Polypharmacy is a significant burden in older patients with cancer. This article reports on the prevalence of polypharmacy in a population of ovarian cancer patients, assessing the evolution of polypharmacy along the cancer care continuum.
Keywords: Ovarian cancer, Polypharmacy, Disparity
Abstract
Objective.
Polypharmacy has been associated with morbidity and mortality in patients with cancer. Data about polypharmacy among patients with ovarian cancer are limited. The primary objective of this study was to evaluate polypharmacy in a cohort of patients with ovarian cancer and to assess the evolution of polypharmacy from initial presentation to 2 years posttreatment. A secondary objective was to evaluate differences in polypharmacy between a subset of patients primarily treated in our comprehensive cancer center (CCC) and our safety net hospital (SNH).
Methods.
Women treated for ovarian cancer between January 1, 2011, and December 31, 2016, were included. Data were abstracted from the electronic medical record. Medication safety was assessed using the established Anticholinergic Burden (ACB) scale and the Beers criteria. Statistical analyses were performed using paired t tests and Cox proportional hazards models, with significance set at p < .05.
Results.
The study included 152 patients. The majority of patients had high‐grade serous carcinoma. Hypertension was the most common medical problem. The mean number of medications at the time of diagnosis was 3.72. Paired testing demonstrated significant patient‐level increases in the number medications at 2 years following initial diagnosis (4.16 vs. 7.01, p < .001). At the CCC, 47.4% of patients met criteria for polypharmacy at diagnosis compared with 19.4% at the SNH (p < .001). By 2 years postdiagnosis, 77.6% of patients at the CCC met criteria for polypharmacy compared with 43.3% at the SNH (p = .001). The use of any medications on the ACB scale (p < .001) increased significantly between initial diagnosis and 2 years for the entire population. Polypharmacy was not a significant predictor of overall survival.
Conclusion.
Polypharmacy worsens as women go through ovarian cancer treatment. Both at initial presentation and at 2 years postdiagnosis, rates of polypharmacy were higher at the CCC. Polypharmacy did not have an effect on survival in this cohort.
Implications for Practice.
Awareness of escalating numbers of medications and potentially adverse interactions is crucial among women with ovarian cancer, who are at high risk for polypharmacy.
Introduction
Polypharmacy, most commonly defined as the use of five or more medications, represents a significant burden in older patients with cancer [1]. It is estimated that more than 50% of older patients with cancer take at least five medications [2], [3]. In fact, some series have suggested that elderly patients with cancer on average take more than nine medications and that between 50% and 70% take complementary and alternative medicines on top of their prescription drugs [4], [5]. Polypharmacy poses a substantial risk to patients and has been associated with impaired physical functioning [2], hospitalization [6], and significant adverse drug‐drug interactions [7].
In specific subpopulations of patients with cancer, polypharmacy has been associated with worse outcomes [8]. For example, in patients with acute myelogenous leukemia, polypharmacy at diagnosis has been strongly associated with lower odds of complete remission and a higher overall mortality [9]. Data are limited in ovarian cancer but are conflicting regarding the prognostic implications of polypharmacy and survival [10]. Similarly, there are limited data outlining disparities in the prevalence of polypharmacy in oncology patients, although studies in other populations have demonstrated that age, education, and race all influence the use of multiple medications [11], [12].
The availability of definitive data on polypharmacy in patients with gynecologic cancer may assist gynecologic oncologists in identifying patients at risk for medication‐associated complications that may lead to increased morbidity and mortality. By defining the scope of this problem, as well as long‐term outcomes, novel interventions to prevent potentially unsafe drug interactions may be considered. The objectives of this study were to determine the baseline prevalence of polypharmacy in a population of patients with ovarian cancer, to assess the evolution of polypharmacy along the cancer continuum in a cohort of women with ovarian cancer from initial presentation to 2 years postdiagnosis, and to identify differences in polypharmacy by treatment center between a comprehensive cancer center (CCC) and a public safety net hospital (SNH).
