Tyrosine kinase inhibitors (TKI) have greatly improved survival for patients diagnosed with chronic myeloid leukemia. It has been shown that patients with a sustained deep molecular response to TKI therapy can stop treatment and remain in a treatment‐free remission. This article explores patient views on TKI discontinuation and the range of factors to be considered when making the decision to discontinue or remain on TKI therapy.
Keywords: Decision making, Leukemia, Myeloid, Chronic phase, Goals, Polypharmacy
Abstract
Background.
The standard treatment for chronic phase chronic myeloid leukemia (CML) is lifelong oral tyrosine kinase inhibitor (TKI) therapy. Multiple clinical trials have demonstrated that some patients with a sustained deep molecular response to TKI therapy can safely stop therapy and remain in a treatment‐free remission. TKI discontinuation is now offered to select patients in routine clinical care. In order to better support patient decision making, we explored patients’ views on TKI discontinuation and the factors patients consider when making this decision.
Materials and Methods.
Patients were recruited from three U.S. academic cancer centers. Qualitative interviews were recorded, transcribed, and content analyzed.
Results.
We interviewed 22 patients, half of whom wanted to try TKI discontinuation. Eleven factors relevant to the decision emerged, and patients weighed these factors differently. Commonly mentioned factors included perceived risk of relapse, TKI side effects, financial considerations, polypharmacy, and willingness to change something that was working (status quo). There were notable differences in patients’ understanding of the likelihood of achieving a treatment‐free remission, with patients who did not want to stop TKIs more accurately reporting the risk of relapse than patients who wanted to stop.
Conclusion.
This is a novel decision that will become more common as the prevalence of patients with well‐controlled CML continues to increase. These results highlight the need for patient education and decision support so that patients and providers can make shared decisions that are informed and values based.
Implications for Practice.
The standard treatment for chronic phase chronic myeloid leukemia (CML) is lifelong oral tyrosine kinase inhibitor (TKI) therapy. Clinical trials have shown that some patients with a sustained deep molecular response to TKI therapy can safely stop therapy and remain in a treatment‐free remission. TKI discontinuation is now being offered to patients outside of clinical trials. This study explored factors that patients who are eligible to try TKI discontinuation considered when making this decision. Factors relevant to the decision included risk of relapse, side effects, financial considerations, polypharmacy, and willingness to change something that was working. This is a novel decision that will become more common as the prevalence of patients with well‐controlled CML continues to increase. These results highlight the need for decision support and outline the factors that should be included so that patients and providers can make shared decisions that are informed and values based.
Introduction
For patients diagnosed with chronic myeloid leukemia (CML), the advent of treatment with tyrosine kinase inhibitors (TKIs) dramatically improved survival and reduced toxicity compared with earlier treatments [1], [2]. There are currently four U.S. Food and Drug Administration‐approved TKIs for frontline treatment of CML [3], [4], [5]. They vary with regard to regimen (i.e., take with or without food, take one or two times per day) and side effect profile. TKI therapy is expensive [6], and the long‐term side effects of TKIs are unknown [7]. Until recently, the standard of care was to continue therapy indefinitely. The French Stop Imatinib (STIM) trial demonstrated that nearly 40% of patients with a sustained deep molecular response to TKI therapy could safely stop therapy and remain in a treatment‐free remission [8]. Additional trials have corroborated this [9], [10], [11], [12], and experts, including in the National Comprehensive Cancer Network clinical practice guidelines, now suggest that TKI discontinuation be offered to select patients in routine clinical practice [13], [14].
Four published surveys have described patient perspectives on TKI discontinuation and treatment‐free remission, but findings are inconsistent regarding the percentage of patients who say they are willing to try discontinuation. A study conducted with 56 patients with CML in Canada presented a scenario modeled after the early STIM trial results and found that 71% of patients would be willing to discontinue TKI therapy if closely monitored [15]. An Italian survey asked over 1,000 patients about whether they would discontinue therapy “if in the future there is a perfect and long‐lasting response to the treatment” [16]. About half endorsed the option “no” for fear of losing the results they had attained, 20% said it would not make a difference because they are used to taking the drug, 16% said they would stop because they do not tolerate the drug well, and 12% said they would stop if doctors said to. A study in China with nearly 900 patients found that most (83%) expected to discontinue TKI therapy in the future, and both younger age and higher out‐of‐pocket expenses were associated with a desire to live treatment free [17]. Finally, a survey of 84 patients from one academic medical center in the U.S. found that 42% would try discontinuation if eligible; another 25% were unsure [18].
