Abstract
Influenza A virus (IAV) poses a constant worldwide threat to human health. Although conventional vaccines are available, their protective efficacy is type or strain specific, and their production is time-consuming. For the control of an influenza pandemic in particular, agents that are immediately effective against a wide range of virus variants should be developed. Although pretreatment of various Toll-like receptor (TLR) ligands have already been reported to be effective in the defense against subsequent IAV infection, the efficacy was limited to specific subtypes, and safety concerns were also raised. In this study, we investigated the protective effect of an attenuated bacterial outer membrane vesicle harboring modified lipid A moiety of lipopolysaccharide (fmOMV) against IAV infection and the underlying mechanisms. Administration of fmOMV conferred significant protection against a lethal dose of pandemic H1N1, PR8, H5N2, and highly pathogenic H5N1 viruses; this broad antiviral activity was dependent on macrophages but independent of neutrophils. fmOMV induced recruitment and activation of macrophages and elicited type I IFNs. Intriguingly, fmOMV showed a more significant protective effect than other TLR ligands tested in previous reports, without exhibiting any adverse effect. These results show the potential of fmOMV as a prophylactic agent for the defense against influenza virus infection.
Key Words: Influenza, Outer membrane vesicle, Antiviral, Macrophages, Type I interferon
Introduction
Currently available influenza vaccines have effectively reduced disease incidence; however, 3–5 million cases of severe illness, with 250,000–500,000 deaths, are reported annually, worldwide [1]. One of the reasons for high incidence is constant genomic changes in the virus, termed as antigenic drift or shift, which allows the virus to evade the antibody response generated by vaccination [2]. These genetic changes elicit new, highly pathogenic strains, which have the potential to cause a pandemic [3]. For these, new seasonal vaccines need to be formulated annually, which is a time-consuming process. Therefore, there is a need for the development of a broad-spectrum antiviral agent that is effective against a large variety of viral strains and elicits antiviral effect immediately.
Innate immunity can confer broad-spectrum and immediate defense against various pathogens including influenza virus. Innate immune responses are initiated mainly by recognition of pathogen-associated molecular patterns (PAMPs) in the pathogen via pattern recognition receptors such as Toll-like receptors (TLRs) [4, 5, 6], and TLR-stimulated innate immune cells protect the host against the pathogen's first attack as well as subsequent reinfections, which is termed “trained immunity” [7, 8]. Based on these findings, various PAMPs such as lipopolysaccharide (LPS), palmitoylated peptides, and the unmethylated CpG oligodeoxynucleotide have been utilized to induce anti-influenza innate immunity [9, 10, 11, 12, 13, 14]. However, the effects achieved so far are insufficient to protect against infection by a wide spectrum of viral strains. For example, LPS protects the host against a lethal dose of PR8 virus, but the effect was significantly reduced in the SpHA/WSN virus infection model [11, 15], and the stimulation of TLR2 also required supplement of a TLR9 ligand for the significant protection [12]. Another study reported that stimulation of TLR3 by synthetic liposomal polyriboinosinic-polyribocytidylic acid resulted in moderate survival rates (70 and 63% after infection by PR8 and H5N1, respectively) [13]. These previous studies indicate the requirement for a sophisticated strategy or novel agent to achieve broad-spectrum anti-influenza efficacy involving activation of innate immunity.
Outer membrane vesicles (OMVs) are phospholipid bilayer vesicles produced naturally by Gram-negative bacteria. Since OMVs contain various bacterial antigens [16], they have been studied as a vaccine candidate against pathogenic bacteria such as Vibrio cholerae and Borrelia burgdorferi [17, 18]. Notably, the OMV from Neisseria meningitidis was the first to be licensed for humans as a vaccine component [19]. In addition to bacterial proteins, these vesicles also contain TLR ligands such as LPS, lipoproteins, and flagellin [16, 20], and in this regard, OMVs augmented adaptive immune response to the coadministered antigens via activation of innate immunity [5, 6]. Previously, we generated fmOMV, which stands for further-modified OMV, of which lipid A is a dephosphorylated and underacylated species that resulted from both expression of the LpxF 4′-phosphatase and deletion of the msbB/pagP lipid A myristoyl/palmitoyltransferase genes. This OMV showed a significant adjuvant effect on a seasonal influenza vaccine exhibiting much attenuated TLR4-stimulating activity in vitro and endotoxicity in vivo [21, 22]. Although the adjuvant effect of OMVs has been reported in various antigen models, the antiviral effects via activation of innate immunity have not yet been addressed.
In this study, we showed that fmOMV elicits host innate immune response against influenza virus infection. Intranasal administration of fmOMV resulted in the recruitment and activation of macrophages in the lung tissue, and these macrophages sufficiently protect infection from different influenza viruses; pH1N1, PR8, H5N2, and highly pathogenic H5N1. These findings suggest the potential of fmOMV as a novel antiviral agent against infection from broad-spectrum influenza viruses.
Materials and Methods
Purification of fmOMV
fmOMV was produced by transforming E. coli W3110 ΔmsbB/ΔpagP mutant strain with pWSK29-LpxF plasmid as previous ly reported [23]. After removing the bacteria by centrifugation (11,000 g), the supernatant was filtered using a 0.22-μm pore filter (Merck, NJ, USA). fmOMV in the filtrate were precipitated in 390 g/L ammonium sulphate solution, and the collected pellets were centrifuged again at 16,000 g for 15 min. The pellets were ultracentrifuged in a sucrose gradient solution for further purification.
TLR Signaling Assay
HEK-BlueTM mTLR2, mTLR3, mTLR4, mTLR5, or mTLR9 (Invivogen, CA, USA) cell lines were maintained in RPMI1640 media (Life technologies, MA) supplemented with 10% fetal bovine serum (FBS; GE Healthcare, UK) and 1× antibiotics (Life Technologies). During activation, each cell line was resuspended in HEK-BlueTM Detection media (Life Technologies), seeded at 5 × 104 cells/well in 96-well plates, and treated with fmOMV or control reagents; Pam3Cys-Ser-(Lys)4 (Pam3, 1.0 µg/mL; Merck Millipore, Germany), poly I:C (10 µg/mL), LPS (100 ng/mL), flagellin (1.0 µg/mL), or CpG ODN 1826 (CpG, 1.0 µg/mL) (Invivogen). After 24-h incubation, the secreted alkaline phosphatase activity was measured at 630 nm.