Materials and Methods
After approval from the institutional review board (Protocol 2016‐0769), a total of 339 women treated for malignant ovarian cancer between January 1, 2011, and December 31, 2016, at both the university's CCC and the county SNH on the same medical campus, were identified. Both the CCC and the SNH provide care for a racially and ethnically diverse patient population, although the SNH treats a much higher proportion of immigrants and women of lower socioeconomic status. All ovarian cancer subtypes and pathologic grades were included in our analyzed cohort. Patients were excluded if they did not have pathologic confirmation of disease, had borderline histology, or had initiated primary treatment elsewhere and transferred care to our institution.
Data were abstracted from the electronic medical record (EMR) and included age of diagnosis, race, ethnicity, treatment location, medical history, stage at diagnosis, histology, debulking status, total number of outpatient medications taken at initial diagnosis and at 2 years postdiagnosis, recurrence status, and status at last follow‐up. The date of diagnosis was defined as the day of pathologic confirmation of cancer. Two years postdiagnosis was defined as the hospital documentation, inpatient or outpatient, nearest to 24 months from date of original diagnosis. Age was recorded as a continuous variable. Patient race was classified as either white or nonwhite, and ethnicity was classified as either Hispanic or non‐Hispanic. Polypharmacy was defined as the use of five or more recorded outpatient medications. No differentiation was made between scheduled and as‐needed medications, as reported medication use was confirmed as either “yes” or “no” on nursing intake for each encounter in the EMR. Chemotherapy drugs were not included in the list of medications contributing to polypharmacy. Medical histories extracted included coronary artery disease/myocardial infarction, hypertension, diabetes mellitus, thyroid dysfunction (hypothyroid/hyperthyroid), rheumatologic disease, osteoarthritis, chronic pain syndrome or fibromyalgia, seizure disorder, dementia, cerebrovascular disease, chronic obstructive pulmonary disease, asthma, psychiatric disease, and prior history of cancer (other than nonmelanoma skin cancer). Each medical problem was considered as a single entity, and for the purposes of analysis were classified categorically as fewer than two medical problems or at least two medical problems based on the median number for the cohort. Histology was classified as serous histology or nonserous histology. Stage at diagnosis was used as a continuous variable. Debulking was classified as either optimal (≤1 cm) or suboptimal (>1 cm). Recurrence was confirmed by either provider documentation or imaging documentation. Overall survival (OS) was measured from the time of diagnosis to death (all‐cause) or last contact.
Numerous tools are available to screen for both drug interactions and polypharmacy. The Anticholinergic Burden (ACB) scale assesses the number of anticholinergic medications and assigns a score based on the risk of significant anticholinergic effects [13]. An overall score of 3 or greater suggests clinically significant anticholinergic risk. All patients were evaluated for polypharmacy using the ACB scale. The Beers criteria for potentially inappropriate medication use in older adults [14] and Zhan's criteria [15] both classify specific medications deemed high risk or inappropriate for elderly individuals. Patients aged ≥65 years were screened for polypharmacy using these criteria. All patient medications were evaluated at both time of diagnosis and 2 years postdiagnosis. During the course of the review, it became clear that no patients in either location or at any time point during their cancer care had any medications listed on Zhan's criteria, so this scale was not used for any further analysis.
Statistical analyses were performed using Stata/IC version 14.2 (StataCorp, College Station, TX). Summary statistics were used to describe the patient cohort. Student's t test and Wilcoxon rank‐sum test were used for continuous variables in parametric and nonparametric calculations, respectively. Paired t tests were used to evaluate the number of medications for individual patients between time of diagnosis and 2 years postdiagnosis. Chi‐square testing (or Fisher's exact test, where appropriate) was used to analyze associations between categorical variables. Univariable and multivariable Cox proportional hazards regression, the log‐rank test, and the Kaplan‐Meier method were utilized to assess survival outcomes. Given that our small sample limited multiple comparisons, stepwise backward multivariable regression analyses only included covariates with p values ≤.05 from the univariable models. All tests were two‐sided, with significance set at p < .05.
