This article reports the results of a study that examined the clinical characteristics and oncologic outcomes of patients with uterine adenosarcoma and cervical adenosarcoma, analyzing prognostic factors and treatment options for these two patient groups.
Keywords: Adenosarcoma of the uterus, Cervix uteri, Fertility preservation
Abstract
Background.
The management of adenosarcoma is based on the limited available data. This study aimed to explore the characteristics and oncologic outcomes of patients with uterine and cervical adenosarcoma.
Materials and Methods.
A total of 21 and 32 cases of cervical and uterine adenosarcoma, respectively, were retrospectively reviewed in Peking Union Medical College Hospital between April 2006 and March 2019.
Results.
The median follow‐up time was 37.5 months (range, 1–153 months). The disease progression rate (DPR) was significantly higher in patients with uterine adenosarcoma compared with those with cervical adenosarcoma (28.1% vs. 4.8%). The curve of progression‐free survival significantly differed. For those with cervical adenosarcoma, the presence of a tumor stalk was a protective factor, whereas heterologous elements (HE) presented a risk factor for disease progression. For those with uterine adenosarcoma, the presence of a tumor stalk was an independent protective factor, whereas lymphovascular space invasion (LVSI) was an independent risk factor for disease progression. Moreover, HE was an independent risk factor for mortality. Fertility‐sparing surgery (FSS) was performed in four and five patients with cervical and uterine adenosarcoma, respectively. Regarding FSS, combined with cases in previous studies, the DPR of patients with uterine adenosarcoma was relatively higher compared with those with cervical adenosarcoma.
Conclusion.
We found that cervical adenosarcoma had a better prognosis than uterine adenosarcoma. The presence of a tumor stalk was a protective factor, whereas HE and LVSI were risk factors for prognosis. For those with uterine adenosarcoma, if FSS was administered, robust evaluation would be necessary. The small sample size limits the ability to make any strong conclusions about FSS.
Implications for Practice.
Uterine cervical adenosarcoma had a better prognosis than uterine adenosarcoma. For patients with cervical adenosarcoma, the presence of a tumor stalk was a protective factor and the presence of heterologous elements (HE) was a risk factor for disease progression. For those with uterine adenosarcoma, the presence of a tumor stalk was a protective factor and lymphovascular space invasion was a risk factor for disease progression. Moreover, HE was a risk factor for mortality. Regarding fertility‐sparing surgery (FSS), the disease progression rate was higher in patients with uterine adenosarcoma compared with those with cervical adenosarcoma. For patients with uterine adenosarcoma, if FSS was administered, hysteroscopy and robust imaging evaluation would be necessary.
Introduction
Adenosarcomas are rare mixed tumors, showing a combination of benign glandular epithelium and malignant stromal elements [1], [2]. Adenosarcomas occur mainly in the uterus but may also occur in the cervix and extrauterine locations [2], [3]. Uterine and cervical adenosarcoma represent 0.43% of uterine and 0.16% of cervical cancers in a National Cancer Database study [3].
The patients with cervical adenosarcoma are younger than those with uterine adenosarcoma [3]. Adenosarcomas usually present as a soft polypoid mass [1]. The majority of patients (73.4%–82%) are diagnosed with stage I disease [4]. The management for adenosarcomas is based on the limited available data for its rarity. Surgery is the mainstay of treatment for adenosarcomas [3]. However, the best surgery procedure and whether fertility‐sparing surgery (FSS) can be used in cervical and uterine adenosarcoma remain controversial [5], [6], [7], [8], [9], [10], [11], [12], [13], [14].
This study aimed to comprehensively review the clinical characteristics and oncologic outcomes, to analyze prognostic factors, and to explore the treatment of uterine and cervical adenosarcoma.
Materials and Methods
Ethics
Our study was approved by the ethics committee of Peking Union Medical College Hospital (PUMCH). The need for written informed consent was waived because of the retrospective nature of the study, and the data set was deidentified in order to protect patient privacy.
Study Design and Patient Information
This was a retrospective study that included information on patients diagnosed and treated at PUMCH, between April 2006 and March 2019. Patient information, including age of onset, clinical features, treatment modality, and outcome associated with treatment, was collected from their medical records. The follow‐up information was obtained from outpatient medical records and via telephone interviews.
Pathological Review
All specimens were reviewed randomly by two independent pathologists from the Department of Pathology at the PUMCH. Tumors that occurred only in the cervix were defined as uterine cervical adenosarcoma, and those that occurred in the corpus with or without involvement of the cervix were defined as uterine adenosarcoma. For uterine adenosarcoma, pathological staging was performed in accordance with the International Federation of Gynecology and Obstetrics (FIGO, 2009) staging system for uterine sarcoma. And for uterine cervical adenosarcoma, staging was conducted using the FIGO 2009 staging system for cervical cancer. Sarcomatous overgrowth was diagnosed when the pure sarcomatous portion of the neoplasm constituted more than 25% of the primary tumor [15].
