Personalized therapy provides patients with the best probability for survival. This review presents an optimized treatment schema and the accompanying diagnostic testing approach for patients with advanced non‐small cell lung cancer.
Keywords: Non‐small cell lung cancer, Molecular diagnostic testing, Personalized medicine
Abstract
Advanced non‐small cell lung cancer (NSCLC) is a complex disease comprising molecularly distinct tumor types, each with a unique biology that is becoming increasingly better characterized. The aim of this review is to present an optimized treatment schema and the accompanying diagnostic testing approach for patients with advanced NSCLC. There are a number of therapies currently approved for patients with advanced NSCLC, including agents that target particular oncogenic drivers, as well as immune checkpoint blockers (ICBs) that elicit an antitumor response. Identification of genetic alterations (e.g., epidermal growth factor receptor, anaplastic lymphoma kinase, reactive oxygen species proto‐oncogene 1, B‐Raf proto‐oncogene) or programmed cell death ligand‐1 expression levels in NSCLC requires diligent molecular testing at initial diagnosis and, in some cases, at disease progression to ensure the most efficacious treatment is delivered. Accurate molecular diagnostic testing, along with the careful selection of currently approved targeted agents, ICBs, or systemic chemotherapy, provides therapy that is personalized according to patients’ needs to achieve the best possible outcome. Enrollment in clinical trials that further the development of tailored therapies is highly recommended at all stages of treatment.
Implications for Practice.
Targeted therapies and immune checkpoint blockers provide effective and tailored options for patients with non‐small cell lung cancer. Careful molecular analysis of tumor samples is necessary to identify the genetic alterations that are present, to ensure that each patient receives the most efficacious treatment for their specific tumor type. Personalized therapy provides each patient with the best probability for prolonged survival. Enrolling patients in clinical trials should be the first consideration before making each treatment decision.
摘要
晚期非小细胞肺癌 (NSCLC) 是一种复杂的疾病,由不同的分子肿瘤类型组成,每一种肿瘤都有其越来越明显的独特生物学特征。本综述的目的在于为晚期NSCLC患者提供优化的治疗方案和相应的诊断测试方法。目前有许多针对晚期NSCLC患者的治疗方法,包括针对特定致癌因素的药物,以及引起抗肿瘤反应的免疫检查点阻断剂 (ICB)。NSCLC的基因改变(如表皮生长因子受体、间变性淋巴瘤激酶、活性氧原癌基因 1、B‐RAF 原癌基因)或程序性细胞死亡配体‐1 的表达水平需要在最初诊断时进行详细的分子测试,有时还需要在疾病进展时进行分子测试,确保提供最有效的治疗。精确的分子诊断测试,以及仔细选择目前批准的靶向药物、ICB或全身化疗,根据患者的需求提供个性化的治疗,以达到最佳的效果。我们强烈建议在各治疗阶段都进行临床试验,以便进一步制定符合患者需求的疗法。
实践意义:靶向治疗和免疫检查点阻断剂为非小细胞肺癌患者提供了有效且量身定制的选择。必须对肿瘤样本进行仔细的分子分析,以便确定是否存在基因改变,确保每位患者均获得针对其特定肿瘤类型的最有效治疗。个性化治疗可最大限度地延长每位患者的生存期。在每次作出治疗决定之前,应首先考虑参与临床试验的患者。
Introduction
Non‐small cell lung cancer (NSCLC) comprises 84% of all lung cancers [1]. Histologically, NSCLC is classified into two subtypes: nonsquamous cell carcinoma and squamous cell carcinoma (SCC). Within the nonsquamous category, adenocarcinoma is the predominant type, followed by large cell (undifferentiated) carcinoma and other rare cell types. Histologic classification of NSCLC is important for selection of therapy. In addition to histologic analysis, molecular characterization of tumors may identify genetic alterations for which targeted therapies or immune checkpoint blockers (ICBs) are available (Table 1). Identifying the genetic alterations that are present ensures that patients with NSCLC receive the most efficacious treatment for their specific tumor type.
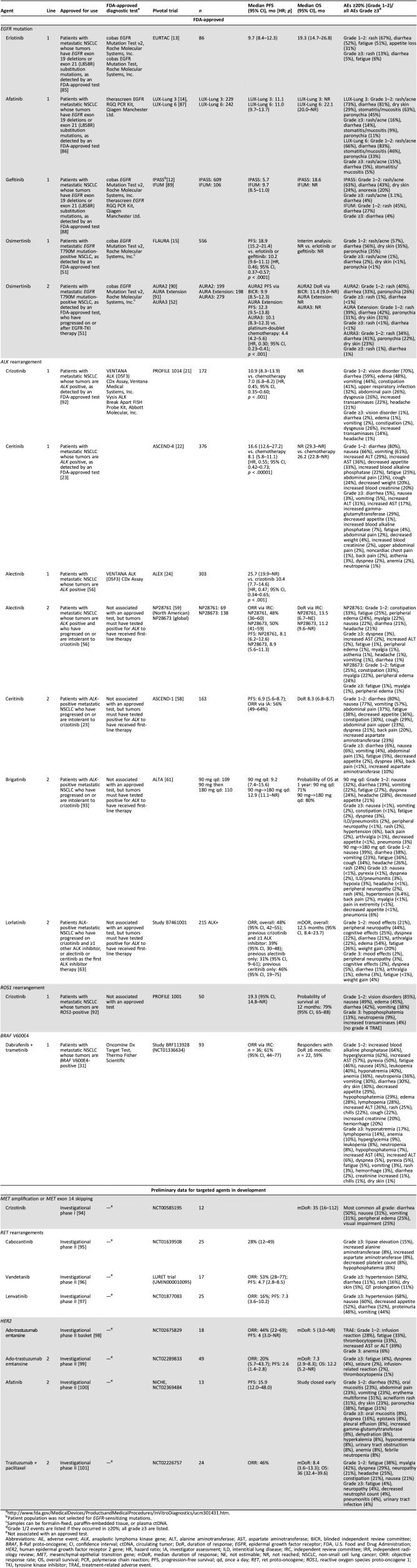
Table 1. Summary of data on efficacy and safety of targeted agents in metastatic NSCLC.
Patient population was not selected for EGFR‐sensitizing mutations.
Samples can be formalin‐fixed, paraffin‐embedded tissue, or plasma ctDNA.
Grade 1/2 events are listed if they occurred in ≥20%; all grade ≥3 are listed.
Not associated with an approved test.
Abbreviations: AE, adverse event; ALK, anaplastic lymphoma kinase gene; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BICR, blinded independent review committee; BRAF, B‐Raf proto‐oncogene; CI, confidence interval; ctDNA, circulating tumor; DoR, duration of response; EGFR, epidermal growth factor receptor; FDA, U.S. Food and Drug Administration; HER2, human epidermal growth factor receptor 2 gene; HR, hazard ratio; IA, investigator assessment; ILD, interstitial lung disease; IRC, independent review committee; IRR, independent radiology review; MET, mesenchymal‐epithelial transition gene; mDoR, median duration of response; NE, not estimable; NR, not reached; NSCLC, non‐small cell lung cancer; ORR: objective response rate; OS, overall survival; PCR, polymerase chain reaction; PFS, progression‐free survival; qd, once a day; RET, ret proto‐oncogene; ROS1, reactive oxygen species proto‐oncogene 1; TKI, tyrosine kinase inhibitor; TRAE, treatment‐related adverse event.
The frequency of molecular alterations in NSCLC varies with tumor histology. In patients with lung adenocarcinoma, ∼60% of tumors contain a driver alteration [2]. Mutations in the epidermal growth factor receptor gene (EGFR) are found in ∼19% of patients with adenocarcinoma and in ∼3% of patients with squamous histology [3]. Rearrangements in the anaplastic lymphoma kinase gene (ALK) and the reactive oxygen species proto‐oncogene 1 (ROS1) occur in ∼5% and 1% of NSCLC cases, respectively [4], [5]. Alterations in EGFR, ALK, ROS1, and the B‐Raf proto‐oncogene (BRAF) are not typically found in the same tumor, and each driver represents a distinct molecular subgroup of NSCLC with unique targeted therapy options. Targeted therapies for other rare genetic alterations are under investigation in the erb‐b2 receptor tyrosine kinase 2 gene (ERBB2), the mesenchymal‐epithelial transition gene (a prototypical receptor tyrosine kinase gene; MET), and the ret proto‐oncogene (a receptor tyrosine kinase gene; RET), each of which is found in ≤3% of patients with lung adenocarcinoma (Table 1) [6]. The most common mutation for which no targeted therapy is available is a Kirsten ras oncogene homolog gene (KRAS) mutation, which occurs in ∼25% of adenocarcinoma [2], [7]. Although targeted agents are available for many patients with adenocarcinoma, targeted therapies are very rarely suitable for patients with SCC [7].
