Abstract
Introduction
In the last few years, immune checkpoint inhibitors (ICPis) have become a common treatment of cancer. ICPis are associated with peculiar immune side effects, termed immune-related adverse events (irAEs). Thyroid disfunction is a common irAE, but clinical manifestation, severity, and pathogenesis can be variable. While destructive thyroiditis and hypothyroidism are the most common thyroid irAEs induced by ICPis, autoimmune hyperthyroidism (Graves' disease) is rare. We describe a case of a Graves' disease induced by anti-PD-1 therapy and we review the previous reports on this issue.
Case Presentation
A 51-year-old man developed an overt autoimmune hyperthyroidism 2 months after he had started nivolumab (anti-PD-1) therapy for a metastatic non-small cell lung cancer. Although TSH-receptor autoantibodies (TRAb) were negative, the persistence of hyperthyroidism, the hypervascular pattern at thyroid ultrasound, and the high uptake at thyroid scintigraphy were all features suggestive of Graves' disease. Methimazole was started with the prompt restoration of euthyroidism. TRAb remained undetectable during the entire follow-up.
Conclusions
Autoimmune hyperthyroidism can be induced by anti-PD-1 treatment. TRAb were negative in both cases of nivolumab-induced Graves' disease described to date. A correct differential diagnosis between destructive thyroiditis and autoimmune hyperthyroidism is crucial for the appropriate treatment.
Keywords: Immune checkpoint inhibitors, Immune-related adverse events, Nivolumab, Graves' disease, Thyrotoxicosis
Introduction
Immune checkpoint inhibitors (ICPis) are powerful new drugs for treatment of cancer. These monoclonal antibodies trigger immune system against cancer cells, blocking inhibitory signals of T-cells, specifically cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1). So far, several randomized clinical trials have demonstrated the efficacy of these molecules in improving the progression-free survival in several types of cancer (e.g., melanoma, non-small cell lung cancer, kidney cancer, breast cancer). ICPis can induce side effects, the so-called immune-related adverse events (irAEs). Among irAEs, endocrinopathies are frequent and include thyroid dysfunction, hypophysitis, insulin-deficient diabetes mellitus, and primary adrenal insufficiency. Thyroid dysfunction is the most common ICPi-induced endocrinopathy, with transient thyrotoxicosis due to destructive thyroiditis and hypothyroidism frequently reported in clinical trials of ICPis. On the contrary, Graves' disease has been described in only eight patients during ICPis treatment, five during anti-CTLA-4 and three during anti-PD-1 treatment. We report the fourth case of Grave's disease induced by anti-PD-1, the second with an overt hyperthyroidism.
Case Presentation
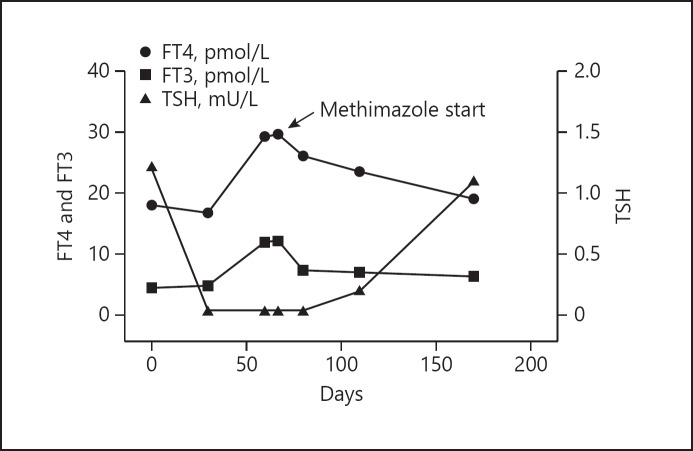
