Honest prognoses discussions with advanced cancer patients are important conversations for clinicians. This article reports on patient preferences for the wording of prognostic information.
Keywords: Cancer, Prognosis, Preferences, Explicitness, Range
Abstract
Introduction.
Although various phrases to communicate prognoses based on a certain concept have been proposed, no study has systematically investigated preferences of patients with cancer for actual phrases. We investigated whether phrases with a wider range and additional “hope for the best, and prepare for the worst” (hope/prepare) statement would be more preferable and explored variables associated with patients’ preferences.
Materials and Methods.
In a cross‐sectional survey, 412 outpatients with cancer self‐assessed their preferences for 13 phrases conveying prognostic information (e.g., phrases with or without median, typical range, and/or best/worst cases, and those with or without a hope/prepare statement) on a 6‐point scale (1 = not at all preferable; 6 = very preferable). We evaluated demographic data and the Coping Inventory for Stressful Situations and conducted multivariate regression analysis.
Results.
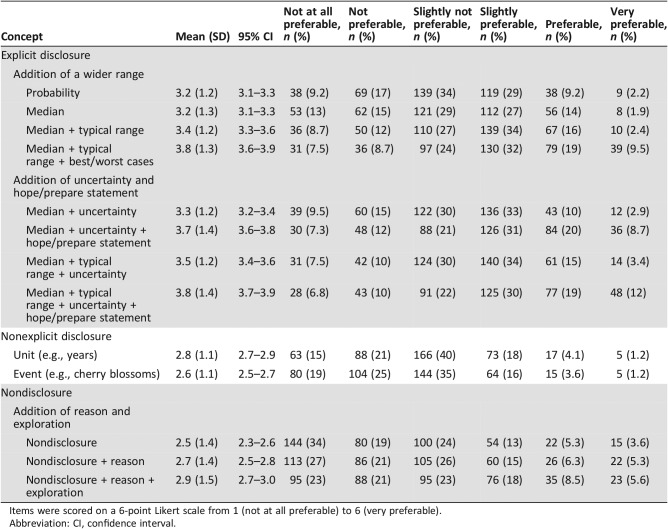
Regarding phrases with various ranges, the one including the median, typical range, and best/worst cases was more preferable (mean ± SD, 3.8 ± 1.3; 95% confidence interval [CI], 3.6–3.9) than the one with the median and typical range (3.4 ± 1.2; 3.3–3.6) or the one with only the median (3.2 ± 1.3; 3.1–3.3). Concerning the hope/prepare statement, the phrase including the median, typical range, uncertainty, and hope/prepare statement was more preferable (3.8 ± 1.4; 3.7–3.9) than the one without the statement (3.5 ± 1.2; 3.4–3.6). In multivariate analyses, task‐oriented coping was significantly correlated with preferences for phrases with explicit information.
Conclusion.
Overall, phrases with a wider range and the hope/prepare statement were preferable to those without them. When patients with cancer ask about prognoses, especially those with task‐oriented coping, clinicians may provide explicit information with a wider range and the hope/prepare statement.
Implications for Practice.
Discussing prognoses with patients with advanced cancer is among the most important conversations for clinicians. In this cross‐sectional survey to systematically investigate preferences of 412 patients with cancer for phrases conveying prognostic information, phrases with the median, typical range, and best/worst cases and those with the “hope for the best and prepare for the worst” (hope/prepare) statement were the most preferred. When patients with cancer ask about prognoses, clinicians may provide explicit information with a wider range and include the hope/prepare statement.
Introduction
Discussing prognoses with patients with advanced cancer is among the most important conversations for clinicians [1]. The majority of patients with cancer want to receive prognostic information [2]. Honest prognostic discussion enables patients to have an accurate understanding of their illness and realistic prognostic awareness and helps patients and their families make informed decisions [3], [4], [5], [6]. Several guidelines have recommended early and honest discussions about communication with patients with advanced cancer [1], [7], [8]. Yet clinicians tend to convey overoptimistic information or never discuss prognoses with patients with advanced cancer [9], [10]. Among the multiple barriers to effective communication are physicians’ discomfort with delicate communication and potentially varying preferences of patients with different coping styles [9], [10], [11]. Thus, systematically understanding preferences of patients with cancer for various phrases in prognostic discussions and factors contributing to their preferences may help oncologists feel more comfortable when communicating prognoses.