In addition to oncogenic driver mutations, tumor cell evasion of the immune system can also lead to carcinogenesis. Some tumor cells can exploit inhibitory immune checkpoints regulated by programmed cell death‐1 receptor (PD‐1) and programmed cell death ligand‐1 (PD‐L1) through expression of PD‐L1 on the tumor cell to prevent T cell activation. An analysis of three global clinical trials involving 4,784 patients with NSCLC found that 66% of all tumors had measurable PD‐L1 expression [8]. When separated by histology, 74% of patients with nonsquamous histology and 81% of patients with squamous histology had measurable PD‐L1 expression [8]. The monoclonal antibodies anti‐PD‐1, anti‐PD‐L1, and anti‐cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) are ICBs that act to resensitize suppressed immune cells and have been a focus of drug development. Recently, anti‐PD‐1 and anti‐PD‐L1 agents were approved for patients with advanced NSCLC (Table 2) [9], [10], [11].
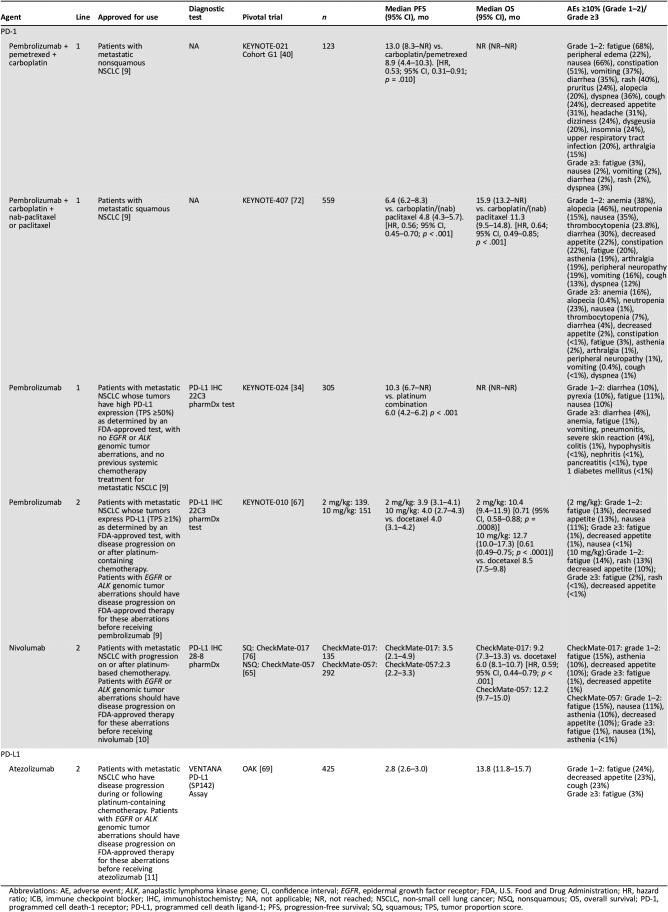
Table 2. Summary of data on efficacy and safety of ICBs approved for use in metastatic NSCLC.
Abbreviations: AE, adverse event; ALK, anaplastic lymphoma kinase gene; CI, confidence interval; EGFR, epidermal growth factor receptor; FDA, U.S. Food and Drug Administration; HR, hazard ratio; ICB, immune checkpoint blocker; IHC, immunohistochemistry; NA, not applicable; NR, not reached; NSCLC, non‐small cell lung cancer; NSQ, nonsquamous; OS, overall survival; PD‐1, programmed cell death‐1 receptor; PD‐L1, programmed cell death ligand‐1; PFS, progression‐free survival; SQ, squamous; TPS, tumor proportion score.
NSCLC Treatment Paradigm
Targeted therapies and ICBs provide effective and tailored options for patients with NSCLC. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Non‐Small Cell Lung Cancer V.1.2019 recommend that all patients with metastatic adenocarcinoma should have their tumor tissue tested for actionable driver mutations [7]. Patients with squamous histology should be tested if the patient has never been a smoker or has mixed histologies or at the discretion of the treating physician. The order of molecular testing and corresponding use of these agents are very important in the treatment of NSCLC. For example, testing for actionable targets (i.e., EGFR, ALK, ROS1, and BRAF V600E) should be conducted first, before PD‐L1 expression, because patients with positive tests for EGFR mutations and ALK rearrangements have high response rates to tyrosine kinase inhibitors (TKIs) but low response rates to PD‐L1 antibodies.
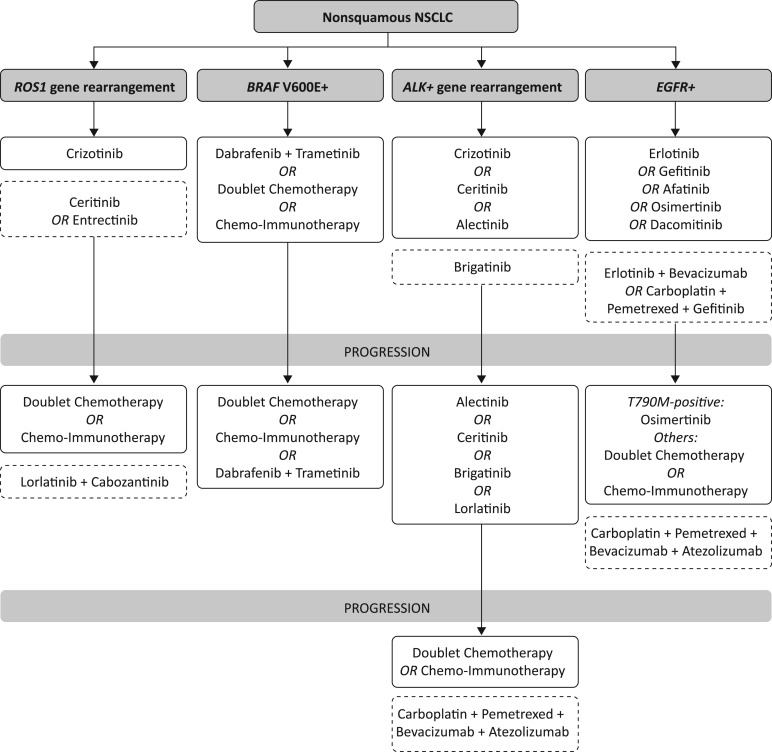
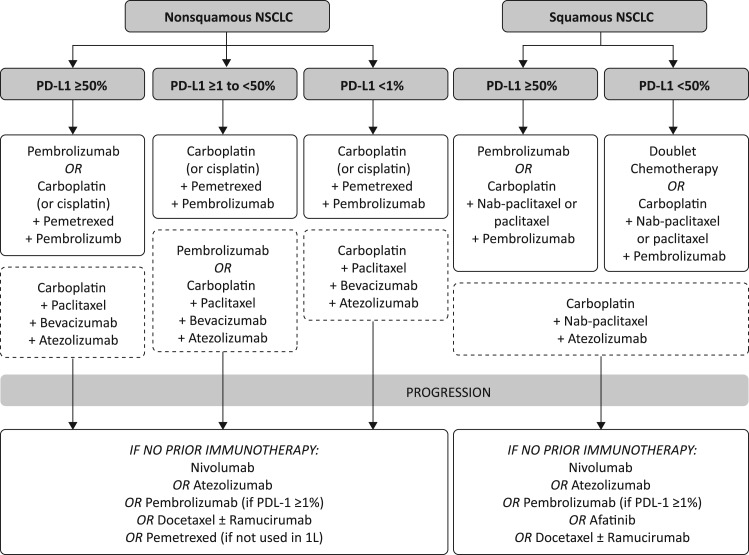
Here, we provide testing and treatment schemata (Figs. 1, 2, 3), inclusive of recent U.S. Food and Drug Administration (FDA) approvals, that summarize how to identify and treat different populations of patients with NSCLC (Fig. 1, targeted therapies; Fig. 2, immunotherapeutic agents; Fig. 3, therapies for patients ineligible for targeted therapy or immunotherapy). Note that some of these agents are not recommended by NCCN for various reasons, including if the drugs have not yet been FDA approved for NSCLC.