Results
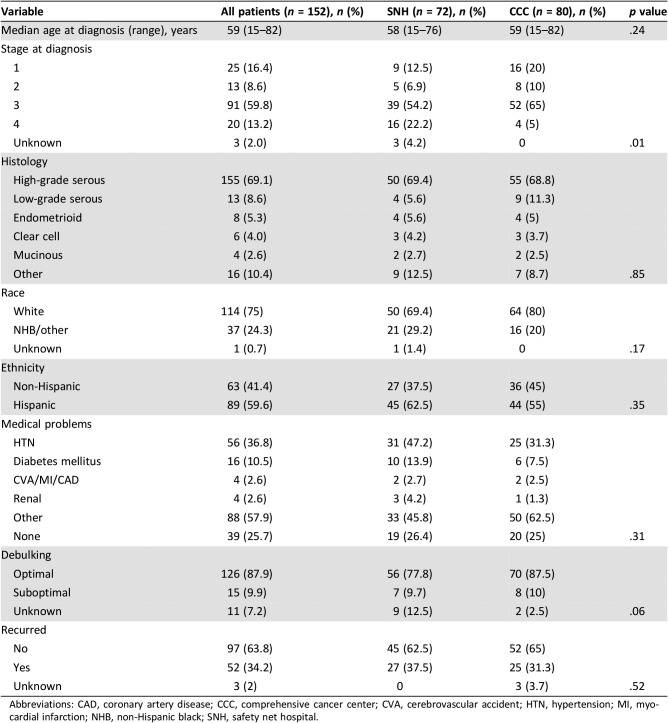
Three hundred thirty‐nine patients diagnosed with ovarian cancer were identified during the study interval. Of these, 152 met criteria for inclusion (80 in the CCC and 72 in the SNH). Demographics of these patients are shown in Table 1. There were no significant differences between the groups by age at diagnosis, race, or ethnicity. Significantly more patients at the SNH presented with stage IV disease at diagnosis compared with the CCC (p = .01). The majority of patients in both cohorts had high‐grade serous carcinoma, and the proportions of patients who recurred during the study interval was equally balanced. Hypertension was the most common medical problem for both groups, although the overall number of medical problems was not significantly different between them. A larger proportion of patients at the CCC had an optimal debulking (87.5% vs. 77.8%), but this did not reach statistical significance (p = .06).
Table 1. Demographic characteristics, with comparison of the SNH and CCC populations.
Abbreviations: CAD, coronary artery disease; CCC, comprehensive cancer center; CVA, cerebrovascular accident; HTN, hypertension; MI, myocardial infarction; NHB, non‐Hispanic black; SNH, safety net hospital.
The mean number of medications at the time of diagnosis for the entire cohort was 3.72, although patients at the CCC were taking, on average, more medications (5.11 vs. 2.13, p < .001). This same finding was maintained at 2 years postdiagnosis, with a greater number of medications used by patients at the CCC (8.46 vs. 3.86, p < .001). Paired testing demonstrated patient‐level increases in the number of medications across the 2 years for the entire cohort (4.16 vs. 7.01, p < .001), as well as at both the CCC (5.11 vs. 8.46, p < .001) and the SNH (2.13 vs. 3.86, p < .001). The proportion of patients with polypharmacy significantly differed by location, with more patients at the CCC meeting criteria for polypharmacy both at time of diagnosis (47.4% vs. 19.4% SNH, p < .001) and at 2 years (77.6% vs. 42.4% SNH, p = .001).
The use of any medications on the ACB scale increased significantly between initial diagnosis and 2 years (30.5% vs. 48.4%, p < .001). The mean ACB score at diagnosis for the entire cohort was 0.55 at diagnosis; by 2 years postdiagnosis, the mean ACB score had increased to 0.88 (p = .03). At the CCC, 32.3% of patients used any ACB medication at diagnosis, compared with 52.3% at 2 years (p < .001), whereas at the SNH, 26.7% used any ACB medication at diagnosis, compared with 40% at 2 years (p = .02). The proportion of patients meeting criteria for severe ACB effect increased at both sites over time, although it only met statistical significance at the CCC (7.7% vs. 18.5%, p = .01; SNH 6.7% vs. 10.0%, p = .63). There were no differences in the mean ACB score between the treatment sites at either diagnosis (CCC 0.63 vs. SNH 0.46, p = .40) or 2 years postdiagnosis (CCC 0.98 vs. SNH 0.67, p = .23). The most commonly used medications on the ACB scale are shown in Table 2.
Table 2. Most common medications on the anticholinergic burden scale by location.
