Abstract
Aims
We aimed to compare the outcome of curative treatment options in localised Prostate Cancer (PCa) amongst HIV positive (HIV+) men.
Methods
A systematic search of the Cochrane Library of Systematic Reviews, the Scopus and PubMed databases was performed (January 1995 to November 2015) using pre-determined search terms. Outcome measures for comparison included the rate of biochemical failure (BCF), survival benefit and complications.
Results
A total of 14 eligible articles were identified for inclusion, representing a total of 202 HIV+ men with PCa. Radical Prostatectomy was performed in 40/153 compared to 109/153 patients undergoing alternative (non-surgical) treatments options. Only 3 studies compared outcomes within their respective study cohort. One study (n = 10) reported BCF results with 1/2 BCF patient in the surgical arm vs. 1/8 BCF positive patients in the non-surgical arm (mean 46 months follow-up), while two other studies reported no occurrences of BCF within both arms of their studies.
Conclusion
Due to paucity in the literature, there is insufficient evidence to support a certain treatment modality arm specifically for HIV+ men with localized PCa. An individualized management algorithm seems feasible within this cohort, until more definitive studies are performed.
Key Words: HIV, Prostate cancer, Radical prostatectomy, Systematic review, Options
Introduction
Due to the effective use of highly active antiretrovi-ral therapy (HAART) the natural progression of human immunodeficiency virus (HIV) to acquired immunodeficiency syndrome (AIDS) has been improved. The net effect of this scientific advancement resulted in HIV-positive (HIV+) patients having a longer life expectancy [1]. Traditionally, in the setting of HIV/AIDS, overwhelming infectious diseases limit patients' survival. The advances in HIV treatment have further resulted in non-AIDs defining cancers playing an increased role in the mortality of this “older” patient cohort [1, 2, 3].
When reviewing the cancer related mortality, prostate and lung malignancies are increased in HIV+ people when compared to non-infected populations [2, 3]. This may be due to HIV-infected men presenting at a relatively younger age, with more advanced disease stage when compared to the general population [4]. The hormonal effect of testosterone in the HIV-infected population due to hypogonadism may also play a role in the pathogen-esis of this malignancy [5]. A trend has been noted that HIV+ men more frequently received radiation for the management of prostate cancer (PCa) and less frequently received surgery [2]. A recent review and meta-analysis have reviewed the outcomes of surgery versus radiotherapy for clinically localized PCa, and observed a higher mortality in the radiotherapy treatment arm [6].
Active treatment for PCa, including prostatectomy, has consistently been shown to provide a survival benefit in men with optimistic life expectancies [7]. Accordingly, as the lifespan of HIV+ patients has been progressively increasing, controversy exists in the optimal treatment option for HIV+ men with PCa. Thus, we aimed to perform a systematic review of the literature to assess whether the current evidence supports a specific treatment modality for localized PCa amongst HIV+ men. Specifically, we aimed to review the rate of biochemical failure (BCF), survival benefit and complications.
Material and Methods
Search Strategy
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-Analysis Guidelines [8]. Scientific literature databases (MEDLINE, Scopus and the Cochrane Library) were systematically searched in December 2015 using several keywords, including: (“prostate” or “prostate cancer” or “prostate neoplasm” or “prostate malignancy”), (“human immunodeficiency virus” or “HIV” or “acquired immunodeficiency disease” or “AIDS”), “prostatectomy”, “radiotherapy”, “active surveillance” and “treatment”.
Both retrospective and prospective studies as well as clinical trials assessing the curative treatment for HIV+ patients with Pca were included in the current study. Articles involving non-curative, metastatic, or palliative treatment were excluded. The concept of active surveillance was considered as a “treatment” modality within the confines of this study. Journal article abstracts were screened for inclusion and suitability. Full-text papers were retrieved for all of the relevant articles. The study selection was independently performed by 2 reviewers (A.A., J.B.). Discrepancies in selection were solved by discussion, or group consensus.
Primary outcomes for the current study included BCF and prevalence of perioperative complications, including death. Heterogeneity in definitions of the PCa risk stratification methods and outcome measures of the included studies precluded meta-analyt-ical assessment. Extracted data were collated in Excel 2007 (Microsoft Corporation, Redmond, CA).