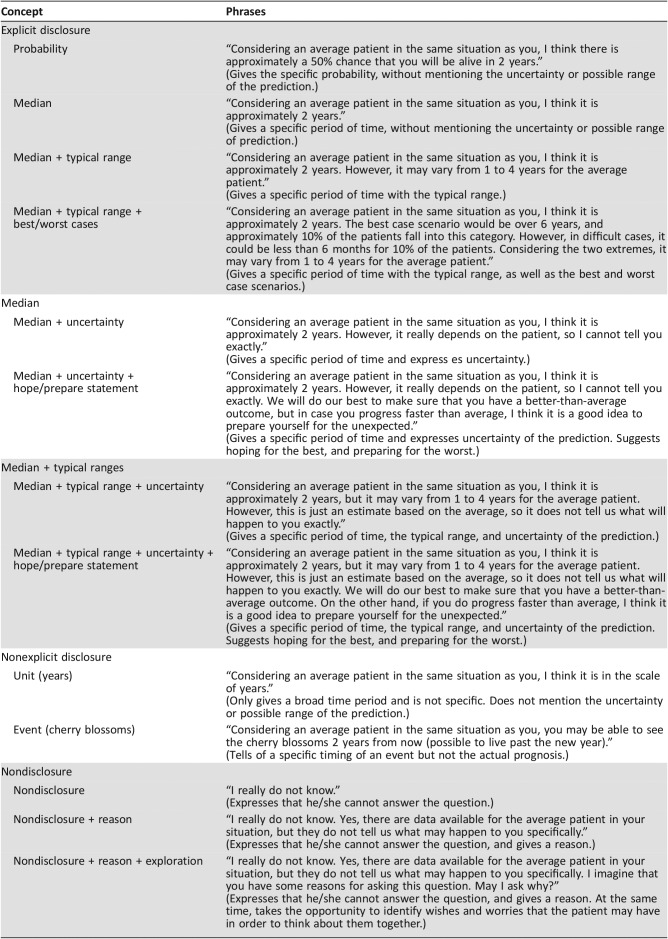
Prior studies have suggested various concepts with or without example phrases when discussing prognoses and examined preferences of patients with cancer for some of them: explicit disclosure such as median survival (temporal), typical range (a half to double the median) [11], [12], [13], best/worst cases (a quarter to 3–4 times as long as the median) [14], [15], [16], [17], [18], or probability of living for a certain period (probabilistic) [19], [20], [21], [22], [23]; nonexplicit disclosure such as possibility of living until a certain event (e.g., birthday, anniversary) [13] and unit of time frames (e.g., months, years) [13], [20]; and nondisclosure [11], [13]. Also suggested were the importance of the exploration of patients’ information needs [1], [8], additional explanation (e.g., uncertainty and limitations involved [13], [20]), and a positive statement (e.g., “hope for the best, and prepare for the worst” [hope/prepare] [8], [24]).
However, to our knowledge, no study has systematically investigated patients’ preferences for actual phrases to communicate prognostic information based on these concepts. Furthermore, little is known about what underlying characteristics determine patient preferences for phrases that provide prognostic information. We hypothesized that phrases conveying explicit information with a wider range and those with the additional hope/prepare statement would be more preferable given their novelty [14], [15], [16], [17], [18] and clinical importance in maintaining hope [8], [24], respectively, and patients’ coping styles would contribute to their preferences for phrases with or without explicitness.
Thus, the primary aim of our study was to systematically investigate preferences of patients with cancer for phrases conveying prognostic information with a variety of concepts. Specifically, we examined whether phrases with explicit information with a wider range and those with the additional hope/prepare statement would be more preferable. We also explored whether patients’ underlying coping styles are associated with their preferences for these phrases.
Subjects, Materials, and Methods
Participants and Procedure
We conducted a cross‐sectional web‐based anonymous survey involving patients with cancer in February 2018. Inclusion criteria were (a) patients with cancer being followed as outpatients, and (b) an age of 20 years or older. A private web‐based survey company (MACROMILL; Tokyo, Japan) recruited potential participants across Japan by convenient sampling and sent questionnaires to them online. Responses were considered consent to participate. Responses to the questionnaire were voluntary, and confidentiality was maintained throughout all investigations and analyses. The participants received an incentive equivalent to 50 cents from the survey company by completing the questionnaire, and no follow‐up was required after the survey completion. The ethical validity of the study was approved by the institutional review board of Seirei Mikatahara General Hospital.
Measurements
Patients’ Preferences for Phrases Conveying Prognostic Information.
The questionnaire included 13 items of various phrases conveying prognostic information (Table 1). These phrases were generated with specific attention to their underlying concept based on a systematic literature review [1], [8], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], in‐depth focus group interviews with 10 oncologists and palliative care physicians, and discussions among the researchers. The instrument was piloted on four people with cancer experiences, who provided feedback on the content, clarity, and format of the items.
Table 1. Underlying concept and actual phrases conveying prognostic information.