To our knowledge, no studies have explored this decision using in‐depth qualitative methods. In this study, we conducted qualitative interviews to explore U.S. patients’ views on TKI discontinuation and the range of factors patients would consider when making the decision to discontinue or remain on TKI therapy. An in‐depth understanding of the factors patients say are important can direct development of patient‐focused decision support. We also explored patients’ understanding of the chance of achieving treatment‐free remission.
Materials and Methods
Recruitment
Three academic medical centers that were participating in a multisite discontinuation study (Life After Stopping TKIs, NCT02269267) recruited patients for this study. Eligible patients were (a) aged 18 or older, (b) able to speak English, (c) diagnosed with CML in chronic phase, and (d) either already eligible to try TKI discontinuation or taking a TKI for at least 1 year with a deep molecular response, such that they would be expected to be in a position to try TKI discontinuation in the near future. Our purposive sampling aimed to recruit patients who wanted to try TKI discontinuation as well as patients who did not; beyond that, participants represent a convenience sample. Physicians provided a printed study flyer to patients that described the researchers’ rationale for the study; interested patients contacted study staff at the Medical College of Wisconsin for screening. Twenty‐nine patients were screened; all were eligible. Twenty‐two patients gave verbal informed consent, enrolled, and completed the interview. We stopped recruiting patients after three consecutive interviews in which no new themes emerged (data saturation) [19]. Patients were given $50 for their time. The study was approved by the institutional review board of the Medical College of Wisconsin (PRO00021095).
Data Collection
The semistructured interview guide included questions about experiences being diagnosed with and treated for CML and patient knowledge about TKI discontinuation. For patients who were not familiar with discontinuation, we included a brief description modeled after the STIM trial results, which included safety data and information about the need for increased monitoring. We then asked whether and why the participant would or would not consider discontinuation. Finally, we assessed their understanding of the chance of achieving a treatment‐free remission, for both themselves and the average patient. Telephone interviews were conducted by an experienced interviewer (J.M.M.) who did not have a previous relationship with the patients. Interviews lasted 20 minutes on average (range, 11–43 minutes) and were audio‐recorded and transcribed.
Data Analysis
A team‐based systematic content analysis was conducted as follows [20]. Two members of the research team (K.E.F. and J.M.M.) read the first five interviews and developed an initial coding scheme, with prespecified themes based on the interview guide (e.g., beliefs about risks of TKI discontinuation) plus open coding to capture themes that arose from the data (e.g., factors in discontinuation decision making). We independently coded two transcripts (∼10%) using the initial scheme and revised the scheme to include the new topics that emerged. An additional two transcripts were coded and compared with ensure consistent use of the codes (A.D. and J.M.M.), resulting in average intercoder agreement of 0.94 across codes and transcripts (range 0.57–0.99). Differences were discussed and revised in an iterative process until perfect agreement was achieved. The remaining transcripts were coded by one team member (J.M.M.). The transcripts were managed and analyzed using NVivo 10 (QSR International, Melbourne, Australia).
Results
Sample Characteristics
The 22 patients represented men and women of diverse ages, although none was younger than 40 years. Most (86%) had been diagnosed at least 4 years previously, with five diagnosed before the TKI era (Table 1). Patients had been on TKIs for 2–15 years, with a median of 7 years. The majority (82%) reported having discussed TKI discontinuation with their doctor.
Table 1. Self‐reported patient characteristics.