Figure 1.
Therapies for patients with NSCLC eligible for targeted therapies. Boxes with the dashed lines contain those drugs which are not yet U.S. Food and Drug Administration (FDA) approved. Please see the text for treatment options for other actionable mutations. Note that some of these agents are not recommended by NCCN for various reasons, including if the drugs have not yet been FDA approved for NSCLC. Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, B‐Raf proto‐oncogene; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; ROS1, reactive oxygen species proto‐oncogene 1.
Figure 2.
Immunotherapeutic agents for patients with NSCLC ineligible for targeted therapy. Boxes with the dashed lines contain those drugs which are not yet U.S. Food and Drug Administration (FDA) approved. Note that some of these agents are not recommended by NCCN for various reasons, including if the drugs have not yet been FDA approved for NSCLC.
Abbreviations: NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand‐1.
Figure 3.
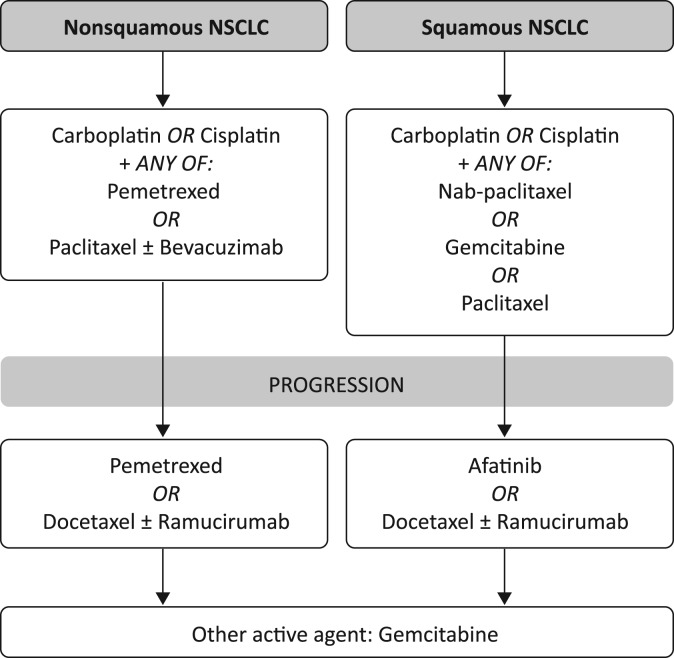
Therapies for patients with NSCLC ineligible for targeted therapy or immunotherapy. Note that NCCN recommends many regimens for metastatic nonsquamous or squamous NSCLC; this is an incomplete list. Some of these agents are not recommended by NCCN.
Abbreviation: NSCLC, non‐small cell lung cancer.
Nonsquamous Histology: First‐line Targeted Therapies
First‐line, FDA‐approved agents for patients with advanced or metastatic NSCLC with mutations in EGFR (exon 19 deletions, exon 21 L858R substitution, as detected by an FDA‐approved test) include the EGFR‐TKIs gefitinib, erlotinib, afatinib, and osimertinib (Table 1). Gefitinib, erlotinib, and afatinib are selective for EGFR‐sensitizing mutations; osimertinib is selective for both EGFR‐sensitizing and EGFR T790M resistance mutations. In the patient population that is EGFR mutation‐positive, these agents have a response rate of approximately 70%, have successfully extended the median progression‐free survival (PFS) by about 1 year, and have a median overall survival (OS) of approximately 19 months [12], [13], [14], [15]. Osimertinib significantly improved PFS compared with gefitinib or erlotinib in FLAURA [15]. Osimertinib is the agent of choice not only because of its superior efficacy but also because of its mild toxicity profile and ability to treat and delay brain metastases. Based on results from the FLAURA trial, osimertinib is recommended by NCCN as the preferred first‐line option in patients with locally advanced or metastatic NSCLC who have sensitizing EGFR mutation [7]. The EGFR‐TKI dacomitinib significantly improved PFS [16] and OS [17] compared with gefitinib in ARCHER 1050. Dacomitinib recently received FDA approval, but this is unlikely to change current practice.
Combination therapies are being assessed. Gefitinib in combination with carboplatin plus pemetrexed increased median OS (52.2 vs. 38.8 months; p = .013) and PFS (20.9 vs. 11.2 months; p < .001), but not PFS2 (20.9 vs. 21.1 months; p = .806), compared with gefitinib alone, in NEJ009 [18]. Addition of bevacizumab to erlotinib therapy increased median PFS (16.0 vs. 9.7 months; p = .0015) [19], but not OS [20], in JO25567. However, with osimertinib being the agent of choice, studies must be conducted with osimertinib combinations versus single‐agent osimertinib.
Patients with ALK rearrangement‐positive NSCLC benefit from ALK TKIs (crizotinib, ceritinib, alectinib; Table 1) [21], [22]. Crizotinib is an ALK, ROS1, and MET inhibitor; ceritinib inhibits ALK and IGF‐R1; and alectinib inhibits ALK and RET. Crizotinib is FDA approved for first‐line use in patients with ALK‐rearranged, locally advanced, or metastatic NSCLC based on the PROFILE 1014 trial that demonstrated significantly longer median PFS with crizotinib compared with chemotherapy (10.9 vs. 7.0 months; p < .001) [21]. Ceritinib was recently approved in the first line in ALK‐rearranged NSCLC based on the phase III ASCEND‐4 trial [23]. Ceritinib‐treated patients had significantly longer median PFS compared with the chemotherapy group (16.6 vs. 8.1 months; p < .00001) [22]. Furthermore, alectinib has recently been FDA approved and is recommended by NCCN as the preferred first‐line option (category 1) in patients with metastatic, ALK‐rearranged NSCLC based on the phase III ALEX study that found a significantly longer median PFS with alectinib compared with patients receiving crizotinib (25.7 vs. 10.4 months; p < .001) and on its ability to treat and delay brain metastases [24]. In a recent first interim analysis from ALTA‐1L, brigatinib increased estimated 12‐month PFS compared with crizotinib (67% vs. 43%; p < .001) in patients with ALK‐rearranged NSCLC who had not previously received ALK inhibitors [25], [26]. Frontline phase III trials of lorlatinib or ensartinib versus crizotinib are ongoing.
For patients with ROS1‐rearranged NSCLC, crizotinib is FDA approved for first‐line use based on the PROFILE 1001 phase I study (Table 1) [27], [28]. Of the 53 patients with ROS1 rearrangements who were treated with crizotinib, the median PFS was 19.3 months, and the probability of survival at 6 and 12 months was 91% and 79%, respectively [27]. In a recent phase II study in 28 patients with NSCLC and ROS1 rearrangements who were treated with ceritinib, the median PFS with ceritinib was 9.3 months [29]. Crizotinib is recommended by NCCN as the preferred first‐line option over ceritinib for patients with ROS1‐rearranged NSCLC, based on the trial data and the FDA approval [7]. Therapy with entrectinib in ROS1‐rearranged NSCLC is being assessed [30].
The BRAF inhibitor dabrafenib in combination with trametinib is FDA approved for first‐line use in patients with advanced or metastatic NSCLC who are BRAF V600E‐mutation positive [31]. In the phase II BRF113928 study, the overall response rate (ORR) was 61% in the first‐line cohort (n = 36) and 63% in patients who had received at least one previous platinum regimen (n = 57) [31].
For driver mutations without corresponding approved targeted therapies for metastatic NSCLC (e.g., MET, RET, and human epidermal growth factor receptor 2 [HER2]), NCCN recommends (category 2A) using targeted agents approved in other indications or a clinical trial for the corresponding agent, if available [7]. Early‐phase clinical trials have shown promising results with the RET inhibitors LOXO‐292 and BLU‐667 [32], [33]. Among 30 patients with RET‐positive NSCLC treated with LOXO‐292 in a phase I/II study, the ORR was 77% [32]. BLU‐667 demonstrated antitumor activity in a phase I study in patients with RET‐altered solid tumors, including NSCLC [33]. The question remains: what is the optimal time to administer targeted therapies that have not yet been approved for use in NSCLC? Most of the data support their use in the second line or beyond.