Participants were asked, “Imagine that you want to know your life expectancy, and you asked your doctor. If your doctor predicts that your life expectancy is approximately 2 years and he/she starts the conversation by saying ‘That is a difficult question,’ to what level do you prefer the following statements as a follow‐up?” They were asked to choose the responses that best reflected how they would like to be informed of their prognosis (scored on a 6‐point Likert scale with accompanying anchors: 1 = not at all preferable; 2 = not preferable; 3 = slightly not preferable; 4 = slightly preferable; 5 = preferable; and 6 = very preferable).
Variables.
Demographic data such as age, sex, employment status, annual household income, marital status, family situation (living with family, having a child, and having a parent requiring care), education level, and family history of cancer death were assessed. Medical data, such as the duration since cancer diagnosis, presence of recurrence and/or metastasis, and Eastern Cooperative Oncology Group performance status were also obtained. In addition, we assessed participants’ coping styles with the Coping Inventory for Stressful Situations (CISS) [25], [26]. The CISS is a 48‐item instrument that distinguishes three basic coping strategies with 16 items per scale: task‐oriented, emotion‐oriented, and avoidance. The score for each item ranges from 1 = not at all to 5 = very much, and scores for all items per scale are summed to form scale scores, with a higher score signifying a greater use of that particular coping strategy. These variables either have been shown to contribute to patients’ preferences for prognostic disclosure previously [11], [17], [19], [27], [28], [29], [30], [31] or are deemed clinically important.
Statistical Analysis
We used descriptive statistics and calculated means, SD, and 95% confidence intervals (CIs) of preference scores. Then, we conducted multivariate linear regression analyses to identify variables contributing to patients’ preferences for each phrase of prognostic information. Demographic and medical data and CISS scores were entered as independent variables. A backward, stepwise selection method was used to remove nonsignificant variables from the models, with p < .05 considered significant.
Assuming that 50%–75% of participants would prefer each phrase, 288–384 patients would be sufficient to calculate the accuracy to within a 10% width with 95% CI. Thus, assuming missing data, 400 subjects would be sufficient. In all statistical evaluations, p < .05 was considered significant. All analyses were performed using SPSS, version 24.0 (IBM Japan Institute; Tokyo, Japan).
Results
Participants
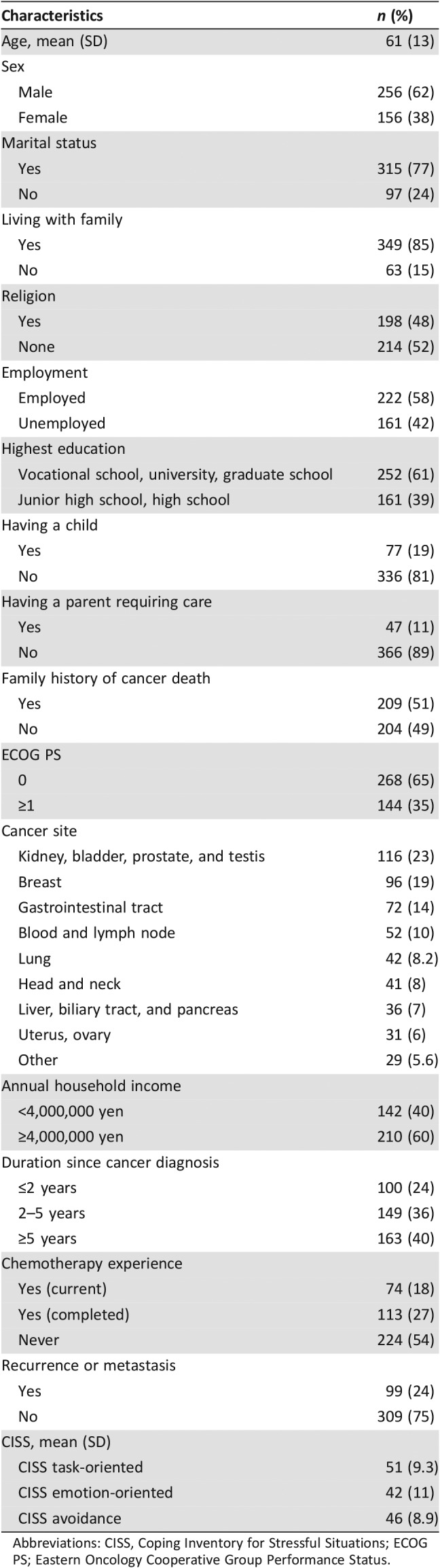
In total, 412 patients from all the eight regions of Japan were included. Table 2 summarizes their baseline characteristics. The average age was 61 (SD, 13), and 256 (62%) were men. The most frequent primary tumor was genitourinary (n = 116, 23%), followed by breast (n = 96, 19%) and gastrointestinal (n = 72, 14%). In total, 99 (24%) had recurrence or metastases. The mean scores ± SD of the CISS task‐oriented, emotion‐oriented, and avoidance scales were 51 ± 9.3, 42 ± 11, and 46 ± 8.9, respectively.