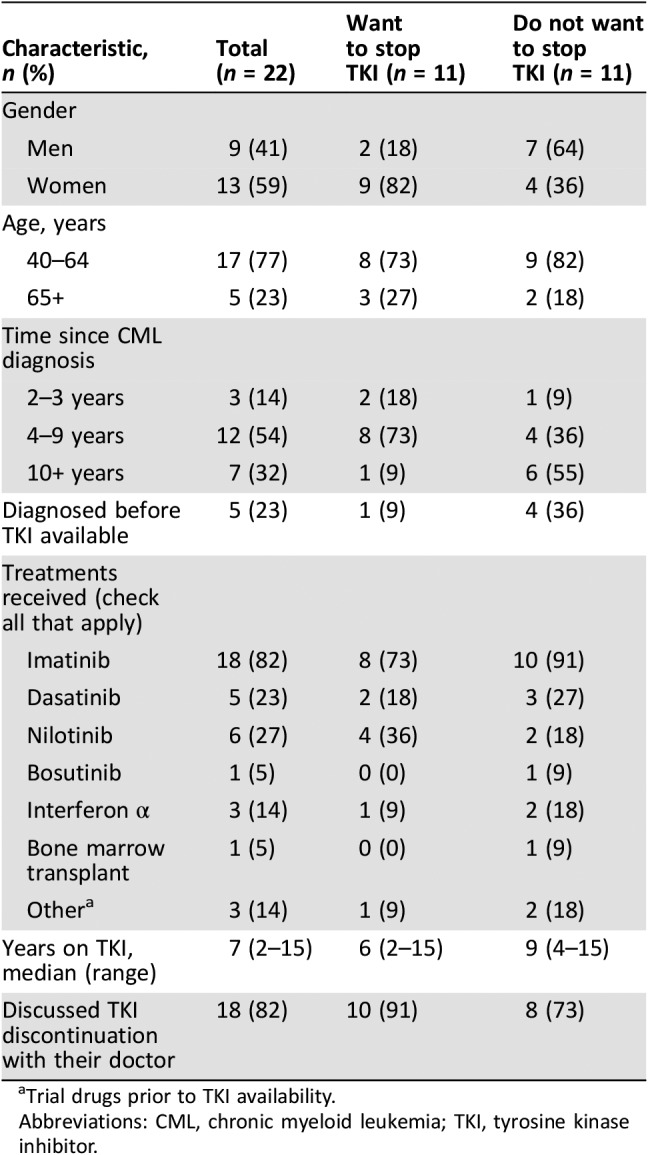
Trial drugs prior to TKI availability.
Abbreviations: CML, chronic myeloid leukemia; TKI, tyrosine kinase inhibitor.
Themes
We asked patients if they would want to try TKI discontinuation and to describe how they would make the decision. (For the three patients who said they were not familiar with TKI discontinuation, we presented a brief description modeled after the STIM trial results.) Patients described multiple factors that they thought were relevant to this decision (Table 2). We present counts of how often each factor was mentioned—not to represent expected prevalence in a larger population but to demonstrate that for most of these factors, the same reasons were given to explain both decisions.
Table 2. Range of factors considered in TKI discontinuation decision making.
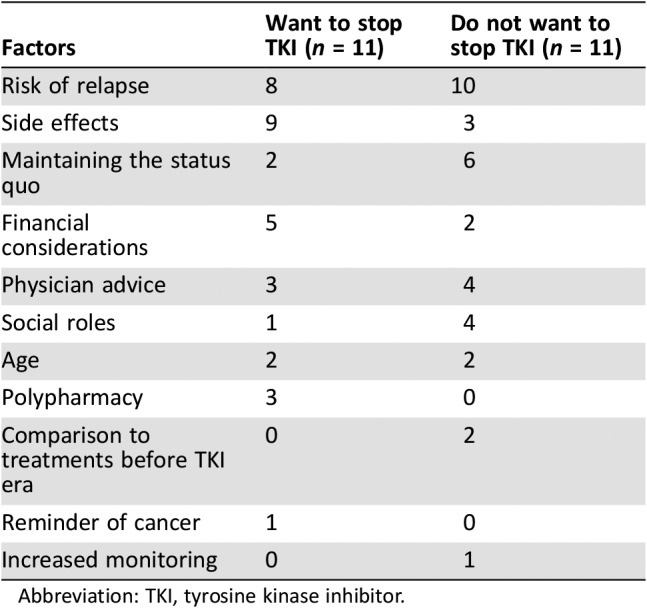
Abbreviation: TKI, tyrosine kinase inhibitor.