Statistical Analysis
The duration of the patients’ overall survival (OS) was calculated from the date of the initial surgery to the date of death or last contact, and their progression‐free survival (PFS) was measured from the date of the initial surgery to the date of first progression or recurrence. Categorical variables were summarized in frequency tables, whereas continuous variables were presented as medians (range). Frequency distributions were compared using chi‐square or Fisher's exact tests, and median values were compared using Mann‐Whitney nonparametric U tests. The product‐limit method of Kaplan and Meier was used to estimate the OS and PFS, and the difference in survival between groups was tested using a log‐rank test. All of the follow‐up information was censored following March 22, 2019. Variables with p values <.1 on univariate analysis were selected for multivariate analysis. A Cox proportion hazards model was used for multivariate regression analysis of survival data. The data were analyzed using SPSS version 22 (IBM, Armonk, NY) or Prism 7 (GraphPad Software, San Diego, CA). A p value <.05 was considered statistically significant using the two‐tailed hypothesis. Some figures were made by OmniGraffle application (The Omni Group).
Results
Clinical Characteristics
A total of 32 and 21 patients were diagnosed with uterine and cervical adenosarcoma, respectively. During this period, approximately 6,221 and 12,450 patients with uterine and cervical malignant tumors, respectively, were diagnosed and treated in our institution. Cases with uterine and cervical adenosarcoma represented 0.51% and 0.17% of uterine and cervical cancers in our institution, respectively.
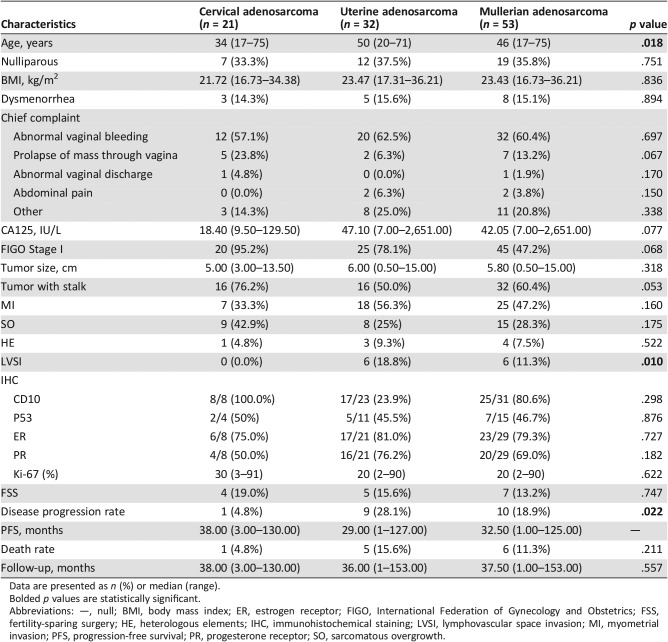
The clinical characteristics of the included patients are summarized in Table 1. The median age at diagnosis was 46 years (range, 17‐75 years). Those with cervical adenosarcoma were significantly younger than those with uterine adenosarcoma (median age 34 vs. 50 years, p = .018). The median body mass index (BMI) was 23.43 kg/m2 (range, 16.73–36.21 kg/m2). The majority of patients (n = 32, 60.4%) presented with abnormal vaginal bleeding. Nineteen (35.8%) patients were nulliparous, and eight (15.1%) patients had dysmenorrhea. The median preoperative value of CA125 was 42.05 U/mL (range, 7–2,651 U/mL). There were no significant differences between the two groups for the above‐mentioned factors.
Table 1. Clinical and pathological characteristics of patients with uterine and cervical adenosarcoma in our institution.
Data are presented as n (%) or median (range).