Table 2. Baseline characteristics of participants (n = 412).
Abbreviations: CISS, Coping Inventory for Stressful Situations; ECOG PS; Eastern Oncology Cooperative Group Performance Status.
Preferences for Phrases Conveying Prognostic Information
Table 3 summarizes descriptive data for each item. The most preferred item (the highest mean) was the phrase including the median, typical range, explanation of uncertainty, and hope/prepare statement (mean ± SD, 3.8 ± 1.4; 95% CI, 3.7–3.9), followed by the one including the median, typical range, and best/worst cases (3.8 ± 1.3; 3.6–3.9). The phrase addressing nondisclosure alone was the least preferred (2.5 ± 1.4; 2.3–2.6).
Table 3. Preferences of patients with cancer for each phrase conveying prognostic information (n = 412).
Items were scored on a 6‐point Likert scale from 1 (not at all preferable) to 6 (very preferable).
Abbreviation: CI, confidence interval.
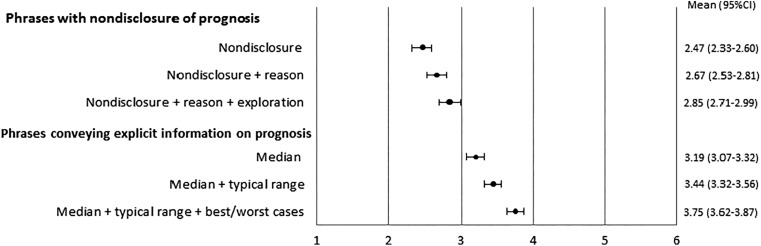
Figure 1 exclusively shows the mean scores and 95% CIs of preferences for phrases that convey explicit temporal prognostic information and nondisclosure. With respect to the phrases conveying explicit temporal prognostic information, the one including the median, typical range, and best/worst cases was more preferred than the one including both the median and typical range, which was in turn more preferred than the one including the median alone. Concerning the phrases of nondisclosure, the one with an additional statement of the reason why the prognosis could not be estimated and exploration of patients’ information need was more preferred than the phrase of nondisclosure and an additional statement of the reason, which was in turn more preferred than the one of nondisclosure alone. However, all the phrases indicating nondisclosure were less preferred than the phrase conveying explicit temporal prognostic information that included the median alone.
Figure 1.
Preferences for phrases addressing explicit prognostic information and nondisclosure. Items were scored on a 6‐point Likert scale from 1 (not at all preferable) to 6 (very preferable).
Abbreviation: CI, confidence interval.
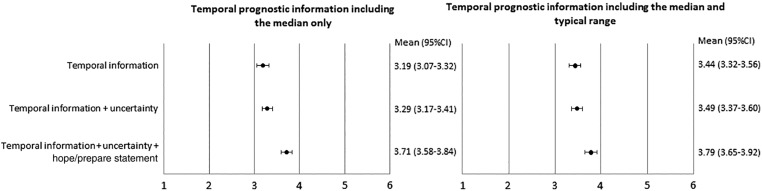
Figure 2 shows the mean scores and 95% CIs of preferences for phrases which had an additional explanation of uncertainty and hope/prepare statement. Whether the baseline temporal prognostic information includes only the median or the median and typical range, the additional explanation of uncertainty had little impact on preference. However, the phrases with an additional hope/prepare statement were more preferred than the ones conveying temporal information without such statement.
Figure 2.
Preferences for phrases conveying temporal information with additional uncertainty and the hope/prepare statement. Items were scored on a 6‐point Likert scale from 1 (not at all preferable) to 6 (very preferable).
Abbreviation: CI, confidence interval.