Risk of Relapse
The most frequently cited factor among those wanting to stay on their TKI and not attempt discontinuation was concern about the risk of relapse. This included uncertainty about sufficiently quick detection of a relapse as well as concern about regaining a deep response (remission).
“When I was first diagnosed, I had no sense of what was going on in my body. I didn't feel bad. So that scares me a little bit knowing that if I go off of it I might not know what's really going on… There's always a question in the back of my head like what if it comes back worse.” [Patient 17]
“I would be hesitant. I would be scared. ‘Scared’ would be the word. I would be afraid that it would come back and I wouldn't be able to get it under control.” [Patient 3]
“You have to catch getting out of remission in time to be able to get back into remission. It's all such a gamble to do it all over again after you've been given this miracle.” [Patient 9]
Patients who wanted to stop also cited risk of relapse as a factor in their decision making, but they described feeling comfortable with the risk.
“I mean if you go out of remission, you just go back on the medication.” [Patient 7]
“The fact that most people go right back into remission… made it seem a lot less life threatening to try. Worst scenario, I'm on a little vacation here, and I'll be no worse off than I was if it comes back.” [Patient 14]
Side Effects
The most frequently cited factor among those wanting to try TKI discontinuation was reducing medication side effects.
“I couldn't stand looking at myself in the mirror… I look like a monster first thing in the morning with my eyes just so puffed out.” [Patient 10]
“I want to get off of [imatinib] just to feel normal again… I think the nausea and the energy are the two things that are very annoying.” [Patient 5]
“We talk about the current side effects, but we don't know what the side effects are going to be… when I'm 60 and 70 and 80.” [Patient 16]
Conversely, not experiencing side effects or finding them manageable was also stated as a reason by patients who preferred to remain on their TKI.
“I would be able to stay on [imatinib] because I know what the side effects are, and I can certainly live with them.” [Patient 12]
Maintaining the Status Quo
Multiple patients reasoned that the TKI was controlling the CML so should be continued.
“I've been living my life like this now, so I just want to keep everything the way it's going. If it ain't broke don't fix it.” [Patient 6]
“Everything is going very well, so I don't want to disrupt it even for one day.” [Patient 21]
Some noted the other factors they weighed in this line of reasoning or how they might make a different decision if they had financial concerns or significant side effects.
“I hate to mess with something that's not necessarily broken… It's not financially a problem. It's not physically a problem. It's not emotionally a problem right now.” [Patient 17]
“If I ever encounter difficulty with taking the drug, for side effects and so forth, at that point I can consider quitting. But there's really not a whole lot of incentive for me to quit now.” [Patient 12]
Financial Considerations
Avoiding the cost of TKIs was mentioned as a reason for TKI discontinuation by four patients who wanted to stop their TKI. Another mentioned the global cost of TKIs.
“The cost, not necessarily even to me, but just to the health care system in general. I think the drug companies are ripping people off charging that much money for it.” [Patient 7]
Two patients had participated in the original clinical trial for ST1571 (imatinib) and continued to receive the drug without cost. Their decision not to try TKI discontinuation was influenced by this; they did not want to lose access to this free medication.
“If I were to stop, I believe my participation in the original study group would be over, and if I did relapse, I would not get the study drug free anymore.” [Patient 12]
Physician Advice
Multiple patients said they would rely on their physician to guide decisions about TKI discontinuation.
“A lot of it would be determined by what [my doctor] would tell me. That would be a big part of it, what his opinion is.” [Patient 3]
However, for some patients, hearing a doctor describe TKI discontinuation seemed at odds with previous advice to be adherent to a TKI regimen.
“I was very surprised because all I kept hearing was ‘don't forget to take your [imatinib]. Don't forget to take your [imatinib].’ When I had an appointment with him I was so surprised when he said I might stop using [it].” [Patient 15]
Another patient understood their physician's prompting about adherence as a tacit recommendation to not try TKI discontinuation.
