This article examines prognostic factors in patients with advanced cancer, comparing validated factors to determine which are optimal.
Keywords: Prognosis, Inflammation, Survival, Cancer, Palliative
Abstract
Background.
The optimal prognostic factors in patients with advanced cancer are not known, as a comparison of these is lacking. The aim of the present study was to determine the optimal prognostic factors by comparing validated factors.
Materials and Methods.
A multicenter, prospective observational cohort study recruited patients over 18 years with advanced cancer. The following were assessed: clinician‐predicted survival (CPS), Eastern Cooperative Oncology Group performance status (ECOG‐PS), patient reported outcome measures (anorexia, cognitive impairment, dyspnea, global health), metastatic disease, weight loss, modified Glasgow Prognostic Score (mGPS) based on C‐reactive protein and albumin, lactate dehydrogenase (LDH), and white (WCC), neutrophil (NC), and lymphocyte cell counts. Survival at 1 and 3 months was assessed using area under the receiver operating curve and logistic regression analysis.
Results.
Data were available on 478 patients, and the median survival was 4.27 (1.86–7.03) months. On univariate analysis, the following factors predicted death at 1 and 3 months: CPS, ECOG‐PS, mGPS, WCC, NC (all p < .001), dyspnea, global health (both p ≤ .001), cognitive impairment, anorexia, LDH (all p < .01), and weight loss (p < .05). On multivariate analysis ECOG‐PS, mGPS, and NC were independent predictors of survival at 1 and 3 months (all p < .01).
Conclusion.
The simple combination of ECOG‐PS and mGPS is an important novel prognostic framework which can alert clinicians to patients with good performance status who are at increased risk of having a higher symptom burden and dying at 3 months. From the recent literature it is likely that this framework will also be useful in referral for early palliative care with 6–24 months survival.
Implications for Practice.
This large cohort study examined all validated prognostic factors in a head‐to‐head comparison and demonstrated the superior prognostic value of the Eastern Cooperative Oncology Group performance status (ECOG‐PS)/modified Glasgow Prognostic Score (mGPS) combination over other prognostic factors. This combination is simple, accurate, and also relates to quality of life. It may be useful in identifying patients who may benefit from early referral to palliative care. It is proposed ECOG‐PS/mGPS as the new prognostic domain in patients with advanced cancer.
Background
“How long have I got?”
This is often the first question patients ask when they are told that their cancer is incurable. Most clinicians find this challenging to answer but rely on their experience, clinical intuition, and the possible outcome of therapies when considering their answer. Of course, some patients may not want to know their likely outcome. Either way, it is important that the clinician has an awareness of the likely survival as this will inform important decisions around appropriateness of anticancer therapy [1], [2] and place of care [3]. It may also relieve patient and family anxiety associated with prognostic uncertainty [3].
In patients with advanced cancer, measures of performance status (e.g., Eastern Cooperative Oncology Group performance status [ECOG‐PS]) remain the most reliable prognostic factor. These are widely used in oncology practice to help inform important decisions but have been criticized as being subjective, inaccurate, and overly optimistic [4].
Since 2005, there has been a clear drive to try and augment prognostic accuracy, with several clinical and biomarkers being identified as having prognostic value [5], [6], [7]. Clinical markers with prognostic value include anorexia, cognitive impairment, dyspnoea, global health, and weight loss in the last 3 months [5], [8]. Biomarkers include lactate dehydrogenase (LDH), white (WCC), neutrophil (NC) and lymphocyte (LC) cell counts, and the modified Glasgow Prognostic Score (mGPS; a combination of C‐reactive protein [CRP] and albumin). The mGPS measures the inflammatory response and has demonstrated the role of the host‐tumour inflammatory response in prognosis [9] in addition to its established role in tumour genesis and quality of life [10], [11].
Although such factors have been identified and advocated, it is not clear which factors are optimal because a comparison has not, to date, been done. In particular, it is not known how established prognostic markers such as performance status compare to newer clinical and biomarkers. Therefore, the aim of the present study was to compare prospectively, validated prognostic factors in a cohort of patients with advanced cancer.
