Sicca syndrome has been reported as a common complication of immune checkpoint therapy. This article describes the clinicopathologic features of sicca syndrome, with the goal of raising awareness to a encourage early recognition and appropriate management.
Keywords: Sicca syndrome, Immune checkpoint inhibitor, Immune‐related adverse event, Sialadenitis, Sjögren's syndrome, Programmed cell death 1/programmed cell death ligand 1 pathway, Xerostomia
Abstract
Background.
The objective of this study was to characterize the clinicopathologic features of sicca syndrome associated with immune checkpoint inhibitor (ICI) therapy.
Subjects, Materials, and Methods.
Consecutive patients with new or worsening xerostomia in the setting of ICI treatment for benign or malignant neoplastic disease were evaluated, including labial salivary gland biopsy (LSGB).
Results.
Twenty patients (14 male; median age 57 years) had metastatic melanoma (n = 10), metastatic carcinoma (n = 6), or recurrent respiratory papillomatosis (n = 4) and were being treated with avelumab (n = 8), nivolumab (n = 5), pembrolizumab (n = 4), nivolumab/ipilimumab (n = 2), and M7824, a biologic targeting programmed cell death ligand 1 (PD‐L1) and transforming growth factor ß (n = 1). Four had pre‐existing autoimmune disease. Nineteen had very low whole unstimulated saliva flow; six had new dry eye symptoms. The median interval between ICI initiation and dry mouth onset was 70 days. Rheumatoid factor and anti‐Sjögren's Syndrome‐related Antigen A (Anti‐SSA) were both positive in two subjects. LSGB showed mild‐to‐severe sialadenitis with diffuse lymphocytic infiltration and architectural distortion. There were lymphocytic aggregates in eight patients, composed mainly of CD3+ T cells with a slight predominance of CD4+ over CD8+ T cells. ICI targets (e.g., programmed cell death 1 and PD‐L1) were variably positive. In direct response to the advent of the sicca immune‐related adverse event, the ICI was held in 12 patients and corticosteroids were initiated in 10. Subjective improvement in symptoms was achieved in the majority; however, salivary secretion remained very low.
Conclusion.
ICI therapy is associated with an autoimmune‐induced sicca syndrome distinct from Sjögren's syndrome, often abrupt in onset, usually developing within the first 3 months of treatment, and associated with sialadenitis and glandular injury. Improvement can be achieved with a graded approach depending on severity, including withholding the ICI and initiating corticosteroids. However, profound salivary flow deficits may be long term.
Implications for Practice.
Sicca syndrome has been reported as an immune‐related adverse event (irAE) of immune checkpoint inhibitor therapy (ICI) for neoplastic diseases. Severe dry mouth (interfering with eating or sleeping) developed abruptly, typically within 90 days, after initiation of ICI therapy. Salivary gland biopsies demonstrated mild‐to‐severe sialadenitis distinct from Sjögren's syndrome, with diffuse T‐cell lymphocytic infiltration and acinar injury. Recognition of the cardinal features of ICI‐induced sicca will spur appropriate clinical evaluation and management, including withholding of the ICI and corticosteroid, initiation. This characterization should help oncologists, rheumatologists, and oral medicine specialists better identify patients that develop ICI‐induced sicca to initiate appropriate clinical evaluation and therapy to reduce the likelihood of permanent salivary gland dysfunction.
摘要
背景。本研究旨在探讨与免疫检查点抑制剂 (ICI) 治疗相关的干燥综合征的临床病理特点。
受试者、材料和方法。评估采用 ICI 治疗新的或恶化的良性或恶性肿瘤连续发病口腔干燥症患者,包括唇腺病理活检 (LSGB)。
结果。20 例(男性患者 14 例;中位年龄 57 岁)出现转移性黑色素瘤 (n = 10)、转移癌 (n = 6) 或复发性呼吸道乳头瘤样增生 (n = 4),之后接受 Avelumab (n = 8)、纳武单抗 (n = 5)、帕博利珠单抗 (n = 4)、纳武单抗 /易普利姆玛 (n = 2) 或 M7824 生物靶向细胞程序性死亡配体 1 (PD‐L1) 和转化生长因子 ß (n = 1) 治疗。其中 4 例患者既往存在自身免疫性疾病。19 名患者整体非刺激性唾液量非常低,6 名患者出现了新的干眼症状。开始 ICI 治疗至口干舌燥的间隔时间中位数为 70 天。类风湿因子和抗Sjögren综合征相关抗原 A(抗 SSA)在两名受试者中均为阳性。LSGB 结果显示轻‐重度涎腺炎,伴有弥漫性淋巴细胞浸润和结构变形。8 例患者淋巴细胞聚集,以 CD3+ T 细胞为主,CD4+ 略高于 CD8+ T 细胞。ICI 靶细胞(如程序性细胞死亡 1 和 PD‐L1)呈变异阳性。针对干燥免疫相关不良事件的发生,12 例患者暂停接受 ICI 治疗,10 例患者开始使用皮质类固醇。大多数患者的主观症状得到了改善,但唾液分泌量仍然很低。
结论。ICI 治疗会引发一种不同于Sjögren综合征的自身免疫性干燥综合征,通常发病突然,常发病于治疗的前 3 个月内,而且会并发涎腺炎和腺体损伤。可通过根据严重程度进行分级的方法改善症状,包括停用 ICI 和开始服用皮质类固醇。但患者长期时间内都会存在严重的唾液量不足。
实践意义:研究已证实在肿瘤疾病的治疗中,使用免疫检查点抑制剂 (ICI)药物会引发干燥综合征这种免疫相关不良事件 (irAE)。通常在开始 ICI 治疗后 90 天内,会突发严重口干(影响饮食或睡眠)。涎腺病理活检结果表明出现了不同于Sjögren综合征的轻‐重度涎腺炎,伴有弥漫性 T淋巴细胞浸润和腺泡损伤。认识 ICI 引起的干燥综合征的主要特征有助于促进相应的临床评估和管理,包括停用 ICI,开始服用皮质类固醇药物。这一特征有助于肿瘤学家、风湿病学家和口腔医学专家更好地确定因 ICI 引起干燥综合征的患者,从而进行适当的临床评估和治疗,降低永久性唾液腺功能障碍的可能性。
Introduction
The advent of biological agents that block immunologic checkpoints has significantly advanced cancer therapy [1]. These immune checkpoint inhibitors (ICI) have remarkable efficacy in the treatment of a variety of tumors, from bladder to head and neck cancer. ICIs augment the antitumor immune response by blocking negative costimulation of T cells [1]. Their targets include cytotoxic T‐lymphocyte antigen 4 (CTLA4; targeted by ipilimumab), programmed cell death 1 (PD‐1; targeted by nivolumab, pembrolizumab, and cemiplimab), and programmed cell death ligand 1 (PD‐L1; targeted by avelumab, atezolizumab, and durvalumab).