“I think I would [stop] if [my doctor] said this to me. But you know, his whole thing with me was, ‘You're taking the [dasatinib] every day right?’ So, ‘yeah, I'm taking it every day.’ ‘Okay, good.’ So that must mean something.” [Patient 6]
Social Roles and Age
Multiple patients mentioned their age or social roles (e.g., caring for children) as a factor in this decision. However, these factors were weighed differently by different people.
“And my age is such that even if it came back I don't think—I might die from something else.” [Patient 11]
“No, I would never stop… I want to retain that for the rest of my life because I'm old.” [Patient 9]
“At this point I'm not sure, but I think if I got older I would take the chance.” [Patient 1]
“I have younger kids, so, I would say, ‘No’.” [Patient 6]
Polypharmacy
Reducing the number of medications a person was on was another factor mentioned by patients who wanted to stop. This was sometimes about the TKI itself and sometimes about the additional medications that were used by patients to manage the side effects of the TKI.
“It's worth a try. One less drug… My kidney function has been a little off. I'm thinking I can't take the [imatinib] without this [esomeprazole], which is bad for your kidneys.” [Patient 1]
“I'm excited about not throwing another big med in my bloodstream and in my body. I've had so much chemotherapy [already] that if I could get rid of one, that's good with me.” [Patient 2]
Previous Treatments
Five patients were diagnosed before TKIs were available. Some of these patients described how they initially faced limited treatment options with serious consequences. These experiences provided a different context compared with patients who had not experienced these other treatments.
“It [bone marrow transplant]. was horrible… I was in the hospital for 32 days. … They told me that I could plan on being off work up to 2 years… I don't want to put myself in a position where I have to go through another bone marrow transplant… They would have to take me off [imatinib] kicking and screaming.” [Patient 13]
“I think everyone who suffered the feeling of every day you don't know whether you're going to live or die… you would never stop taking [imatinib]… Because nowadays, if you're diagnosed they say, ‘Take this pill and you're going to be fine.’ For me they said, ‘You’ve got 3 years to live. Take this interferon, and it will last for a while and you're going to feel awful.’” [Patient 9]
Reminder of Cancer
For one patient, taking a TKI was a regular reminder of having cancer; thus discontinuation was attractive.
“If you're not taking the medication twice a day, you kind of can get more of a mindset where you don't have to think all the time about the fact that you have CML.” [Patient 7]
Increased Monitoring
Patients who are well‐controlled on a TKI need close monitoring with periodic blood tests, commonly every 3–6 months. In most of the TKI discontinuation trials, monitoring was more frequent—monthly for the first 6 months, then every 2–3 months after that. This increased monitoring was mentioned as a reason to not try discontinuation (although the patient misunderstood the required frequency of the increased monitoring).
“If I were to go off it that I would have to come and get my blood checked every week or frequently which sounds really petty, but in my time in my life right now… it would be a lot to put on my plate and in my schedule to come every week to have my blood drawn.” [Patient 17]
Patients’ Perceptions of Risk of TKI Discontinuation
We asked patients (a) what they thought their personal chances would be of remaining in a treatment‐free remission after TKI discontinuation and (b) what they thought the average patient's chances would be. Despite having either discussed TKI discontinuation with their physician or the interviewer having described the results of the STIM trial, about one third of patients stated they did not know and declined to answer. Of those who did respond, there were notable differences between patients who did and did not want to stop in how they understood risk of relapse (Table 3). Patients who wanted to discontinue their TKI were confident about remaining in treatment‐free remission following discontinuation, with a median of 90% confidence (range, 50%–100%). Comparatively, patients who did not want to stop their TKI expressed less confidence that they would retain remission if they tried TKI discontinuation, with a median of 50% confidence (range, 0%–60%). Patients in both groups were generally accurate in estimating the average patient's chance of maintaining a treatment‐free remission (median of 50%). However, the range among those who wanted to stop was notably higher (40%–90%) compared with those who did not want to stop (2%–50%).
Table 3. Patient perceptions of risk of TKI discontinuation.