Materials and Methods
Study Population
Adult patients (≥18 years) were recruited from either one of nine regional cancer centers or one of seven specialist palliative care units in the U.K. (listed in Acknowledgments). Patients had cancer that was defined as incurable. This encompassed metastatic cancer (histological, cytological, or radiological evidence), nondisseminated cancer (e.g., glioblastoma multiforme), locally advanced cancer (e.g., pancreatic cancer), or hematological malignancies that were being treated/previously been treated with anticancer therapy with palliative intent. Eligible patients also were able to complete study questionnaires, provide a venous blood sample, and have performance status of 1–4 (ECOG) as agreed by their treating clinician. Patients were excluded if they had breast or prostate carcinoma with only bone metastases, as in some cases their survival may be many years. Patients were either inpatients or outpatients, undergoing anticancer therapy or not. Adjustments were made for age, sex, and having lung, gastrointestinal, or other cancers and the analysis was adjusted accordingly. There were no protocol modifications. The study had ethics committee approval (U.K.–12/SS/0181) and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The study adhered to the STROBE guidelines for cohort studies.
Centers were opened on a staggered basis. At each center, consecutive patients who met the eligibility criteria were invited to participate (sequential sampling) and consented, reducing selection bias. All assessments, including blood sampling, were done on the day of consent.
Prognostic Markers
Patient's age, sex, and demographics were recorded, as were details of underlying disease including metastases. The prognostic tools and factors examined in the present prospective study were those previously identified from a systematic review undertaken by our group [6]. In brief, these prognostic tools and factors had been validated in adult patients with advanced cancer (n > 100).
Clinical Markers.
Clinician Predicted Survival (CPS) and ECOG‐PS, the presence of metastases, and weight loss (in the previous 3 months) were assessed by the treating clinician. The patient reported outcome measures (PROMs) dyspnea, global health, cognitive impairment, and anorexia were assessed by the patient using the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C‐30 (EORTC QLQ‐C30) [12].
Biomarkers.
Biomarkers were CRP and albumin (combined in the mGPS), LDH, WCC, NC and LC.
Statistical Analysis
The primary outcome was to compare the prognostic value of the aforementioned factors. The secondary outcomes were to assess if such factors had independent prognostic value and could be combined to improve prediction of survival at 1 and 3 months from study entry. These time points were chosen as clinically relevant for the management of patients with advanced cancer [6], [13], [14].
The primary outcome was to compare the prognostic value of the aforementioned factors on survival at 1 and 3 months using the area under the receiver operator curve method. The sample size was based on a systematic review to identify key prognostic markers in patients with advanced cancer [6]. Based on this information, a sample size of approximately 500 patients would have the power to reliably assess the prognostic value of approximately 10 variables [15].
The following were grouped according to specific thresholds. The mGPS was grouped as CRP ≤10 mg/L = 0, CRP >10 mg/L = 1, CRP >10 mg/L, and albumin <35 g/L = 2 [16]. Weight loss was grouped as <2.5%, 2.5%–5.9%, 6%–10.9%, 11%–14.9%, and >15% according to thresholds described by Martin et al. [17]. WCC, NC, and LC were categorized as above or below/equal to normal limits [18]. LDH was classified as abnormal if >250 U/L [19]. EORTC QLQ‐C30 scores were calculated using scoring procedures as described by Aaronson et al. [12]. EORTC QLQ‐C30 scales were analyzed as discrete categories representing underlying continuous constructs, and PROMs (symptoms and quality of life variables) were defined as being present if the score was greater than 50 [20]. CPS was categorized into days (≤14 days), weeks (15–56 days), or months (≥57 days). For categorical variables with more than two categories (e.g., mGPS, ECOG‐PS, weight loss, CPS), these were treated as continuous variables in terms of hazard ratios in line with their proven prognostic value [5].
All patients were followed up until death or study censoring, which was the date of the end of the study (March 7, 2015) and 9 months after the last patient was recruited. The survival time was defined as the number of months from study entry until death, or censored if patients were alive at follow‐up date. Area under the receiver operating curve (AUC) was measured to assess survival at 1 and 3 months. Univariate logistic regression was used to examine whether the prognostic factors were predictive of death at 1 month and 3 months postconsent. Multivariate survival analysis was done using a stepwise backward conditional procedure to derive a final model of prognostic factors that had a significant independent relationship with survival at 1 month and 3 months. Only variables with a univariate p < .1 were considered in the model.
To examine the relationship between ECOG‐PS, mGPS, and quality of life a series of X2 tests for trend were used. Quality of life was calculated using the summary score of the EORTC QLQ‐C30 with a maximum score of 100 [21].