ICI therapy is associated with the development of inflammatory lesions of specific organ systems in up to 60% of patients, with a frequency dependent in part on the specificity and mode of action of the agent [2], [3], [4], [5]. These lesions most commonly affect endocrine glands, skin, gastrointestinal tract, and liver and are thought to arise from activation of autoreactive T cells or other autoimmune pathways [6]. Accordingly, they have been categorized as “immune‐related adverse events” (irAEs) [7]. They may occur de novo or as exacerbations of previously diagnosed organ‐specific or systemic autoimmune diseases.
Sicca syndrome with severe salivary hypofunction was first reported as a complication of ICI therapy in four patients treated with ipilimumab and/or nivolumab [8]. Since that publication, there have been other reports of sicca syndrome, inflammatory arthritis, polymyalgia rheumatica, and myositis induced or exacerbated by ICI [9], [10], [11]. Dry eye and dry mouth have been reported respectively in three and four clinical trials, with an incidence ranging from 3% to 24% [12]. In these reports, there is insufficient characterization of the sicca syndrome and associated glandular histopathology. Because sicca symptoms are common in the population, with a prevalence reaching up to 30% in persons >65 years of age, their occurrence in a clinical trial may not be attributed to study drug, leading to under‐reporting [13].
Herein, we provide a comprehensive description of the clinical phenotype of 20 patients, the largest cohort to date, who developed severe dry mouth after initiation of ICI therapy. Our report provides evidence for a novel immune‐mediated sialadenitis that is clinically and histologically distinct from Sjögren's syndrome (SS).
Subjects, Materials, and Methods
Study Approval
All studies were carried out in accordance with approved NIH guidelines conforming to the standards of the Declaration of Helsinki. All participants provided informed consent prior to the initiation of any study procedures. Human samples were obtained from NIH Institutional Review Board‐approved protocols (ClinicalTrials.gov Identifier: NCT00001196) in the Sjögren's Syndrome Clinic at the National Institute of Dental and Craniofacial Research at the NIH in Bethesda, MD.
Patient Cohort
We evaluated 20 consecutive patients referred to us by intramural and extramural oncologists with new‐onset or worsening dry mouth symptoms in the setting of ICI treatment. One of these patients has been previously reported [8]. The evaluation included a comprehensive oral examination by an oral medicine specialist and a medical history and physical examination by a rheumatologist. Sialometry, labial salivary gland biopsy (LSGB), salivary gland ultrasonography (SGUS), and assessment of lacrimal function (Schirmer's test) were conducted. Clinical laboratory studies at NIH included assays for antinuclear antibodies (ANA), antibodies to extractable nuclear antigens (anti‐SSA, ‐SSB, ‐Sm, ‐RNP, ‐Scl‐70, ‐Jo‐1), anti‐dsDNA, and rheumatoid factor. In one patient (patient 1), serologic testing was performed at the Johns Hopkins Hospital and was limited to ANA, anti‐SSA, anti‐SSB, and rheumatoid factor. Ultrasonography was performed by the study investigators, using a GE Logiq e portable ultrasound machine (GE Healthcare, Wauwatosa, WI) with a high‐resolution linear scanner (4–13 MHz). Follow‐up evaluations were performed on six patients.
Outcome Measures
Dry mouth severity was rated according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5). The scoring included grade 1: symptomatic (e.g., dry or thick saliva) without significant dietary alteration; unstimulated saliva flow >0.2 mL/minute; grade 2: moderate symptoms; oral intake alterations (e.g., copious water, other lubricants, diet limited to purees and/or soft, moist foods); unstimulated saliva 0.1–0.2 mL/minute; and grade 3: inability to adequately aliment orally; tube feeding or total parenteral nutrition indicated; unstimulated saliva <0.1 mL/minute (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf, last accessed July 22, 2018).
Patient‐reported response to management of salivary hypofunction was defined as (a) significant: near‐complete resolution of dry mouth symptoms; (b) moderate: improvement to the point that symptoms were tolerable but still present; and (c) minimal: symptoms remained severe despite treatment [14].
Ocular dryness was assessed with the Schirmer test. Wetting of 5 mm or less in either eye over a 5‐minute period was considered indicative of dry eye disease, commensurate with the definition used in the classification criteria for Sjögren's syndrome [15].
Response of solid tumors to ICI therapy was rated using the RECIST 1.1 [16]. The therapeutic response to avelumab in the patients with recurrent respiratory papillomatosis is still under study.
Histopathologic Analyses
Slides were scanned at ×40 with a NanoZoomer S360 slide scanner (Hammamatsu Photonics, Hammamatsu‐city, Japan), and digital photomicrographs at ×5 resolution were captured using NDP.view2 software (Hammamatsu Photonics). LSGB were interpreted by the same board‐certified surgical pathologist (D.K.), blinded to study treatment. Salivary gland inflammation and fibrosis were graded according to Greenspan et al. [17] and Tarpley et al. [18]. For LSGB with Greenspan grade 3 or 4 sialadenitis, a focus score was calculated according to Daniels et al. [19]. A limited panel of histochemical and immunohistochemical studies (e.g., hematoxylin and eosin [H&E], Masson, CD20, CD3, CD4, CD8, PD‐1, transparenteral nutrition PD‐L1) was conducted by the Anatomic Pathology Laboratory of the National Cancer Institute.