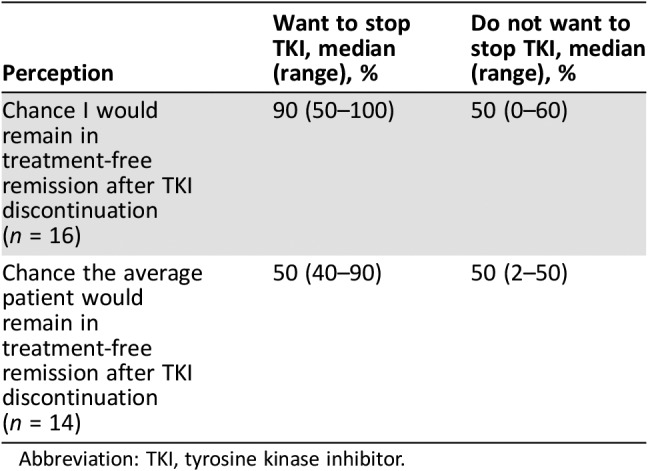
Abbreviation: TKI, tyrosine kinase inhibitor.
Discussion
This study represents the first in‐depth qualitative study to examine patient decision making about discontinuation of TKI therapy for well‐controlled CML. This is a novel medical decision that is expected to receive increasing attention given the high cost of TKIs and the need for lifelong treatment for some, but not all, patients. The findings are consistent with previous patient surveys in that the most commonly mentioned reason for wanting to discontinue TKI therapy was to reduce side effects, whereas concern about relapse was most commonly mentioned as the reason for not wanting to try TKI discontinuation. The findings extend previous survey studies, providing new insights into this decision that can guide development of decision support tools. Using open‐ended questions in an interview format allowed us to hear additional reasons from patients’ personal experiences. Of particular importance, patients’ responses frequently included references to their age or social roles, as well as to their interest in their physician's advice in making decisions about TKI discontinuation.
Our findings confirm the importance of a shared decision‐making approach for TKI discontinuation, in which patients are given information, patient values are elicited and discussed, and patients, who will bear the consequences of the decision, are given an equal voice in making it. First, the majority of the reasons given were mentioned to support both decisions, that is, to stop and not to stop, suggesting that values clarification will be an important component of supporting patients’ decisions about TKI discontinuation. Second, the patients in this study were patients of CML experts who were participating in a national TKI discontinuation trial, and the majority of patients said they had discussed discontinuation with their doctors. Yet in many cases, these patients did not know basic information about risk of relapse, and others misunderstood key details around TKI discontinuation. Future work to develop decision support materials is warranted to help patients and their physicians make this decision. Of note, when we began this study, TKI discontinuation was still experimental, but it is now part of practice guidelines. Our findings should be confirmed in this postguideline era, in a larger sample that includes both academic and community sites.
Patients were recruited from three U.S. cancer centers, including men and women across the typical age range for patients with CML. That said, they represent a convenience sample of patients from academic sites, and generalizability to all patients is unknown. We did not return transcripts to participants for comment or corrections, nor did we solicit participant feedback on the findings. This is a qualitative study and hypothesis testing or statistical comparisons are inappropriate. However, the data allowed us to explore a variety of themes in depth, providing a roadmap for the development of decision support.
Conclusion
The prevalence of patients with well‐controlled CML continues to increase because of the unprecedented success of TKI treatment. As such, more and more patients will be in a position to attempt TKI discontinuation. This decision is influenced by multiple factors, and many patients rely on their physicians for advice. Our results highlight the need for patient education and decision support so that patients and providers can make shared decisions that are informed and values based.