There was a greater proportion of patients over the age of 65, and thus subject to evaluation by the Beers criteria, at the CCC compared with the SNH (33.8% vs. 13.9%, p = .004). The overall proportion of patients using at least two medications on the Beers criteria was 35.1% at diagnosis, increasing to 37.0% at 2 years (p = .91). At diagnosis, 44.4% of patients at the CCC used at least two medications on the Beers criteria, compared with 11.9% at the SNH (p = .07). At 2 years, the proportional differences were nonsignificant (p = .31), although there was attrition of 27% of this subcohort of patients aged at least 65 years.
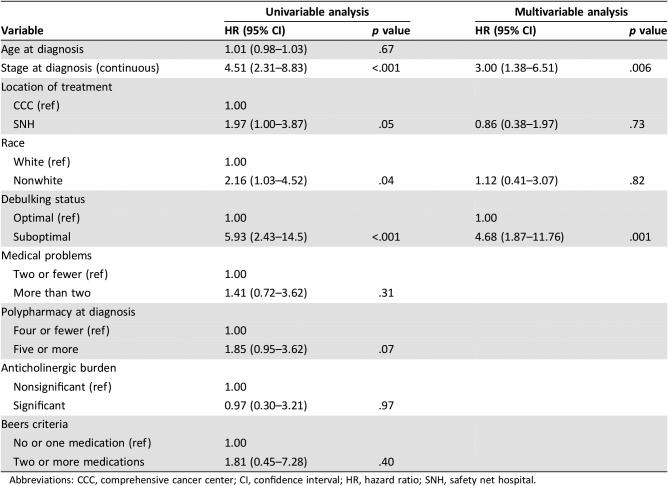
Table 3 shows the Cox proportional hazards models for OS for the entire cohort. Advanced stage at diagnosis, treatment at the SNH, nonwhite race, and suboptimal debulking were all associated with worse OS in the univariable model. Polypharmacy was not a significant predictor (hazard ratio [HR] 1.85 [0.95–3.62], p = .07). In the multivariable model, only advanced stage (HR 3.00 [1.38–6.51], p = .006) and suboptimal debulking (HR 4.68 [1.87–11.76], p = .001) remained independently predictive of poorer OS.
Table 3. Cox analysis of factors affecting overall survival in the entire cohort.
Abbreviations: CCC, comprehensive cancer center; CI, confidence interval; HR, hazard ratio; SNH, safety net hospital.
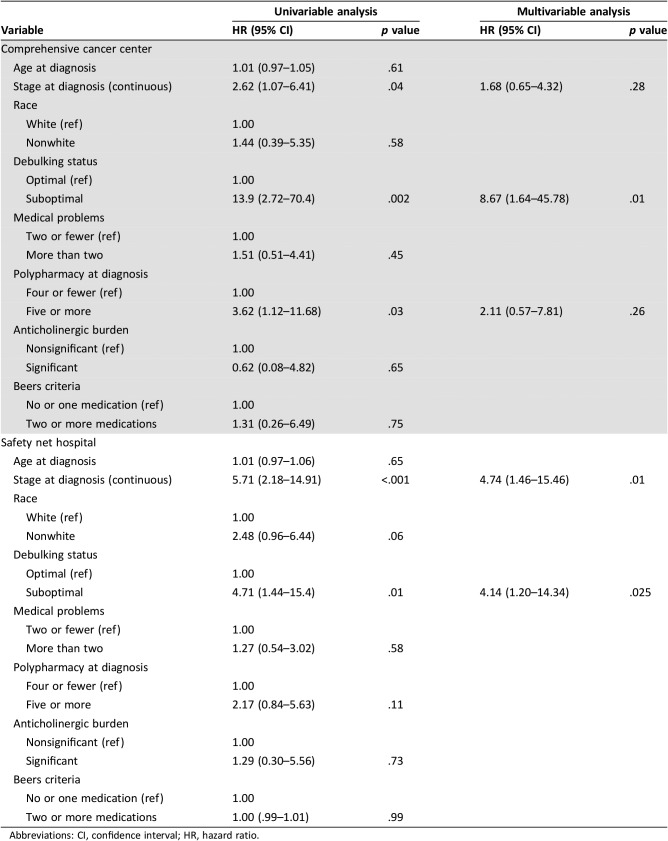
Table 4 shows the Cox proportional hazards models for OS for each individual location. At the CCC, advanced stage at diagnosis, suboptimal debulking, and polypharmacy were each associated with worse OS, although only suboptimal debulking remained significant in the multivariable model (HR 8.67 [1.64–45.8], p = .01). At the SNH, advanced stage at diagnosis and suboptimal debulking were associated with worse OS in the univariable model. Nonwhite race trended toward significance (HR 2.48 [0.96–6.44], p = .06). In the multivariable model, only advanced stage (HR 4.74 [1.46–15.46], p = .01) and suboptimal debulking (HR 4.14 [1.20–14.34], p = .025) remained independent predictors of survival.