A 51-year-old male was referred to us because he had experienced palpitations, heat intolerance, and insomnia after the fourth infusion of nivolumab (3 mg/kg every 14 days) for a metastatic non-small cell lung cancer. Thyroid function tests before the start of nivolumab treatment showed euthyroidism, with free thyroxine (FT4) 18.1 pmol/L (normal range 9.0–21.8), free triiodothyronine (FT3) 4.5 pmol/L (4.1–8.7), thyroid-stimulating hormone (TSH) 1.22 mU/L (0.4–4.0) (Fig. 1), associated with negative thyroperoxidase autoantibodies (TPOAb), negative thyroglobulin autoantibodies (TgAb), and unremarkable thyroid ultrasound. In the 3 months prior to referral, no administration of iodinated contrast media was reported. At physical examination, we observed increased heart rate and sweaty skin and no signs of Graves' ophthalmopathy. Thyroid function tests were suggestive of overt thyrotoxicosis: FT4 29.3 pmol/L, FT3 11.2 pmol/L, TSH 0.04 mU/L (Fig. 1). TSH-receptor autoantibodies (TRAb) as well as TPOAb and TgAb turned out negative one more time. We re-evaluated the patient 1 week later. Thyroid function tests were comparable (FT4 29.6 pmol/L, FT3 11.4 pmol/L), and at ultrasound, the thyroid was enlarged (20 mL), hypoechoic, and with a mild hypervascularity at Doppler. 131I thyroid scintigram showed an increased and diffuse uptake (3 h uptake 40%; normal range 15–25%).
Fig. 1.
Time course of thyroid function of the patient during treatment with nivolumab. Time 0: first infusion of anti-PD-1 (Nivolumab).
The patient started methimazole, 20 mg/day. At the following observation, 30 days later, symptoms of thyrotoxicosis had disappeared and thyroid function tests had significantly improved: FT4 25.7 pmol/L, FT3 7.4 pmol/L, TSH 0.2 mU/L (Fig. 1). Within 60 days of treatment with methimazole, euthyroidism was restored: FT4 19 pmol/L, FT3 6.29 pmol/L, TSH 1.1 mU/L. Methimazole was therefore tapered down to 5 mg/day, with maintenance of euthyroidism. Thyroid autoantibodies remained negative throughout the entire follow-up (Fig. 1). The patient died 4 months later because of cancer progression.
Discussion
In clinical trials and subsequent clinical practice, thyroid dysfunction has been reported as a common adverse effect during ICPi treatment [1]. A similar overall incidence of hypothyroidism (7–10%) has been estimated for the three types of drugs. At variance, the incidence of thyrotoxicosis differs according to the ICPi regimen, being lower (3.4%) during the anti-CTLA-4 treatment and higher (13.0%) when anti-CTLA-4 and anti-PD-1 are combined [1]. Among cases of thyrotoxicosis, the large majority are transient forms due to destructive thyroiditis, which are usually followed by persistent hypothyroidism, while Graves' disease (hyperthyroidism and/or Graves' ophthalmopathy) has been rarely reported [1, 2].
Graves' disease is typically characterized by diffuse goiter, hyperthyroidism, and, in 50% of patients, ophthalmopathy [3]. Including the present, a total of nine cases of Graves' disease, five after anti-CTLA-4 and four after anti-PD-1 treatment, have been reported (Table 1). It is worth noting that four patients (two after anti-CTLA-4 and two after anti-PD-1 treatment) developed isolated hyperthyroidism and five (three after anti-CTLA-4 and two after anti-PD-1 treatment) euthyroid Graves' ophthalmopathy (Table 1). At variance with the common presentation of spontaneous Graves' disease, no patient developed both autoimmune hyperthyroidism and Graves' ophthalmopathy. Of note, euthyroid Graves' ophthalmopathy is very uncommon after other immunotherapy drugs (e.g., interferon and alemtuzumab); in these settings, ophthalmopathy is usually associated with hyperthyroidism [4, 5].
Table 1.