Viruses
Influenza A/California/04/2009 (pandemic H1N1, pH1N1), influenza A/Puerto Rico/8/1934 (PR8), influenza A/aquatic bird/Korea/CN2-MA/2009 (H5N2), and influenza A/Environment/Korea/W149/2006 (H5N1) viruses were cultivated in the allantoic cavities of embryonated chicken eggs. Viruses were titrated by calculating the 50% egg infectious dose (EID50) and stored at −80°C until use.
Animals and Experimental Schedule
Seven-week-old, female C57BL/6 mice were kept in pathogen-free, biosafety level-2 or -3 facilities at Korea Research Institute of Bioscience and Biotechnology (KRIBB) or Chungbuk National University, respectively. Mice were injected intranasally with fmOMV (10 µg/mouse) once. Three, 7, or 14 days after fmOMV injection, mice were challenged with a 10 lethal dose 50 (LD50) of PR8, pH1N1, H5N2, or highly pathogenic H5N1 viruses and their mortality rates were monitored for 2 weeks. The trivalent split influenza vaccine containing A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Massachusetts/2/2012 (1.0 μg of HA/mouse, Green Cross, Korea) was immunized either intranasally (Fig. 2) or intramuscularly (Fig. 5) twice at a 2-week interval. The vaccine-immunized groups were challenged 2 weeks after the booster injection. For intranasal injection, the total volume was adjusted to 30 µL/mouse by using phosphate-buffered saline (PBS). A humane endpoint of 25% weight loss was used for this challenge study.
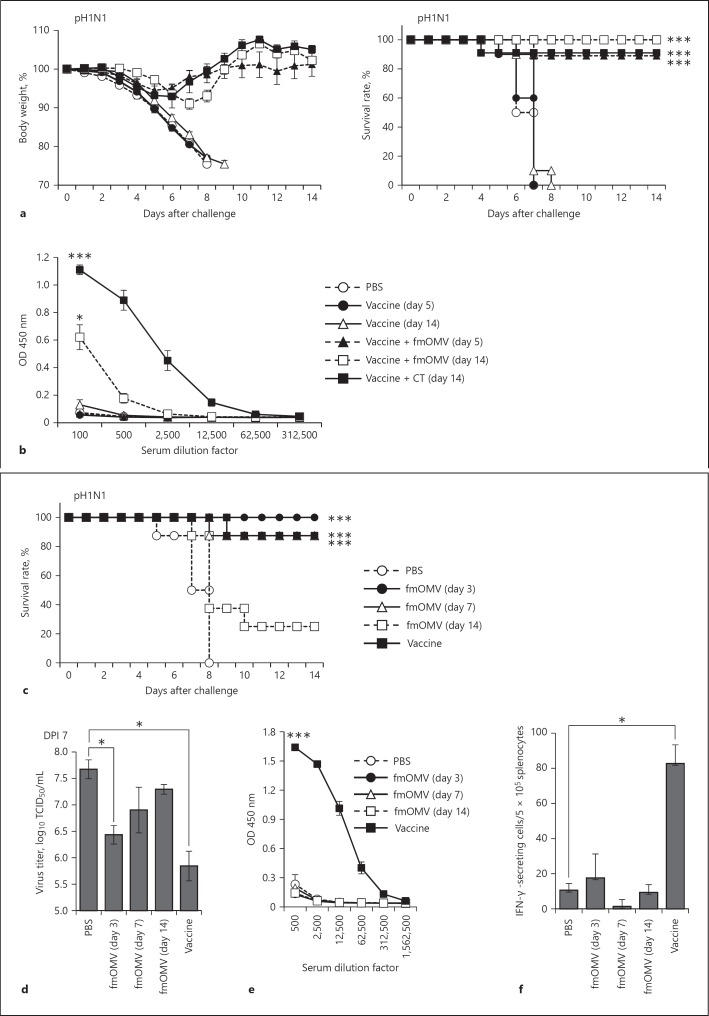
Fig. 2.
Antiviral activity of fmOMV is independent of the adaptive immune response. Mice (n = 10) were intranasally immunized with the trivalent split influenza vaccine mixed with fmOMV or cholera toxin, and then challenged with pH1N1 virus at 5 and 14 days after the injection. a After challenge, the mice were monitored for changes in body weight and survival rate for 14 days. b Sera were collected before virus challenge, and the virus-specific antibody response was determined by ELISA. c–f Mice (n = 8) were injected with fmOMV alone. At 3, 7, or 14 days after the injection, the mice were infected with pH1N1 virus. The vaccinated group was immunized intramuscularly with pH1N1 split vaccine followed by a booster injection after 2 weeks (n = 8). Two weeks after the second injection, the mice were challenged with the pH1N1 virus. c The survival rates were monitored for 2 weeks after the pH1N1 viral challenge. d The lung tissues were collected at 7 days after the viral challenge and virus titers were determined. e Influenza-specific IgG antibodies were measured by ELISA using sera collected on the day of virus challenge. f IFN-γ-secreting T cells were estimated by enzyme-linked immunospot assay, using splenocytes harvested on the day of virus challenge. Data are presented as mean ± standard error of mean. *** p < 0.001, * p < 0.05.