Quality Appraisal
Articles were quality assessed based on nine closed ended questions within the Critical Appraisal Skills Programme (CASP) cohort study checklist [9]. This tool is a validated checklist that assesses 3 domains for each study: the validity of the results, the precision of the results and the usefulness of the results. The closed ended question of the relevant CASP checklist was utilized.
Results
Search Strategy
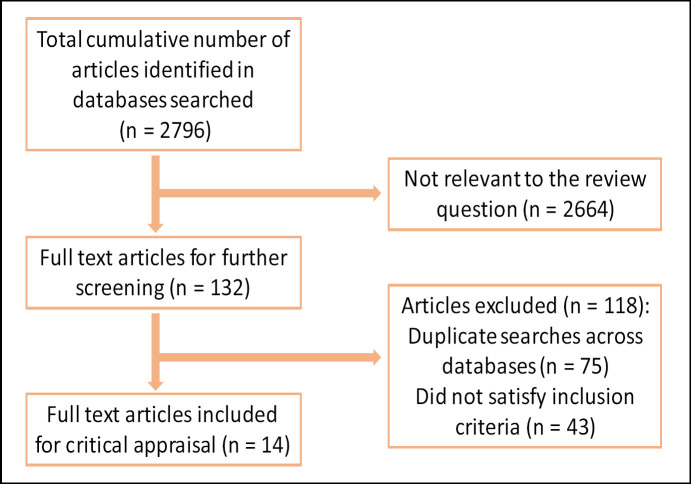
Using the systematic search strategy outlined, 2,796 articles were identified, of which 2,664 were not suitable for full-text review. Of the remaining 132, 75 were duplicate publications and 43 did not meet the inclusion criteria of the study (Fig. 1). Thus, 14 relevant studies were included in this review. The relevant study inclusion categories, grade, and treatment modalities utilized and subsequent outcomes are summarized in Table 1 (demographic data) and Table 2 (outcome stratification).
Fig. 1.
Flow diagram outlining the study review process.
Table 1.
Tabulation of the demographic constituents of the relevant studies assessed
| Author | Country | n | Race | Age | Mean CD4 cells/ml | Viral load copies/ml | HARRT, n | PSA at diagnosis ng/mls | Gleason score | T stage | Risk categories | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ong et al.[10] | Victoria, Australia | 12 | NS | 62.7 | 559.4 | 8/12 UD20 | 12 | mean (11.1) | 3 + 3 | 4 | Tlc | 7 | D'Amico | |
| 4/12 26–56,200 | 3+4 | 1 | T2a | 1 | L | 1 | ||||||||
| 4 + 3 | 3 | T2b | 2 | I | 7 | |||||||||
| 4+4 | 1 | T4 | 2 | H | 4 | |||||||||
| 5+4 | 3 | |||||||||||||
| Riedel et al. [4] | Baltimore, UK | 49 | mixed | 60.7 | 391 | 37/49 ≤ 400 | 47 | mean (82) | mean | 7 | T1 | 20 | NS | |
| 45 AA | 8/49 > 400 | MD (7.7) | median | 7 | T2 | 14 | ||||||||
| 4 CA | range | 5–10 | T3 | 10 | ||||||||||
| T4 | 5 | |||||||||||||
| Murphy et al. [11] | Chicago, US | 43 | mixed | 59.2 | 459.5 | 37/43 < 500 | 41 | MD (5.9) | NS | T1 | 24 | NCCN | ||
| 15 AA | (MD) | 6/43 >500 | T2a | 12 | L | 27 | ||||||||
| 24 CA | T2b | 0 | I | 7 | ||||||||||
| 4 UN | T2c | 3 | H | 4 | ||||||||||
| T3a | 3 | M | 5 | |||||||||||
| T3b | 1 | |||||||||||||
| Schreiber et al. [12] | Brooklyn, US | 15 | mixed | 65 | 464.2 | 11/15 UDU | 13 | <10 (11) | 2 to 6 | 3 | Tlc | 12 | D'Amico | |
| 12 AA | 4/15 90–70,276 | 10.1 to 20 (3) | 7 | 7 | T2a | 1 | L | 2 | ||||||
| 1CA | >20 (1) | 8 to 10 | 5 | T2b | 1 | I | 7 | |||||||