All statistical testing was done at the 5% significance level with 95% confidence intervals reported. In order to adjust for multiple comparisons in the present study a p value of <.01 was considered significant. All analyses were performed using IBM SPSS Version 23.0 (SPSS, Chicago, IL). Where appropriate, mean and SD or median and interquartile range (IQR) are reported. Patients who have missing survival time endpoint (i.e., last known date alive or date of death) are not included in the analyses.
The study design was developed in conjunction with lay members of the National Cancer Research Institute palliative care clinical studies development group, including cancer survivors.
Results
Between 24 January, 2013, and 25 September, 2014, 563 patients were screened, with 539 recruited, and core data on ECOG‐PS was available for 478 (88.7%). The clinicopathological characteristics of patients are shown in Table 1. The mean (SD) age was 67.04 (12.08) years, and 256 (54%) patients were female. The minimum and median (IQR) follow‐up for survivors was 0 days and 198 (137–273) days, respectively. When study data collection stopped, 194 (41%) patients were alive. The median (IQR) survival was 4.27 (1.86–7.03) months. The most common cancer type was lung, present in 177 (37%) patients, and metastases were present in 377 (85%) patients.
Table 1. Clinicopathological characteristics of patients with advanced cancer (n = 478).
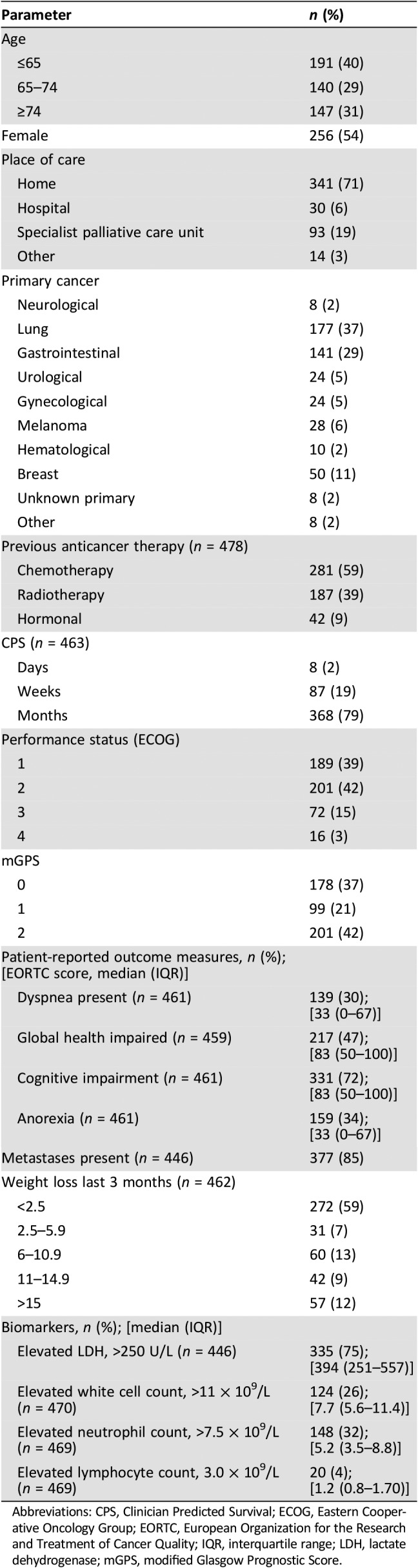
Abbreviations: CPS, Clinician Predicted Survival; ECOG, Eastern Cooperative Oncology Group; EORTC, European Organization for the Research and Treatment of Cancer Quality; IQR, interquartile range; LDH, lactate dehydrogenase; mGPS, modified Glasgow Prognostic Score.