Variables Associated with Patient Preferences
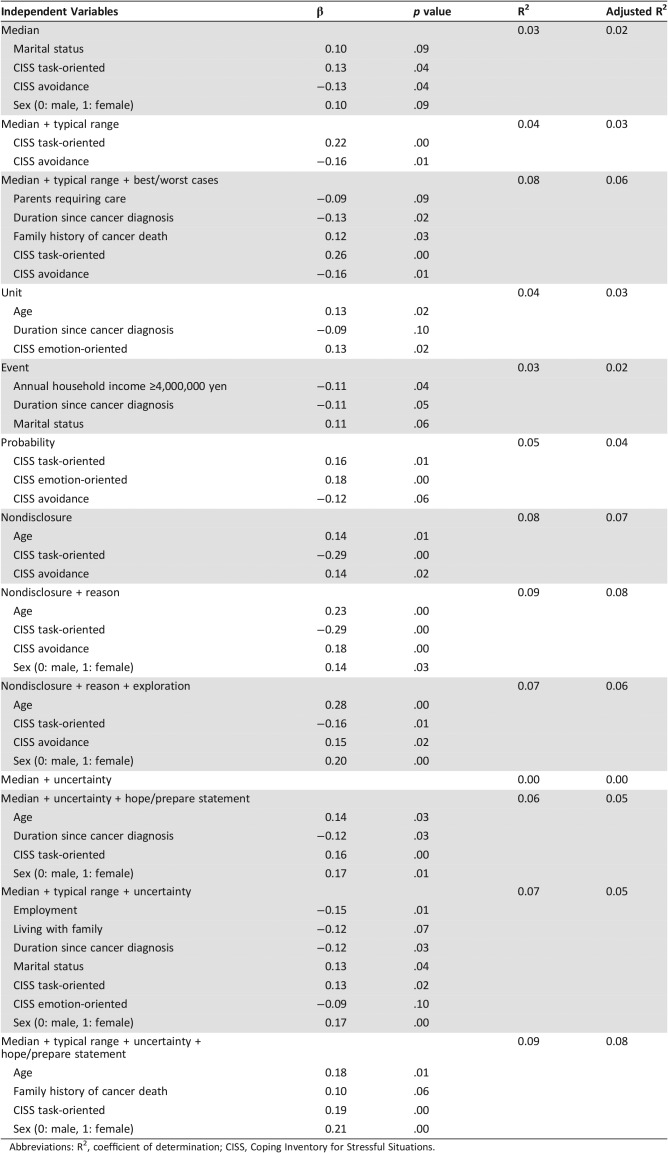
Table 4 lists the variables associated with patients’ preferences for all the phrases conveying prognostic information. Patients’ underlying coping styles for stressful situations were shown to be independent and significant factors that consistently contributed to their preferences for phrases. Overall, patients with task‐oriented coping significantly preferred phrases providing explicit prognostic information that included either temporal or probabilistic information, and those with avoidance coping significantly preferred phrases of nondisclosure.
Table 4. Variables associated with patients’ preferences for phrases ceonveying prognostic information.
Abbreviations: R2, coefficient of determination; CISS, Coping Inventory for Stressful Situations.
Discussion
Main Findings
This is, to the best of our knowledge, the first large cross‐sectional survey to systematically investigate preferences of patients with cancer for phrases conveying prognostic information. The first and most important finding was that explicit prognostic information was more preferred when a wider range of survival was added. This was consistent with prior studies showing that the majority of people with cancer experience wished to know the best case, worst case, and typical scenarios for survival to explain life expectancy [17]. The potential interpretation is that the inclusion of the best case with a wide‐ranging prognosis could convey hope and reassurance, whereas information on the worst case and typical range could help patients better understand their survival time and make plans for the future [17]. In contrast, nondisclosure of prognostic information was not generally preferred even with the additional reasons and exploration of a patient's information need. Likewise, implicit information such as “years” and a specific event was not preferred overall. These are consistent with prior studies indicating that patients value explicit prognostic information [2], [11]. These findings suggest that clinicians should be encouraged to disclose explicit prognostic information if deemed appropriate and provide both best/worst cases and the typical range in addition to the median survival time.
The second important finding was that the majority of patients with cancer preferred the hope/prepare statement. The two phrases including the hope/prepare statement were the first and third most preferred phrases presented in this study. This finding supports the prior proposal of utilizing such a statement [24]. Embracing a dual approach of hoping for the best and preparing for the worst helps clinicians not only join with patients and families but also gently introduce advance care planning (ACP), and hence potentially strengthens the patient‐clinician relationship [24]. Although the importance of ACP has been increasingly recognized worldwide [32], [33], [34], initiating end‐of‐life (EOL) discussions remains challenging among patients with life‐threatening illnesses such as cancer because of various factors associated with patients, families, clinicians, and health care systems [10], [35]. Yet the majority of patients with cancer wish for their clinician to initiate such discussions [36]. Future clinical trials should explore if clinicians’ hope/prepare statement to provide prognostic information could actually help patients engage in ACP and improve short‐term and long‐term outcomes.