Bolded p values are statistically significant.
Abbreviations: —, null; BMI, body mass index; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; FSS, fertility‐sparing surgery; HE, heterologous elements; IHC, immunohistochemical staining; LVSI, lymphovascular space invasion; MI, myometrial invasion; PFS, progression‐free survival; PR, progesterone receptor; SO, sarcomatous overgrowth.
Diagnosis
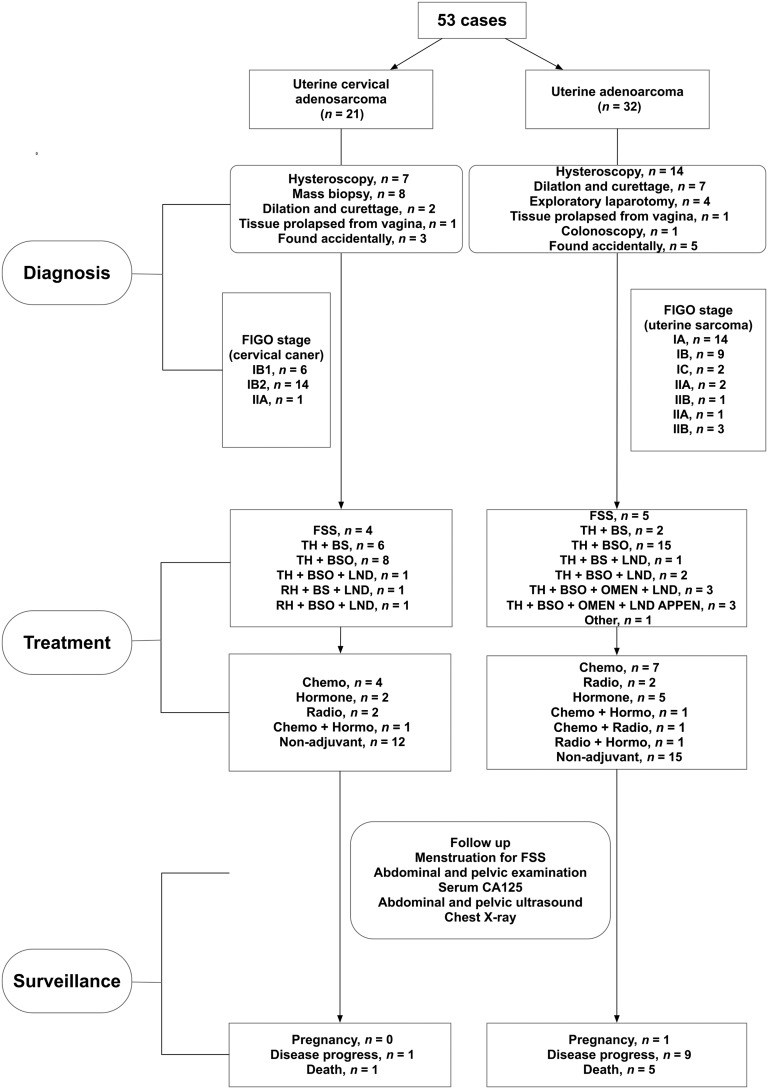
As is shown in Figure 1, 7 (33.3%) and 14 (43.8%) cases of cervical and uterine adenosarcoma, respectively, were diagnosed using hysteroscopy. For uterine cervical adenosarcoma, 6 (28.6%), 14 (66.7%), and 1 (4.8%) patients were of stages IB1, IB2, and IIA, respectively. For uterine adenosarcoma, 14 (43.8%), 9 (28.1%), 2 (6.3%), 2 (6.3%), 1 (3.1%), 1 (3.1%), and 3 (9.4%) patients were of stages IA, IB, IC, IIA, IIB, IIIA, and IIIB, respectively. Although without significant difference (p = .068), the proportion of patients with stage I in cervical adenosarcoma (n = 20, 95.2%) was higher than that of those with uterine adenosarcoma (n = 25, 78.1%).
Figure 1.
The information regarding diagnosis, treatment, and surveillance in this study.
Abbreviations: APPEN, appendicectomy; BS, bilateral salpingectomy; BSO, bilateral salpingo‐oophorectomy; Chemo, chemotherapy; FIGO, International Federation of Gynecology and Obstetrics; FSS, fertility‐sparing surgery; Hormo, hormone therapy; LND, lymph node dissection; OMEN, omentectomy; Radio, radiotherapy; RH, radical hysterectomy; TH, total hysterectomy.
Pathologic Characteristics
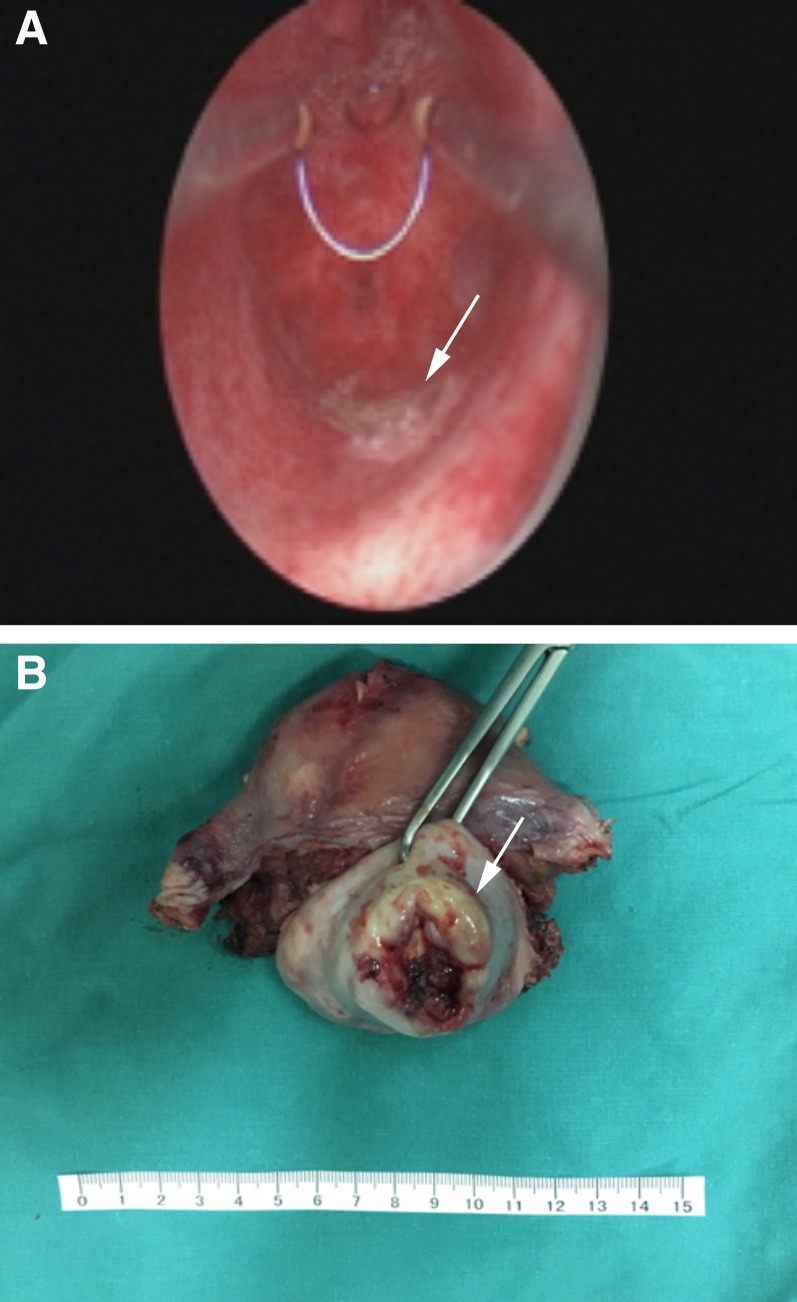
Overall, 32 (60.4%) patients were polypoid with a stalk and 21 (39.6%) patients showed extensive tumor without an obvious stalk. Figure 2 shows uterine adenosarcoma with a tumor stalk and cervical adenosarcoma without a tumor stalk. The proportion of patients with the presence of tumor stalks (n = 16, 76.2%) was relatively higher in cervical adenosarcoma than that in uterine adenosarcoma (n = 16, 50.0%), although the difference was not significant (p = .053). The median maximum diameter of tumors among those with cervical and uterine adenosarcoma was 5 and 6 cm, respectively. The proportion of patients with myometrium invasion, sarcomatous overgrowth, heterologous elements (HE), and lymphovascular space invasion (LVSI) in cervical and uterine adenosarcoma is summarized in Table 1. Moreover, the proportion of patients with LVSI was significantly higher among those with uterine adenosarcoma than among those with cervical adenosarcoma (18.8% and 0.0%, respectively, p = .010).