| 2 HI | T3b | 1 | H | 6 | ||||||||||
| Kahn et al. [13] | Atlanta, US | 13 | mixed | 55 | 412.5 | 5/13 UDU | 9 | <10 (11) | <7 | 6 | Tlc | 10 | NS | |
| 8/13 418–20,718 | 10 to 20 (2) | 7 | 6 | T2a | 2 | |||||||||
| > 20 (0) | >7 | 1 | T2c | 1 | ||||||||||
| Silberstein et al. [14] | San Diego, US | 8 | 5 AA | 54.5 | 634 | 8/8 UD50 | 8 | mean (6) | <6 | 2 | T1b-c | 3 | D'Amico | |
| 3 CA | MD (5.6) | 7 | 6 | T2a-c | 5 | L | 4 | |||||||
| >8 | 0 | T3a-b | 0 | I | 3 | |||||||||
| H | 1 | |||||||||||||
| Wosnitzer et al. [15] | New York, US | 4 | NS | 53 | 543 | 138 | 2 | mean (7.9) | NS | NS | Tlc | 4 | NS | |
| New York, US | 3 | NS | NS | 437 | 255 | 3 | mean (8) | NS | NS | Tlc | 2 | NS | ||
| T2a | 1 | |||||||||||||
| New York, US | 4 | NS | NS | 1417 | 12.5 | 2 | mean (10) | NS | NS | Tlc | 3 | NS | ||
| T2b | 1 | |||||||||||||
| Ng et al. [16] | New York, US | 14 | NS | 61 | 523 | 9/14 UDU | 11 | mean (14.3) | <7 | 8 | Tlc | 6 | NS | |
| 5/14 1,600–27,000 | 7 | 5 | T2b | 5 | ||||||||||
| >7 | 1 | T2c | 3 | |||||||||||
| Pantanowitz et al. [5] | Burlington, | 16 | 3 AA | 59 | 336 | 17319 | 14 | mean (30) | mean | 6.8 | T1 | 7 | NS | |
| Chicago, | 8 CA | T2 | 2 | |||||||||||
| Boston, | 2 HI | T3 | 1 | |||||||||||
| Seattle, | 1 HA | N1 | 1 | |||||||||||
| US | 2 UN | M | 1 | |||||||||||
| NS | 4 | |||||||||||||
| Walters et al. [17] | London, UK | 1 | NS | 59 | 300 | UD50 | 1 | mean (7.9) | 4 + 3 | 1 | T2 | 1 | NS | |
| Huang et al.[18] | New York, US | 5 | NS | 52 | 617.4 | 3/5 UD50 | 3 | mean (11.26) | 3+3 | 1 | Tlc | 3 | NS | |
| 2/5 3,600–18,700 | 3+4 | 4 | T2a | 2 | ||||||||||
| O'Connor et al. [19] | New Orleans, US | 3 | 2 AA | 55.3 | 331 | 2/3 400–37,000 | 3 | mean (5.1) | 6 | 2 | T1c | 1 | NS | |
| 1 CA | 1/3 NS | 7 | 1 | T2a | 2 | |||||||||
| Quatan et al. [20] | London, UK | 2 | NS | 61 | 420.5 | 1/2 1242 | 2 | mean (2,821.3) | 3+2 | 1 | NS | 1 | NS | |
| 1/2 NS | NS | NS | T4 | 1 | ||||||||||
| Levinson et al. [21] | New York, US | 10 | 6 AA | 54 | 417 | UD50 | 9 | mean (9.2) | 5 | 2 | T1c | 7 | NS | |
| 2 CA | 6 | 6 | T2a | 2 | ||||||||||
| 2 HI | 7 | 2 | T2b | 1 |
AA = African American; CA = Caucasian; H = high; HA = Haitian; HI = Hispanic; I = intermediate; L = low; M = metastatic; MD = median; NS = Not stated; UD20 = undetectable < 20 copies/ ml; UD30 = undetectable < 50 copies/ml; UDU = undetectable not defined; UN = unknown.
Table 2.