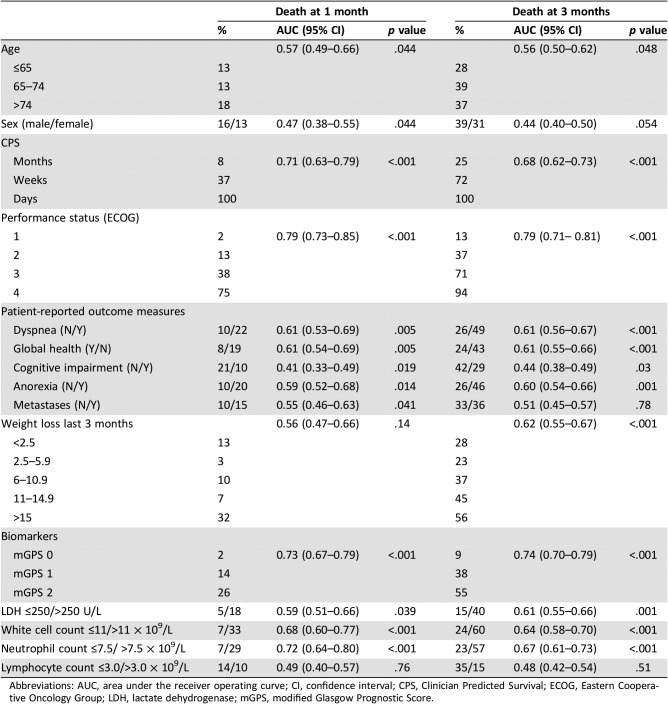
The relationship between clinical markers and biomarkers and survival at 1 and 3 months, using the AUC, is shown in Table 2. ECOG‐PS and mGPS had the highest AUC for survival at 1 month: ECOG‐PS 0.79 (95% confidence interval [CI], 0.73–0.85; p < .001) and mGPS 0.73 (95% CI, 0.67–0.79; p < .001). ECOG‐PS and mGPS had the highest AUC for survival at 3 months: ECOG‐PS 0.79 (95% CI, 0.71–0.81; p < .001) and mGPS 0.74 (95% CI, 0.70–0.79; p < .001).
Table 2. The relationship between prognostic factors and survival (1 month and 3 months) in patients with advanced cancer.
Abbreviations: AUC, area under the receiver operating curve; CI, confidence interval; CPS, Clinician Predicted Survival; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; mGPS, modified Glasgow Prognostic Score.
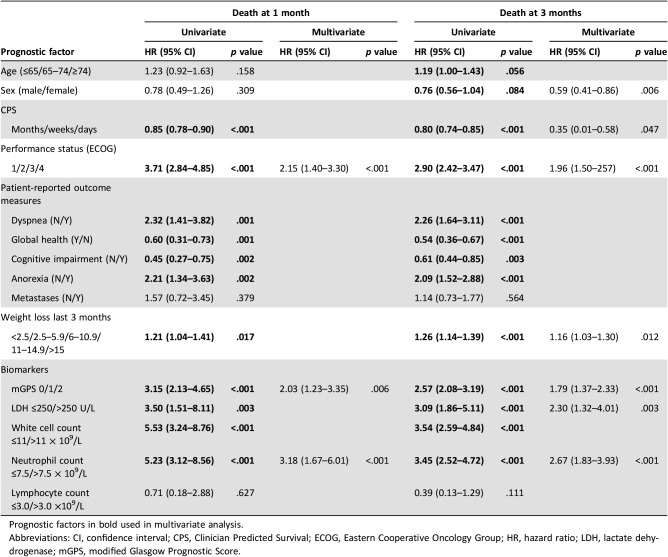
The univariate and multivariate analysis of survival at 1 and 3 months is shown in Table 3. The following independently predicted death at 1 month: ECOG‐PS (hazard ratio [HR], 2.15; 95% CI, 1.40–3.30; p < .001), mGPS (HR, 2.03; 95% CI, 1.23–3.35; p = .006), and NC (HR, 3.18; 95% CI, 1.67–6.01; p < .001). The following independently predicted death at 3 months: ECOG‐PS (HR, 1.91; 95% CI, 1.47–2.49; p < .001), mGPS (HR, 1.77; 95% CI, 1.36–2.31; p < .001), weight loss (HR, 1.15; 95% CI, 1.03–1.29; p = .013), LDH (HR, 2.00; 95% CI, 1.15–3.47; p = .013), and WCC (HR, 2.50; 95% CI, 1.71–3.66; p < .001).
Table 3. The relationship between prognostic factors and survival (1 and 3 months) in patients with advanced cancer: univariate and multivariate analysis.
Prognostic factors in bold used in multivariate analysis.
Abbreviations: CI, confidence interval; CPS, Clinician Predicted Survival; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; mGPS, modified Glasgow Prognostic Score.
The percentage survival ± SE at 1 and 3 months, as per the factors which are the strongest predictors of survival on multivariate analysis, is shown in Table 4. To illustrate, survival at 1 month ranges from 98% ± 1 ECOG‐PS 1 to 25% ± 11 ECOG‐PS 4. It is of note that the factors with the greatest discrimination in survival at 1 and 3 months are ECOG‐PS and the mGPS.