Results
Patient Characteristics
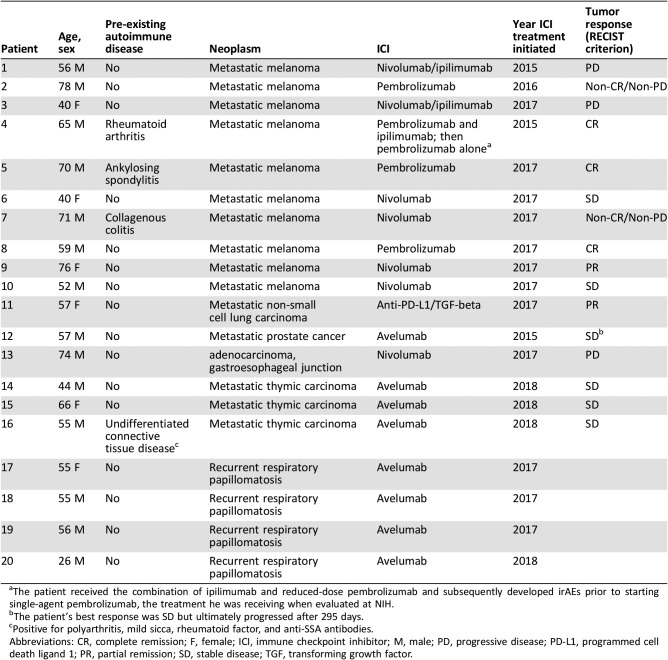
The patient cohort consisted of 14 men and 6 women, with a median age of 57 years (range 26–78; Table 1). ICI were used in this cohort to treat advanced malignancies (n = 16; Table 1) and recurrent respiratory papillomatosis (RRP; n = 4). Four patients had evidence of autoimmune disease prior to ICI therapy (Table 1). When seen at NIH, the patients were undergoing treatment with avelumab (n = 8), nivolumab (n = 5), pembrolizumab (n = 4), the combinations of nivolumab and ipilimumab (n = 2), and M7824, a bifunctional fusion protein targeting PD‐L1 and transforming growth factor ß (n = 1). One patient (patient 4) received combination pembrolizumab/ipilimumab initially but was only receiving pembrolizumab at the time of his sicca evaluation. The ICI was given in combination with enzalutamide (patient 12), a proprietary combinatorial PD‐1‐based therapy (patient 10), and epacadostat or placebo (patient 5). Two patients had a prior course of ICI treatment; one with ipilimumab (patient 3), and one with pembrolizumab (patient 9), in each stopped at least 1 year before the current one.
Table 1. Clinical features of patients, underlying neoplasm, ICI treatment, and tumor response.
The patient received the combination of ipilimumab and reduced‐dose pembrolizumab and subsequently developed irAEs prior to starting single‐agent pembrolizumab, the treatment he was receiving when evaluated at NIH.
The patient's best response was SD but ultimately progressed after 295 days.
Positive for polyarthritis, mild sicca, rheumatoid factor, and anti‐SSA antibodies.
Abbreviations: CR, complete remission; F, female; ICI, immune checkpoint inhibitor; M, male; PD, progressive disease; PD‐L1, programmed cell death ligand 1; PR, partial remission; SD, stable disease; TGF, transforming growth factor.
The RECIST tumor responses to ICI therapy in the 16 patients with metastatic disease included complete remission in 3, partial remission in 2, stable disease in 6, progressive disease in 3, and noncomplete remission/nonprogressive disease in 2.
Sicca Syndrome
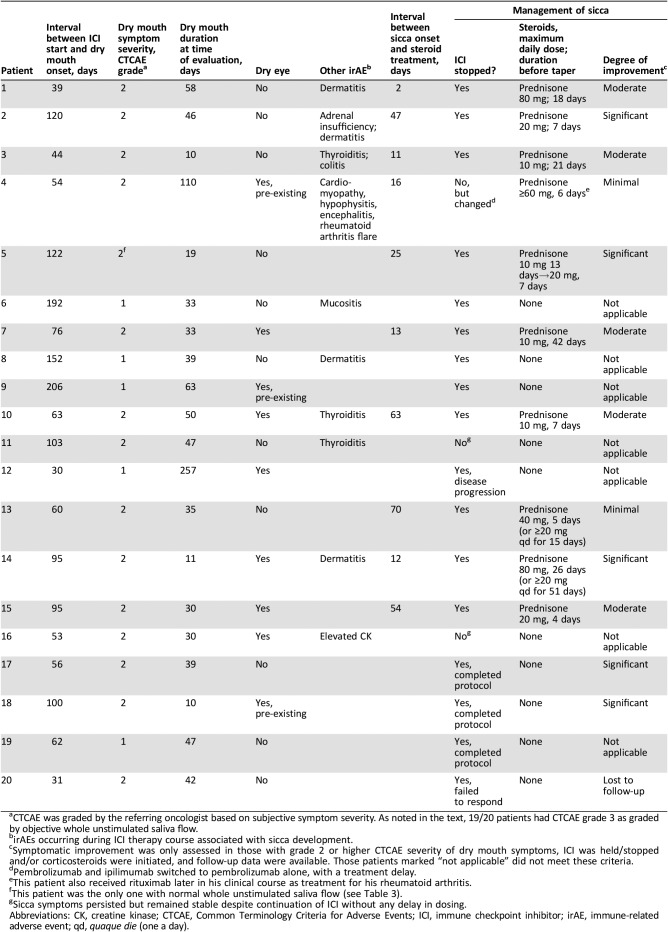
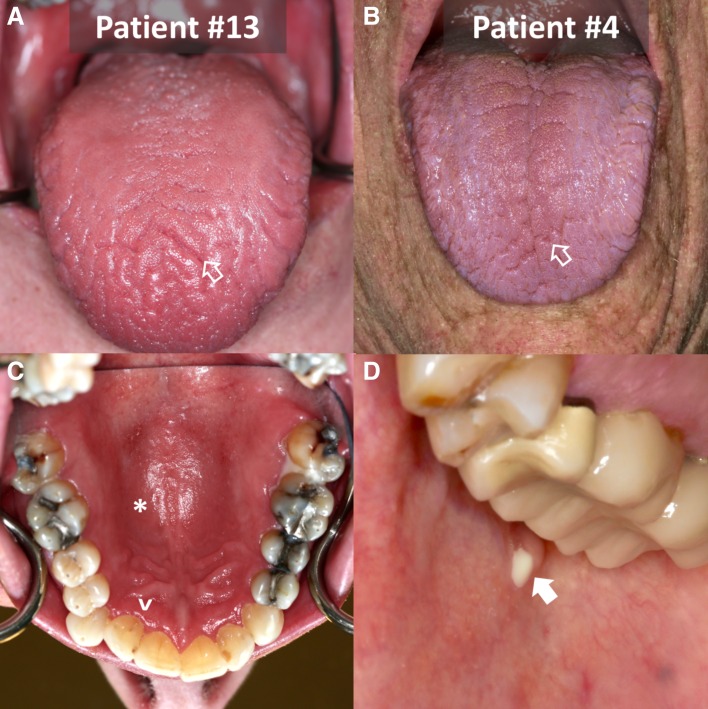
Dry mouth was a new symptom in 18 and an exacerbation of an existing one in 2 patients. The dry mouth was typically abrupt in onset and, in most, required that the patient drink water to chew and swallow dry foods. Physician‐reported CTCAE severity for “dry mouth” was grade 2 in 15 patients and grade 1 in 5 patients, as rated by symptom criteria alone (Table 2). The symptoms were often worse with physical exertion or at night, with patients awakening at night with their tongue stuck to their teeth or hard palate and needing to drink water. Other symptoms included thick or sticky saliva, dryness of the throat with hoarseness, altered taste, and sensitivity to spicy and acidic foods. Changes in oral mucosa indicative of salivary gland hypofunction were evident in the majority (Fig. 1); this included findings in several patients that were consistent with chronic erythematous candidiasis. Several patients reported oral mucosal ulcerative lesions and/or mucosal burning. None reported acute salivary gland enlargement; one patient reported tender parotid glands at onset of dryness. None of the patients had findings of oral herpes.