The outcomes within the studies reviewed
| Author | Palliative treatment/non-localized disease | Curative treatment modality used | Mean follow-up | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RP | Non-RP | Total | BCF RP positive | BCF Non-RP positive | RP | Non-RP | ||||||
| Onget al.[10] | 1 WW 1metastatic |
2 RP | 1 BT 5 EBRT 1AS 1 EBRT&ADT |
12 | 46 | 1/2 | 1/8 | 2/2 RP clear surgical margins 1/2 RP, put on salvage EBRT. |
1/5 curative EBRT pts developed BCF (based on phoenix definition). Three deaths reported, none of which were PCa-related deaths | |||
| Riedelet al.[4] | NS | NS | NS | NS | 32.4 | NS | NS | By the end of the study period, 13 (27%) patients had died (8 patients [16%] of the cancer they had). Cumulative 5-year mortality was 22%, whereas CSS was 74% at 5 years. Mortality was higher in patients with stage III-IV disease compared with that in patients with stage I—II disease. | ||||
| Schreiberet al. [12] | 0 | 0 | 10 EBRT&ADT 5 EBRT |
15 | 52.67 | 0 | 2/15 | 2 BCF (28 and 63 months). 0/2 had evidence of metastatic disease. BCF negative (5-year) 92.3%. Acute GU toxicity: 4 Grade 1, 4 Grade 2, 1 Grade 3. Late GU toxicity: 2 Grade 2,1 Grade 3. Acute GI toxicity: 4 Grade 2 Late GI toxicity: 1 Grade 3 |
||||
| Kahnet al. [13] | 0 | 0 | 1 EBRT&ADT 12 EBRT |
13 | 39 | 0 | 2/13 | 4-year BCF -ve survival rate = 87% in the HIV+ group elevated pre- and post-EBRT VL were found to be significant risk factors for BCF. Acute GU toxicity: 3 Grade 1, 4 Grade 2 Late GU toxicity: 4 Grade 1, 1 Grade 3 Acute GI toxicity: 2 Grade 1, 5 Grade 2 Late GI Toxicity: 2 Grade 1 HIV positivity and the use of intensity-modulated radiation therapy were found to be protective of acute GU toxicities. 11/13 pts CD4 declined post EBRT. |
||||
| Silbersteinet al. [14] | 0 | 8RALP | 0 | 8 | 5.6 | 0 | 0 | 8/8 pts had negative margins. No significant differences in the prevalence of overall, low-grade, or high-grade complications between HIV+ and HIV-. | ||||
| Wosnitzer et al. [15] | 0 | 4 3 RP 1 MIRP |
0 | 4 | 18.5 (MD) |
0 | 0 | All patients achieved undetectable serum PSA levels; no recurrence | ||||
| 0 | 3ERBT | 3 | 60 | 0 | 0 | All patients had stable post EBRT PSA levels; no recurrence. No major complications. | ||||||
| 0 | 4 BT | 4 | 75 | NS | NS | Post BT: 2/4 serum PSA of < 0.1 ng/ml 1/4 had a stable PSA level of 3.56 ng/ml 1/4 lost to follow-up, no recurrences | ||||||
| Ng et al. [16] | 0 | 0 | 4 BT 8ERBT& BT 2 EBRT (± ADT) |
14 | 26 | 0 | 1/14 | 13/14 pts PSA decline to 1.1 ng/ml or below. 1/14 PSA elevation and suspicious bone lesion Acute GU toxicity: 6 Grade 2 Late GU toxicity: 2 Grade 2 |
||||
| Author | Palliative treatment/non-localized disease | Curative treatment modality used | Mean follow-up | Outcome | ||||||||
| RP | Non-RP | Total | BCF RP positive | BCF Non-RP positive | RP | Non-RP | ||||||
| Acute GI toxicity: 6 Grade 2 | ||||||||||||
| Late GI toxicity: 2 Grade 2 | ||||||||||||
| 7/14 reported ED | ||||||||||||
| Pantanowitz | 1 | 3 | 7 ADT & EBRT | 16 | NS | 0 | 0 | Complete response in all patients treated. Undetectable PSA and the absence of tumour recurrence. | Hot flushes reported in four men after hor- monal therapy. | |||
| et al. [5] | 2 ADT | |||||||||||
| 3 BT | ||||||||||||
| Walters et al. | 0 | 0 | 1 ADT | 1 | 34 | 0 | 0 | Patient was initially managed conservatively. | ||||
| [17] | The PSA rose to 24 ng/ml over the next 6 months. Therefore, he was commenced on bicalutamide in July 2005, resulting in a fall in his PSA to 0.46 ng/ml. | |||||||||||
| Huang et al. | 0 | 5 | 0 | 5 | 26 | 0 | 0 | 1/5 positive lymph node and extracapsular extension 0/5 BCF at follow-up. | ||||
| [18] | ||||||||||||
| 2/5 Wound infections | ||||||||||||
| O'Connor et | 0 | 0 | 2 EBRT | 3 | 1 | 0 | 0 | Limited follow-up duration. | ||||
| al. [19] | 1 EBRT & ADT | 3/3 experienced PSA decline at 1 month | ||||||||||