Case reports on Graves' disease induced by ICPis
| First author, year | ICPis | Hyperthyroidism | GO | Cycle/ time | FT4 (pmol/L) | FT3 (pmol/L) | TRAb | Iodine/99TC uptake | Vascular pattern | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CTLA-4 | ||||||||||
| Borodic [8], 2011 | Ipilimumab | No | Yes | II/6 weeks | na | na | Positive | na | na | Glucocorticoids |
| Min [9], 2011 | Ipilimumab | No | Yes | IV/12 weeks | 14.1 | na | Positive | na | na | Glucocorticoids |
| McElnea [10], 2014 | Ipilimumab | No | Yes | III/6 weeks | 23.7 | na | Negative | na | na | Glucocorticoids |
| Azmat [11], 2016 | Ipilimumab | Yes | No | II/6 weeks | 46.8 | 15.1 | Positive | High | na | Methimazole Thyroidectomy |
| Gan [12], 2017 | Tremelimumab | Yes | No | XX/8 years | 35.5 | 13.0 | Positive | na | na | Carbimazole |
| Anti-PD-1 | ||||||||||
| Park [13], 2018 | Pembrolizumab | No | Yes | III/9 weeks | na | na | na | na | na | Glucocorticoids |
| Campredon [14], 2018 | Nivolumab | No | Yes | III/6 weeks | 15.4 | 4.3 | Negative | na | na | Glucocorticoids |
| Iadarola [15], 2019 | Nivolumab | Yes | No | II/4 weeks | 17.5 | 8.7 | Negative | High | Normal | Methimazole |
| Brancatella (present case), 2019 | Nivolumab | Yes | No | IV/8 weeks | 29.3 | 11.1 | Negative | High | Hypervascular | Methimazole |
ICPis, immune checkpoint inhibitors; GO, Graves' ophthalmopathy; na, not available.
The patient we describe herein developed an overt and symptomatic thyrotoxicosis 2 months after nivolumab was started. The characteristics that allow the clinician to differentiate between thyrotoxicosis due to destructive thyroiditis and autoimmune hyperthyroidism ensuing during ICPi treatment are: severity (mild to moderate vs. moderate to severe), duration (self-limited vs. persistent), lag time (weeks vs. weeks to years), 131I uptake (low vs. high), FT3/FT4 ratio (low vs. high), and vascular pattern at color Doppler (low vs. high) (Table 2).
Table 2.
Destructive thyroiditis and Graves' hyperthyroidism during ICPi therapy: differential diagnosis
| Destructive thyroiditis | Graves' hyperthyroidism | |
|---|---|---|
| Duration | Transient (days to weeks) | Persistent (months to years) |
| Lag time from ICPi start | Short (weeks) | Variable (weeks to years) |
| Iodine uptake | Low | High |
| FT3/FT4 ratio | Low | High |
| TgAb and TPOAb | +/− | +/− |
| TRAb | - | +/− |
| Treatment | No/symptomatic | Anti-thyroid drugs (radioiodine thyroidectomy) |
ICPi, immune checkpoint inhibitor.
In our patient, TPOAb, TgAb, and TRAb were undetectable during the entire follow-up. The role of thyroid autoantibodies in the pathogenesis of ICPi-related thyroid dysfunction is debated. While Osorio and colleagues [6] reported an association between positive TPOAb and positive TgAb and thyroid dysfunction induced by ICPi therapy, this association was not observed in other studies.
The pathogenic role of TRAb in Graves' disease is well established and TRAb are positive in up to 95% of spontaneous cases [7]. In the clinical practice, TRAb are a useful tool in the differential diagnosis of thyrotoxicosis. Anti-CTLA-4-induced Graves' disease was reported to be associated with positive TRAb (Table 1). In our patient, as well as in the other cases of nivolumab-induced Graves' disease, TRAb were persistently negative (Table 1). The finding that all the cases of anti-PD-1-induced Graves' disease are associated with negative TRAb is surprising.
Destructive thyroiditis is usually self-limited and symptoms can be managed by beta blockers. In case of severe thyrotoxicosis or in patients with high cardiovascular risk, treatment with glucocorticoids can be required [1]. Conversely, Graves' hyperthyroidism is persistent and requires anti-thyroid treatment. Anti-thyroid drugs showed a good efficacy in restoring and maintaining euthyroidism in all cases of ICPi-induced Graves' hyperthyroidism. Treatment with thyroidectomy or radioiodine remains an option when hyperthyroidism is not manageable with anti-thyroid drugs. One patient underwent total thyroidectomy for hyperthyroidism during neck dissection for residual melanoma (Table 1). In all cases, Graves' ophthalmopathy was successfully treated with high dose of glucocorticoids (Table 1).