Table 2. Sicca syndrome features and management response.
CTCAE was graded by the referring oncologist based on subjective symptom severity. As noted in the text, 19/20 patients had CTCAE grade 3 as graded by objective whole unstimulated saliva flow.
irAEs occurring during ICI therapy course associated with sicca development.
Symptomatic improvement was only assessed in those with grade 2 or higher CTCAE severity of dry mouth symptoms, ICI was held/stopped and/or corticosteroids were initiated, and follow‐up data were available. Those patients marked “not applicable” did not meet these criteria.
Pembrolizumab and ipilimumab switched to pembrolizumab alone, with a treatment delay.
This patient also received rituximab later in his clinical course as treatment for his rheumatoid arthritis.
This patient was the only one with normal whole unstimulated saliva flow (see Table 3).
Sicca symptoms persisted but remained stable despite continuation of ICI without any delay in dosing.
Abbreviations: CK, creatine kinase; CTCAE, Common Terminology Criteria for Adverse Events; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; qd, quaque die (one a day).
Figure 1.
Examples of patients’ oral cavities with dry mouth and salivary hypofunction induced by immune checkpoint inhibitors. Patient 13 (A, C) exhibits significant papillary atrophy with erythema and fissuring (open arrow). Evidence of dryness (reflectance) and inflammation (erythema) of the mucosa of the palate (*) and gingiva adjacent to the teeth (^) can be appreciated. The general erythematous appearance of the palatal and lingual mucosa is strongly indicative of erythematous candidiasis; he was treated with antifungal therapy. Patient 4 (B, D), with a history of pre‐existing rheumatoid arthritis, also exhibits papillary atrophy and fissuring (open arrow) of the dorsum of the tongue. During therapy, the patient developed strictures of the Stensen's duct and subsequent recurrent infectious parotitis (closed arrow) with Staphylococcus aureus and Candidiasis spp.; he was treated with antibiotics, irrigation of the parotid ducts, and eventual sialoendoscopic dilation of his ductal strictures. Rituximab was also used for management of his rheumatoid arthritis and possibly the sialadenitis.
Acute dry eye symptoms and signs developed in six patients concurrently with dry mouth (Table 2). In one (patient 14), the dry eye symptoms were severe, leading to photophobia; in the others, the symptoms were milder. Three additional patients had dry eye prior to ICI treatment and noted no worsening during its course.
The median interval between onset of ICI treatment and that of dry mouth was 70 days (range 30–206). The patients were evaluated a median of 39 days (range 10–257) following the onset of their dry mouth symptoms. Concomitant medications with the potential to cause dry mouth included antihistamines (n = 4), sertraline (n = 1), and diuretics (n = 3). No patient was taking more than one medication with this potential.
Other irAEs occurred in 10 patients in association with the same course of ICI treatment (Table 2); these occurred contemporaneously with the sicca in 8 of them.
Saliva and Tear Flow
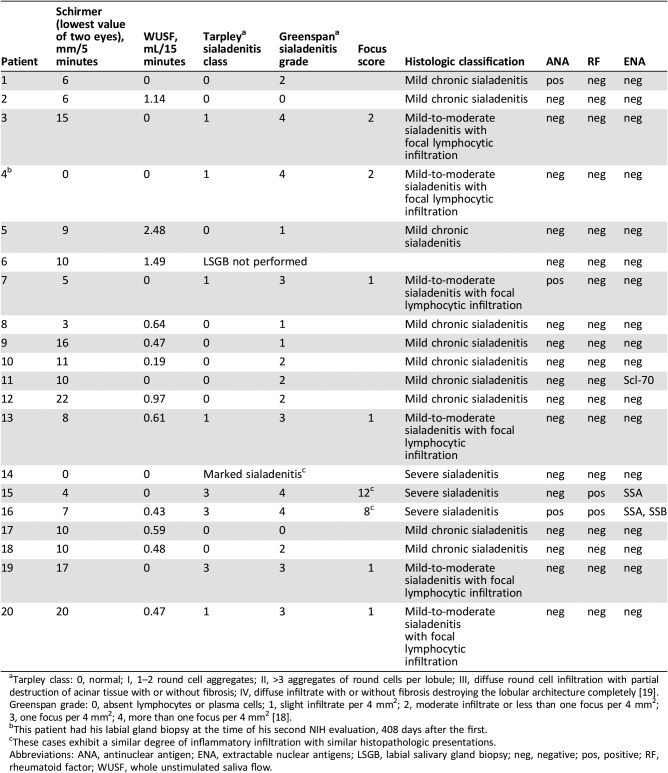
Nineteen subjects had salivary hypofunction with whole unstimulated saliva flow (WUSF) less than 1.5 mL/15 minutes; eight of these patients exhibited no WUSF (Table 3) [20], [21]. The median WUSF was 0.45 mL/15 minutes (range 0–2.49). Thus, CTCAE severity for “dry mouth” was grade 3 in 19 patients and grade 2 in 1 patient, as judged by WUSF. Only six patients had any measurable citric acid‐stimulated saliva flow in either parotid gland, the amount being <0.3 mL/minute/gland in 10/12 glands [22]. Unstimulated submandibular/sublingual salivary flow was measurable in only 3 patients and stimulated flow with citric acid in 14.
Table 3. Clinical, laboratory, and pathologic assessments.
Tarpley class: 0, normal; I, 1–2 round cell aggregates; II, >3 aggregates of round cells per lobule; III, diffuse round cell infiltration with partial destruction of acinar tissue with or without fibrosis; IV, diffuse infiltrate with or without fibrosis destroying the lobular architecture completely [19]. Greenspan grade: 0, absent lymphocytes or plasma cells; 1, slight infiltrate per 4 mm2; 2, moderate infiltrate or less than one focus per 4 mm2; 3, one focus per 4 mm2; 4, more than one focus per 4 mm2 [18].