| Quatan et al. | 1 | 0 | 1 EBRT & ADT | 1 | 36 | 0 | 0 | In the patient with localized disease the PSA remained stable at 1.1 ng/ml, patient had CVA. | ||||
| [20] | ||||||||||||
| 0 | 0 | 0 | 1 | NS | One patient had metastatic disease at presentation. | |||||||
| Levinson et | 1 MIRP | 1 CRY | 10 | 27.6 | 0 | 0 | 0/1 BCF at follow-up | 1/1 gynecomastia due to ADT | ||||
| al. [21] | 1 ADT | |||||||||||
| 3 EBRT | ||||||||||||
| 1 BT | ||||||||||||
| 1 BT &ADT | ||||||||||||
| 2 WW | ||||||||||||
ADT = Androgen deprivation therapy; AS = active surveillance; BT = brachytherapy; CRY = cryo-surgery; CSS = cancer specific survival; EBRT = external beam radiotherapy; MD = median; MIRP = minimally-invasive radical prostatectomy; NS = not stated; RP = radical prostatectomy; WW = watchful waiting.
This review, represented a total of 153 individual patients, since an additional 49 patients from Riedel et al. [4] could not be included in Table 2 due to a paucity of treatment details. A total of 2 studies evaluating 13 men, assessed the surgical outcome. A further 6 studies assessed the non-surgical modalities, assessing 48 men in total. Five studies involved both surgical and non-surgical management and included 92 patients.
Quality Appraisal Results
Each relevant study was scored using the CASP cohort study checklist [9]. Scoring was performed by 2 evalua-tors (A.A., J.B.) and any discrepancies were resolved by consensus. For ease of reference, results of the quality appraisal have been tabulated (Table 3).
Table 3.
Quality appraisal results tabulated, utilizing the closed ended questions of the CASP cohort study checklist [9]
| Question | Ongetal. [11] | Riedelet al. [4] | Murphy etal. [11] | Schreiberet al. [12] | Kahn et al. [13] | Silberstein et al. [14] | Wosnitzer etal. [15] | Ng et al. [16] | Pantanowitz etal. [5] | Walters et al. [17] | Huanget al. [18] | O'Connor etal. [19] | Quatan et al. [20] | Levinsonet al. [21] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Did the study address a clearly focused issue? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N |
| 2 | Was the cohort recruited in an acceptable way? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | N |
| 3 | Was the exposure accurately measured to minimize bias? | Y | Y | Y | Y | Y | N | N | Y | N | N | Y | N | N | N |
| 4 | Was the outcome accurately measured to minimize bias? | Y | Y | Y | Y | -– | - | N | N | N | N | N | N | N | N |
| 5a | Have the authors listed all confounding factors? | Y | Y | Y | Y | N | N | N | Y | Y | N | Y | Y | N | N |
| 5b | Have the authors taken account of all the confounding factors? | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 6a | Was the follow-up complete enough? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y |
| 6b | Was the follow-up long enough*? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Do you believe the results? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 8 | Can the results be applied to a local population? | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 9 | Do the results of the study fit with other avail- | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Patient Demographics and HIV/AIDS Status
Of the included 14 studies, the respective mean ages of the cohort ranged between 52 and 65 years. Regarding the patient's HIV/AIDS status, mean CD4 count of the included studies ranged between 300 and 1,417 cells/ml. Of the included 202 patients, the proportion of patients actively receiving HAART was 89.1%. Mean duration of HAART was poorly reported across all studies. These findings are summarized in Table 1.
PCa Characteristics
Of the included studies, mean serum prostate-specific antigen ranged between 5.1 and 82 ng/ml. PCa risk stratification was heterogeneous and poorly reported across the studies, with several publications utilizing the National Comprehensive Cancer Network risk tool [11] or D'Amico scoring system [10, 12, 14].