Figure 2.
Uterine corpus and cervical adenosarcoma. (A): Uterine adenosarcoma with a tumor stalk, the tumor root after tumor resection (arrow). (B): Uterine cervical adenosarcoma without a stalk (arrow).
The information regarding immunohistochemical staining available is summarized in Table 1. The estrogen receptor (ER) and progesterone receptor (PR) immunohistochemical staining were performed in 29 patients. The ER and PR positivity rates were 79.3% and 69.0%, respectively. The information regarding Ki‐67, CD10, and P53 is also summarized in Table 1. There was no significant difference between those with uterine and cervical adenosarcoma.
Treatment
For those with cervical adenosarcoma, FSS, total hysterectomy (TH), and radical hysterectomy was performed in 4 (19.0%), 15 (71.4%), and 2 (9.6%) patients, respectively. Of these 17 patients with hysterectomy, bilateral salpingo‐oophorectomy (BSO) was performed in 10 (58.8%) patients and lymph node dissection (LND) in 3 (17.6%) patients (Fig. 1).
For those with uterine adenosarcoma, FSS was performed in five (15.6%) patients. TH was conducted in 26 (81.3%) patients, of whom 13 (50.0%), 9 (34.6%), 2 (7.7%), and 3 (11.5%) underwent oophorectomy, LND, omentectomy, and appendectomy, respectively (Fig. 1). Moreover, one (3.1%) patient experienced rapid disease progression after diagnosis and did not receive further treatment.
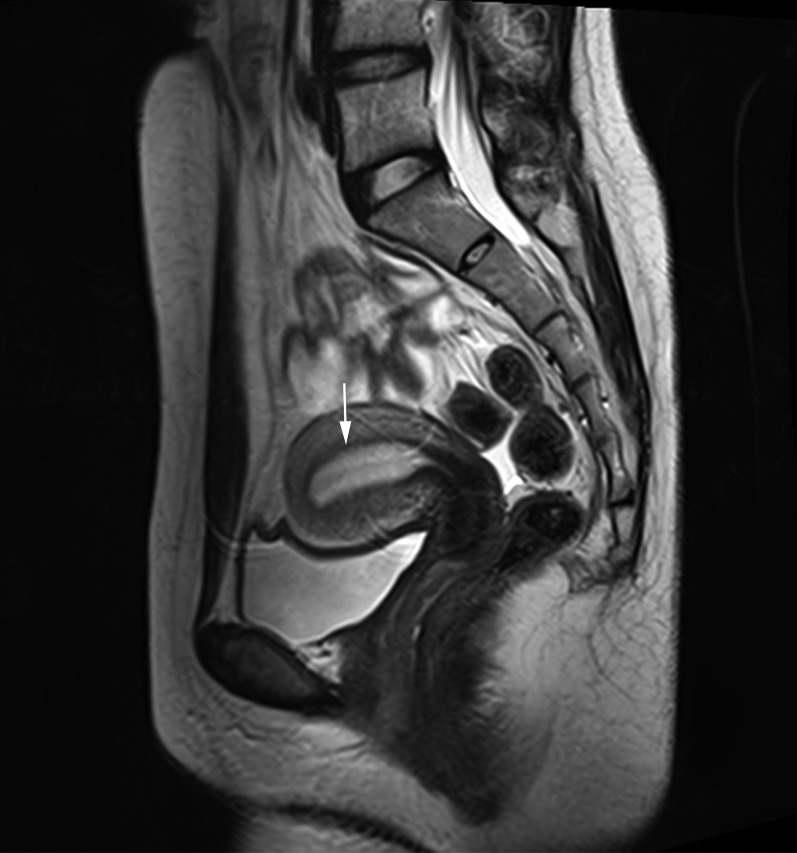
For all the patients for whom FSS was conducted, magnetic resonance imaging (MRI) of the pelvis was performed prior to fertility‐sparing treatment. As shown in Figure 3, the junctional zone was clearly identified. Moreover, computed tomography (CT) of the chest and abdomen was also conducted for the detection of local and distant metastasis. Hysteroscopy was also conducted for both cervical and uterine adenosarcoma. For those with uterine adenosarcoma, hysteroscopy was used to confirm the complete tumor resection, whereas for those with cervical adenosarcoma, hysterectomy was used not only to confirm the complete tumor resection but also to eliminate the presence of endometrial lesion. Figure 2A shows the uterine adenosarcoma resected during hysteroscopy. Among these four and five patients with cervical and uterine adenosarcoma, respectively, two (50.0%) patients with cervical adenosarcoma and one (20.0%) with uterine adenosarcoma had sarcomatous overgrowth; even after being informed adequately of the risks, these patients still required fertility preservation and underwent FSS.