This patient had his labial gland biopsy at the time of his second NIH evaluation, 408 days after the first.
These cases exhibit a similar degree of inflammatory infiltration with similar histopathologic presentations.
Abbreviations: ANA, antinuclear antigen; ENA, extractable nuclear antigens; LSGB, labial salivary gland biopsy; neg, negative; pos, positive; RF, rheumatoid factor; WUSF, whole unstimulated saliva flow.
Five patients had aqueous tear deficiency, as determined by a Schirmer's test result of ≤5 mm wetting/5 minutes in at least one eye (Table 3). Only four of the nine patients with dry eye symptoms had an abnormal Schirmer test at the time of our initial evaluation, suggesting that some may have had evaporative rather than aqueous deficient dry eye [23].
Laboratory and Imaging Results
Three patients had a positive test for ANA, two with prior autoimmune disease (Table 1). Rheumatoid factor and anti‐SSA antibodies were positive in two; in one, these antibodies were also present 3 years before ICI therapy. One subject had anti‐Scl‐70 antibodies without clinical stigmata of scleroderma.
SGUS revealed consistent, generally mild changes in the major glands, including parenchymal heterogeneity with hyperechogenic bands and scattered ovoid hypoechoic lesions, and these persisted on follow‐up (supplemental online Fig. 1).
Glandular Histopathology
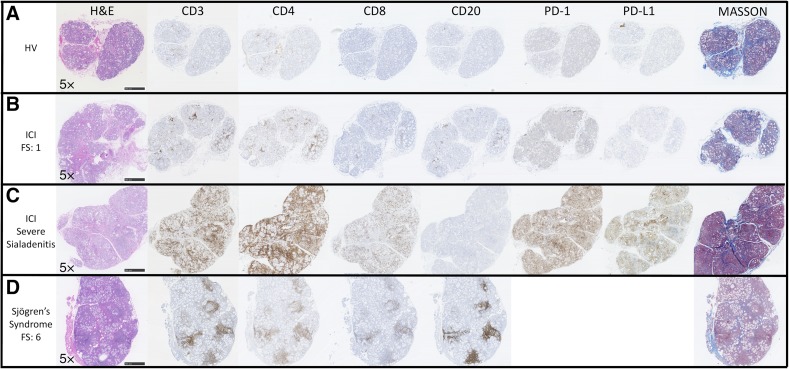
Nineteen patients underwent LSGB. Three general histopathologic patterns were present: mild nonspecific chronic sialadenitis with acinar atrophy and fibrosis (focus score [FS] = 0; n = 10); mild‐to‐moderate sialadenitis with focal lymphocytic sialadenitis (FLS) with atrophy and fibrosis (FS = 1, n = 4; FS = 2, n = 2); and severe sialadenitis with injury to the ducts and acini, nuclear enlargement, anisonucleosis, and irregular distribution, apoptosis, fibrosis, acinar atrophy, luminal mucin inspissation, and rupture mucin extravasation (“marked sialadenitis,” n = 1; FS = 8, n = 1; FS = 12, n = 1; Table 3; Fig. 2). Of the nine patients with FLS, three had known pre‐existing autoimmune diseases (patients 4, 7, and 16; Table 1). On H&E‐stained sections, LSGB with mild‐to‐moderate FLS exhibited a pattern of lymphocytic infiltration indistinguishable from SS. However, for the cases exhibiting marked sialadenitis, the pattern of infiltration (and epithelial injury) was noteworthy. Immunohistochemistry demonstrated that the infiltrate in ICI patients’ LSGB consisted of increased numbers of CD3+ T cells, a slight predominance of CD4+ compared with CD8+ T cells, and a paucity of CD20+ B cells in contrast with the immune cell infiltrates of SS (Fig. 2). PD‐1 was variably positive in the lymphocytes in ICI patients’ LSGB, whereas PD‐L1 was positive in the epithelium in areas of dense inflammation, and in some inflammatory cells in only the most severe sialadenitis cases (Fig. 2).
Figure 2.
ICI‐induced sicca exhibits histopathological changes distinct from Sjögren's syndrome. Histological and immunohistochemical comparison of representative labial salivary gland biopsy (LSGB) from a healthy volunteer, FS = 0 (A), a patient (patient 7) with ICI‐induced sicca with focal lymphocytic sialadenitis, FS = 1 (B), a patient (patient 14) with ICI‐induced sicca with severe sialadenitis (C), and a non‐ICI‐treated sicca patient who meets diagnostic criteria for Sjögren's syndrome, FS = 6 (D). The ICI‐induced sicca patients’ (B, C) biopsies demonstrate variably dense sialadenitis composed mainly of CD3+ T cells with a slight predominance of CD4+ over CD8+ T cells and a virtual absence of B cells. In contrast, the lymphocytic infiltrate in Sjögren's syndrome is composed mainly of CD20+ B cells, with variable germinal center formation, admixed with variable number of CD3+ T cells and a slight predominance of CD4+ over CD8+ T cells. In patients treated with ICI, PD‐1 was variably positive in a subset of the lymphocytes, mainly in areas of aggregation. PD‐1 was rarely positive in glands from healthy volunteers (A). Interestingly, the patients with the most profound sialadenitis (patients 14–16) are the only patients whose LSGB exhibited PD‐L1 positivity (C), typically in the epithelium in areas of dense inflammation, and rarely in some inflammatory cells. Increased inter‐ and intralobular fibrosis as highlighted by Masson trichrome staining (blue) relative to the healthy volunteer is evident. Acinar atrophy and disorganization is evident in both ICI (B, C) and Sjögren's syndrome (D) patients. However, epithelial injury with sloughing into the lumens, the presence of necrotic and apoptotic epithelial cells, and prominent exocytosis of lymphocytes into the epithelium is distinctly present in the severe sialadenitis patients (D).
Abbreviations: FS, focus score; H&E, hematoxylin and eosin; HV, healthy volunteer; ICI, immune checkpoint inhibitor; PD‐1, programmed cell death 1; PD‐L1, programmed cell death ligand 1.
There was no correlation between ICI treatment response and degree of salivary gland inflammation in LSGB (data not shown). In addition, the three observed histopathologic patterns did not correlate with ICI agent.