Including the demographic data from Riedel et al. [4], of the 202 patients, histopathologically, a vast majority represented pT1 disease (54.5%) with the remainder pT2 (30.2%), pT3 (7.9%), pT4 (4.0%) or unknown (3.4%). Of the included patients, tumors were characterized as Gleason < 7 in 17.3%, Gleason 7 in 17.8%, Gleason > 7 in 5.4% and unknown in 59.9%. These findings are summarized in Table 1.
PCa Treatment and Oncological Outcomes
Regarding treatment, 40/153 underwent radical prostatectomy with curative attempt. Studies reported this as being performed as robotic-assisted laparoscopic prostatectomy (RALP), minimally-invasive radical prostatectomy or otherwise unspecified. The remainder of the patients received either external beam radiotherapy, brachytherapy or active surveillance.
Mean follow-up ranged 1-90 months post-operatively. During this follow-up period, 7 patients experienced BCF. Of these 1/40 was from the prostatectomy group and 6/109 were from patients that underwent other forms of curative treatment for PCa. A lack of comparative series precluded the calculation of pooled hazards ratios.
Discussion
Recent therapeutic advancements in the management of HIV/AIDs has resulted in a significant improvement in life expectancy in these patients. As a result of less toxic antiretroviral drugs, improved adherence and management of comorbid disease, it is thought that previously described prognostic models and life expectancy estimates for HIV+ patients should be revised [22]. Accordingly, treatment of relatively indolent cancers, such as low and intermediate risk PCa are becoming more relevant. In addition, the increased use of prebiopsy mul-tiparametric magnetic resonance imaging is resulting in more clinically relevant PCa in newly diagnosed patients [23, 24] and thus possibly more HIV+ patients requiring intervention. Our systematic review has highlighted the fact that such patients may exhibit acceptable oncological outcomes when treated with curative measures.
Traditionally, curative treatment for PCa is reserved for patient with a life expectancy of greater than 10 years [25]. The rapid improvement in HAART has improved the life expectancy of HIV+ patients, to the point where they may be considered for curative treatment for PCa. Indeed, the current review identified that of the treated patients, a majority were clinically low-to-intermediate risk. Specifically, of the reporting studies, patients were predominantly D'Amico or NCCN low or intermediate risk. Similarly, a majority of the patients were clinically stage T1. Such patient demographics highlight the nature of early intervention for HIV-infected patients with concurrent PCa.
The incidence of PCa is increasing globally and there is increased PCa mortality in all but the higher resource countries [26, 27]. With this fact in mind it is important to critically assess the available treatment options available for localized PCa in the growing number of HIV-infected men with PCa. Our study has highlighted that, while there is limited data to date, surgical and non-surgical treatment of PCa in this cohort results in acceptable oncological outcomes. This empiric data does not deter the surgical option in this select cohort of patients, as short-term data have not shown a significant difference in outcome based on HIV status. The data, although not reaching statistical significance, does seem to suggest a lower rate of radical prostatectomies being performed in the HIV+ population. The paucity of available evidence also suggests the dire need for further research into this population subgroup, which will increase in number and significance due to the efficacy of HAART therapy.
The differences in the management of PCa in HIV+ infected men when compared to HIV- men may indicate treatment disparities, although some studies have shown most HIV+ men were in fact treated in accordance with guidelines [11]. Some factors that may influence the decision to perform prostatectomy may include risk to the surgical team and risk of operative complications. Furthermore, it is known that PCa decision aids may shift patients' preference from prostatectomy to radiotherapy [28]. Despite the low risk of HIV transmission with needle stick injuries (< 0.09%) [29], it remains a concern for the treating clinicians. Some studies have suggested that RALP may limit the surgical teams exposure to blood and needle stick injury [18, 21]. It has also been shown that RALP has comparable oncological outcomes when compared to open surgery [30].
Silberstein et al. [14] found that HIV-infected individuals had a higher rate of peri-operative transfusion and ileus/small bowel obstruction, but had similar oncological outcomes and other peri-operative complications when compared to non-infected patients. Careful patient selection and assessment is suggested for this patient group including CD4+ counts, viral load, albumin levels, and clinical HIV staging [18]. Furthermore, focal therapy of PCa may be beneficial in this population of patients given the potential increased risk of more invasive treatment modalities, as it may be associated with reduced morbidity [24].