Table 4. Cox analysis of factors affecting overall survival in the comprehensive cancer center versus the safety net hospital.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
Polypharmacy was prevalent in our cohort, and we demonstrated significant worsening of polypharmacy over the course of treatment at both hospital sites, with between 48% and 78% of patients using at least five drugs at 2 years postdiagnosis. Although such proportions seem high, our population is not unique. In a large cross‐sectional analysis of oncology patients in Europe, the average number of drugs taken was 7.8, with more than a quarter of the patients taking ten or more medications [16]. Others have reported similar figures, with the mean number of medications taken by elderly patients undergoing outpatient cancer treatment to be 7, with a range of 3–19 [17].
Two main concerns have been proposed in patients with cancer as a result of polypharmacy: significant adverse drug reactions and compromise in survival. In 2018, Goh et al. reported on a group of elderly patients with cancer, noting that the use of five or more medications doubles the risk of a drug complication, such as potential drug‐drug interaction and subtherapeutic dosage, independent of other patient comorbidities [17]. Risk for medication complications has been reported to triple in similar cohorts [18]. A large, multinational study evaluating the use of unneeded drugs in patients with cancer found that 45% of patients received unnecessary drugs, 7% of which were duplicates. In this study, nearly 50% of patient were using a combination of medications known to exacerbate sedation; 53% took medications associated with increased risk for neuropsychiatric syndromes, such as delirium; and 20% took drugs associated with increased risk for cardiac arrhythmia [16].
Although the data are definitive about polypharmacy and increased risk of adverse drug‐drug interactions, data on cancer‐specific survival outcomes are less clear and appear to vary by disease site. For example, a retrospective study evaluating the effect of polypharmacy on outcomes in patients with acute myelogenous leukemia noted lower rates of complete remission and a higher overall mortality rate (HR 2.13 [1.15–3.92]) [9]. Interestingly, inappropriate use of medication by the Beers criteria was not independently associated with clinical outcomes, a finding echoed by our data in patients with ovarian cancer. Other authors have reported no difference in outcomes, although these data do not include patients with gynecologic cancer [19]. In fact, only two studies specifically address polypharmacy in ovarian cancer, and with conflicting results. Freyer et al. [10] reported on a cohort of women undergoing treatment for recurrent ovarian cancer between 1998 and 2003, 41% of whom took at least four medications. Of the subset of women who took six or greater medications, there was a significant negative association with overall survival. In this paper, however, the confidence interval is not reported, and the small number of women taking at least six medications (n = 7) limits the strength of the findings. In contrast, Woopen et al. [20] described a cohort of women who received weekly topotecan for recurrent, platinum‐resistant ovarian cancer. Although these authors reported a high level of polypharmacy (56.3%) with associated increased risk for both hematologic and nonhematologic toxicities, there was no association between polypharmacy and overall survival. However, these were patients with recurrent cancer, and the overall survival assessment was from time of study enrollment, thus making their outcomes incomparable with our own. In our present study, polypharmacy was not an independent predictor of survival in proportional hazards models. However, when included in a multivariable model with such strong predictors as stage and debulking status, the effect of polypharmacy would need to be very significant to have an identifiable effect.