Figure 3.
Magnetic resonance imaging evaluation for patients with FSS. The clearly identified junctional zone (arrow).
For those with cervical adenosarcoma, five (23.8%), three (14.3%), and two (9.5%) patients received chemotherapy, hormone therapy, and radiotherapy after the treatment surgery, respectively. Twelve (57.1%) patients chose to observe without adjuvant therapy. For those with uterine adenosarcoma, nine (28.1%), seven (21.9%), and four (12.5%) patients received chemotherapy, hormone therapy, and radiotherapy, respectively (12.5%). Another 15 (46.9%) patients chose to proceed without adjuvant therapy (Fig. 1). The chemotherapy regiment included PEI (cisplatin+ epirubicin + ifosfamide), PE (cisplatin + epirubicin), PI (cisplatin + ifosfamide), and others (gemcitabine + docetaxel). The hormone therapy regimen included medroxyprogesterone acetate, megestrol, and gonadotropin‐releasing hormone analogues.
Surveillance
All patients had regular follow‐ups in the outpatient department. The median follow‐up time was 37.5 months (range, 1–153 months) for all patients. Information regarding menstruation of patients with FSS was obtained (Fig. 1). Abdominal and gynecologic examinations, serum CA125 levels, and ultrasonography of the pelvis and abdomen were also performed. Chest X‐ray was performed every year.
Oncologic Outcomes
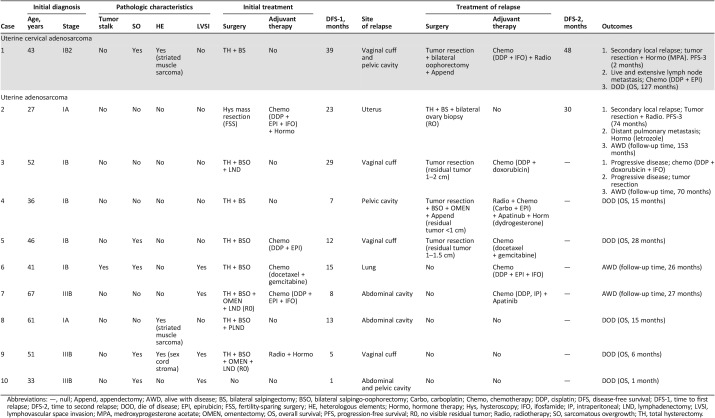
Of the 53 patients included, 1 patient died of a gastrointestinal malignant tumor 1 year after the initial surgery, without evidence of the uterine adenosarcoma having relapsed; this patient was considered to be censored. Disease progression was found in one (4.8%) and nine (28.1%) patients from the cervical and uterine adenosarcoma groups, respectively. The characteristics regarding disease progression are detailed in Table 2. For those with cervical adenosarcoma, the recurrence was local, whereas for those with uterine adenosarcoma, local relapse occurred in seven (77.8%) patients and distant relapse in the lung occurred in one (11.1%) patient. One (11.1%) patient experienced rapid disease progression after diagnosis and did not attain complete remission. For the one case with cervical adenosarcoma, the patient (case 1) experienced relapse three times and then died of disease. The overall survival was 127 months. For the treatment of relapse, among those eight (88.9%) patients with uterine adenosarcoma, four (57.1%) underwent surgery, followed by adjuvant chemotherapy, radiotherapy, hormone therapy, or just observation. Of these four patients, one (25.0%) patient (case 2) experienced relapse three times but was still alive with disease by the end of the study period; one (25.0%) patient (case 3) underwent disease progression during the period of chemotherapy, underwent surgery again, and was still alive with disease by the end of the study period; two (50.0%) patients (cases 4 and 5) experienced disease progression after the surgery and died. For the treatment of relapse among those with uterine adenosarcoma, two (25.0%) patients (cases 6 and 7) received chemotherapy without surgery and were still alive with disease by the end of the study period, but the follow‐up time was relatively short (26 and 27 months). The remaining two (25.0%) patients (case 8 and case 9) opted for palliative care and died soon thereafter.
Table 2. Details of patients with disease progression.
Abbreviations: —, null; Append, appendectomy; AWD, alive with disease; BS, bilateral salpingectomy; BSO, bilateral salpingo‐oophorectomy; Carbo, carboplatin; Chemo, chemotherapy; DDP, cisplatin; DFS, disease‐free survival; DFS‐1, time to first relapse; DFS‐2, time to second relapse; DOD, die of disease; EPI, epirubicin; FSS, fertility‐sparing surgery; HE, heterologous elements; Hormo, hormone therapy; Hys, hysteroscopy; IFO, ifosfamide; IP, intraperitoneal; LND, lymphadenectomy; LVSI, lymphovascular space invasion; MPA, medroxyprogesterone acetate; OMEN, omentectomy; OS, overall survival; PFS, progression‐free survival; R0, no visible residual tumor; Radio, radiotherapy; SO, sarcomatous overgrowth; TH, total hysterectomy.