Table 4. Percentage of survival ± SE at 1 and 3 months as per CPS, ECOG‐performance status, modified Glasgow Prognostic Score, lactate dehydrogenase, and neutrophil count categories.
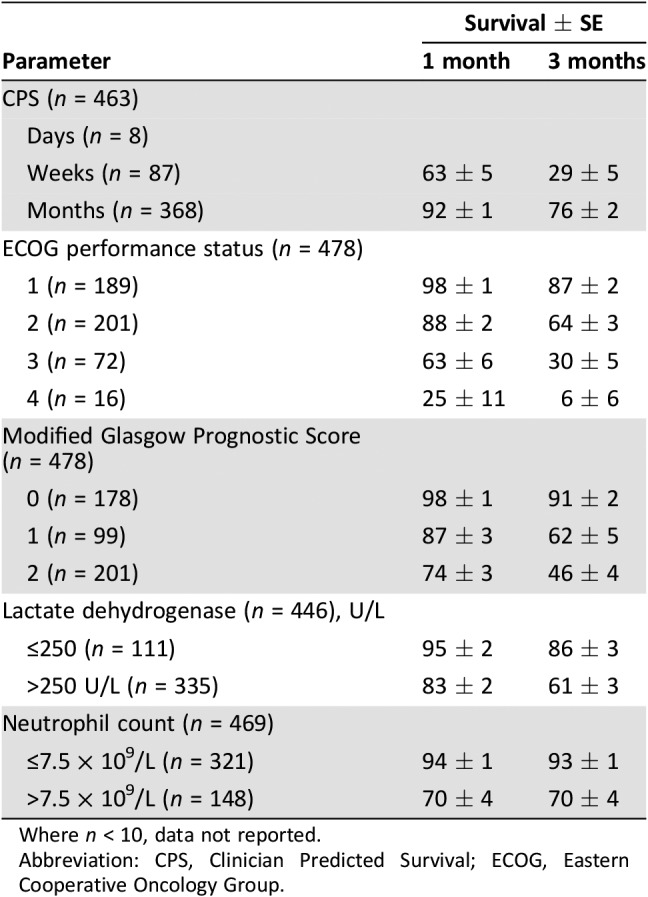
Where n < 10, data not reported.
Abbreviation: CPS, Clinician Predicted Survival; ECOG, Eastern Cooperative Oncology Group.
The relationship between ECOG‐PS, mGPS, and quality of life was also examined (data not shown). Decreasing PS and increasing inflammation were independently associated with deteriorating quality of life. In combination mean quality of life scores ranged from 80 (ECOG‐PS 1, mGPS 0) to 46 (ECOG‐PS 3, mGPS 2), p < .001; the combination providing a greater differentiation of quality of life scores than each component in isolation (ECOG‐PS or mGPS) in isolation.
Discussion
A number of clinical and biomarkers predict 1 and 3 month survival in patients with advanced cancer. The present study compares these prospectively and shows the superior prognostic value of several key prognostic factors, including performance status and biomarkers of the inflammatory response (e.g., mGPS). It is of interest that markers of the inflammatory response compare favorably to performance status in terms of survival prediction. In the present study, the prognostic value of ECOG‐PS and mGPS was retained when adjusted for age, sex, and cancer location. Given that both ECOG‐PS and mGPS have been extensively validated in both observational and randomized clinical trial settings, it is likely that this prognostic framework will be clinically useful in the majority of common solid tumors.
The results of the present study should reassure clinicians of the value of performance status in prognostication. However, the findings should also alert clinicians that patients who have a good performance status and systemic inflammation are at increased risk of poorer quality of life and survival. The ECOG‐PS/mGPS framework reported herein has been validated previously by our group and could be used to stratify patients who may benefit from referral to palliative care services [11], [13].
In patients with advanced cancer, the decision is often not “can we treat with anticancer therapy?” but rather “should we treat with anticancer therapy?” and accurate assessment of prognosis is a key consideration in this regard. Using robust prognostic factors (e.g., biomarkers of the inflammatory response) in combination with clinical judgement may help inform decisions regarding appropriate place for end of life care (e.g., hospice admission), whereas the latter may inform the decision to continue with anticancer therapy (e.g., radiotherapy for painful bone metastases) or clinical trial participation.