Patients treated at the SNH took fewer medications both at diagnosis and at 2 years, and the medications they most commonly took were different from patients treated at the CCC. One explanation may be that these patients had not been routinely seen within the health care system prior to their diagnoses of cancer. Large, population‐based studies have previously shown that nonwhite patients less frequently have a primary care doctor, have insurance, or seek preventative care visits because of cost [21], [22]. Additionally, minority populations have been shown to vary significantly in self‐perceived health status, knowledge of risk factors for disease, and utilization of screening services [22]. In toto, these data suggest that the lack of medications taken is more a reflection of prior engagement with the health care system. Significant disparities have also been reported in medication acquisition by minority patients, as well, potentially mediated by decreased access to medication by geography. Pharmacy “deserts,” or areas of low pharmacy‐to‐population density, have been reported in a number of neighborhoods with low socioeconomic conditions, thus potentially limiting the ease of filling prescriptions, even if care is sought [23], [24]. Even when pharmacies are accessible, medications may be poorly stocked, thus further limiting medication pick‐up [25]. The finding that patients treated at the SNH did not have antidepressants contributing to polypharmacy at all time points is also not necessarily unexpected, as Hispanics and non‐Hispanic blacks are less likely to be prescribed medication to treat depression than non‐Hispanic white patients and have reported lower rates of utilization even when these drugs are prescribed [26], [27], [28]. In contrast, other data suggest that variables associated with affluence actually increase the risk of polypharmacy, which may explain greater medication use at the CCC. In a cross‐sectional survey reported by Pappa et al. [11], patients with a university education who have multiple annual visits with a physician were more likely to demonstrate polypharmacy. As many of our SNH patients are immigrants, additional confounders may be contributing to medication use or acquisition. Data on immigrant patients and polypharmacy are scarce, although it was been reported in immigrant populations in Australia that confusion about medications is common because of difficulties locating a pharmacist who speaks an immigrant's native language and because of perceived lack of support from practitioners to address medication concerns [29]. It is clear that the social determinants of polypharmacy and equity in health care access are quite complicated, and further investigation is warranted.
Our study has several strengths. The patient population was cared for on the same university campus, by the same group of gynecologic oncologists, thus limiting significant practice pattern variation, which may affect survival outcomes. The population is also diverse, with significant minority representation. Additionally, this is the largest and only study dedicated solely to polypharmacy in patients with ovarian cancer and is unique in that it assesses this variable in a paired fashion at two time points. However, as a retrospective study, our analysis has limitations. First, the electronic medical record systems at the SNH and CCC are different. Although medications are reconciled at every clinic visit, this may introduce a degree of information bias. Secondly this report did not evaluate complementary and alternative medicine (CAM) use. These medications are difficult to track in the EMR and are not listed on either the ACB scale or the Beers criteria for potential toxicity. This is significant because as many as 44% of women with ovarian cancer report taking CAM on a regular basis [30]. An additional limitation is that the number of medications a patient takes is fluid. Medications are commonly started and stopped. Although we assessed medication use at 2 years postdiagnosis, we did not correlate this to active treatment. Medications used on an as‐needed basis were also not able to be classified for this investigation in terms of frequency of use. This may result in an overestimation of polypharmacy, which unfortunately cannot be to be reconciled, as actual medication use is recorded as “yes” or “no” in the EMR, whereas frequency of use is not recorded. The populations were similar regarding proportion of patients who recurred, so it is unlikely that recurrence itself affected medication use. Given the complexity and variety of treatment regimens that can be utilized to treat recurrent disease, there may be some degree of unintended confounding in those patients in active treatment. However, as the most common medications on both the ACB scale and the Beers criteria do not include very many agents used to mediate the effects of chemotherapy (Table 2), we do not feel that this omission detracts from the results. The number of included patients (152 of 339 potentially eligible) may introduce a degree of selection bias. All included patients received their entire treatments at our facilities, and so the patterns of medication prescribing and documentation are consistent among them. We consciously excluded patients who were transfers of care, as the outside medical records for these patients were inconsistently available and would have potentially introduced further information bias. Finally, although we used objective measures of outcome to assess the effects of polypharmacy, we were unable to capture any aberration of quality of life which may result from polypharmacy. It has previously been shown that elderly patients on multiple medications have lower health‐related quality of life [31]. In a population of patients with ovarian cancer, many of whom will succumb to their disease, appreciating what medical interventions may be affecting quality of life is crucial and should be further evaluated.
Conclusion
As the care for patients with ovarian cancer evolves in the era of targeted therapeutics, we will continue to see improvements in survival. Optimizing care in these women is crucial, and policies to address medication safety should be routinely implemented. Aggressive de‐escalation of prescription drug use has been encouraged, with novel approaches to such medication evaluation suggested by a number of authors. For example, Whitman et al. [32] reported on a program in which patients were individually assessed by a pharmacist for medication interaction. Using a three‐tool assessment, 73% of potentially inappropriate medications were deprescribed, an average of three medications per patient, and for an overall estimated savings of $4,282 per patient. The engagement of palliative care physicians, who may better streamline medication prescription in the setting of recurrent disease, may be another avenue by which to reduce polypharmacy. Many EMRs, including those at our institutions, now have flags that alert providers to potential drug‐drug interactions and may also abrogate the risk of inappropriate prescribing.
