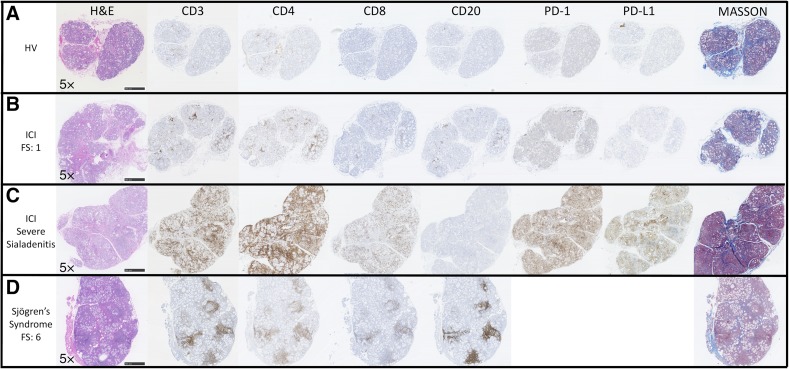
Figure 2.
ICI‐induced sicca exhibits histopathological changes distinct from Sjögren's syndrome. Histological and immunohistochemical comparison of representative labial salivary gland biopsy (LSGB) from a healthy volunteer, FS = 0 (A), a patient (patient 7) with ICI‐induced sicca with focal lymphocytic sialadenitis, FS = 1 (B), a patient (patient 14) with ICI‐induced sicca with severe sialadenitis (C), and a non‐ICI‐treated sicca patient who meets diagnostic criteria for Sjögren's syndrome, FS = 6 (D). The ICI‐induced sicca patients’ (B, C) biopsies demonstrate variably dense sialadenitis composed mainly of CD3+ T cells with a slight predominance of CD4+ over CD8+ T cells and a virtual absence of B cells. In contrast, the lymphocytic infiltrate in Sjögren's syndrome is composed mainly of CD20+ B cells, with variable germinal center formation, admixed with variable number of CD3+ T cells and a slight predominance of CD4+ over CD8+ T cells. In patients treated with ICI, PD‐1 was variably positive in a subset of the lymphocytes, mainly in areas of aggregation. PD‐1 was rarely positive in glands from healthy volunteers (A). Interestingly, the patients with the most profound sialadenitis (patients 14–16) are the only patients whose LSGB exhibited PD‐L1 positivity (C), typically in the epithelium in areas of dense inflammation, and rarely in some inflammatory cells. Increased inter‐ and intralobular fibrosis as highlighted by Masson trichrome staining (blue) relative to the healthy volunteer is evident. Acinar atrophy and disorganization is evident in both ICI (B, C) and Sjögren's syndrome (D) patients. However, epithelial injury with sloughing into the lumens, the presence of necrotic and apoptotic epithelial cells, and prominent exocytosis of lymphocytes into the epithelium is distinctly present in the severe sialadenitis patients (D).
Abbreviations: FS, focus score; H&E, hematoxylin and eosin; HV, healthy volunteer; ICI, immune checkpoint inhibitor; PD‐1, programmed cell death 1; PD‐L1, programmed cell death ligand 1.