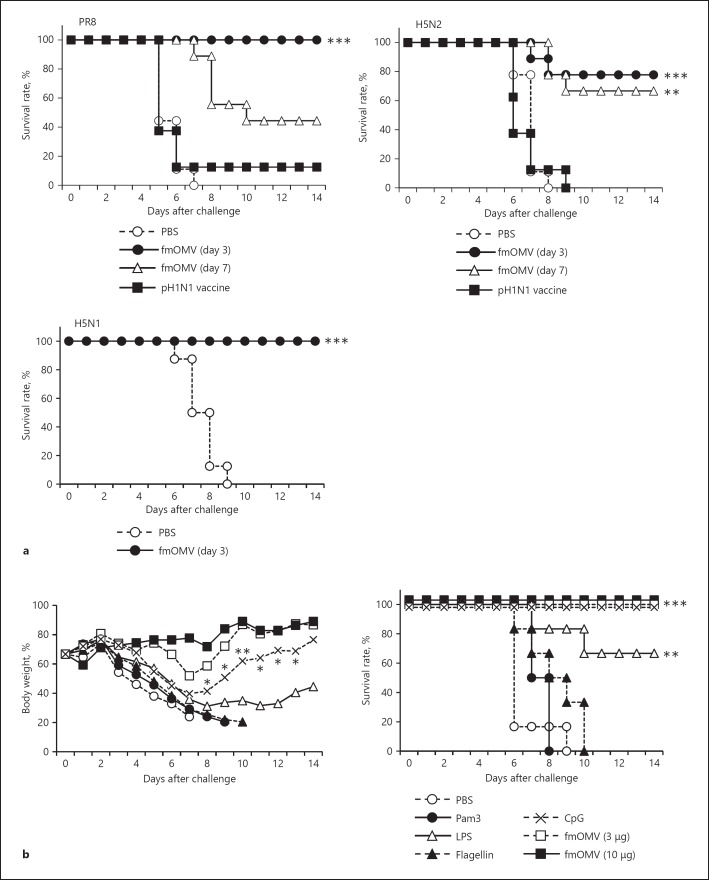
Fig. 5.
fmOMV provide broad and potent protection against diverse influenza A viruses. a Mice (n = 8–9) were injected with fmOMV and challenged with 10 LD50 of PR8, H5N2, and highly pathogenic H5N1 influenza viruses at the indicated time points. The vaccinated group was immunized intramuscularly with the trivalent split influenza vaccine containing pH1N1 antigen twice at a 2-week interval (n = 8). Two weeks after the second injection, the mice were challenged with each virus. b Mice (n = 8) were intranasally injected with each indicated TLR ligand (3 µg) and challenged with 10 LD50 of the pH1N1 virus. a, b The survival rates were monitored for 2 weeks after the viral challenge. These data are representative of two or three independent experiments. *** p < 0.001, ** p < 0.01, * p < 0.05.
Enzyme-Linked Immunosorbent Assay
ELISA plates (ThermoFisher Scientific, MA, USA) were coated with vaccine antigen (200 ng/well), and then incubated with the serum samples. Bound antibodies were detected by sequential incubation with peroxidase goat anti-mouse total IgG (Cell Signaling Technology, MA, USA) and 3,3′,5,5′-tetramethylbenzidine substrate (BD Bioscience, CA, USA). Optical density was measured at 450 nm wavelength using VICTOR3TM (PerkinElmer, MA, USA).
Enzyme-Linked Immunospot (ELISPOT) Assay
Influenza-specific IFN-γ-producing cells were quantified using a mouse IFN-γ enzyme-linked immunospot set (BD Biosciences) on the day of virus challenge. Briefly, splenocytes (5 × 105 cells/well) were incubated with inactivated pH1N1 virus on ELISPOT plates coated with IFN-γ capture antibody. After 40-h incubation, the plates were further incubated with biotinylated IFN-γ detection antibody and then horseradish peroxidase-conjugated streptavidin. Spots were visualized by adding 3-amino-9-ethyl-carbazole substrate solution and counted using the BioSpot analyzer (Cellular Technology, OH, USA).
Virus Titration
Total lung homogenate samples were obtained at indicated time points, and then added to Madin-Darby canine kidney cells with 10-fold serial dilution. Three days after infection, virus titer was determined with a hemagglutinin test and calculated by the method of Reed and Muench, as previously described [24]. Virus titer was expressed as log10 of the 50% tissue culture-infective dose (TCID50) per milliliter.
Fluorescent Labeling of fmOMV
fmOMV was labeled with Alexa Fluor 488 fluorescent dye (Alexa488) according to the manufacturer's instructions (ThermoFisher Scientific). In brief, 1 M sodium bicarbonate buffer was added to fmOMV diluted in PBS, and then Alexa488 tetrafluorophenyl ester was added to the mixture. After incubation for 15 min at room temperature, unreacted dye was removed using a spin filter containing Bio-Gel P-6 fine resin.
Flow Cytometry
Samples were resuspended in fluorescence-activated cell sorting buffer (PBS containing 2.5% FBS and 0.1% sodium azide) and incubated with Fc-block (anti-CD16/CD32; eBioscience, CA, USA). After washing, the cells were stained for CD11b, CD11c, SiglectF, CD40, CD45, CD80, CD86, MHC class II, F4/80, and Ly6G (eBioscience). Samples were acquired on GalliosTM (Beckman Coulter, CA, USA) and analyzed using FlowJo software (Tree Star, OH, USA).
Depletion of Neutrophils and Macrophages
Neutrophils were depleted by intraperitoneal injection of anti-mouse Ly6G antibody (500 μg/mouse, 1A8 clone; Biolegend, CA, USA) 24 h before fmOMV treatment. For macrophage depletion, empty or clodronate-encapsulating liposomes (FormuMax, CA, USA) were administered via intravenous routes (1.0 mg/mouse) at 2 days before fmOMV administration.
Quantitative RT-PCR
RNA was isolated from the lung tissue using TRIzol (Invitrogen) and was reverse-transcribed with Moloney murine leukemia virus reverse transcriptase and oligo-d(T) according to the manufacturer's instructions. Quantitative RT-PCR was performed using specific primers (IFN-α: sense, 5′-ATGGCTAGGCTCTGTGCTTTCCT-3′, antisense, 5′-AGGGCTCTC CAGACTTCTGCTCTG-3′; IFN-β: sense, 5′-CCCTATGGAGATGACGGAGA-3′, antisense, 5′-TCCCACGTCAATCTT TCCTC-3′; HPRT: sense, 5′-CAGACTGAAGAG CTACTGTAATGATCA-3′, antisense, 5′-TCA ACA ATCAAGACATTCTTTCCA-3′) and SYBR Premix Ex Taq (Takara Bio) on a Dice TP800 Thermal Cycler (Takara Bio). The mRNA levels were normalized relative to the expression of Hprt mRNA.