Our results demonstrate that polypharmacy increases after a diagnosis of ovarian cancer, but we failed to definitively show that polypharmacy has an effect on overall survival. However, understanding what is already known about the potentially detrimental effects of polypharmacy on general health, efforts to optimize safe prescribing practices and to eliminate disparities in medication use and acquisition among patients with ovarian cancer should be undertaken. A simple intervention, such as thorough review of a patient's medications by a member of the care team, may significantly affect outcomes. Further evaluation of sociodemographic factors and their effects on cancer polypharmacy should also be encouraged.
Author Contributions
Conception/design: Matthew Schlumbrecht
Provision of study material or patients: Sean Oldak, Stephanie Ioannou, Priyanka Kamath, Matthew Schlumbrecht
Collection and/or assembly of data: Sean Oldak, Stephanie Ioannou, Priyanka Kamath, Matthew Schlumbrecht
Data analysis and interpretation: Sean Oldak, Matthew Schlumbrecht
Manuscript writing: Sean Oldak, Stephanie Ioannou, Priyanka Kamath, Marilyn Huang, Sophia George, Matthew Schlumbrecht
Final approval of manuscript: Sean Oldak, Stephanie Ioannou, Priyanka Kamath, Marilyn Huang, Sophia George, Matthew Schlumbrecht
Disclosures
The authors indicated no financial relationships.
References
- 1.Gnjidic D, Hilmer S, Blyth F et al. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995. [DOI] [PubMed] [Google Scholar]
- 2.Turner JP, Shakib S, Singhal N et al. Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer 2014;22:1727–1734. [DOI] [PubMed] [Google Scholar]
- 3.Prithviraj GK, Koroukian S, Margevicius S et al. Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. J Geriatr Oncol 2012;3:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol KC, Knudsen JF, Li MM. Polypharmacy in older oncology patients and the need for an interdisciplinary approach to side‐effect management. J Clin Pharm Ther 2007;32:169–175. [DOI] [PubMed] [Google Scholar]
- 5.Hanigan MH, Dela Cruz BL, Thompson DM et al. Use of prescription and nonprescription medications and supplements by cancer patients during chemotherapy: Questionnaire validation. J Oncol Pharm Pract 2008;14:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sganga F, Landi F, Ruggiero C, Corsonello A et al. Polyphamacy and health outcomes among older adults discharged from the hospital: Results from the CRIME study. Geriatr Gerontol Int 2015;15:141–146. [DOI] [PubMed] [Google Scholar]
- 7.Popa MA, Wallace KJ, Brunello A et al. Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 2014;5:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nightingale G, Skonecki E, Boparai M. The impact of polypharmacy on patient outcomes in older adults with cancer. Cancer J 2017;23:211–218. [DOI] [PubMed] [Google Scholar]
- 9.Elliot K, Tooze JA, Geller R et al. The prognostic importance of polypharmacy in older adults treatment with acute myelogenous leukemia (AML). Leuk Res 2014;38:1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freyer G, Geay JF, Touzet S et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol 2005;16:1795–1800. [DOI] [PubMed] [Google Scholar]
- 11.Pappa E, Kontodimopoulos N, Papadopoulos AA et al. Prescribed drug‐utilization and polypharmacy in a general population in Greece: Association with sociodemographic, health needs, health‐services utilization, and lifestyle factors. Eur J Clinc Pharmacol 2011;67:185–192. [DOI] [PubMed] [Google Scholar]
- 12.Karter AJ, Laiteerapong N, Chin MH et al. Ethnic differences in geriatric conditions and diabetes complications among older, insured adults with diabetes: The Diabetes & Aging Study. J Aging Health 2015;27:894–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aging Brain Program of the Indiana University Center of Aging Research , Anticholinergic Cognitive Burden Scale, 2012 Update. https://www.uea.ac.uk/mac/comm/media/press/2011/June/Anticholinergics+study+drug+list. Accessed May 17, 2015.