Overall, the disease progression rate (DPR) was significantly higher among those with uterine adenosarcoma than among those with cervical adenosarcoma (28.1% vs. 4.8%, p = .022), although the mortality rate in the two groups did not statistically differ (15.6% vs. 4.8%, p = .211). Moreover, the survival curve of PFS significantly differed (p = .039; supplemental online Fig. 1).
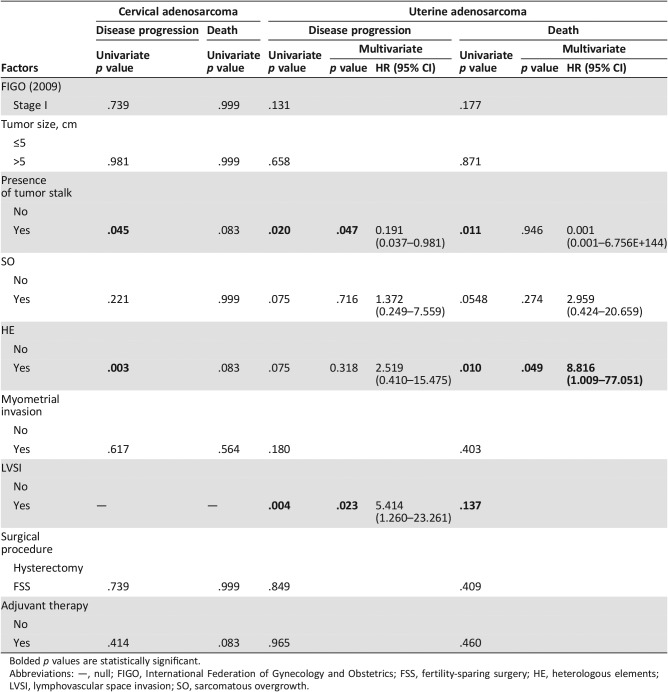
Table 3 shows the risk factors for disease progression and mortality for those with cervical adenosarcoma and uterine adenosarcoma, respectively. For those with cervical adenosarcoma, in the univariate analysis, PFS was significantly associated with the presence of a tumor stalk (p = .045) and HE (p = .003). The number of patients with cervical adenosarcoma was not enough to conduct a multivariate Cox regression analysis. For those with uterine adenosarcoma, in the univariate analysis, PFS was significantly associated with the presence of a tumor stalk (p = .020) and LVSI (p = .004). Moreover, OS was significantly associated with the presence of a tumor stalk (p = .011) and HE (p = .010). In the multivariate analysis, the presence of a tumor stalk remained an independent protective factor (hazard ration [HR] = 0.191, 95% confidence interval [CI] = 0.037–0.981, p = .047) and LVSI remained an independent risk factor (HR = 5.414, 95% CI = 1.260–23.261, p = .023) for disease progression. Moreover, HE remained an independent risk factor for mortality (HR = 8.816, 95% CI = 1.009–77.051, p = .049).
Table 3. The risk factors for disease progression and mortality among patients with uterine and cervical adenosarcoma.
Bolded p values are statistically significant.
Abbreviations: —, null; FIGO, International Federation of Gynecology and Obstetrics; FSS, fertility‐sparing surgery; HE, heterologous elements; LVSI, lymphovascular space invasion; SO, sarcomatous overgrowth.
The Oncologic Outcomes of Patients with Fertility‐Sparing Surgery
For the four patients with cervical adenosarcoma with FSS, none experienced relapse. All the patients had normal menstruation, and none experienced a pregnancy. The median follow‐up time was 19 months (range, 15–62 months). For the 20 patients with stage I cervical adenosarcoma, FSS was not significantly associated with PFS and OS (p = .724 and .999, respectively; supplemental online Fig. 2).
For the five patients with uterine adenosarcoma, one (20.0%) experienced relapse and was alive with disease at the end of the study period (Table 2, case 2) and the remaining four (80.0%) were without evidence of disease. All the four patients had normal menstruation, and one patient got pregnant, 12 months after the surgery, and delivered a healthy infant. The median follow‐up time was 32 months (range, 5–153 months). For the 25 patients with stage I uterine adenosarcoma, FSS was not significantly associated with PFS and OS (p = .947 and .446, respectively; supplemental online Fig. 2).
Because of the rarity of this disease, we combined the cases in our institution with those from previously published studies and then analyzed the outcomes (supplemental online Table 1). A literature search was performed via PubMed, EMBASE and Web of Science. A total of 16 and 13 cases of cervical and uterine adenosarcoma, respectively, were included [6], [7], [8], [9], [10], [11], [12], [13], [24], [25], [26]. One case (case 13) was excluded from the analysis as a result of being lost to follow‐up. The DPR of cervical and uterine adenosarcoma were 1/15 (6.7%) and 6/13 (46.2%), respectively. Additionally, the DPR of uterine adenosarcoma was significantly higher than that of cervical adenosarcoma (p = .029). No deaths were reported.