Multiplex Cytokine Immunoassay
Bronchoalveolar lavage fluid (BALF) samples were harvested 3 days after fmOMV treatment. The cytokine levels were measured using a multiplex cytokine immunoassay system (Bio-Rad Laboratories, CA, USA) according to the manufacturer's instructions.
Statistical Analysis
Statistical differences among groups were assessed using a 2-tailed Student's t test or a log-rank test with GraphPad Prism software. p values of < 0.05 were considered statistically significant.
Results
fmOMV Stimulates Diverse TLRs
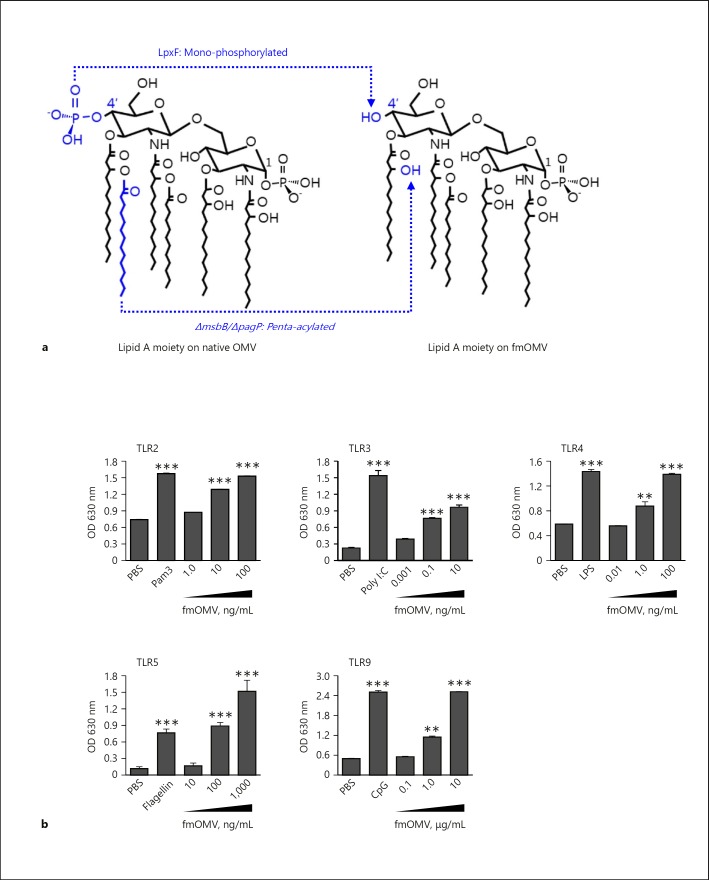
Activation of various TLR signaling has been reported to initiate antiviral innate immunity [4]. Based on the fact that OMVs contain diverse bacterial components which can stimulate TLRs [23, 25, 26], we previously generated a modified OMV harboring less endotoxic LPS (fmOMV, Fig. 1a) and investigated its safety and adjuvant effect using an influenza vaccine model [21]. In this study, we tested whether fmOMV initiates TLR 2, 3, 4, 5, and 9 signaling simultaneously, using HEK-Blue cells expressing individual TLRs. Treatment with fmOMV induced TLR2, TLR3, TLR4, TLR5, and TLR9 signaling in a dose-dependent manner (Fig. 1b). These data suggest that fmOMV treatment elicits innate immunity required to generate antiviral host immunity via simultaneous triggering of various TLR signaling.
Fig. 1.
Modified outer membrane vesicle (fmOMV) stimulates diverse Toll-like receptors (TLRs). a Structure of lipid A moiety on fmOMV. b HEK293 cells expressing mouse TLR2, TLR4, TLR5, or TLR9 were cultured with indicated amounts of fmOMV or corresponding ligand (PAM3 100 ng/mL; LPS 100 ng/mL; flagellin 100 ng/mL; or CPG 100 ng/mL) for 24 h. The extent of fmOMV- or TLR ligand-induced TLR stimulation was determined by measuring secreted embryonic alkaline phosphatase activity using QUANTI-Blue colorimetric enzyme assay. Data are presented as mean ± standard deviation from triplicate culture wells, at optical density (OD) of 570 nm. *** p < 0.001, ** p < 0.01.
Antiviral Activity of fmOMV Is Independent of the Adaptive Immune Response
Previously, we reported that OMV injection with a vaccine antigen increases vaccine-induced antibody responses using ovalbumin and influenza vaccine antigens [21, 23]. Since rapid induction of protective immunity in particular is required for vaccines against highly transmissible infectious diseases such as influenza, we first tested whether fmOMV could induce protective efficacy of an influenza vaccine at early time points after the injection. When mice were challenged both 5 and 14 days after immunization, the mice coimmunized with fmOMV exhibited reduced body weight loss and an increased survival rate, which were significantly higher than those achieved by vaccination without fmOMV (Fig. 2a). Intriguingly, the antigen-specific antibody was not detected in sera 5 days after immunization (Fig. 2b). This discrepancy between the protection rate and antibody response at day 5 after vaccination led to the possibility that fmOMV induces a protective response before the vaccine-induced antibody response is generated. To verify this hypothesis, mice were inoculated intranasally with fmOMV alone and then challenged with pH1N1 influenza virus at 3, 7, or 14 days after fmOMV administration. All fmOMV-injected mice survived at 3 days after fmOMV injection (Fig. 2c); however, survival rates decreased with an increase in the interval between fmOMV injection and pH1N1 virus challenge (day 7: 80%, day 14: 20%). Additionally, the viral titer in the lungs was the lowest at 3 days after fmOMV injection (Fig. 2d). To investigate whether the protective effect of fmOMV was associated with adaptive immunity, we measured antigen-specific antibody and T cell responses on the day of virus challenge. As expected, neither the antibody response nor the interferon (IFN)-γ-secreting T cell response specific for the vaccine were detected at 3, 7, or 14 days after fmOMV injection (Fig. 2e, f), indicating that the fmOMV-induced antiviral effect is not associated with adaptive immunity.