Of note is that patients’ underlying coping styles were independent factors contributing to their preferences for most phrases. Overall, patients with task‐oriented coping were more likely to prefer explicit prognostic disclosure, whereas those with avoidant coping were more likely to prefer nondisclosure. These findings suggest that clinicians should pay attention to patients’ underlying coping style and ask them how much information they wish to know when communicating prognoses. The prior literature provides no consistent evidence regarding how patients’ coping styles could contribute to their satisfaction about communication with clinicians [11], [37], [38]. Future prospective studies should elucidate the most effective ways for prognostic disclosure depending on patients’ underlying coping styles.
Clinical and Research Implications
When patients with cancer ask about the prognosis, clinicians may provide explicit information with the median, typical range, and best/worst cases and include the hope/prepare statement. In real life, however, we often do not have the ability to estimate survival accurately [39]. If explicit prognostic disclosure is not considered appropriate, for example in situations where the prognosis could markedly vary based on the response to future treatment, or patients actively adopt avoidance coping strategies, then clinicians may refrain from the disclosure of explicit information. At the same time, however, clinicians should give the reason why accurate prognostication is difficult at that time, explore the patient's information need, discuss what can be done together in the face of uncertainty, and re‐evaluate the appropriateness of prognostic communication periodically.
Our study may lay a foundation for future intervention studies. Specifically, several hypotheses need future confirmation. Does the addition of wider ranges of explicit prognostic information improve prognostic awareness of patients with cancer while conveying more compassion? Does a hope/prepare statement help patients better engage in ACP without causing emotional distress? Can explicit prognostic disclosure with a wide range and the addition of the hope/prepare serve as an effective trigger for patients with advanced cancer to better prepare for their EOL and life completion? Randomized, video‐vignette studies and clinical trials will be promising to generate confirmatory findings to answer these important clinical questions.
Strengths and Limitations
The strengths of our study included a relatively large sample size and systematic comparison of various phrases developed based on an existing concept. However, our study has some limitations. First, as we used a convenience sampling and analyzed the first 412 respondents through a web‐based survey company, we could not extract a response rate or the characteristics of nonresponders. Second, the patients with cancer who participated in our study were relatively young, had a good performance status, and might have some computer literacy. Therefore, they may not represent patients with cancer in the real world. Third, this was essentially a descriptive study, and we used a preference scale that had not been clearly validated or had predetermined, clinically meaningful magnitude of differences. Thus, we could not strictly compare the patients’ various preferences with clinical and statistical significance. Interestingly, a majority of patients only expressed slight preference to one statement versus another, which might have reflected the tendency of Asian patients to exhibit acquiescent response style while avoiding extreme responses [40]. Fourth, as both the “wide ranges” and hope/prepare statement give a generic prognostic information, it may be difficult to say that such a prognostic disclosure allows a tailored therapeutic approach. Furthermore, our study was based on hypothetical scenario in which the estimated survival was 2 years. The findings may be affected by different vignette time frames and patient population. Clinicians are thus encouraged to use our findings as a general guide and modify their communication to better address individual patient's needs and situations. Fifth, our study was a cross‐sectional survey and could not determine the effects of these phrases conveying prognostic information. Future intervention studies are warranted to elucidate their effects on clinically important outcomes such as trust in the clinician, patient‐perceived clinician compassion, satisfaction with communication, and anxiety, as well as long‐term outcomes related to ACP. Finally, prognostic communication may require several encounters and should take into account individual and cultural differences. Thus, caution should be exercised when generalizing our findings.
Conclusion
We demonstrated that phrases conveying explicit prognostic information with a wider range and the hope/prepare statement were more preferred by patients with cancer than were phrases without them, and patients with task‐oriented coping were significantly more likely to prefer phrases with explicit information. When patients with cancer, especially those with task‐oriented coping, ask about the prognosis, clinicians may provide explicit information with the median, typical range, and best/worst cases and include a hope/prepare statement. Future prospective studies are strongly warranted to confirm our findings.
Acknowledgments
The authors thank Dr. Kengo Imai, Dr. Isseki Maeda, Dr. Takashi Yamaguchi, and Dr. Naosuke Yokomichi for their participation in our focus group discussions. We would also like to thank staff members of Cancer Solutions, Inc. (Naomi Sakurai, Chizuko Komagata, Wakana Shirahama, and Midori Takahashi) for their valuable input during pilot testing. This study was supported by a Health, Labour, and Welfare Sciences Research Grant: Research for Promotion of Cancer Control Programmes (H29‐Cancer Control‐general‐017).