In conclusion, Graves' disease has been reported during ICPis. Both anti-CTLA-4 and anti-PD-1 can induce Graves' hyperthyroidism, although the presentation can be different. A correct differential diagnosis between destructive thyroiditis and Graves' hyperthyroidism is required because treatment of the two conditions is different.
Statement of Ethics
All diagnostic and therapeutic procedures were in accordance with ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from the individual participant included in the report.
Disclosure Statement
The authors have no conflict of interest.
Funding Sources
None of the authors has received funding for this publication.
Author Contributions
Both Alessandro Brancatella and Francesco Latrofa contributed to the final version of the manuscript. All authors discussed the results and conclusions of this work.
References
- 1.Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019 Feb;40((1)):17–65. doi: 10.1210/er.2018-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab. 2017 Aug;102((8)):2770–80. doi: 10.1210/jc.2017-00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menconi F, Marcocci C, Marinò M. Diagnosis and classification of Graves' disease. Autoimmun Rev. 2014 Apr-May;13((4-5)):398–402. doi: 10.1016/j.autrev.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Minelli R, Spagnoli F, Marchesi E, Venturi N, Marina M, Orlandini A, et al. Course of graves disease in interferon-treated patients with chronic hepatitis C virus infection and in uninfected patients. J Investig Med. 2013 Dec;61((8)):1173–7. doi: 10.2310/JIM.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 5.Heath G, Airody A, Peter R, Gale RP. Drugs. Springer International Publishing; 2017. The Ocular Manifestations of Drugs Used to Treat Multiple Sclerosis. [DOI] [PubMed] [Google Scholar]
- 6.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-Mediated Thyroid Dysfunction During T-cell Checkpoint Blockade in Patients with Non-Small Cell Lung Cancer. Ann Oncol. 2017 Jan;28((3)):583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N. TSH receptor autoantibody immunoassay in patients with Graves' disease: improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun Rev. 2012 Dec;12((2)):107–13. doi: 10.1016/j.autrev.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011 Jul-Aug;27((4)):e87–8. doi: 10.1097/IOP.0b013e3181ef72a1. [DOI] [PubMed] [Google Scholar]
- 9.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011 Feb;164((2)):303–7. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElnea E, Ní Mhéalóid A, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014 Dec;33((6)):424–427. doi: 10.3109/01676830.2014.949792. [DOI] [PubMed] [Google Scholar]
- 11.Azmat U, Liebner D, Joehlin-price A, Agrawal A, Nabhan F, Report C. Case Report Treatment of Ipilimumab Induced Graves ' Disease in a Patient with Metastatic Melanoma. 2016;2016((Table 1)):1–5. doi: 10.1155/2016/2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan EH, Mitchell AL, Plummer R, Pearce S, Perros P. Tremelimumab-Induced Graves Hyperthyroidism. 2017:167–170. doi: 10.1159/000464285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park ES, Rabinowits G, Hamnvik OR, Dagi LR. A case of Graves' ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J AAPOS. 2018 Aug;22((4)):310–2. doi: 10.1016/j.jaapos.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Campredon P, Imbert P, Mouly C, Grunenwald S, Mazières J, Caron P. Severe Inflammatory Ophthalmopathy in a Euthyroid Patient during Nivolumab Treatment. Eur Thyroid J. 2018 Mar;7((2)):84–87. doi: 10.1159/000485742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iadarola C, Croce L, Quaquarini E, Teragni C, Pinto S, Bernardo A, et al. Nivolumab Induced Thyroid Dysfunction: Unusual Clinical Presentation and Challenging Diagnosis. Front Endocrinol (Lausanne) 2019;17(9):813. doi: 10.3389/fendo.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]