Nonsquamous Histology: First‐Line ICBs
When no actionable alterations are detected, ICBs have a role in the treatment of metastatic NSCLC (Table 2). Monotherapy with the PD‐1 receptor inhibitor pembrolizumab gained first‐line approval based on the phase III KEYNOTE‐024 study of patients with metastatic NSCLC and high PD‐L1 expression (≥50% tumor proportion score [TPS]), with no EGFR or ALK alterations (n = 305; Table 2) [9], [34]. First‐line pembrolizumab significantly extended median PFS (10.3 vs. 6.0 months; p < .001) and OS (estimated 6‐month OS rate of 80.2% vs. 72.4%; p = .005) compared with platinum‐based chemotherapy [34]. Significance was maintained within the nonsquamous subset (n = 249) [34]. Pembrolizumab lacks efficacy in TKI‐naive patients with EGFR mutations [35]. Recent results from the KEYNOTE‐042 trial confirm OS benefits with pembrolizumab in patients with PD‐L1 expression levels ≥1% (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.71–0.93; p = .0018) [36]. This is of particular interest in light of the CheckMate‐026 results that showed that patients with NSCLC and PD‐L1 expression levels ≥5% who were treated with nivolumab monotherapy did not improve their PFS compared with those treated with chemotherapy (4.2 vs. 5.9 months) [37]. However, the benefit in KEYNOTE‐042 was driven by the >50% PD‐L1 expression group. The role of tumor mutation burden (TMB) as a predictive biomarker is being assessed as part of the CheckMate 227 trial, with initial results showing increased PFS with nivolumab plus ipilimumab versus platinum‐doublet chemotherapy in patients with 10 or more mutations per megabase (mut/Mb) compared with those with fewer than 10 mut/Mb [38]. More data are needed to assess whether TMB is a viable biomarker for predicting beneficiaries of therapy; currently, it is neither FDA approved nor ready for clinical use.
Pembrolizumab was granted accelerated approval for use in metastatic nonsquamous NSCLC in combination with pemetrexed and carboplatin, independent of PD‐L1 expression [39]. The approval is based on results from KEYNOTE‐021 in which 123 patients received either carboplatin plus pemetrexed plus pembrolizumab or carboplatin plus pemetrexed alone as a first‐line regimen and showed that the addition of pembrolizumab significantly extended PFS (13.0 vs. 8.9 months; p = .010) [40]. However, OS data are pending, and it remains unclear if giving an ICB concurrently is superior to using a sequential approach. Pembrolizumab in combination with pemetrexed and platinum was recently granted FDA approval as first‐line therapy, based on KEYNOTE‐189 trial data, which showed PFS and OS benefits with pembrolizumab plus pemetrexed plus carboplatin (or cisplatin) compared with pemetrexed plus carboplatin (or cisplatin) alone [41]. Many other ICBs are being evaluated in phase III clinical trials in the first‐line setting as monotherapy or dual therapy, or in combination with chemotherapy (Table 3). Atezolizumab in combination with carboplatin plus paclitaxel plus bevacizumab significantly increased PFS compared with carboplatin plus paclitaxel plus bevacizumab alone in IMpower150 (19.2 vs. 14.7 months; HR, 0.78; 95% CI, 0.64–0.96; p = .016) [42], and the combination was recently granted priority FDA review.
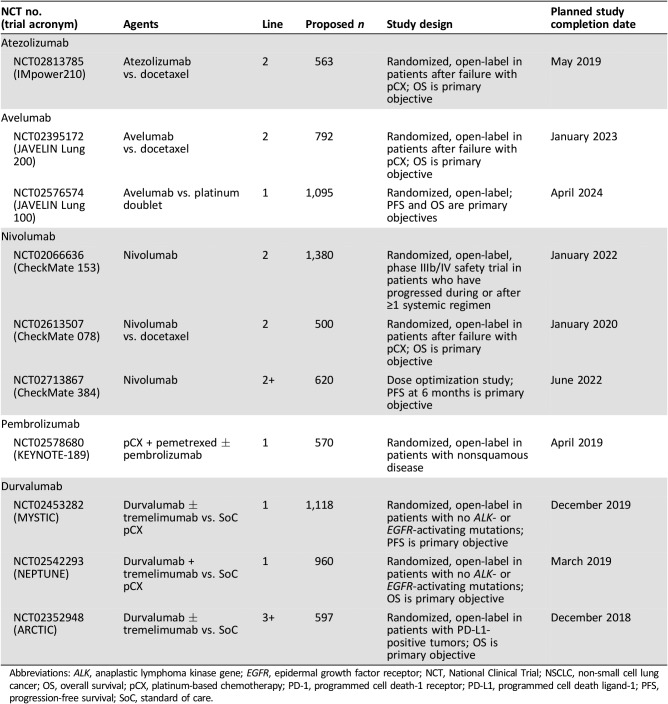
Table 3. Ongoing or planned phase III clinical trials of immune checkpoint blockers in metastatic NSCLC.
Abbreviations: ALK, anaplastic lymphoma kinase gene; EGFR, epidermal growth factor receptor; NCT, National Clinical Trial; NSCLC, non‐small cell lung cancer; OS, overall survival; pCX, platinum‐based chemotherapy; PD‐1, programmed cell death‐1 receptor; PD‐L1, programmed cell death ligand‐1; PFS, progression‐free survival; SoC, standard of care.
Nonsquamous Histology: First‐Line Chemotherapy
Chemotherapy is a first‐line intervention when no actionable biomarkers are detected and when pembrolizumab is not suitable for the patient [7]. Pemetrexed plus a platinum‐based chemotherapy is the most common regimen, but other platinum doublets are available. This combination is recommended based on a phase III study of patients with advanced NSCLC, in which the subanalysis of 1,000 patients with nonsquamous histology found a statistically significant improvement in OS in patients who received the cisplatin plus pemetrexed combination versus cisplatin plus gemcitabine (11.8 vs. 10.4 months; HR, 0.81; p = .005) [43]. Pemetrexed maintenance therapy is also used in this population based on the PARAMOUNT study, which found a significant reduction in the risk of disease progression (HR, 0.62; p < .0001), improved OS (HR, 0.78; p = .0195), and increased PFS, 4.1 months (95% CI, 3.2–4.6) versus 2.8 months (95% CI, 2.6–3.1), compared with placebo [44], [45]. A combination of carboplatin, paclitaxel, and bevacizumab can be used in selected patients with nonsquamous NSCLC based on significant improvements in the median OS (12.3 vs. 10.3 months; HR, 0.79; p = .003) and PFS (6.2 vs. 4.5 months; HR, 0.66; p < .001) compared with chemotherapy alone [46]. Bevacizumab should be considered for patients without hemoptysis, cavitary, or central tumors; however, patients with treated brain metastases can be included for treatment with bevacizumab.
Nonsquamous Histology: Second‐Line Targeted Therapies
Rebiopsy is recommended for patients who progress on a first‐line targeted therapy, as it may indicate the mechanism of drug resistance. The most commonly acquired resistance mechanism to first‐ and second‐line EGFR‐TKIs is the EGFR T790M mutation, which occurs in ∼60% of cases [47], [48], [49], [50]. Only osimertinib is approved for use in patients with metastatic EGFR T790M mutation‐positive NSCLC, as detected by an FDA‐approved liquid biopsy or tumor test, who have progressed on or after EGFR‐TKI therapy with erlotinib, afatinib, or gefinitib [51]. The AURA3 phase III trial found osimertinib to improve PFS significantly (10.1 vs. 4.4 months; p < .001) versus a platinum‐based doublet chemotherapy [51], [52]. Osimertinib is also a category 1 recommendation for patients with metastatic NSCLC who have EGFR T790M mutation and symptomatic brain metastases [7]. As third‐line treatment in EGFR T790M mutated NSCLC, PFS with osimertinib was 10.20 months compared with 2.95 months with docetaxel‐bevacizumab in a recently completed phase III trial [53].