Management
In addition to supportive measures (including the use of cevimeline or pilocarpine in some), the salivary gland dysfunction was managed by stopping the ICI therapy in direct response to the advent of this irAE in 12 patients. ICI therapy was also stopped because of disease progression (patient 12) 8.5 months after the onset of dry mouth. In patient 4, therapy with pembrolizumab and ipilimumab was switched to pembrolizumab alone, with a treatment delay, after the onset of sicca and other irAEs; the pembrolizumab was later stopped because of meningoencephalitis and a flare of underlying rheumatoid arthritis. In the patients with RRP, the ICI was stopped per protocol after completing six infusions (n = 3) or three infusions (n = 1), in each patient at a time point coincidental with clinical recognition of the dry mouth irAE. Ten patients were treated with corticosteroids; the dose was equivalent to prednisone 20 mg daily or higher in seven. We assessed the outcome of grade 2 sicca symptoms in 12 patients in whom ICI were held/stopped and/or corticosteroids administered and follow‐up data were available. Among these 12 patients, there was significant, moderate, and minimal improvement in the dry mouth symptoms in five, five, and two patients, respectively (Table 3). In each category, patients improved with or without corticosteroids with cessation of ICI; however, symptoms did not resolve in any patient. One patient (patient 4) with pre‐existing rheumatoid arthritis developed Stensen's duct strictures (Fig. 1D). The severe dry eye symptoms of patient 14 improved dramatically with steroids; the response of the others was hard to assess because they were either mild or not sufficient to be reported as an irAE by the health professionals evaluating the patient at follow‐up visits. Response to treatment was assessed with serial salivary flow measurements in six patients (patients 4, 11, 14, 15, 18, and 19); all exhibited objective improvements in WUSF (median increment: 0.89 mL/15 minutes, range 0.19–4.39) with a median follow‐up of 3.8 months (range 1–13.4 months). Only one patient reached a WUSF value above 1.5 mL/15 minutes (patient 14). Three of the six patients were on a corticosteroid for sicca or another irAE at the time of their second evaluation, with a median of 52 days (range 4–502).
ICI therapy was resumed in seven patients (patients 1, 5, 6, 7, 9, 10, and 15). In five patients (patients 5, 6, 7, 10, and 15), this was done within 3 months of their last dose, allowing them to remain in their given clinical trial. This rechallenge was done while four patients (patients 5, 10, 14, and 15) were either maintained or started on prednisone to prevent worsening of their sicca. The two other patients (patients 1 and 9) were started on a different ICI regimen after the original ICI had been held because of sicca, after an interval of 120–872 days. Sicca symptoms remained stable with rechallenge in all but one patient (patient 1) who was started on a different ICI 2.4 years after the original ICI course and had renewed dry mouth symptoms.
Discussion
We report 20 patients who developed sicca syndrome early in the course of ICI therapy, manifested by new or abruptly worse dry mouth symptoms in all and dry eye symptoms in 6. Dry mouth was the predominant symptom, but this may have reflected the referral bias of our oral medicine clinic. Dry mouth and dry eye symptoms are common in the population at large, particularly in older individuals, and is a common symptom during cancer therapy. However, the dry mouth symptoms reported in this cohort were notable for their abrupt onset and severity. Overall, the observed deficits in salivary function were profound and not consistent with those observed with anticholinergic medication effects.
Damage to the major salivary glands was assessed using SGUS (supplemental online Fig. 1) and LSGB (Fig. 2). The majority of our cases exhibited mild parenchymal changes by SGUS, and these typically persisted or worsened with follow‐up imaging. A minority of LSGB exhibited FLS (Table 3; Fig. 2), the histopathology associated with SS [17]. The majority had mild chronic sialadenitis with findings indicative of chronic salivary gland injury. In addition to mild sialadenitis, atrophy of the terminal acini, periductal and perilobular fibrosis, and mucous inspissation and extravasation were found. Three cases exhibited severe sialadenitis with epithelial injury and effacement of the gland architecture. By immunohistochemistry, there was a predominant T‐lymphocytic infiltrate, with a slight majority of CD4+ over CD8+ T cells and few CD20+ B cells. This pattern is different from characteristic SS infiltrates where B cells represent 20%–62% of all lymphocytes and FS directly correlating with B‐cell ratios [24]. The targets (e.g., PD‐1, PD‐L1) of ICI therapies were variably positive in the patients with ICI with scattered infiltrating PD‐1‐positive T cells and epithelial PD‐L1 positivity exhibited only in the most severely infiltrated cases (Fig. 2). These patients were treated with ICI for thymic epithelial neoplasms; the significance of this finding is intriguing but may reflect underlying thymic dysfunction‐related autoimmunity, which was exacerbated by an ICI‐augmented immune response [25].
This irAE occurred in association with ICI agents of different specificities, including those targeting CTLA‐4, PD‐1, and PD‐L1, and in patients with a variety of tumor types. Our series was too small and subject to referral biases to allow us to state whether this irAE was more prevalent with one ICI agent versus another. This may have reflected the referral pattern of patients to our study, as opposed to differences in propensity to cause salivary gland dysfunction.
The sicca syndrome was managed in accordance with practices employed for other irAEs [26]. In the majority, the ICI was discontinued, either temporarily or permanently, and corticosteroids were instituted as is recommended generally for grade 3 and higher irAEs by oncology guidelines [27], [28]. We observed improvement in the sicca syndrome when the type of therapeutic intervention (e.g., symptomatic measures, cessation of ICI therapy, institution of corticosteroids) was guided by the severity of the sicca.
We recommend determining whether any patient being considered for ICI treatment has a history of a systemic autoimmune disease, including Sjögren's syndrome, rheumatoid arthritis, systemic lupus, and myositis. The presence of a pre‐existing autoimmune disease does not preclude ICI treatment, but the patient should be made fully aware of the risk of a flare and should be observed closely for such an occurrence, best in concert with the patient's rheumatologist [29]. We do not recommend screening patients for the presence of rheumatic disease autoantibodies in the absence of a history of a prior autoimmune systemic rheumatic disease.