To our knowledge, this is the first report to prospectively compare validated prognostic factors for use in patients with advanced cancer. It was of interest that a number of validated prognostic factors did not, on multivariate analysis, retain independent prognostic value at 1 and 3 months. For example, weight loss was less prominent as an independent prognostic factor than might be anticipated from the literature pertaining to the cachexia associated with cancer. Indeed, the prominence of weight loss as the cornerstone of palliation in patients with advanced cancer has been questioned [22], [23], [24]. Furthermore, PROMs often associated with reduced survival (dyspnea, global health, cognitive impairment, and anorexia) were less prominent suggesting that their prognostic value is dependent at least in part, on physical function and the systemic inflammatory response. Indeed, significant associations have been reported between such PROMs, physical function, and systemic inflammation in large independent cohorts [8], [11]. If this were to be the case, then improvement in physical function [25] and moderation of the systemic inflammatory response [26] should result in an improvement of such PROMs.
This would be in keeping with observations that the systemic inflammatory response has prognostic value in nonmalignant conditions with recent work supporting the inflammatory hypothesis of atherosclerosis [27]. Although in cancer the use of anti‐inflammatory therapies in a tumoricidal role is increasing, the sound argument of targeting the inflammatory response to influence survival in cancer, has by in large been neglected. The results from the present study support the hypothesis that the inflammatory response is a key driving factor in survival in cancer and the need for an increased recognition its importance as a therapeutic target for palliation in patients with advanced cancer [11], [28].
Recently, a number of groups have carried out a prospective comparison of validated prognostic factors in patients in the palliative care setting. For example, Baba and coworkers reported, in a multicenter study of approximately 2,500 patients in a variety of palliative care settings, the feasibility and accuracy of the PaP score, D‐PaP score, PPI, and modified PiPS model, including patients receiving chemotherapy [7]. The most important finding was that all prediction tools investigated in the study, PaP score, D‐PaP score, PPI, and modified PiPS model, can differentiate subgroups with different survival profiles in all palliative care settings. Similarly, Hui and coworkers reported, in a study of approximately 200 patients with advanced cancer, that PaP score was more accurate than CPS, and the addition of CPS to the prognostic model reduced its accuracy [29].
Therefore, it is clear that the prognostic models PPI and PaP have indeed reliable prognostic value. However, these prognostic models include ECOG‐PS and the PaP also includes CPS. Indeed, the prognostic value of these models depend largely on the assessment of functional status as a core component and ECOG‐PS, compared with the sparse use of PPI and PaP tools, is used extensively in routine clinical practice [6]. Therefore, in addition to ECOG‐PS, a number of core prognostic variables that were identified in a recent systematic review, including the mGPS, were included in the present analysis. [6] This approach has the advantage of identifying core prognostic variables that add substantially to ECOG‐PS and thereby simplifying the patient assessment.
In the present study, after ECOG‐PS and mGPS, CPS was the third and fourth most significant factor predicting death at 1 and 3 months, respectively. Although because of its subjective nature CPS is often reported as being inaccurate, the present results confirm the clinical utility of the CPS. In the context of the integration of palliative care and oncology the combination of CPS and either ECOG‐PS or mGPS was not examined in the present study
The primary outcome was a direct comparison of variables previously identified to have prognostic value in patients with advanced cancer. It was clear from the present analysis that a number of prognostic variables had AUC with overlapping 95% CI. This perhaps is not surprising because, for example, performance status is a key component of the CPS and a number of prognostic tools in patients with advanced cancer [6]. With the increasing integration of oncology and palliative care [30], it is likely that performance status will increasingly form the cornerstone of outcome prediction.
Limitations
The present study had a number of limitations. As the majority of patients were under the care of palliative care services, it may be assumed they had a high symptom burden, which itself may be an indicator of a shorter prognosis. Furthermore, although recruitment was across 16 centers, the present cohort may not be entirely representative but was well defined in terms of the components of validated prognostic scores. This will allow comparison with other populations in future studies. Another limitation is that the recruitment/sampling strategy was opportunistic; however, the heterogeneity of the primary cancer types herein would support the findings in multiple tumor types. Patients with delirium based on clinical assessment were not included in the present study. However, formal screening for delirium was not carried out as part of eligibility assessment, and it is possible that patients with hypoactive delirium were not identified and therefore may have been recruited as part of the study. In the present study, the EORTC QLQ‐30 was used to assess symptoms and cognitive impairment. Although this has been used and validated extensively, there are now more targeted and sensitive tools available. Further studies are required to examine whether such tools enhance the ECOG‐PS/mGPS framework.