Macrophages but Not Neutrophils Are Associated with Anti-Influenza Effect by fmOMV
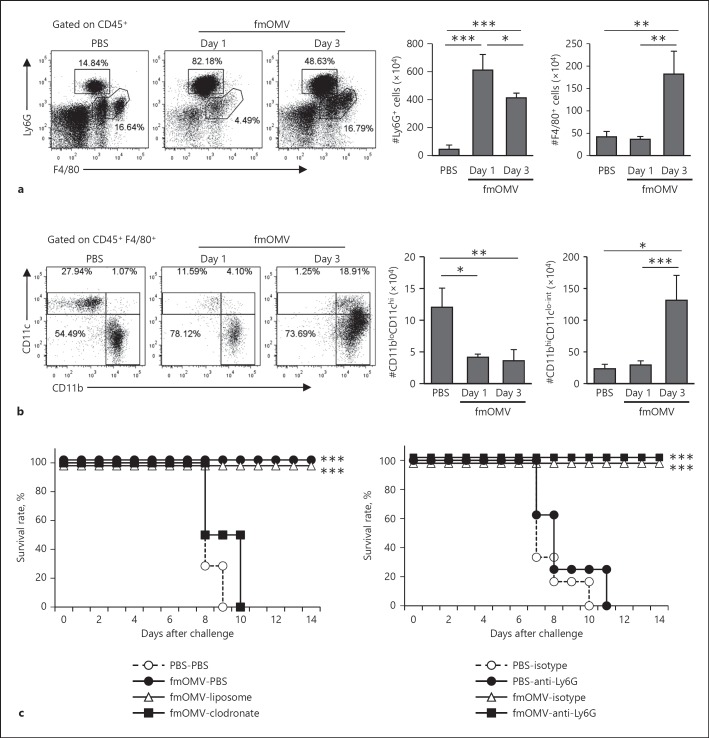
Given that the fmOMV-induced antiviral effect was independent of adaptive immunity, we hypothesized that the antiviral activity is associated with innate immunity. Since previous reports showed that macrophages and neutrophils comprise the majority of innate immune cells responsible for the antiviral response in an influenza infection model [27], we measured the change in counts of macrophages and neutrophils in the lungs after fmOMV injection. Both cell counts significantly increased after fmOMV injection (Fig. 3a). Additional analysis of the change in macrophage phenotypes showed that major population of F4/80+ cells in the lungs was CD11bhiCD11clo-int (Fig. 3b) [28].
Fig. 3.
Macrophages but not neutrophils are associated with anti-influenza effect by fmOMV. a Macrophages and neutrophils were measured by fluorescence-activated cell sorting analysis of lung tissue at 1 and 3 days after fmOMV injection. b The CD45+F4/80+ macrophage population was subdivided in terms of CD11b and CD11c expression. c The survival rate of macrophage- or neutrophil-depleted mice infected with pH1N1. After mice were injected with clodronate-liposome or anti-Ly6G, they were injected with fmOMV intranasally and infected with 10 LD50 of pH1N1 at 3 days after fmOMV injection. Data are presented as mean ± SEM and representative of at least three independent experiments. *** p < 0.001, ** p < 0.01, * p < 0.05.
To identify which innate immune cells mainly contributed to the antiviral activity by fmOMV, we monitored the survival rate of fmOMV-injected mice under neutrophil- or macrophage-depleted conditions. Depletion of macrophages by clodronate-liposome injection completely abrogated the antiviral effect by fmOMV administration (Fig. 3c), indicating that macrophages play an important role in the antiviral effect by fmOMV against influenza virus infection (Fig. 3c, left). After neutrophil depletion by anti-Ly6G antibody injection, the antiviral effect by fmOMV was not affected, showing that neutrophils are not associated with antiviral effect by fmOMV (Fig. 3c, right). These data suggest that the antiviral effect by fmOMV injection is associated with an increase in macrophages in the lungs.
fmOMV Activates Macrophages and Induces Antiviral Cytokines in the Lung Tissue
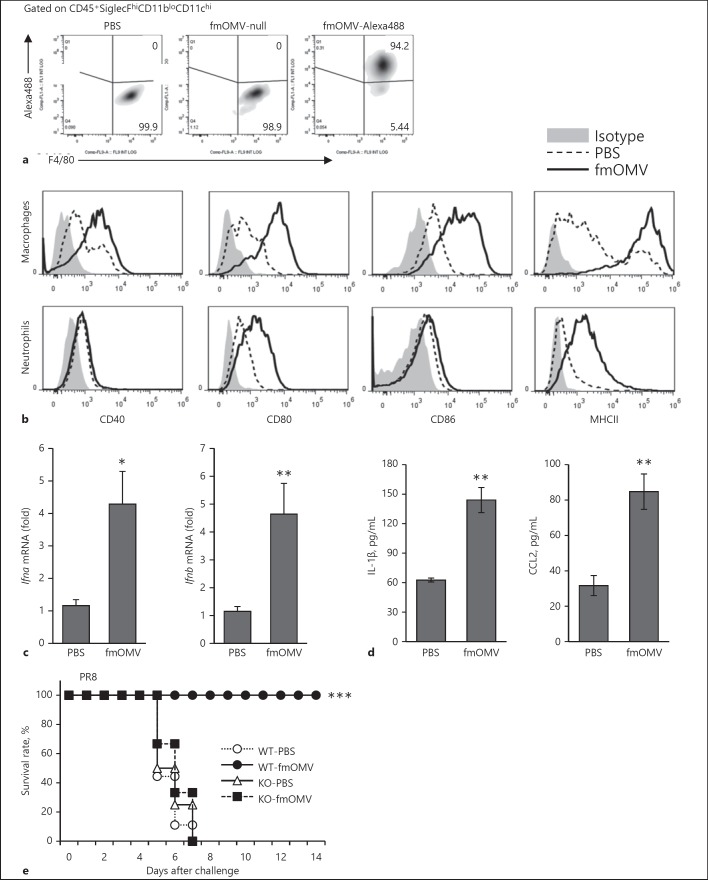
It has been known that alveolar macrophages (AMs) are the major population that firstly recognizes foreign materials and induces subsequent immune activation in the lungs. To investigate whether fmOMV-induced antiviral effect was mediated by direct interaction of fmOMV with AMs, we injected Alexa488-labelled fmOMV (fmOMV-Alexa488) and quantified the fluorescence signal in AMs. At 3 h after fmOMV-Alexa488 injection, 94.2% of CD45+SiglecFhiCD11bloCD11chiF4/80+ cells were Alexa488-positive (Fig. 4a), showing direct interaction of fmOMV with AMs.