Acknowledgments
Financial support for this study was provided in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Author Contributions
Conception/design: Kathryn E. Flynn, Ehab Atallah
Provision of study material or patients: Charles A. Schiffer, James E. Thompson, Ehab Atallah
Collection and/or assembly of data: Kathryn E. Flynn, Judith M. Myers, Charles A. Schiffer, James E. Thompson, Ehab Atallah
Data analysis and interpretation: Kathryn E. Flynn, Judith M. Myers, Anita D'Souza, Charles A. Schiffer, James E. Thompson, Ehab Atallah
Manuscript writing: Kathryn E. Flynn, Judith M. Myers, Anita D'Souza, Charles A. Schiffer, James E. Thompson, Ehab Atallah
Final approval of manuscript: Kathryn E. Flynn, Judith M. Myers, Anita D'Souza, Charles A. Schiffer, James E. Thompson, Ehab Atallah
Disclosures
Charles A. Schiffer: Astellas, Ambit, Pfizer, Takeda, Pharmacyclics, Juno, Celgene, Puma, Genentech (C/A), Celgene, Novartis, Bristol‐Myers Squibb, Ariad, Micromedex, Pharmacyclics (RF); Ehab Atallah: Bristol‐Myers Squibb, Novartis, Takeda, Pfizer (C/A), Novartis, Bristol‐Myers Squibb, Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Druker BJ, Fo Guilhot, O'Brien SG et al. Five‐year follow‐up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408–2417. [DOI] [PubMed] [Google Scholar]
- 2.Hahn EA, Glendenning GA, Sorensen MV et al. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low‐dose cytarabine: Results from the IRIS Study. J Clin Oncol 2003;21:2138–2146. [DOI] [PubMed] [Google Scholar]
- 3.Cortes JE, Saglio G, Kantarjian HM et al. Final 5‐year study results of DASISION: The dasatinib versus imatinib study in treatment‐naïve chronic myeloid leukemia patients trial. J Clin Oncol 2016;34:2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Saglio G, Hughes TP et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia 2016;30:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Gambacorti‐Passerini C, Deininger MWN et al. Bosutinib (BOS) versus imatinib (IM) for newly diagnosed chronic myeloid leukemia (CML): Initial results from the BFORE trial. J Clin Oncol 2017;35:7002–7002. [Google Scholar]
- 6.Experts in Chronic Myeloid Leukemia . The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood 2013;121:4439–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steegmann JL, Cervantes F, le Coutre P et al. Off‐target effects of BCR‐ABL1 inhibitors and their potential long‐term implications in patients with chronic myeloid leukemia. Leuk Lymphoma 2012;53:2351–2361. [DOI] [PubMed] [Google Scholar]
- 8.Etienne G, Guilhot J, Rea D et al. Long‐term follow‐up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol 2017;35:298–305. [DOI] [PubMed] [Google Scholar]
- 9.Shah NP, Paquette R, Müller MC et al. Treatment‐free remission (TFR) in patients with chronic phase chronic myeloid leukemia (CML‐CP) and in stable deep molecular response (DMR) to dasatinib ‐ The Dasfree Study. Blood 2016;128:1895–1895. [Google Scholar]
- 10.Atallah E, Schiffer CA, Radich JP et al. Results from the U.S. Life After Stopping TKIs (LAST) Study. Blood 2017;130:2903–2903. [Google Scholar]
- 11.Hochhaus A, Masszi T, Giles FJ et al. Treatment‐free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017;31:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saussele S, Richter J, Guilhot J et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO‐SKI): A prespecified interim analysis of a prospective, multicentre, non‐randomised, trial. Lancet Oncol 2018;19:747–757. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology, Version I . 2018. July 26, 2017. [Google Scholar]
- 14.Khoury HJ, Williams LA, Atallah E et al. Chronic myeloid leukemia: What every practitioner needs to know in 2017. Am Soc Clin Oncol Educ Book 2017;37:468–479. [DOI] [PubMed] [Google Scholar]
- 15.Sanford D, Kyle R, Lazo‐Langner A et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Oncol 2014;21:e241–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breccia M, Efficace F, Sica S et al. Adherence and future discontinuation of tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia. A patient‐based survey on 1133 patients. Leuk Res 2015;39:1055–1059. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Liu ZC, Zhang SX et al. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol 2016;142:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg S, Hamarman S. Patients with chronic myelogenous leukemia may not want to discontinue tyrosine kinase inhibitor therapy. Blood 2015;126:1584–1584. [Google Scholar]
- 19.Saunders B, Sim J, Kingstone T et al. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual Quant 2018;52:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–1288. [DOI] [PubMed] [Google Scholar]