Another limitation of the present study is that some of the variables studied (e.g., symptoms) were dichotomized based on 50% because the ideal cutoff was not clear from the literature. This was a pragmatic approach to examine whether the variable had prognostic value.
The median survival of the studied population was 4 months and therefore the efficacy of the ECOG‐PS/mGPS framework may not be useful in other cohorts of patients with advanced care that have a different survival profile. However, given the simplicity and objective nature of the framework, it is likely that it will tested extensively in different patient cohorts [31].
Conclusion
In the first prospective comparison of validated prognostic factors, most factors predicted survival; however, the superior value of performance status and biomarkers of the inflammatory response (mGPS, neutrophil count) were demonstrated. Moreover, combining clinical factors with biomarkers (e.g., performance status with the mGPS) has been reported to have a differential impact on quality of life [11], [13]. This framework should alert clinicians to patients who are at increased risk of dying but may also have a higher symptom burden.
Acknowledgments
The IPAC Study Group was composed of Dr. G. Lingesan, Dr. A. Franks, Dr. V. Chaitanya, Dr. A. Chauhan, Prof. N. Stuart, Dr. C. Ross, Dr. R. Isherwood, Prof. M. Johnson, Dr. H. Zacharias, Dr. A. Gould, Dr. C. Turner, and Dr. Durrani.
We thank the staff and patients in the following centers, where the study was conducted. Scotland: Beatson West of Scotland Cancer Centre, Glasgow; Edinburgh Cancer Centre, Edinburgh; St Andrews Hospice, Airdrie; Strathcarron Hospice, Denny; Prince and Princes of Wales Hospice, Glasgow; England: St Gemma's Hospice, Leeds; Nightingale MacMillan Unit, Royal Derby Hospital, Derby; John Eastwood Hospice, Mansfield,; Hayward House MacMillan Specialist Palliative Care Unit, Nottingham; University Hospitals Coventry and South Warwickshire NHS Trust, Coventry; Scarborough General Hospital, Scarborough; Wales: Glan Clywyd Hospital, Bodelwyddan, Wales; Wrexham Maelor Hospital, Wrexham; Ysbyty Gwynedd, Penrhorsgarnedd, Bangor; Neath Port Talbot Hospital, Abertawe; Bronglais General Hospital, Aberystwyth, Wales; We also thank lay members of the National Cancer Research Institute palliative care clinical studies development group (including Tom Haswell and Sharon Paradine) who informed the design of the study.
This work was supported by Medical Research Scotland (Grant Number 487 FRG).
Deceased.
Contributed equally.
Joint senior authors.
Contributor Information
Barry J. Laird, Email: barry.laird@ed.ac.uk.
Collaborators: on behalf of the IPAC Study Group, Dr. G. Lingesan, Dr. A. Franks, Dr. V. Chaitanya, Dr. A. Chauhan, Prof. N. Stuart, Dr. C. Ross, Dr. R. Isherwood, Prof. M. Johnson, Dr. H. Zacharias, Dr. A. Gould, Dr. C. Turner, and Dr. Durrani
Author Contributions
Conception/design: Donald C. McMillan, Kenneth C. Fearon, Marie Fallon, Barry J. Laird
Collection and/or assembly of data: Claribel Simmons
Manuscript writing: Donald C. McMillan, Alistair McKeown, Mike Bennett, Claire O'Neill, Andrew Wilcock, Caroline Usborne, Barry J Laird
Final approval of manuscript: Claribel Simmons, Donald C. McMillan, Sharon Tuck, Cat Graham, Alistair McKeown, Mike Bennett, Claire O'Neill, Andrew Wilcock, Caroline Usborne, Kenneth C. Fearon, Marie Fallon, Barry J. Laird
Provided statistical support: Sharon Tuck, Cat Graham
Principal investigators: Marie Fallon, Barry J. Laird
Disclosures
The authors indicated no financial relationships.