Patient anxiety based on the severity grade of the underlying cancer has been proven to increase in men with more severe PCa [31], however, the impact of concurrent HIV infection within this cohort corrected for grade, has not yet been specifically explored.
Our literature review does not support or refute the surgical treatment of localized PCa within HIV+ men. This is further confounded by the ongoing debate in the literature regarding the best practice in the treatment of high risk PCa in HIV- men [32]. However, an individualized treatment approach seems logical, with the following factors and risks taken into consideration; the CD4 count, viral load, presence of AIDS defining disease, cancer factors and histological subtype, patient comorbid status, patient life expectancy, the surgeon experience and hazardous risk exposure and the availability of (equally effective) alternative therapeutic options. With the improvements in HAART, urologists may find themselves managing HIV+ patients with concurrent PCa more frequently. Further advances in the management of PCa, such as focal therapies [33], may further add to the armamentarium of treating clinicians within the near future.
Conclusion
The current available data reviewed does not support or discourage a particular treatment arm in localized PCa amongst HIV+ men, thus an optimal treatment modality can not be supported within this cohort of patients. Until better more defining studies are published, an individualized approach seems logical.
Acknowledgments
The authors wish to acknowledge the research assistance from Ms. Tshifhiwa Nkwenika, BSc, and Prof. Samuel Manda, PhD, from the Biostatistics Unit, Medical Research Council, Pretoria, South Africa. The authors are also indebted to Mrs. Anna Welman, Department of Surgery at the Helen Joseph Hospital, University of the Witwatersrand, Johannesburg, South Africa, for her secretarial support in the construction of this review.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus Infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Marcus JL, Chao C, Leyden WA, Xu L, Yu J, Horberg MA, Klein D, Towner WJ, Quesen-berry CP, Abrams DI, Silverberg MJ. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev. 2015;24:1167–1173. doi: 10.1158/1055-9965.EPI-14-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedel DJ, Cox ER, Stafford KA, Gilliam BL. Clinical presentation and outcomes of prostate cancer in an urban cohort of predominantly African American, human immunodeficiency virus-infected patients. Urology. 2015;85:415–421. doi: 10.1016/j.urology.2014.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantanowitz L, Bohac G, Cooley TP, Aboula-fia D, Dezube BJ. Human immunodeficiency virus-associated prostate cancer: clinico-pathological findings and outcome in a multi -institutional study. BJU Int. 2008;101:1519–1523. doi: 10.1111/j.1464-410X.2008.07474.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallis CJ, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, Shah PS, Dan-joux C, Nam RK. Surgery versus radiotherapy for clinically-localized prostate aancer: a systematic review and meta-analysis. Eur Urol. 2016;70:21–30. doi: 10.1016/j.eururo.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, Nordling S, Häggman M, Andersson SO, Spångberg A, Andrén O, Palmgren J, Steineck G, Adami HO, Johansson JE. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Critical Appraisal Skills Programme (CASP) 2014 CASP Checklists. http://www.casp-uk.net/casp-tools-checklists. [Google Scholar]
- 10.Ong WL, Manohar P, Millar J, Royce P. Clinicopathological characteristics and management of prostate cancer in the human immunodeficiency virus (HIV)-positive population: experience in an Australian major HIV centre. BJU Int. 2015;116((suppl 3)):5–10. doi: 10.1111/bju.13097. [DOI] [PubMed] [Google Scholar]