- 14.Fick DM, Semla TP, Steinman M et al.; 2019. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Zhan C, Sangl J, Bierman AS et al. Potentially inappropriate medication use in the community‐dwelling elderly: Findings from the 1996 Medical Expenditure Panel Survey. JAMA 2001;286:2823–2829. [DOI] [PubMed] [Google Scholar]
- 16.Kotlinska‐Lemieszek A, Paulsen O, Kaasa S et al. Polypharmacy in patients with advanced cancer and pain: A European cross‐sectional study of 2282 patients. J Pain Symptom Manage 2014;48:1145–1159. [DOI] [PubMed] [Google Scholar]
- 17.Goh I, Lai O, Chew L. Prevalence and risk of polypharmacy among elderly patients receiving chemotherapy in an ambulatory oncology setting. Curr Oncol Rep 2018;20:38. [DOI] [PubMed] [Google Scholar]
- 18.Yeoh TT, Tay XY, Si P et al. Drug‐related problems in elderly patients with cancer receiving outpatient chemotherapy. J Geriatr Oncol 2015;6:280–287. [DOI] [PubMed] [Google Scholar]
- 19.Nieder C, Mannsăker B, Pawinski A et al. Polypharmacy in older patients ≥70 years receiving palliative radiotherapy. Anticancer Res 2017;37:795–799. [DOI] [PubMed] [Google Scholar]
- 20.Woopen H, Richter R, Ismaeel F et al. The influence of polypharmacy on grade III/IV toxicity, prior discontinuation of chemotherapy and overall survival in ovarian cancer. Gynecol Oncol 2016;140:554–558. [DOI] [PubMed] [Google Scholar]
- 21.Lau M, Lin H, Flores G. Racial/ethnic disparities in health and health care among U.S. adolescents. Health Serv Res 2012;47:2031–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Y, Bang D, Cosgrove S et al. Surveillance of health status in minority communities ‐ Racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) risk factor survey, United States, 2009. MMWR Surveill Summ 2011;60:1–44. [PubMed] [Google Scholar]
- 23.Amstislavski P, Matthews A, Sheffield S et al. Medication deserts: Survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr 2012;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chisholm‐Burns MA, Spivey CA, Gatwood J et al. Evaluation of racial and socioeconomic disparities in medication pricing and pharmacy access and services. Am J Health Syst Pharm 2017;74:653–668. [DOI] [PubMed] [Google Scholar]
- 25.Morrison RS, Wallenstein S, Natale DK et al. “We don't carry that”—Failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med 2000;342:1023–1026. [DOI] [PubMed] [Google Scholar]
- 26.Lal LS, Miller LA, Arbuckle R et al. Disparities in outpatient antidepressant prescribing patterns and determinants of resource utilization at a tertiary care cancer center. J Support Oncol 2009;7:237–244. [PubMed] [Google Scholar]
- 27.Bengtson AM, Pence BW, Crane HM et al. Disparities in depressive symptoms and antidepressant treatment by gender and race/ethnicity among people living with HIV in the United States. PLoS One 2016;11:e0160738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Rizzo JA. Racial and ethnic disparities in antidepressant use. J Ment Health Policy Econ 2008;11:155–165. [PubMed] [Google Scholar]
- 29.White L, Klinner C. Medicine use of elderly Chinese and Vietnamese immigrants and attitude to home medicines review. Aust J Prim Health 2012;18:50–55. [DOI] [PubMed] [Google Scholar]
- 30.Helpman L, Ferguson SE, Mackean M et al. Complementary and alternative medicine use among women receiving chemotherapy for ovarian cancer in 2 patient populations. Int J Gynecol Cancer 2011;21:587–593. [DOI] [PubMed] [Google Scholar]
- 31.Naveiro‐Rilo JC, Diez‐Juárez D, Flores‐Zurutuza ML et al. Quality of life in the elderly on polymedication and with multiple morbidities [in Spanish]. Rev Esp Geriatr Gerontol 2014;49:158–164. [DOI] [PubMed] [Google Scholar]
- 32.Whitman A, DeGregory K, Morris A et al. Pharmacist‐led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: A pilot study. Support Care Cancer 2018;26:4105–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]