References
- 1.Blanke CD, Fromme EK. Chemotherapy near the end of life: First–and third and fourth (line)–do no harm. JAMA Oncol 2015;1:785–786. [DOI] [PubMed] [Google Scholar]
- 2.Earle CC, Neville BA, Landrum MB et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–321. [DOI] [PubMed] [Google Scholar]
- 3.Steinhauser KE, Christakis NA, Clipp EC et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284:2476–2482. [DOI] [PubMed] [Google Scholar]
- 4.Fairclough DL, Cella DF. Eastern Cooperative Oncology Group (ECOG). J Natl Cancer Inst Monogr 1996:73–75. [PubMed] [Google Scholar]
- 5.Maltoni M, Caraceni A, Brunelli C et al. Prognostic factors in advanced cancer patients: Evidence‐based clinical recommendations—A study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol 2005;23:6240–6248. [DOI] [PubMed] [Google Scholar]
- 6.Simmons CPL, McMillan DC, McWilliams K et al. Prognostic tools in patients with advanced cancer: A systematic review. J Pain Symptom Manage 2017;53:962–970.e910. [DOI] [PubMed] [Google Scholar]
- 7.Baba M, Maeda I, Morita T et al. Survival prediction for advanced cancer patients in the real world: A comparison of the palliative prognostic score, delirium‐palliative prognostic score, palliative prognostic index and modified prognosis in palliative care study predictor model. Eur J Cancer 2015;51:1618–1629. [DOI] [PubMed] [Google Scholar]
- 8.Laird BJ, McMillan DC, Fayers P et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. The Oncologist 2013;18:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan R, McSorley S, Horgan PG et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta‐analysis. Crit Rev Oncol Hematol 2017;116:134–146. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill FR, Mantovani A. Cancer‐related inflammation: Common themes and therapeutic opportunities. Semin Cancer Biol 2012;22:33–40. [DOI] [PubMed] [Google Scholar]
- 11.Laird BJ, Fallon M, Hjermstad MJ et al. Quality of life in patients with advanced cancer: Differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 13.Laird BJ, Kaasa S, McMillan DC et al. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation‐based prognostic system. Clin Cancer Res 2013;19:5456–5464. [DOI] [PubMed] [Google Scholar]
- 14.Gwilliam B, Keeley V, Todd C et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: Prospective cohort study. BMJ Support Palliat Care 2015;5:390–398. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE. Regression modelling strategies. New York: Springer‐Verlag, 2001. [Google Scholar]
- 16.McMillan DC. The systemic inflammation‐based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 17.Martin L, Senesse P, Gioulbasanis I et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 18.Leitch EF, Chakrabarti M, Crozier JE et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer 2007;97:1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Yao YH, Li BG et al. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta‐analysis. Sci Rep 2015;5:9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayers P, Aaronson N, Bjordal K et al. The EORTC QLQ‐C30 Scoring Manual. Brussels, Belgium: European Organisation for the Research and Treatment of Cancer, 2001:5–15.11432756 [Google Scholar]
- 21.Giesinger JM, Kieffer JM, Fayers PM et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ‐C30 is robust. J Clin Epidemiol 2016;69:79–88. [DOI] [PubMed] [Google Scholar]
- 22.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–226. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald N. Terminology in cancer cachexia: Importance and status. Curr Opin Clin Nutr Metab Care 2012;15:220–225. [DOI] [PubMed] [Google Scholar]
- 24.Bozzetti F, Santarpia L, Pironi L et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: A multi‐centre observational study with prospective follow‐up of 414 patients. Ann Oncol 2014;25:487–493. [DOI] [PubMed] [Google Scholar]
- 25.Idorn M, Thor Straten P. Exercise and cancer: From “healthy” to “therapeutic”? Cancer Immunol Immunother 2017;66:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diakos CI, Charles KA, McMillan DC et al. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Everett BM, Thuren T et al; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 28.Roxburgh CS, McMillan DC. Cancer and systemic inflammation: Treat the tumour and treat the host. Br J Cancer 2014;110:1409–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui D, Park M, Liu D et al. Clinician prediction of survival versus the palliative prognostic score: Which approach is more accurate? Eur J Cancer 2016;64:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaasa S, Loge JH, Aapro M et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol 2018;19:e588–e653. [DOI] [PubMed] [Google Scholar]
- 31.Pantano Nde P, Paiva BS, Hui D et al. Validation of the modified Glasgow Prognostic Score in advanced cancer patients receiving palliative care. J Pain Symptom Manage 2016;51:270–277. [DOI] [PubMed] [Google Scholar]