With osimertinib moving to the first‐line setting, many mechanisms of resistance are emerging. If an actionable mutation is found, the appropriate inhibitor may be tried, or, in the case of transformation to small cell lung cancer, the patient should be treated with etoposide plus platinum. Otherwise, combination chemotherapy is the treatment of choice.
With osimertinib moving to the first‐line setting, many mechanisms of resistance are emerging. If an actionable mutation is found, the appropriate inhibitor may be tried, or, in the case of transformation to small cell lung cancer, the patient should be treated with etoposide plus platinum. Otherwise, combination chemotherapy is the treatment of choice. In an EGFR/ALK mutation subgroup analysis of IMPower150, OS was not reached in patients treated with atezolizumab plus bevacizumab plus chemotherapy, compared with an OS of 17.5 months for those treated with bevacizumab plus chemotherapy [42]. Upon progression, disease flare occurs in 9%–23% of patients when the EGFR‐TKI therapy is discontinued [54], [55]. Therefore, continuation of EGFR‐TKI therapy until immediately before starting an appropriate second‐line regimen is recommended.
The ALK inhibitors ceritinib, alectinib, and brigatinib are three FDA‐approved, second‐line options for patients with ALK rearrangements who have progressed and are crizotinib‐resistant [23], [56], [57]. The open‐label ASCEND‐1 trial showed that ceritinib benefits this population, with an ORR of 56%, median duration of response (DoR) of 8.3 months, and median PFS of 6.9 months [58]. Alectinib was tested in two phase II studies in crizotinib‐resistant patients with measurable ALK‐positive NSCLC [59], [60]. The NP28761 trial of 69 evaluable patients treated with alectinib showed an ORR of 48%, an estimated median PFS of 8.1 months, and a DoR of 13.5 months [60]. The NP28673 trial of 122 patients demonstrated similar efficacy of alectinib, with an ORR of 50% and a median PFS of 8.9 months [59]. Brigatinib was approved based on the ALTA trial, a global, phase II registration study of patients with ALK‐positive NSCLC who were previously treated with crizotinib [57]. Patients who received brigatinib (180 mg once a day with 7‐day, 90 mg lead‐in) had a median PFS of 12.9 months [61]. Lorlatinib was recently granted FDA approval for patients previously treated with at least one ALK TKI, based on results from a global, multicohort phase II study [62], [63]. In 215 patients who were ALK‐positive and who had received at least one previous ALK TKI, the ORR was 48% and the intracranial response rate in 89 patients with CNS lesions at baseline was 60% [63]. These targeted therapies are currently being evaluated in phase III clinical trials for use in the first‐ second‐, and third‐line settings (Table 4). In addition to these TKIs, other agents are in development. The National Clinical Trials Network ALK Master Protocol aims to tailor treatment based on the molecular mechanism of resistance. This basket trial will be opening soon.
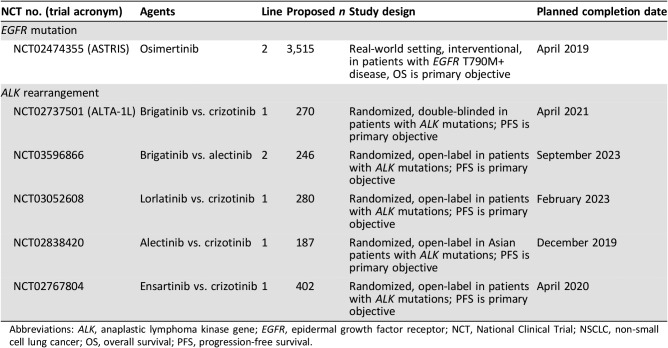
Table 4. Ongoing or planned phase III clinical trials of targeted therapy in locally advanced or metastatic NSCLC.
Abbreviations: ALK, anaplastic lymphoma kinase gene; EGFR, epidermal growth factor receptor; NCT, National Clinical Trial; NSCLC, non‐small cell lung cancer; OS, overall survival; PFS, progression‐free survival.
Therapy with lorlatinib in ROS1‐rearranged NSCLC is being assessed [64]. Emerging evidence suggests that cabozantinib is effective in patients with ROS1‐positive NSCLC that has become resistant to crizotinib or ceritinib.
Nonsquamous Histology: Second‐Line ICBs
With pembrolizumab moving to the first‐line treatment setting, the role of ICBs in the second line has diminished; however, many important questions need to be addressed regarding the optimal placement of an ICB in the treatment life of a patient. Nivolumab is indicated for second‐line use, regardless of PD‐L1 status [10], [65], [66]. Nivolumab was tested against docetaxel in CheckMate‐057, a phase III study that showed that nivolumab improved OS compared with docetaxel in patients with nonsquamous NSCLC (12.2 vs. 9.4 months). Although all patients derived clinical benefit from nivolumab, when data were stratified by PD‐L1 expression, the magnitude of clinical benefit increased with increasing PD‐L1 expression [65]. A PD‐L1 complementary diagnostic biomarker test to measure PD‐L1 expression is not required for prescribing nivolumab in patients with nonsquamous NSCLC; however, the results of the complementary PD‐L1 test may aid clinicians in deciding if nivolumab is appropriate for patients [7].
Pembrolizumab is FDA approved in the second‐line setting for patients with metastatic, nonsquamous NSCLC and PD‐L1 expression (TPS ≥1%) as determined by an FDA‐approved, companion diagnostic test, with disease progression on or after platinum‐containing chemotherapy, based on the phase II/III trial, KEYNOTE‐010 [67]. In this trial, in which 70% of patients had nonsquamous histology, pembrolizumab (2 mg/kg group) significantly improved OS over docetaxel (10.4 vs. 8.5 months; p = .0008) [67]. The presence of microsatellite instability‐high (MSI) tumors in NSCLC is rare and is not as routinely tested for as EGFR, ALK, ROS, BRAF, or PD‐L1 alterations; however, when the patient obtains full molecular profiling and the results show presence of MSI, then pembrolizumab is indicated and is the correct choice of therapy.
The anti‐PD‐L1 antibody atezolizumab has gained FDA approval for second‐line use, regardless of PD‐L1 status, based on the phase III OAK and phase II POPLAR trials [68], [69]. Among the 74% of patients with nonsquamous NSCLC, atezolizumab treatment (n = 313) was associated with longer median OS (15.6 months; 95% CI, 13.3–17.6) versus docetaxel‐treated patients (n = 315; 11.2 months; 95% CI, 9.3–12.6); the OS benefit associated with atezolizumab was statistically significant (HR, 0.73; 95% CI, 0.60–0.89; p = .0015) [69], [70]. Among patients with the highest PD‐L1 expression (PD‐L1 expression on ≥50% tumor cells or ≥10% immune cells), OS was 59% greater in patients treated with atezolizumab versus docetaxel (p < .0001). Even in patients with no PD‐L1 expression, atezolizumab provided a significant 25% improvement in OS versus docetaxel (p = .0215) [69]. Therefore, atezolizumab is approved regardless of PD‐L1 expression levels, although a complementary PD‐L1 test may provide useful information to guide treatment decisions [7], [11].
Nonsquamous Histology: Chemotherapy Second‐Line Therapy
For patients who have received the pembrolizumab‐carboplatin‐pemetrexed combination in the first line, several options are recommended for second‐line therapy, including docetaxel with or without the anti‐VEGF antibody ramucirumab [7].
Nonsquamous Histology: Chemotherapy Third‐Line Therapy
There is no standard therapy beyond second line, but single‐agent chemotherapy is a reasonable approach.
SCC: First‐Line Targeted Therapy and ICBs
Squamous histology requires different considerations for therapy selection. NCCN Guidelines recommend that molecular testing can be considered, including for EGFR and ALK genetic alterations, in patients with SCC if (a) the patient has never smoked, (b) small biopsy samples were used to assess histology, or (c) mixed histology was reported [7], [71]. ROS1 and BRAF testing can also be considered. Molecular profiling can also be employed at the discretion of the treating physician. If actionable driver mutations are identified, treatment with the corresponding targeted therapy is appropriate [7].