Author Contributions
Conception/design: Masanori Mori, Maiko Fujimori, Hiroto Ishiki, Tomohiro Nishi, Jun Hamano, Hiroyuki Otani, Yu Uneno, Akira Oba, Tatsuya Morita, Yosuke Uchitomi
Provision of study material or patients: Masanori Mori
Collection and/or assembly of data: Masanori Mori
Data analysis and interpretation: Masanori Mori, Maiko Fujimori
Manuscript writing: Masanori Mori, Maiko Fujimori, Hiroto Ishiki, Tomohiro Nishi, Jun Hamano, Hiroyuki Otani, Yu Uneno, Akira Oba, Tatsuya Morita, Yosuke Uchitomi
Final approval of manuscript: Masanori Mori, Maiko Fujimori, Hiroto Ishiki, Tomohiro Nishi, Jun Hamano, Hiroyuki Otani, Yu Uneno, Akira Oba, Tatsuya Morita, Yosuke Uchitomi
Disclosures
The authors indicated no financial relationships.
References
- 1.Gilligan T, Coyle N, Frankel RM et al. Patient‐clinician communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol 2017;35:3618–3632. [DOI] [PubMed] [Google Scholar]
- 2.Johnson M, Tod AM, Brummell S et al. Prognostic communication in cancer: A critical interpretive synthesis of the literature. Eur J Oncol Nurs 2015;19:554–567. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AS, Prigerson HG, O'Reilly EM et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol 2016;34:2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton JM, Butow PN, Arnold RM et al. Discussing end‐of‐life issues with terminally ill cancer patients and their carers: A qualitative study. Support Care Cancer 2005;13:589–599. [DOI] [PubMed] [Google Scholar]
- 5.Weeks JC, Cook EF, O'Day SJ et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–1714. [DOI] [PubMed] [Google Scholar]
- 6.Enzinger AC, Zhang B, Schrag D et al. Outcomes of prognostic disclosure: Associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol 2015;33:3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrijvers D, Cherny NI, ESMO Guidelines Working Group . ESMO Clinical Practice Guidelines on palliative care: Advanced care planning. Ann Oncol 2014;25(suppl 3):iii138‐42. [DOI] [PubMed] [Google Scholar]
- 8.Clayton JM, Hancock KM, Butow PN et al. Clinical practice guidelines for communicating prognosis and end‐of‐life issues with adults in the advanced stages of a life‐limiting illness, and their caregivers. Med J Aust 2007;186(suppl 12):S77, S79, S83–108. [DOI] [PubMed] [Google Scholar]
- 9.Mori M, Shimizu C, Ogawa A et al. A national survey to systematically identify factors associated with oncologists’ attitudes toward end‐of‐life discussions: What determines timing of end‐of‐life discussions? The Oncologist 2015;20:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernacki RE, Block SD, American College of Physicians High Value Care Task Force . Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern Med 2014;174:1994–2003. [DOI] [PubMed] [Google Scholar]
- 11.van Vliet LM, van der Wall E, Plum NM et al. Explicit prognostic information and reassurance about nonabandonment when entering palliative breast cancer care: Findings from a scripted video‐vignette study. J Clin Oncol 2013;31:3242–3249. [DOI] [PubMed] [Google Scholar]
- 12.Morita T, Akechi T, Ikenaga M et al. Communication about the ending of anticancer treatment and transition to palliative care. Ann Oncol 2004;15:1551–1557. [DOI] [PubMed] [Google Scholar]
- 13.Clayton JM, Butow PN, Arnold RM et al. Discussing life expectancy with terminally ill cancer patients and their carers: A qualitative study. Support Care Cancer 2005;13:733–742. [DOI] [PubMed] [Google Scholar]
- 14.Kiely BE, Tattersall MH, Stockler MR. Certain death in uncertain time: Informing hope by quantifying a best case scenario. J Clin Oncol 2010;28:2802–2804. [DOI] [PubMed] [Google Scholar]
- 15.Stockler MR, Tattersall MH, Boyer MJ et al. Disarming the guarded prognosis: Predicting survival in newly referred patients with incurable cancer. Br J Cancer 2006;94:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiely BE, Alam M, Blinman P et al. Estimating typical, best‐case and worst‐case life expectancy scenarios for patients starting chemotherapy for advanced non‐small‐cell lung cancer: A systematic review of contemporary randomized trials. Lung Cancer 2012;77:537–544. [DOI] [PubMed] [Google Scholar]
- 17.Kiely BE, McCaughan G, Christodoulou S et al. Using scenarios to explain life expectancy in advanced cancer: Attitudes of people with a cancer experience. Support Care Cancer 2013;21:369–376. [DOI] [PubMed] [Google Scholar]