Fig. 4.
fmOMV activates macrophages and induces antiviral cytokines in the lung tissue. a Mice were intranasally administered with 10 µg of Alexa Fluor 488 (Alexa488)-labeled fmOMV. After 3 h, the population of Alexa488 and F4/80-positive cells were determined by flow cytometry, gated on CD45+SiglecFhiCD11bloCD11chi bronchoalveolar lavage cells. b The expression of activation markers on macrophages (upper panels) and neutrophils (lower panels) in the lungs were analyzed at 3 days after fmOMV injection. c, d The levels of type I interferons, IL-1β, and C-C motif chemokine ligand 2 (CCL2) in the lungs were determined by RT-PCR and multiplex cytokine immunoassay, respectively. e Type I IFN receptor 1 knockout (IFNAR1KO) mice (n = 4 or 6) were injected with fmOMV. Three days after the injection, the mice were infected with PR8 virus, and the survival rates were monitored for 2 weeks after the viral challenge. Data are presented as mean ± SEM and representative of at least three independent experiments. *** p < 0.001, ** p < 0.01, * p < 0.05.
Upon activation, AMs secrete proinflammatory chemokines which in turn lead to recruitment of diverse immune cells ([29]), as shown in Figure 3a, b. Given that macrophages are indispensable for the fmOMV-mediated antiviral effect (Fig. 3c, left), we next investigated the characteristics of infiltrated macrophages in lung tissue 3 days after fmOMV injection. The activation markers, such as CD40, CD80, CD86, and MHC class II on macrophages, were upregulated after fmOMV injection (Fig. 4b). We also analyzed the levels of antiviral cytokines and chemokines after fmOMV injection. The level of IFN-α and β, IL-1β, and C-C motif chemokine ligand 2 (CCL2, MCP-1) in the lung tissue was increased by fmOMV injection (Fig. 4c, d), indicating that fmOMV activated the innate immune system leading to antiviral soluble factors. Given that fmOMV induces significant production of type I IFNs, we further investigated whether type I IFNs contributed to fmOMV-mediated protection using mice lacking a type I IFN receptor (IFNAR1KO). When IFNAR1KO mice were treated with fmOMV and then challenged with a lethal dose of PR8 virus, all mice lost weight and eventually died (Fig. 4e), indicating that type I IFNs play an important role in the fmOMV-mediated antiviral effect.
fmOMV Provide Broad and Potent Protection against Diverse Influenza A Viruses
To further examine whether fmOMV protects against a broad spectrum of influenza virus subtypes, we additionally challenged fmOMV-injected mice with PR8, H5N2, and highly pathogenic H5N1 viruses. Eighty to one hundred percent of the fmOMV-injected mice survived the PR8, H5N2, and H5N1 viral challenge at 3 days after fmOMV injection (Fig. 5a). The protective effect of fmOMV was compared with other TLR ligands: Pam3, LPS, flagellin, and CpG, which have been reported to protect against influenza viruses [11, 12, 30]. Interestingly, while the TLR ligands except CpG were ineffective, fmOMV (3 and 10 µg) completely protected against lethal influenza virus challenge (Fig. 5b). Although CpG completely protected the viral challenge in terms of the survival rate (Fig. 5b, right), the body weight of mice administered CpG significantly declined upon viral challenge compared to that of mice administered fmOMV (Fig. 5b, left). Consistent with a previous report [21], the mobility, food intake, and body weight of the mice were normal, and inflammation in the lung tissue was not observed when fmOMV was intranasally injected (data not shown). These data indicate that fmOMV provide broad and potent protection against diverse influenza A viruses (IAVs) without endotoxic inflammation.
Discussion
Emerging infectious diseases caused by yet unidentified viruses or new variants of known viruses are a constant threat to human health, as exemplified by Middle East respiratory syndrome coronavirus and influenza viruses. These pandemic or epidemic outbreaks necessitate the availability of therapeutics and vaccines. However, factors such as lack of information regarding the virus and the time-consuming developmental process have resulted in an urgent need for an effective antiviral agent that protects against a broad spectrum of viruses immediately. In this study, we demonstrated that intranasal injection of fmOMV not only protects against infection by a broad spectrum of influenza viral strains, but also elicits this antiviral effect as early as 3 days after administration. Cell depletion and subtype analysis studies revealed that an increase and activation of macrophages by fmOMV injection provided broad-spectrum antiviral effects.
Previous reports showed that AMs play an indispensable role in the protection against airway infection with influenza viruses [31, 32, 33, 34]. Upon infection with influenza viruses, AMs primarily produce type I IFNs that play an important role in the protection against influenza viruses by both inhibiting viral replication and suppressing excessive tissue inflammation [35, 36]. In this study, we observed activation of TLR3 and 4, which are known to induce type I IFNs via interferon regulatory factor 3, in vitro (Fig. 1b), and significant increase in type I IFNs in vivo after fmOMV treatment (Fig. 4c). In addition to LPS, OMVs contain bacteria-derived RNA molecules and activate TLR3, which coincides well with our observation [25, 37]. It is possible that type I IFNs induced by fmOMV promote antiviral molecular milieu in lung epithelial cells, and these “pre-armed” epithelial cells effectively suppressed influenza virus replication regardless of the evading mechanism of influenza virus in this study.