In patients with SCC who have high PD‐L1 expression (≥50% TPS), pembrolizumab is recommended for first‐line intervention based on the KEYNOTE‐024 trial described previously [34]. For the squamous subgroup (n = 56), there was a clear benefit of pembrolizumab to the risk of disease progression or death (HR, 0.35; 95% CI, 0.17–0.71) [34]. Pembrolizumab in combination with chemotherapy (carboplatin with paclitaxel or nab‐paclitaxel) increased the objective response rate compared with chemotherapy alone (58.4% vs. 35.0%; p = .0004) in a first interim analysis from KEYNOTE‐407 [72]. The median OS and PFS were also significantly improved with pembrolizumab plus chemotherapy combination over chemotherapy alone (OS, 15.9 vs. 11.3 months; p = .0008. PFS, 6.4 vs. 4.8 months; p < .0001) [72]. Pembrolizumab in combination with carboplatin and either paclitaxel or nab‐paclitaxel was recently granted FDA approval as first‐line therapy in patients with metastatic squamous NSCLC, based on KEYNOTE‐407 trial data. Recent primary analyses from IMpower131 showed significantly increased PFS with atezolizumab in combination with carboplatin plus nab‐paclitaxel compared with carboplatin plus nab‐paclitaxel alone (6.3 vs. 5.6 months; HR, 0.71; 95% CI, 0.60–0.85; p = .0001) [73]. The OS data are immature.
SCC: First‐Line Chemotherapy
In the absence of a genetic driver mutation or expression of PD‐L1, NCCN recommends several chemotherapy options, including cisplatin plus carboplatin plus gemcitabine, or a taxane for first‐line intervention [7]. SWOG S0819 reconfirmed that paclitaxel plus carboplatin remains a treatment option for unselected patients [74]. In this trial, which evaluated paclitaxel plus carboplatin with or without cetuximab, there was no benefit to the addition of cetuximab regardless of histology. In patients with squamous histology, the median OS was 8.0 months. Gemcitabine plus cisplatin is also a popular regimen based on a subgroup analysis of a noninferiority trial in which first‐line cisplatin plus gemcitabine was compared with cisplatin plus pemetrexed in patients with advanced NSCLC. For patients with SCC, the OS was significantly improved with cisplatin plus gemcitabine versus cisplatin plus pemetrexed (10.8 vs. 9.4 months; HR, 1.23; 95% CI, 1.00–1.51; p = .05) [43]. This trial led to the removal of treatment of patients with squamous histology from the pemetrexed label. Combination carboplatin plus nab‐paclitaxel is a reasonable option for SCC, based on a phase III subset analysis of patients with SCC that demonstrated a significantly higher ORR of 41% (95% CI, 34.7–47.4) with carboplatin plus nab‐paclitaxel versus carboplatin plus/solvent‐based paclitaxel (ORR, 24%; 95% CI, 18.8–30.1) [75]. Both continuation maintenance with gemcitabine and switch maintenance with docetaxel are category 2B recommendations in NCCN Guidelines for patients with SCC; both approaches have shown significant PFS benefits, although with no significant changes in OS [7].
Necitumumab plus cisplatin plus gemcitabine was removed from the NCCN Guidelines as a regimen for patients with metastatic SCC based on a lack of safety or efficacy benefit when compared with cisplatin plus gemcitabine and other available agents [7].
SCC: Second‐Line ICBs
Nivolumab first received FDA approval for the second‐line treatment of patients with squamous NSCLC, with no EGFR or ALK genomic tumor aberrations, based on CheckMate‐017 [76]. Nivolumab significantly increased OS compared with docetaxel (9.2 vs. 6.0 months; p < .001) [76]. PD‐L1 expression was neither prognostic nor predictive of efficacy in this patient population.
Pembrolizumab gained FDA approval for the second‐line treatment of patients with metastatic NSCLC with squamous histology and PD‐L1 expression (≥1% TPS), based on the phase II/III KEYNOTE‐010 trial previously summarized [67]. In this trial, 21% of patients had squamous histology, and the improvement in OS associated with pembrolizumab was not statistically significant (HR, 0.74; 95% CI, 0.50–1.09), in part owing to the small population size involved.
Atezolizumab has FDA approval regardless of PD‐L1 status based on the phase III OAK trial and the phase II POPLAR trial [11], [68], [69]. In the 26% of patients with squamous NSCLC in the OAK study, atezolizumab significantly increased OS versus docetaxel (8.9 months [95% CI, 7.4–12.8] vs. 7.7 months [95% CI, 6.3–8.9]; HR, 0.73 [95% CI, 0.54–0.98]; p = .0383) [69].
Given the positive data obtained when ICBs are combined with chemotherapy, second‐line ICB usage will lessen.
Use of docetaxel plus or minus ramucirumab or afatinib should be entertained.
SCC: Second‐Line Therapy in Patients Ineligible for Targeted Therapy or Immunotherapy
Afatinib has FDA approval for the treatment of patients with advanced squamous cell lung carcinoma whose disease progressed after treatment with platinum‐based chemotherapy, based on LUX‐Lung 8 trial results [77]. Docetaxel alone or in combination with ramucirumab is also an approved second‐line option.
SCC: Third‐Line Therapy
Therapeutic interventions following progression on a second‐line treatment are at the discretion of the patient and doctor.
Diagnostic Testing
A biomarker test may help to identify patients who would benefit most from targeted therapy, ICBs, or a combination of therapies. Diagnostic tests require sufficient tissue and appropriate timing [78]. As the number of tailored therapies increases, there will be an increased need for testing algorithms that ensure all necessary molecular tests have been performed.
Molecular Testing
The joint recommendation issued by the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology strongly recommends molecular testing for EGFR and ALK in patients with advanced lung adenocarcinoma at initial diagnosis and at progression in those with lower‐stage disease. Additionally, it is strongly recommended that physicians employ EGFR and ALK testing for tumors with histologies other than adenocarcinoma, if there are clinical features that suggest a higher probability of detecting an oncogenic driver [79]. The updated guidelines emphasize the importance of testing not only for EGFR and ALK but also for ROS1, BRAF, and other driver mutations [79]. NCCN Guidelines also strongly endorse broad molecular profiling [7]. Movement away from single testing to multiplex testing is encouraged to gain a comprehensive biological analysis of a patient's tumor.
Circulating Tumor Testing
Although tumor biopsy is considered the gold standard for molecular analysis, “liquid” biopsies obtained from peripheral blood or urine present an opportunity for a less invasive method to be used [80]. There are a number of clinically validated methods available for circulating tumor (ctDNA) testing [80], [81]. Although ctDNA testing has great promise, it does have limitations. The amount of ctDNA a tumor sheds is variable and can affect assay sensitivity [82]. The sensitivities of liquid‐based assays are typically lower than those of tissue‐based tests, and therefore there is a possibility of false negatives [83]. For example, a negative plasma test result for EGFR T790M warrants a rebiopsy to avoid missing an important actionable mutation [7], [83].
PD‐L1 Testing
It is recommended that patients with metastatic NSCLC be tested for PD‐L1 expression before first‐line treatment [7]. The Dako PD‐L1 IHC 22C3 pharmDX companion diagnostic for pembrolizumab is recommended for use in the first‐line setting to identify patients with a TPS of ≥50% who are suitable for pembrolizumab monotherapy; however, institutions may use their own PD‐L1 immunohistochemistry (IHC) testing platforms. The inherent variability in the use of different PD‐L1 IHC testing platforms has been recognized by both industry and academia and has brought about the Blueprint PD‐L1 IHC Assay Comparison Project that evaluated these tests for clinical comparability [84]. This study found that the assays cannot be considered to be interchangeable for the determination of PD‐L1 status [84]. Of the four assays tested, all but the Ventana SP142 assay had comparable tumor cell staining, and immune cell staining was more variable than tumor cell staining [84]. PD‐L1 testing is an imperfect biomarker because of its dynamic expression, which may be influenced by a variety of factors; thus, there is an ongoing and intensive search for other predictive biomarkers, such as TMB.
PD‐L1 testing is an imperfect biomarker because of its dynamic expression, which may be influenced by a variety of factors; thus, there is an ongoing and intensive search for other predictive biomarkers, such as TMB.
Conclusion
Both targeted therapies and ICB agents have an important role in the treatment of NSCLC. In order to personalize therapy appropriately, careful molecular analysis of tumor samples is necessary to provide each patient with the best probability for prolonged survival. However, there is still much work to be done, and enrolling patients in clinical trials should be considered when making each treatment decision.
