 2015;40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA. 2004;101:2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strandberg KL, Richards SM, Gunn JS. Cathelicidin antimicrobial peptide expression is not induced or required for bacterial clearance during Salmonella enterica infection of human monocyte-derived macrophages. Infect Immun. 2012;80:3930–3938. doi: 10.1128/IAI.00672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stocks CJ, Schembri MA, Sweet MJ, Kapetanovic R. For when bacterial infections persist: Toll-like receptor-inducible direct antimicrobial pathways in macrophages. J Leukoc Biol. 2018;103:35–51. doi: 10.1002/JLB.4RI0917-358R. [DOI] [PubMed] [Google Scholar]
- 63.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 64.Das K, Garnica O, Dhandayuthapani S. Modulation of host miRNAs by intracellular bacterial pathogens. Front Cell Infect Microbiol. 2016;6:79. doi: 10.3389/fcimb.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- 70.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 71.Nakano M, Nakajima M. Significance of A-to-I RNA editing of transcripts modulating pharmacokinetics and pharmacodynamics. Pharmacol Ther. 2018;181:13–21. doi: 10.1016/j.pharmthera.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Heale BS, Eulalio A, Schulte L, Vogel J, O'Connell MA. Analysis of A to I editing of miRNA in macrophages exposed to Salmonella. RNA Biol. 2010;7:621–627. doi: 10.4161/rna.7.5.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 74.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui WW, Hercik K, Belsare S, Alugubelly N, Clapp B, Rinaldi C, Edelmann MJ. Salmonella enterica serovar Typhimurium alters the extracellular proteome of macrophages and leads to the production of proinflammatory exosomes. Infect Immun. 2018;86:e00386-17. doi: 10.1128/IAI.00386-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haghjoo E, Galan JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci USA. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guidi R, Levi L, Rouf SF, Puiac S, Rhen M, Frisan T. Salmonella enterica delivers its genotoxin through outer membrane vesicles secreted from infected cells. Cell Microbiol. 2013;15:2034–2050. doi: 10.1111/cmi.12172. [DOI] [PubMed] [Google Scholar]
- 78.Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 79.Hu GQ, Song PX, Chen W, Qi S, Yu SX, Du CT, Deng XM, Ouyang HS, Yang YJ. Critical role for Salmonella effector SopB in regulating inflammasome activation. Mol Immunol. 2017;90:280–286. doi: 10.1016/j.molimm.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Guiney DG. The role of host cell death in Salmonella infections. Curr Top Microbiol Immunol. 2005;289:131–150. doi: 10.1007/3-540-27320-4_6. [DOI] [PubMed] [Google Scholar]
- 81.Lage SL, Buzzo CL, Amaral EP, Matteucci KC, Massis LM, Icimoto MY, Carmona AK, D'Imperio Lima MR, Rodrigues MM, Ferreira LC, Amarante-Mendes GP, Bortoluci KR. Cytosolic flagellin-induced lysosomal pathway regulates inflammasome-dependent and -independent macrophage responses. Proc Natl Acad Sci USA. 2013;110:E3321–E3330. doi: 10.1073/pnas.1305316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reimann T, Buscher D, Hipskind RA, Krautwald S, Lohmann-Matthes ML, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 83.Hueffer K, Galan JE. Salmonella-induced macrophage death: multiple mechanisms, different outcomes. Cell Microbiol. 2004;6:1019–1025. doi: 10.1111/j.1462-5822.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 84.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163:1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herrero-Fresno A, Olsen JE. Salmonella typhimurium metabolism affects virulence in the host - a mini-review. Food Microbiol. 2018;71:98–110. doi: 10.1016/j.fm.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, Balaji KN, Chakravortty D. Cationic amino acid transporters and Salmonella typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One. 2010;5:e15466. doi: 10.1371/journal.pone.0015466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 89.Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14:171–182. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Ann Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613864. doi: 10.1155/2013/613864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma AZ, Zhang Q, Song ZY. TNFa alter cholesterol metabolism in human macrophages via PKC-theta-dependent pathway. BMC Biochem. 2013;14:20. doi: 10.1186/1471-2091-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfeifhofer-Obermair C, Albrecht-Schgoer K, Peer S, Nairz M, Siegmund K, Klepsch V, Haschka D, Thuille N, Hermann-Kleiter N, Gruber T, Weiss G, Baier G. Role of PKC theta in macrophage-mediated immune response to Salmonella typhimurium infection in mice. Cell Commun Signal. 2016;14:14. doi: 10.1186/s12964-016-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutcliffe EL, Bunting KL, He YQ, Li J, Phetsouphanh C, Seddiki N, Zafar A, Hindmarsh EJ, Parish CR, Kelleher AD, McInnes RL, Taya T, Milburn PJ, Rao S. Chromatin-associated protein kinase C-theta regulates an inducible gene expression program and microRNAs in human T lymphocytes. Mol Cell. 2011;41:704–719. doi: 10.1016/j.molcel.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 95.Roop RM, 2nd, Caswell CC. Bacterial persistence: finding the “sweet spot.”. Cell Host Microbe. 2013;14:119–120. doi: 10.1016/j.chom.2013.07.016. [DOI] [PubMed] [Google Scholar]