We observed that F4/80+CD11bhiCD11clo-int cells massively infiltrated into the lungs 3 days after fmOMV injection (Fig. 3b) and that these macrophages are indispensable for the antiviral effect of fmOMV (Fig. 3c, left). When AMs are activated, they produce CCL2, resulting in the recruitment of bone marrow-derived macrophages to the lungs [29]. In this study, we showed that fmOMV increased the level of CCL2 in BALF (Fig. 4d, right), suggesting that massive infiltration of macrophages was induced by AM-derived chemokines. Inflammatory macrophages suppress viral replication after influenza virus infection [38]. In particular, stimulation of various TLRs using LPS, imiquimod, peptidoglycan, or β-glucan is known to transform macrophages to a state called “trained immunity” that is hyperresponsive to pathogenic re-infection [8, 39]. Specifically, TLR stimulation induces a decrease in repressive histone H3K9me2 marks via phosphorylation of ATF7. This epigenetic change causes an increase in basal expression of genes required for protection against pathogens by macrophages. Considering that fmOMV can stimulate various TLRs (Fig. 1b), fmOMV injection may transform recruited macrophages to the hyperresponsive state presumably via epigenetic changes.
Injection of fmOMV induced IL-1β secretion in the lung tissue (Fig. 4d, left). In contrast to the case that LPS or flagellin located outside of the cells can only activate corresponding TLRs, the ligands introduced into the intracellular compartment activate NLRP3 and the NLRC4-dependent inflammasome, respectively [40, 41]. Detection of IL-1β in BALF indicates that fmOMV enters the target cells, leading to a sequential activation of the inflammasome and proteolytic cleavage of IL-1β precursor [42]. Consistent with our interpretation, it was recently reported that OMVs mediate cytosolic localization of LPS and activate caspase-11 [43].
Neutrophils have a protective role in influenza virus infection [44, 45]. In this study, however, depletion of neutrophils did not decrease survival rate in fmOMV-injected and influenza-challenged mice, suggesting that neutrophils did not participate in antiviral activities provided by fmOMV in our experimental model (Fig. 3c, right). Antiviral activities of neutrophils are mainly associated with secretion of antiviral molecules such as long-chain pentraxin (PTX3), defensin, and formation of neutrophil extracellular traps after influenza virus infection [46, 47, 48]. These molecules are secreted from neutrophils after activation via interaction directly with influenza virus or virus-induced molecular inflammatory milieu. It is speculated that in fmOMV-injected mice, infiltrated macrophages have sufficiently suppressed disease severity after influenza virus infection. Therefore, viral load and inflammatory cytokines may have been insufficient to activate neutrophils to produce antiviral molecules after influenza virus infection in fmOMV-injected mice. Meanwhile, neutrophil activation is known to intensify disease severity during influenza infection [49]; however, this was not observed in the present study, in terms of change in body weight and survival rates of fmOMV-injected mice (Fig. 2c, 3c, and 5a). This suggests that there was nil or relatively low activation of neutrophils upon influenza virus infection in fmOMV-injected mice, coinciding well with our observation (Fig. 3c, right).
In our study, fmOMV pretreatment successfully protected the mice against diverse IAVs; PR8, pH1N1, H5N2, and highly pathogenic H5N1 (Fig. 2a and 5a). It has been reported that pretreatment with a single TLR ligand provided differential protective efficacy depending on the challenging influenza subtypes or strains [9, 11, 14, 30]. TLR4 activation alone was sufficient to protect H5 subtype, whereas simultaneous activation of TLR2 and 4 was required for the protection against PR8 (H1) subtype [11]. Pam2Cys, a TLR2 ligand, has been reported to elicit 80% survival rate after PR8 virus infection [9, 30]. In addition, costimulation of TLR2 and TLR9 protects the host against H3N2 virus infection completely, but the effect was reduced up to 50% in an H1N1 virus infection model [12]. Since fmOMV used in this study is a complex of various TLR ligands and presumably capable of simultaneously activating TLRs and cytosolic pattern recognition receptors, it is probable that fmOMV leads to the higher protective efficacy against diverse IAVs. Further investigation is necessary to determine the underlying mechanisms for differential protective efficacy of each TLR signal against different virus strains.
In conclusion, we showed that fmOMV injection protected against infection by a wide spectrum of IAV strains and this antiviral effect was mediated by infiltrated macrophages. Our findings show potential for development of an efficient and broad-spectrum antiviral agent against influenza as well as emerging infectious diseases.
Statement of Ethics
All animal experiments were approved by the Institutional Animal Use and Care Committee of KRIBB and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Disclosure Statement
The authors declare that they have no competing interests.
Funding Sources
This work was supported by a grant of the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT (2016M3A9B6918675) and the KRIBB Initiative programs (KGM4691713 and KGM9411711). This research was also supported by Basic Research Program of the NRF funded by the Ministry of Science & ICT (2017R1C1B1005137). Work in S.-H. Kim's laboratory was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Research Center Support Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (716002-7).