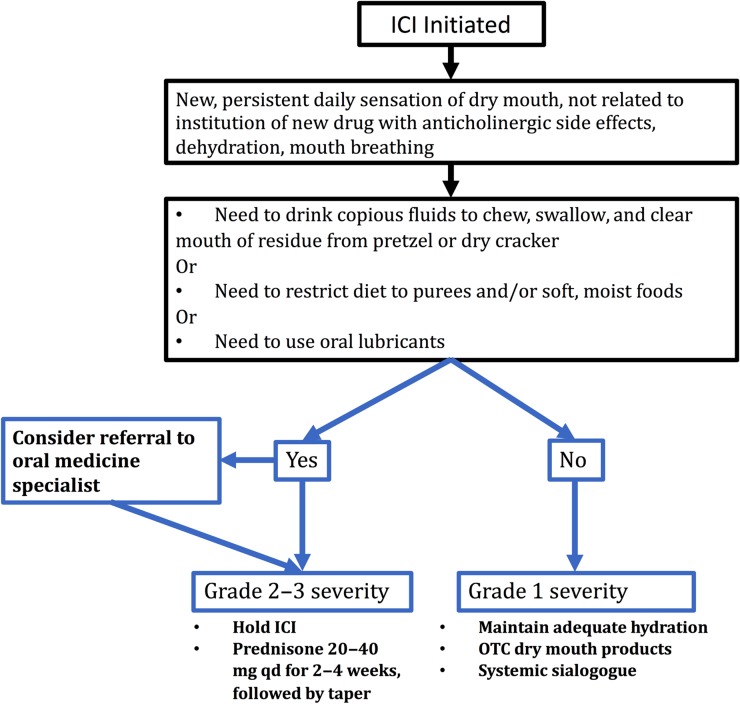
Our experience supports the following protocol for management of sicca that arises during the course of ICI therapy (Fig. 3). First, this irAE should be suspected when a patient reports new oral symptoms, with inadequate saliva to chew and swallow a dry cracker, pasty saliva, or prominent nocturnal oral dryness. Second, alternative etiologies (e.g., a recent prescription of a drug with anticholinergic side effects) should be excluded. A comprehensive oral examination should be done to exclude mucositis, which can be a distinct form of irAE. Referral to an oral medicine specialist for comprehensive oral exam, saliva assessment, and LSGB should be considered in those with CTCAE grade 2 xerostomia symptoms. This should include an evaluation for an infectious etiology in patients with mucositis, burning mouth, or oral ulcers. Third, a stepwise approach to management is recommended, depending on the sicca severity. Mild (grade 1) symptoms can be managed with topical therapies (e.g., sips of water, artificial tear and saliva supplements) and sialogogues (e.g., pilocarpine and cevimeline). For patients with grade 2 or 3 symptoms, ICI therapy should be withheld and prednisone therapy initiated. Initial prednisone doses of 20–40 mg per day for 2–3 weeks, followed by a taper, proved most beneficial in our patients. Upon improvement of the sicca, the ICI can be resumed, ideally after an interval of 3 months. In patients with persistent and severe sicca, the ICI therapy must be permanently discontinued.
Figure 3.
Assessment and management of sicca developing during course of ICI therapy.
Abbreviations: ICI, immune checkpoint inhibitor; OTC, over‐the‐counter; qd, quaque die (one a day).
Although many of our patients reported subjective improvement, few patients returned to normal salivary flow rates and none reported complete resolution of their symptoms. Importantly, the sicca remained grade 3 when assessed by CTCAE objective criteria (<0.1 mL/minute whole unstimulated saliva flow). Serial SGUS of the major salivary glands did not demonstrate consistent improvement, and in some cases demonstrated worsening. We project that many of these patients will experience long‐term salivary hypofunction, with increased risk of dental sequelae (e.g., caries, recurrent candidiasis infections) and reduced quality of life [30]. For patients with this irAE, we recommend increased contact (e.g., every 3–4 months) with dentists or oral medicine specialists, including more frequent dental prophylaxis and topical fluoride treatments, to maintain the oral tissues and encourage continual management of their dry mouth symptoms.
Our data suggest that the mechanism of salivary gland hypofunction is distinct from other diseases affecting the salivary glands. We hypothesize that ICI therapy may break immune tolerance locally leading to activation of cytotoxic T cells damaging the salivary epithelium. The robust T‐cell infiltration of the salivary glands, with acinar destruction, is akin to ICI‐induced tissue injury seen in cases of fulminant myocarditis [31], acute interstitial nephritis [32], and hepatitis [33]. It is distinct from the histopathology of other forms of immune‐mediated sialadenitis, such as SS [24], graft‐versus‐host disease [34], and IgG4‐related sialadenitis [35].
Our study was limited by our bias in studying salivary hypofunction rather than ocular dryness; thus, the full clinical spectrum of ICI‐induced sicca syndrome may not have been captured. Furthermore, we performed limited dry eye evaluation and subjective measures of dry eye complaints before and after sicca management were not taken. Patients in this cohort were treated with one or multiple ICIs, some in combination with standard agents, for both malignant and benign conditions; this may have introduced confounding. Strengths of our study include the cohort size as well as the comprehensive evaluation of each patient including SGUS, histopathology, and immunohistochemistry studies.
Conclusion
We have described the cardinal features of a sicca syndrome induced by ICI therapy for neoplastic disease and likely arising from a dysregulated immune response. In the majority, it was induced de novo and developed abruptly within the first 3 months of such therapy. In some patients, the sicca syndrome may have represented an exacerbation of a prior autoimmune rheumatic disease, including incipient SS. Histopathology distinct from that of SS was observed, exhibiting acinar and ductal damage and a predominant T‐cell infiltrate. Improvement can be achieved with a graded approach depending on severity, including withholding the ICI and initiating corticosteroids. However, full recovery of salivary function was not observed and resolution of symptoms was not reported. Interestingly, our study illustrates a consistent difference in ICI sicca when using either subjective (grade 1 or 2 in 20/20) or objective (grade 3 in 19/20) CTCAE criteria. This discrepancy may lead to under‐reporting and reduced management of the patient's sicca symptoms. Further investigation of this entity promises to reveal novel mechanisms of autoimmune salivary and lacrimal gland dysfunction. Such research would facilitate development of novel therapeutics for this side effect while preserving the antitumor response.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the patients for agreeing to participate in this study. We thank the NIH Dental Clinic staff for their support in caring for the participating patients. Lastly, we acknowledge and thank Dr. Stuart Selonick for referring a patient to the study.
This research was fully supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research Grants: 1ZIADE000704‐15, 1ZIEDE000727‐08.
The NIH therapeutic clinical trials (i.e., NCT03076554, NCT01772004, NCT02517398, NCT02682667) that the patients were treated on were conducted at, and supported by, the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Dr. Alan N. Baer receives support from NIH Grant R01 DE12345‐15A1 and the Jerome L. Greene Foundation.
Preliminary data from this study were presented at the 14th International Sjögren's Syndrome Symposium, Washington DC, April 21, 2018.
Contributed equally.