Discussion
In this study, we comprehensively reviewed the demographics, clinicopathological characteristics, and oncologic results of 53 patients with adenosarcomas, comparing the characteristics and oncologic results of those with cervical and uterine adenosarcoma, especially of those patients with FSS. To the authors’ knowledge, this is the first and largest study to compare cervical and uterine adenosarcoma, especially on FSS, at a single institution.
The frequency of cervical and uterine adenosarcoma in our institution was 0.17 % and 0.51%, The median age of those with cervical adenosarcoma was 34 years, whereas that among those with uterine adenosarcoma was 50 years. The patients with uterine cervical adenosarcoma were younger than those with uterine adenosarcoma. Moreover, 95.2% and 78.1% of the patients were FIGO stage I for cervical and uterine adenosarcoma, respectively. The majority of tumors presented with a tumor stalk to the cervix or uterine corpus (76.2% and 50%, respectively). All the above‐mentioned data were consistent with previous studies [1], [3], [4].
Few cases of cervical adenosarcoma have been reported in the literature; the majority are included in case series of uterine adenosarcoma [5]. The majority of the guidance regarding the management of cervical adenosarcoma is extrapolated from the experience managing uterine adenosarcoma. Surgical procedures, including excisional biopsy, tumor resection, conization, trachelectomy, hysterectomy, TH, and radical hysterectomy, have been reported [3], [9], [10], [13], [16]. To the authors’ knowledge, there were three relatively larger studies regarding the management of cervical adenosarcoma. In one study, 0/2 and 1/3 patients with radical hysterectomy and TH experienced disease progression, respectively [13]. In another study, 0/7 of patients with radical hysterectomy experienced disease progression [14]. In the final study, 1/9 patients with TH experienced disease progression [10]. In our institution, 0/2 and 1/15 patients with radical hysterectomy and TH underwent disease progression. In total, 0/11 (0.0%) and 3/27 (11.1%) patients with radical hysterectomy and TH underwent disease progression. There is no statistical difference of DPR (p = .542). There was no significant association between the surgery procedure used and survival. Because of the rarity of cervical adenosarcoma, there is no consensus regarding the optimal surgery procedure. However, positive or unknown surgical margins have been shown to be associated with an increased hazard of mortality [3]. Thus, obtaining a negative margin is essential. Also, there is no common agreement on BSO and LND during primary surgery [3], [17].
Regarding the surgery of uterine adenosarcoma, in our institution, 46.8% of patients with uterine adenosarcoma underwent TH and BSO. TH and BSO have been recommended for the majority of patients with uterine adenosarcoma [5], [18]. However, BSO may not be necessary for premenopausal women; it is not associated with more survival benefits [3]. Previous studies have shown that uterine adenosarcoma has the lowest incidence of lymph node metastasis among the sarcoma subtypes [19]. Moreover, there is no significant difference in prognosis between those who underwent lymphadenectomy and those who did not [5].
In this study, 42.9% and 53.1% of patients with cervical and uterine adenosarcoma, respectively, received adjuvant therapy, including chemotherapy, radiotherapy, and hormonotherapy. There was no significant difference in PFS and OS between the adjuvant and nonadjuvant groups for both cervical and uterine adenosarcoma. These findings are consistent with those from previous studies; no survival benefit was observed for radiotherapy, chemotherapy, and hormonotherapy for uterine [3], [4], [5], [20] and cervical adenosarcoma [3]. There is no strong evidence here to recommend adjuvant therapy. Whether the adjuvant therapy should be given, to which patients it should be given, and what regiments should be given remains to be investigated.
For cervical adenosarcoma, there were few studies that had investigated prognostic factors. One study, using the National Cancer Database, found that increased age at diagnosis, increased tumor size, and positive or unknown surgical margins were associated with an increased hazard of a poor prognosis [3]. In our study, we identified that the presence of a tumor stalk was protective against disease progression. This finding can be explained by the fact that tumors with stalks may be easier to completely resect, with a lower possibility of any residual tumor. Other previous studies reported that tumors with pedunculated tumors and uninvolved stalks can be curative by local excision [1], [21]. We also found that HE was a risk factor for disease progression and that the HE was striated muscle sarcoma differentiation in our study. Our findings are consistent with others in that HE, in particular striated muscle sarcoma differentiation, of the cervical adenosarcoma may be a more aggressive histologic type [15].
For uterine adenosarcoma, a review has suggested that sarcomatous overgrowth, myometrial invasion, size, mitosis, age, race, FIGO stage, resection status, necrosis, cellular atypia, HE, and rhabdomyosarcoma element are possible prognostic factors [4]. In our study, we found that the presence of a tumor stalk was an independent protective factor for disease progression, whereas LVSI was an independent risk factor for disease progression and HE was an independent risk factor for mortality. We believe that the reason the presence of tumor stalks may be protective against the poor prognosis for uterine adenosarcoma is similar to that for cervical adenosarcoma. For those with uterine adenosarcoma, the tumor resection status or negative surgical margins were also important prognostic factors [4], [5]. Although insignificant, there was a trend toward worse PFS and OS in patients with sarcomatous overgrowth; those findings are consistent with previous studies [22]. The more aggressive treatment performed in patients with sarcomatous overgrowth and the limited number of patients may have biased the analysis in our study. However, upon univariate and multivariate analysis, LVSI and HE remained independent factors for a poor prognosis, which is consistent with the findings from previous studies [4], [5].