- 11.Murphy AB, Bhatia R, Martin IK, Klein DA, Hollowell CM, Nyame Y, Dielubanza E, Achenbach C, Kittles RA. Are HIV-infected men vulnerable to prostate cancer treatment disparities? Cancer Epidemiol Biomarkers Prev. 2014;23:2009–2018. doi: 10.1158/1055-9965.EPI-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber D, Chhabra A, Rineer J, Nabhani T, Katsoulakis E, Morkos R, Rotman M, Schwartz D. Outcomes and tolerance of human immunodeficiency virus-positive U.S. veterans undergoing dose-escalated external beam radiotherapy for localized prostate cancer. Clin Genitourin Cancer. 2014;12:94–99. doi: 10.1016/j.clgc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Kahn S, Jani A, Edelman S, Rossi P, Godette K, Landry J, Anderson C. Matched cohort analysis of outcomes of definitive radiotherapy for prostate cancer in human immunodeficiency virus-positive patients. Int J Radiat Oncol Biol Phys. 2012;83:16–21. doi: 10.1016/j.ijrobp.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 14.Silberstein JL, Parsons JK, Palazzi-Churas K, Downs TM, Sakamoto K, Derweesh IH, Woldrich J, Kane CJ. Robot-assisted laparoscopic radical prostatectomy in men with human immunodeficiency virus. Prostate Cancer Prostatic Dis. 2010;13:328–332. doi: 10.1038/pcan.2010.35. [DOI] [PubMed] [Google Scholar]
- 15.Wosnitzer MS, Lowe FC. Management of prostate cancer in HIV-positive patients. Nat Rev Urol. 2010;7:348–357. doi: 10.1038/nrurol.2010.61. [DOI] [PubMed] [Google Scholar]
- 16.Ng T, Stein NF, Kaminetsky J, Berman S, Marans HY, McDermott B, Berson AM. Preliminary results of radiation therapy for prostate cancer in human immunodeficiency virus-positive patients. Urology. 2008;72:1135–1138. doi: 10.1016/j.urology.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Walters Z, Shamash J, Powles T. Prostate cancer in HIV-positive individuals: what we know and what we don't. J HIV Ther. 2007;12:66–67. [PubMed] [Google Scholar]
- 18.Huang WC, Kwon EO, Scardino PT, Eastham JA. Radical prostatectomy in patients infected with human immunodeficiency virus. BJU Int. 2006;98:303–307. doi: 10.1111/j.1464-410X.2006.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor JK, Nedzi LA, Zakris EL. Prostate adenocarcinoma and human immunodeficiency virus: report of three cases and review of the literature. Clin Genitourin Cancer. 2006;5:85–88. doi: 10.3816/CGC.2006.n.023. [DOI] [PubMed] [Google Scholar]
- 20.Quatan N, Nair S, Harrowes F, Hay P. Should HIV patients be considered a high risk group for the development of prostate cancer? Ann R Coll Surg Engl. 2005;87:437–438. doi: 10.1308/003588405X60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levinson A, Nagler EA, Lowe FC. Approach to management of clinically localized prostate cancer in patients with human immunodeficiency virus. Urology. 2005;65:91–94. doi: 10.1016/j.urology.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting an-tiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlaw KR, Woo HH. Evaluation of the changing landscape of prostate cancer diagnosis and management from 2005 to 2016. Prostate Int. 2017;5:130–134. doi: 10.1016/j.prnil.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall S, Taneja S. Focal therapy for prostate cancer: the current status. Prostate Int. 2015;3:35–41. doi: 10.1016/j.prnil.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, Van den Broeck T, van der Poel HG, van der Kwast TH, Rou-vière O, Schoots IG, Wiegel T, Cornford P. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Tol-Geerdink JJ, Willem Leer J, Weijerman PC, Oort IM, Vergunst H, Lin EN, Alfred Witjes J, Stalmeier PF. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU Int. 2013;111:564–573. doi: 10.1111/j.1464-410X.2012.11402.x. [DOI] [PubMed] [Google Scholar]
- 29.Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102((5B)):9–15. doi: 10.1016/s0002-9343(97)89441-7. [DOI] [PubMed] [Google Scholar]
- 30.Vora AA, Marchalik D, Kowalczyk KJ, Nis-sim H, Bandi G, McGeagh KG, Lynch JH, Ghasemian SR, Verghese M, Venkatesan K, Borges P, Uchio EM, Hwang JJ. Robotic-assisted prostatectomy and open radical retro-pubic prostatectomy for locally-advanced prostate cancer: multi-institution comparison of oncologic outcomes. Prostate Int. 2013;1:31–36. doi: 10.12954/PI.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johanes C, Monoarfa RA, Ismail RI, Umbas R. Anxiety level of early- and late-stage prostate cancer patients. Prostate Int. 2013;1:177–182. doi: 10.12954/PI.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung BH. The role of radical prostatectomy in high-risk prostate cancer. Prostate Int. 2013;1:95–101. doi: 10.12954/PI.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13:641–653. doi: 10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]