Author Contributions
Conception/design: Blake M. Warner, Alan N. Baer, Ilias Alevizos
Provision of study material or patients: Evan J. Lipson, Alan N. Baer, Clint Allen, Christian Hinrichs, Arun Rajan, James L. Gulley, Ravi A. Madan, Josephine Feliciano, Laura Cappelli
Collection and/or assembly of data: Blake M. Warner, Alan N. Baer, Evan J. Lipson, Clint Allen, Christian Hinrichs, Arun Rajan, Eileen Pelayo, Margaret Beach, James L. Gulley, Ravi A. Madan, Josephine Feliciano, Margaret Grisius, Lauren Long, Astin Powers, David E. Kleiner, Ilias Alevizos
Data analysis and interpretation: Blake M. Warner, Alan N. Baer, Eileen Pelayo, Margaret Beach, Astin Powers, David E. Kleiner, Ilias Alevizos
Manuscript writing: Blake M. Warner, Alan N. Baer, Evan J. Lipson, Eileen Pelayo, Margaret Beach, James L. Gulley, David E. Kleiner, Ilias Alevizos
Final approval of manuscript: Blake M. Warner, Alan N. Baer, Evan J. Lipson, Clint Allen, Christian Hinrichs, Arun Rajan, Eileen Pelayo, Margaret Beach, James L. Gulley, Ravi A. Madan, Josephine Feliciano, Margaret Grisius, Lauren Long, Astin Powers, David E. Kleiner, Laura Cappelli, Ilias Alevizos
Disclosures
Evan J. Lipson: National Cancer Institute (P30 CA006973), Bristol‐Myers Squibb, Sysmex, Merck (RF), Array BioPharma, Bristol‐Myers Squibb, EMD Serono, Macrogenics, Merck, Millennium, Novartis (C/A), Patent pending: Method of preventing organ transplant rejections using agonists to the PD‐1 checkpoint pathway (IP); James L. Gulley: EMD Serono (RF); Josephine Feliciano: Merck, AstraZeneca, Genentech (C/A); Laura Cappelli: Bristol‐Myers Squibb (RF), Regeneron/Sanofi (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutros C, Tarhini A, Routier E et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473–486. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQM et al. Safety and activity of anti–PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS. Overcoming immunological tolerance to melanoma: Targeting CTLA‐4. Asia Pac J Clin Oncol 2010;6(suppl 1):S16–S23. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Drake CG, Pardoll DM. Targeting the PD‐1/B7‐H1(PD‐L1) pathway to activate anti‐tumor immunity. Curr Opin Immunol 2012;24:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tocut M, Brenner R, Zandman‐Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018;17:610–616. [DOI] [PubMed] [Google Scholar]
- 7.Cappelli LC, Shah AA, Bingham CO. Cancer immunotherapy‐induced rheumatic diseases emerge as new clinical entities. RMD Open 2016;2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelli LC, Gutierrez AK, Baer AN et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese C, Kirchner E, Kontzias K et al. Rheumatic immune‐related adverse events of checkpoint therapy for cancer: Case series of a new nosological entity. RMD Open 2017;3:e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narváez J, Juarez‐López P, LLuch J et al. Rheumatic immune‐related adverse events in patients on anti‐PD‐1 inhibitors: Fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev 2018;17:1040–1045. [DOI] [PubMed] [Google Scholar]
- 11.Richter MD, Pinkston O, Kottschade LA et al. Brief report: Cancer immunotherapy in patients with preexisting rheumatic disease: The Mayo Clinic experience. Arthritis Rheumatol 2018;70:356–360. [DOI] [PubMed] [Google Scholar]
- 12.Cappelli LC, Gutierrez AK, Bingham CO 3rd et al. Rheumatic and musculoskeletal immune‐related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care Res (Hoboken) 2017;69:1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc 2002;50:535–543. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese L, Mariette X. The evolving role of the rheumatologist in the management of immune‐related adverse events (irAEs) caused by cancer immunotherapy. Ann Rheum Dis 2018;77:162–164. [DOI] [PubMed] [Google Scholar]
- 15.Shiboski CH, Shiboski SC, Seror R et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan JS, Daniels TE, Talal N et al. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 1974;37:217–229. [DOI] [PubMed] [Google Scholar]
- 18.Tarpley TM, Anderson LG, White CL. Minor salivary gland involvement in Sjögren's syndrome. Oral Surg Oral Med Oral Pathol 1974;37:64–74. [DOI] [PubMed] [Google Scholar]
- 19.Daniels TE, Silverman S, Michalski JP et al. The oral component of Sjögren's syndrome. Oral Surg Oral Med Oral Pathol 1975;39:875–885. [DOI] [PubMed] [Google Scholar]
- 20.Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res 1992;71:1363–1369. [DOI] [PubMed] [Google Scholar]
- 21.Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjögren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren's syndrome. Ann Rheum Dis 1994;53:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RE, Ship JA. Major salivary gland flow rates in young and old, generally healthy African Americans and whites. J Natl Med Assoc 1995;87:131–135. [PMC free article] [PubMed] [Google Scholar]
- 23.Craig JP, Nelson JD, Azar DT et al. TFOS DEWS II Report Executive Summary. Ocul Surf 2017;15:802–812. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J Autoimmun 2010;34:400–407. [DOI] [PubMed] [Google Scholar]
- 25.Shelly S, Agmon‐Levin N, Altman A et al. Thymoma and autoimmunity. Cell Mol Immunol 2011;8:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eigentler TK, Hassel JC, Berking C et al. Diagnosis, monitoring and management of immune‐related adverse drug reactions of anti‐PD‐1 antibody therapy. Cancer Treat Rev 2016;45:7–18. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson JA. New NCCN Guidelines: Recognition and management of immunotherapy‐related toxicity. J Natl Compr Canc Netw 2018;16:594–596. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen SB, Pedersen AM, Vissink A et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support Care Cancer 2010;18:1039–1060. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortazar FB, Marrone KA, Troxell ML et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johncilla M, Misdraji J, Pratt DS et al. Ipilimumab‐associated hepatitis: Clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol 2015;39:1075–1084. [DOI] [PubMed] [Google Scholar]
- 34.Imanguli MM, Atkinson JC, Mitchell SA et al. Salivary gland involvement in chronic graft‐versus‐host disease: Prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transplant 2010;16:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baer AN, Gourin CG, Westra WH et al. Rare diagnosis of IgG4‐related systemic disease by lip biopsy in an international Sjögren's syndrome registry. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:e34–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]