- 18.West TA, Kiely BE, Stockler MR. Estimating scenarios for survival time in men starting systemic therapies for castration‐resistant prostate cancer: A systematic review of randomised trials. Eur J Cancer 2014;50:1916–1924. [DOI] [PubMed] [Google Scholar]
- 19.Hagerty RG, Butow PN, Ellis PA et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 2004;22:1721–1730. [DOI] [PubMed] [Google Scholar]
- 20.Umezawa S, Fujimori M, Matsushima E et al. Preferences of advanced cancer patients for communication on anticancer treatment cessation and the transition to palliative care. Cancer 2015;121:4240–4249. [DOI] [PubMed] [Google Scholar]
- 21.Hui D, Kilgore K, Nguyen L et al. The accuracy of probabilistic versus temporal clinician prediction of survival for patients with advanced cancer: A preliminary report. The Oncologist 2011;16:1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez‐Cruz PE, Dos Santos R, Silva TB et al. Longitudinal temporal and probabilistic prediction of survival in a cohort of patients with advanced cancer. J Pain Symptom Manage 2014;48:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui D. Prognostication of survival in patients with advanced cancer: Predicting the unpredictable? Cancer Control 2015;22:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Back AL, Arnold RM, Quill TE. Hope for the best, and prepare for the worst. Ann Intern Med 2003;138:439–443. [DOI] [PubMed] [Google Scholar]
- 25.Edler N, Parker J. Coping Inventory for Stressful Situations (CISS). Toronto, Canada: Multi‐Health Systems, Inc.; 1990. [Google Scholar]
- 26.Furukawa T, Suzuki‐Moor A, Saito Y et al. Reliability and validity of the Japanese version of coping inventory for stressful situations (CISS): A contribution to the cross‐cultural studies of coping. Seishin Shinkeigaku Zasshi 1993;95:602–620. [PubMed] [Google Scholar]
- 27.Hagerty RG, Butow PN, Ellis PM et al. Communicating prognosis in cancer care: A systematic review of the literature. Ann Oncol 2005;16:1005–1053. [DOI] [PubMed] [Google Scholar]
- 28.Parker SM, Clayton JM, Hancock K et al. A systematic review of prognostic/end‐of‐life communication with adults in the advanced stages of a life‐limiting illness: Patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage 2007;34:81–93. [DOI] [PubMed] [Google Scholar]
- 29.Kaplowitz SA, Campo S, Chiu WT. Cancer patients’ desires for communication of prognosis information. Health Commun 2002;14:221–241. [DOI] [PubMed] [Google Scholar]
- 30.Butow PN, Maclean M, Dunn SM et al. The dynamics of change: Cancer patients’ preferences for information, involvement and support. Ann Oncol 1997;8:857–863. [DOI] [PubMed] [Google Scholar]
- 31.Cassileth BR, Zupkis RV, Sutton‐Smith K et al. Information and participation preferences among cancer patients. Ann Intern Med 1980;92:832–836. [DOI] [PubMed] [Google Scholar]
- 32.Brinkman‐Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end‐of‐life care: A systematic review. Palliat Med 2014;28:1000–1025. [DOI] [PubMed] [Google Scholar]
- 33.Houben CHM, Spruit MA, Groenen MTJ et al. Efficacy of advance care planning: A systematic review and meta‐analysis. J Am Med Dir Assoc 2014;15:477–489. [DOI] [PubMed] [Google Scholar]
- 34.Rietjens JAC, Sudore RL, Connolly M et al. Definition and recommendations for advance care planning: An international consensus supported by the European Association for Palliative Care. Lancet Oncol 2017;18:e543–e551. [DOI] [PubMed] [Google Scholar]
- 35.You JJ, Downar J, Fowler RA et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: A multicenter survey of clinicians. JAMA Intern Med 2015;175:549–556. [DOI] [PubMed] [Google Scholar]
- 36.Johnson S, Butow P, Kerridge I et al. Advance care planning for cancer patients: A systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology 2016;25:362–386. [DOI] [PubMed] [Google Scholar]
- 37.Timmermans LM, van Zuuren FJ, van der Maazen RW et al. Monitoring and blunting in palliative and curative radiotherapy consultations. Psychooncology 2007;16:1111–1120. [DOI] [PubMed] [Google Scholar]
- 38.Steptoe A, Sutcliffe I, Allen B et al. Satisfaction with communication, medical knowledge, and coping style in patients with metastatic cancer. Soc Sci Med 1991;32:627–632. [DOI] [PubMed] [Google Scholar]
- 39.Amano K, Maeda I, Shimoyama S et al. The accuracy of physicians’ clinical predictions of survival in patients with advanced cancer. J Pain Symptom Manage 2015;50:139–146.e1. [DOI] [PubMed] [Google Scholar]
- 40.Lee JW, Jones PS, Mineyama Y et al. Cultural differences in responses to a Likert scale. Res Nurs Health 2002;25:295–306. [DOI] [PubMed] [Google Scholar]