Acknowledgments
Alanna Kennedy, Ph.D., of The Lockwood Group (Stamford, CT) provided medical writing support, which was in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and funded by AstraZeneca (Wilmington, DE). Anja Becher, Ph.D., of Oxford PharmaGenesis (Oxford, UK), provided additional medical writing support, which was in accordance with GPP3 and funded by AstraZeneca (Wilmington, DE).
Author Contributions
Conception/design: Nagla Abdel Karim, Karen Kelly
Manuscript writing: Nagla Abdel Karim, Karen Kelly
Final approval of manuscript: Nagla Abdel Karim, Karen Kelly
Disclosures
Nagla Abdel Karim: AstraZeneca, Bristol‐Myers Squibb (Other‐speaker bureau participation); Karen Kelly: AbbVie, AstraZeneca, G1 Therapeutics, Janssen, Eli Lilly & Co, Regeneron (C/A), Merck & Co. (H), AbbVie, Celgene, EMD Serono, Five Prime, Genentech, Eli Lilly & Co, Lycera, Novartis, Regeneron, Transgene (RF), UpToDate (Other‐author royalties).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society . Cancer Facts & Figures. 2017. Available at: https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/annual‐cancer‐facts‐and‐figures/2017/cancer‐facts‐and‐figures‐2017.pdf Accessed July 20, 2017.
- 2.Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dearden S, Stevens J, Wu YL et al. Mutation incidence and coincidence in non small‐cell lung cancer: meta‐analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007;448:561–566. [DOI] [PubMed] [Google Scholar]
- 5.Gainor JF, Shaw AT. Novel targets in non‐small cell lung cancer: ROS1 and RET fusions. The Oncologist 2013;18:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson D, Capelletti M, Yelensky R et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) . Non‐Small Cell Lung Cancer V1.2019. Available at https://www.nccn.org. Accessed October 30, 2018.
- 8.Aggarwal C, Rodriguez Abreu D, Felip E et al. Prevalence of PD‐L1 expression in patients with non‐small cell lung cancer screened for enrollment in KEYNOTE‐001, ‐010, and ‐024. Ann Oncol 2016;27(suppl 6):1060Pa. [Google Scholar]
- 9.KEYTRUDA for injection [prescribing information]. Whitehouse Station, NJ: Merck & Co.; 2018.
- 10.OPDIVO [prescribing information]. Princeton, NJ: Bristol‐Myers Squibb; 2016.
- 11.TECENTRIQ [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2016.
- 12.Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 15.Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Cheng Y, Zhou X et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): A randomised, open‐label, phase 3 trial. Lancet Oncol 2017;18:1454–1466. [DOI] [PubMed] [Google Scholar]
- 17.Mok T, Cheng Y, Zhou X et al. Dacomitinib (daco) versus gefitinib (gef) for first‐line treatment of advanced NSCLC (ARCHER 1050): Final overall survival (OS) analysis. J Clin Oncol 2018;36(suppl):9004a. [Google Scholar]
- 18.Nakamura A, Inoue A, Morita S et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non‐small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol 2018;36(suppl):9005a. [Google Scholar]
- 19.Seto T, Kato T, Nishio M et al. Erlotinib alone or with bevacizumab as first‐line therapy in patients with advanced non‐squamous non‐small‐cell lung cancer harbouring EGFR mutations (JO25567): an open‐label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–1244. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto N, Seto T, Nishio M et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first‐line treatment for advanced EGFR mutation‐positive non‐squamous non‐small‐cell lung cancer (NSCLC): Survival follow‐up results of JO25567. J Clin Oncol 2018;36(suppl):9007a. [DOI] [PubMed] [Google Scholar]
- 21.Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Tan DSW, Chiari R et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): A randomised, open‐label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 23.ZYKADIA [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017.
- 24.Peters S, Camidge DR, Shaw AT et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 25.Camidge R, Kim HR, Ahn MJ et al. PL02.03. Brigatinib vs crizotinib in patients with ALK inhibitor‐naive advanced ALK+ NSCLC: First report of a phase 3 trial (ALTA‐1L). Presented at: International Association for the Study of Lung Cancer 19th World Conference on Lung Cancer, September 23–26, 2018; Totonto, Canada.
- 26.Camidge DR, Kim HR, Ahn MJ et al. Brigatinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2018;379:2027–2039. [DOI] [PubMed] [Google Scholar]
- 27.Shaw A, Riley GJ, Bang Y et al. Crizotinib in advanced ROS1‐rearranged non‐small cell lung cancer (NSCLC): Updated results from PROFILE 1001. Ann Oncol 2016;27(suppl):1206PDa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw AT, Ou SH, Bang YJ et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SM, Kim HR, Lee JS et al. Open‐label, multicenter, phase II study of ceritinib in patients with non‐small‐cell lung cancer harboring ROS1 rearrangement. J Clin Oncol 2017;35:2613–2618. [DOI] [PubMed] [Google Scholar]
- 30.Ahn M, Cho BC, Siena S et al. OA 14.06 Entrectinib in patients with locally advanced or metastatic ROS1 fusion‐positive non‐small cell lung cancer (NSCLC). J Thorac Oncol 2017;12:S1783a. [Google Scholar]
- 31.TAFINLAR [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017.
- 32.Oxnard GR, Subbiah V, Park K et al. OA12.07. Clinical activity of LOXO‐292, a highly selective RET inhibitor, in patients with RET fusion+ non‐small cell lung cancer. J Thorac Oncol 2018;13:S349–S350a. [Google Scholar]
- 33.Subbiah V, Taylor M, Lin J et al. Highly potent and selective RET inhibitor, BLU‐667, achieves proof of concept in a phase I study of advanced, RET‐altered solid tumors. Cancer Res 2018;78:CT043a. [Google Scholar]
- 34.Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 35.Lisberg AE, Cummings AL, Goldman JW et al. A phase II study of pembrolizumab in EGFR‐mutant, PD‐L1+, tyrosine kinase inhibitor (TKI) naïve patients with advanced NSCLC. J Clin Oncol 2018;36(suppl):9014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes G, Wu YL, Kudaba I et al. Pembrolizumab (pembro) versus platinum‐based chemotherapy (chemo) as first‐line therapy for advanced/metastatic NSCLC with a PD‐L1 tumor proportion score (TPS) ≥1%: Open‐label, phase 3 KEYNOTE‐042 study. J Clin Oncol 2018;36(suppl):LBA4a. [Google Scholar]
- 37.Carbone DP, Reck M, Paz‐Ares L et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017;376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellmann MD, Ciuleanu TE, Pluzanski A et al. Abstract CT077: Nivolumab (nivo) + ipilimumab (ipi) vs platinum‐doublet chemotherapy (PT‐DC) as first‐line (1L) treatment (tx) for advanced non‐small cell lung cancer (NSCLC): Initial results from CheckMate 227. In: Proceedings of the 109th Annual Meeting of the American Association for Cancer Research; Apr 14–18 2018; Chicago, Illinois.
- 39.Merk. FDA approves Merck's KEYTRUDA (pembrolizumab) as first‐line combination therapy with pemetrexed and carboplatin for patients with metastatic nonsquamous non‐small cell lung cancer (NSCLC), irrespective of PD‐L1 expression [press release]. Available at http://investors.merck.com/news/press‐release‐details/2017/FDA‐Approves‐Mercks‐KEYTRUDA‐pembrolizumab‐as‐First‐Line‐Combination‐Therapy‐with‐Pemetrexed‐and‐Carboplatin‐for‐Patients‐with‐Metastatic‐Nonsquamous‐Non‐Small‐Cell‐Lung‐Cancer‐NSCLC‐Irrespective‐of‐PD‐L1‐Expression/default.aspx. Published May 10, 2017. Accessed July 20, 2017.