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004 Sep;292((11)):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Martcheva M. An evolutionary model of influenza A with drift and shift. J Biol Dyn. 2012;6((2)):299–332. doi: 10.1080/17513758.2011.573866. [DOI] [PubMed] [Google Scholar]
- 3.Russell CA, Fonville JM, Brown AE, Burke DF, Smith DL, James SL, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012 Jun;336((6088)):1541–7. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014 Mar;426((6)):1246–64. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis TN, Leiman SA, Kuehn MJ. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun. 2010 Sep;78((9)):3822–31. doi: 10.1128/IAI.00433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pore D, Mahata N, Chakrabarti MK. Outer membrane protein A (OmpA) of Shigella flexneri 2a links innate and adaptive immunity in a TLR2-dependent manner and involvement of IL-12 and nitric oxide. J Biol Chem. 2012 Apr;287((15)):12589–601. doi: 10.1074/jbc.M111.335554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012 Oct;109((43)):17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida K, Maekawa T, Zhu Y, Renard-Guillet C, Chatton B, Inoue K, et al. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol. 2015 Oct;16((10)):1034–43. doi: 10.1038/ni.3257. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Careem MF, Firoz Mian M, Gillgrass AE, Chenoweth MJ, Barra NG, Chan T, et al. FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antiviral Res. 2011 Nov;92((2)):346–55. doi: 10.1016/j.antiviral.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May;11((5)):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Shinya K, Okamura T, Sueta S, Kasai N, Tanaka M, Ginting TE, et al. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol J. 2011 Mar;8((1)):97. doi: 10.1186/1743-422X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuvim MJ, Gilbert BE, Dickey BF, Evans SE. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS One. 2012;7((1)):e30596. doi: 10.1371/journal.pone.0030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong JP, Christopher ME, Salazar AM, Dale RM, Sun LQ, Wang M. Nucleic acid-based antiviral drugs against seasonal and avian influenza viruses. Vaccine. 2007 Apr;25((16)):3175–8. doi: 10.1016/j.vaccine.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Wong JP, Christopher ME, Viswanathan S, Dai X, Salazar AM, Sun LQ, et al. Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr Pharm Des. 2009;15((11)):1269–74. doi: 10.2174/138161209787846775. [DOI] [PubMed] [Google Scholar]
- 15.Shinya K, Ito M, Makino A, Tanaka M, Miyake K, Eisfeld AJ, et al. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012 Jan;86((1)):19–24. doi: 10.1128/JVI.06168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005 Nov;19((22)):2645–55. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 17.Roy N, Barman S, Ghosh A, Pal A, Chakraborty K, Das SS, et al. Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model. FEMS Immunol Med Microbiol. 2010 Oct;60((1)):18–27. doi: 10.1111/j.1574-695X.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Shang ES, Champion CI, Wu XY, Skare JT, Blanco DR, Miller JN, et al. Comparison of protection in rabbits against host-adapted and cultivated Borrelia burgdorferi following infection-derived immunity or immunization with outer membrane vesicles or outer surface protein A. Infect Immun. 2000 Jul;68((7)):4189–99. doi: 10.1128/iai.68.7.4189-4199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009 Jun;27(Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 20.Nevot M, Deroncelé V, Messner P, Guinea J, Mercadé E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ Microbiol. 2006 Sep;8((9)):1523–33. doi: 10.1111/j.1462-2920.2006.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TY, Kim CU, Bae EH, Seo SH, Jeong DG, Yoon SW, et al. Outer membrane vesicles harboring modified lipid A moiety augment the efficacy of an influenza vaccine exhibiting reduced endotoxicity in a mouse model. Vaccine. 2017 Jan;35((4)):586–95. doi: 10.1016/j.vaccine.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim SM, Song EJ, Song D, Lee TY, Kim DJ, Nam JH, et al. Nontoxic outer membrane vesicles efficiently increase the efficacy of an influenza vaccine in mice and ferrets. Vaccine. 2017 Jun;35((30)):3741–8. doi: 10.1016/j.vaccine.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Kim SH, Kang W, Choi YS, Lee SH, Lee SR, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011 Oct;29((46)):8293–301. doi: 10.1016/j.vaccine.2011.08.102. [DOI] [PubMed] [Google Scholar]
- 24.Kim EH, Lee JH, Pascua PN, Song MS, Baek YH, Kwon HI, et al. Prokaryote-expressed M2e protein improves H9N2 influenza vaccine efficacy and protection against lethal influenza A virus in mice. Virol J. 2013 Apr;10((1)):104. doi: 10.1186/1743-422X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen F, Boog CJ, van Putten JP, van der Ley P. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect Immun. 2007 Dec;75((12)):5939–46. doi: 10.1128/IAI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fransen F, Stenger RM, Poelen MC, van Dijken HH, Kuipers B, Boog CJ, et al. Differential effect of TLR2 and TLR4 on the immune response after immunization with a vaccine against Neisseria meningitidis or Bordetella pertussis. PLoS One. 2010 Dec;5((12)):e15692. doi: 10.1371/journal.pone.0015692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathi S, White MR, Hartshorn KL. The amazing innate immune response to influenza A virus infection. Innate Immun. 2015 Jan;21((1)):73–98. doi: 10.1177/1753425913508992. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Kwon HS, Park DE, Woo YD, Kim HY, Kim HR, et al. Thalidomide inhibits alternative activation of macrophages in vivo and in vitro: a potential mechanism of anti-asthmatic effect of thalidomide. PLoS One. 2015 Apr;10((4)):e0123094. doi: 10.1371/journal.pone.0123094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, et al. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011 Sep;184((5)):547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan AC, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, et al. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012 Sep;9((9)):2710–8. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 31.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014 Apr;10((4)):e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumpey TM, García-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005 Dec;79((23)):14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HM, Lee YW, Lee KJ, Kim HS, Cho SW, van Rooijen N, et al. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008 May;82((9)):4265–74. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010 Aug;84((15)):7569–80. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, et al. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013 Sep;99((3)):230–7. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007 Aug;27((2)):240–52. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 May;171((5)):2499–505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Londrigan SL, Short KR, Ma J, Gillespie L, Rockman SP, Brooks AG, et al. Infection of Mouse Macrophages by Seasonal Influenza Viruses Can Be Restricted at the Level of Virus Entry and at a Late Stage in the Virus Life Cycle. J Virol. 2015 Dec;89((24)):12319–29. doi: 10.1128/JVI.01455-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011 May;9((5)):355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011 Sep;477((7366)):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 41.Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015 Oct;45((10)):2911–7. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011 Aug;22((4)):189–95. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, et al. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell. 2016 May;165((5)):1106–19. doi: 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011 Mar;6((3)):e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009 Dec;183((11)):7441–50. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 46.Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J Immunol. 2006 Jun;176((11)):6962–72. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- 47.Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, et al. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008 Mar;180((5)):3391–8. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 48.Tripathi S, Verma A, Kim EJ, White MR, Hartshorn KL. LL-37 modulates human neutrophil responses to influenza A virus. J Leukoc Biol. 2014 Nov;96((5)):931–8. doi: 10.1189/jlb.4A1113-604RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, et al. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol. 2000 Mar;74((5)):2472–6. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]