- 40.Langer CJ, Gadgeel SM, Borghaei H et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol 2016;17:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 42.Socinski MA, Jotte RM, Cappuzzo F et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol 2018;36(suppl):9002a. [Google Scholar]
- 43.Scagliotti GV, Parikh P, von Pawel J et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008;26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 44.Paz‐Ares L, de Marinis F, Dediu M et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): A double‐blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–255. [DOI] [PubMed] [Google Scholar]
- 45.Paz‐Ares LG, de Marinis F, Dediu M et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013;31:2895–2902. [DOI] [PubMed] [Google Scholar]
- 46.Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2005;352:786–792. [DOI] [PubMed] [Google Scholar]
- 48.Oxnard GR, Arcila ME, Sima CS et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sequist LV, Waltman BA, Dias‐Santagata D et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu HA, Arcila ME, Rekhtman N et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013;19:2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.TAGRISSO [prescribing information]. Wilmington, DE: AstraZeneca; 2018.
- 52.Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie K, Zhang Z, Zhang C et al. Osimertinib compared docetaxel‐bevacizumab as third‐line treatment in EGFR T790M mutated non‐small‐cell lung cancer. Lung Cancer 2018;121:5–11. [DOI] [PubMed] [Google Scholar]
- 54.Chaft JE, Oxnard GR, Sima CS et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR‐mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res 2011;17:6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen HJ, Yan HH, Yang JJ et al. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non‐small cell lung cancer. Pathol Oncol Res 2013;19:833–838. [DOI] [PubMed] [Google Scholar]
- 56.ALECENSA [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2016.
- 57.U.S. Food and Drug Administration . Brigatinib. Available at https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555841.htm. Accessed May 8, 2017.
- 58.Kim DW, Mehra R, Tan DSW et al. Activity and safety of ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐1): Updated results from the multicentre, open‐label, phase 1 trial. Lancet Oncol 2016;17:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ou SH, Ahn JS, De Petris L et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: A phase II global study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 60.Shaw AT, Gandhi L, Gadgeel S et al. Alectinib in ALK‐positive, crizotinib‐resistant, non‐small‐cell lung cancer: A single‐group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim DW, Tiseo M, Ahn MJ et al. Brigatinib in patients with crizotinib‐refractory anaplastic lymphoma kinase‐positive non‐small‐cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 62.Solomon BJ, Besse B, Bauer TM et al. Lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer: Results from a global phase 2 study. Lancet Oncol 2018;19:1654–1667. [DOI] [PubMed] [Google Scholar]
- 63.LORBRENA [prescribing information]. New York, NY: Pfizer Inc.; 2018.
- 64.Shaw AT, Felip E, Bauer TM et al. Lorlatinib in non‐small‐cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open‐label, single‐arm first‐in‐man phase 1 trial. Lancet Oncol 2017;18:1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters S, Cappuzzo F, Horn L et al. Analysis of early survival in patients with advanced non‐squamous NSCLC treated with nivolumab vs docetaxel in CheckMate 057. J Thorac Oncol 2017;12(suppl):S253a. [Google Scholar]
- 67.Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 68.Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 69.Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barlesi F, Park K, Ciardiello F et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol 2016;27(suppl):LBA44_PRa. [Google Scholar]
- 71.Paik PK, Varghese AM, Sima CS et al. Response to erlotinib in patients with EGFR mutant advanced non‐small cell lung cancers with a squamous or squamous‐like component. Mol Cancer Ther 2012;11:2535–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 73.Jotte RM, Cappuzzo F, Vynnychenko I et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab‐paclitaxel vs carboplatin + nab‐paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36(suppl):LBA9000a. [Google Scholar]
- 74.Herbst RS, Redman MW, Kim ES et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): A randomised, phase 3 study. Lancet Oncol 2018;19:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socinski MA, Bondarenko I, Karaseva NA et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: Final results of a phase III trial. J Clin Oncol 2012;30:2055–2062. [DOI] [PubMed] [Google Scholar]
- 76.Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soria JC, Felip E, Cobo M et al. Afatinib versus erlotinib as second‐line treatment of patients with advanced squamous cell carcinoma of the lung (LUX‐Lung 8): An open‐label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897–907. [DOI] [PubMed] [Google Scholar]
- 78.Reijans M, Vandercruyssen G, Keuleers I et al. Fully automated and sensitive detection of EGFR exon 18, 19, 20 and 21 mutational status in less than 2.5 hours from a single FFPE slice. Ann Oncol 2016;27(suppl):1173Pa.27002104 [Google Scholar]
- 79.Lindeman NI, Cagle PT, Aisner DL et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323–358. [DOI] [PubMed] [Google Scholar]
- 80.Sorber L, Zwaenepoel K, Deschoolmeester V et al. Circulating cell‐free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer 2017;107:100–107. [DOI] [PubMed] [Google Scholar]
- 81.Cree IA, Deans Z, Ligtenberg MJ et al. Guidance for laboratories performing molecular pathology for cancer patients. J Clin Pathol 2014;67:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sacher AG, Paweletz C, Dahlberg SE et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol 2016;2:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Normanno N, Denis MG, Thress KS et al. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non‐small‐cell lung cancer. Oncotarget 2017;8:12501–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirsch FR, McElhinny A, Stanforth D et al. PD‐L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 85.TARCEVA [prescribing information]. South San Francisco, CA: Genetech, Inc.; 2015.
- 86.GILOTRIF [prescribing information]. Ridgefield, CT: Boehringer Ingelheim; 2014.
- 87.Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 88.IRESSA [prescribing information]. Wilmington, DE: AstraZeneca; 2015.
- 89.Douillard JY, Ostoros G, Cobo M et al. First‐line gefitinib in Caucasian EGFR mutation‐positive NSCLC patients: A phase‐IV, open‐label, single‐arm study. Br J Cancer 2014;110:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goss G, Tsai CM, Shepherd FA et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): A multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol 2016;17:1643–1652. [DOI] [PubMed] [Google Scholar]
- 91.Yang JC, Ahn MJ, Kim DW et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017;35:1288–1296. [DOI] [PubMed] [Google Scholar]
- 92.XALKORI [prescribing information]. New York, NY: Pfizer Inc; 2017.
- 93.ALUNBRIG [prescribing information]. Cambridge, MA: Ariad Pharmaceuticals; 2017.
- 94.Camidge DR, Ou SHI, Shapiro G et al. Efficacy and safety of crizotinib in patients with advanced c‐MET‐amplified non‐small cell lung cancer (NSCLC). J Clin Oncol 2014;32(suppl):8001a. [Google Scholar]
- 95.Drilon A, Rekhtman N, Arcila M et al. Cabozantinib in patients with advanced RET‐rearranged non‐small‐cell lung cancer: An open‐label, single‐centre, phase 2, single‐arm trial. Lancet Oncol 2016;17:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoh K, Seto T, Satouchi M et al. Vandetanib in patients with previously treated RET‐rearranged advanced non‐small‐cell lung cancer (LURET): An open‐label, multicentre phase 2 trial. Lancet Respir Med 2017;5:42–50. [DOI] [PubMed] [Google Scholar]
- 97.Velcheti V, Hida T, Reckamp KL et al. Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion‐positive adenocarcinoma of the lung. Ann Oncol 2016;27(suppl):1204PDa. [DOI] [PubMed] [Google Scholar]
- 98.Li BT, Shen R, Buonocore D et al. Ado‐trastuzumab emtansine in patients with HER2 mutant lung cancers: Results from a phase II basket trial. J Clin Oncol 2017;35(suppl):8510a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stinchcombe T, Stahel RA, Bubendorf L et al. Efficacy, safety, and biomarker results of trastuzumab emtansine (T‐DM1) in patients (pts) with previously treated HER2‐overexpressing locally advanced or metastatic non‐small cell lung cancer (mNSCLC). J Clin Oncol 2017;35(suppl):8509a. [DOI] [PubMed] [Google Scholar]
- 100.Smit EF, Peters S, Dziadziuszko R et al. A single‐arm phase II trial of afatinib in pretreated patients with advanced NSCLC harboring a HER2 mutation: The ETOP NICHE trial. J Clin Oncol 2017;35(suppl):9070a. [Google Scholar]
- 101.De Langen J, Kuiper JL, Thunnissen E et al. Trastuzumab and paclitaxel in patients (pts) with EGFR mutated non‐small‐cell lung cancer (NSCLC) that express HER2 after progression on EGFR TKI treatment. J Clin Oncol 2017;35(suppl):9042a. [DOI] [PMC free article] [PubMed] [Google Scholar]