The prognosis of cervical uterine adenosarcoma was less characterized. In our study, the DPR was 4.8%, being local recurrence. For uterine adenosarcoma, previous studies have reported that DPR was 14.3%–46.0% of patients, with the local relapse being the most common [22], [23]. In our study, the DPR was 28.1%, and 87.5% was local relapse. Interestingly, the DPR was relatively higher in those with uterine adenosarcoma than in those with cervical adenosarcoma, and the survival curve for PFS significantly differed. This may be explained by the fact that cervical adenosarcoma is more liable to cause abnormal vaginal bleeding, resulting in earlier diagnosis and treatment. Moreover, the proportion of patients with cervical adenosarcoma with the presence of a tumor stalk and stage I disease was higher than that of patients with uterine adenosarcoma, making tumor complete resection relatively easier. Additionally, no LVSI was found among those with cervical adenosarcoma. All of the above‐mentioned factors may explain the better prognosis among patients with cervical adenosarcoma.
Whether FSS can be used in cervical and uterine adenosarcoma remains controversial. Some authors claimed that FSS should not be a preferred approach because of its high risk of recurrence [5]. Alternatively, some authors have reported that local tumor excision may been curative in some cases [6], [7], [8], [9], [10], [11], [12], [13], [14]. In this study, FSS was performed in four patients with cervical adenosarcoma and five with uterine adenosarcoma. FSS was not significantly associated with PFS and OS when patients with stage I cervical adenosarcoma and patients with stage I uterine adenosarcoma were analyzed separately. The lack of the difference between PFS and OS among the FSS versus the non‐FSS groups may be due to the extremely small numbers of enrolled patients and the rarity of recurrence. The small sample size limits the ability to make any strong conclusions about FSS in these patients.
Moreover, combining the cases included in our study and those with FSS from previously reported studies (supplemental online Table 1), the DPR of patients with uterine adenosarcoma was higher than that of those with cervical adenosarcoma. The reason why the DPR for patients with uterine adenosarcoma was higher than that for those with cervical adenosarcoma could be that, for those with uterine adenosarcoma, especially those with myometrium invasion (stage IB), the complete resection may not be easy. Moreover, evaluating whether there was any residual tumor using imaging may not be as accurate as that for cervical adenosarcoma using the pathologic diagnosis of cervical conization or other procedures. As was reported, of the three patients with stage IB uterine adenosarcoma, two experienced disease progression [24]. Therefore, for those with uterine adenosarcoma, one must take caution to preserve fertility. If FSS was administered, hysteroscopy and robust imaging evaluation, including MRI of pelvis and CT of chest and abdomen, are necessary.
This study was limited by the small sample size and its retrospective nature, which could have possibly introduced some degree of bias. Despite these limitations, our study observed several important factors. The primary finding was regarding the different prognosis of cervical and uterine adenosarcoma. The second important finding was regarding the risk and protective factors for prognosis. Finally, the third important finding was regarding comparison of oncologic outcomes of FSS in patients with cervical and uterine adenosarcoma.
Conclusion
Uterine cervical adenosarcoma had a lower DPR and better prognosis than uterine adenosarcoma. For those with cervical adenosarcoma, PFS was significantly associated with the presence of a tumor stalk and HE. For those with uterine adenosarcoma, the presence of a tumor stalk remained an independent protective factor and LVSI an independent risk factor for disease progression. Moreover, HE remained an independent risk factor for mortality. Regarding FSS, the DPR of patients with uterine adenosarcoma was higher than that of those with cervical adenosarcoma. For uterine adenosarcoma, if FSS was administered, hysteroscopy and robust imaging evaluation would be necessary. The small sample size limits the ability to make any strong conclusions about FSS in these patients.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81572576 [Keng Shen]) and CAMS Initiative for Innovative Medicine (CAMS‐2018‐12M‐1‐002 [Keng Shen]).
Author Contributions
Conception/design: Zhen Yuan, Dongyan Cao, Mei Yu, Keng Shen
Provision of study material or patients: Zhen Yuan, Dongyan Cao, Mei Yu
Collection and/or assembly of data: Zhen Yuan, Dongyan Cao, Mei Yu, Yonglan He
Data analysis and interpretation: Zhen Yuan, Dongyan Cao, Mei Yu
Manuscript writing: Zhen Yuan, Mei Yu
Final approval of manuscript: Zhen Yuan, Dongyan Cao, Mei Yu, Keng Shen, Yonglan He
Disclosures
The authors indicated no financial relationships.
References
- 1.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: A clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol 1990;21:363–381. [DOI] [PubMed] [Google Scholar]
- 2.Gallardo A, Prat J. Mullerian adenosarcoma: A clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol 2009;33:278–288. [DOI] [PubMed] [Google Scholar]
- 3.Seagle BL, Kanis M, Strohl AE et al. Survival of women with Mullerian adenosarcoma: A National Cancer Data Base study. Gynecol Oncol 2016;143:636–641. [DOI] [PubMed] [Google Scholar]
- 4.Nathenson MJ, Ravi V, Fleming N et al. Uterine adenosarcoma: A review. Curr Oncol Rep 2016;18:68. [DOI] [PubMed] [Google Scholar]
- 5.Nathenson MJ, Conley AP, Lin H et al. The importance of lymphovascular invasion in uterine adenosarcomas: Analysis of clinical, prognostic, and treatment outcomes. Int J Gynecol Cancer 2018;28:1297–1310. [DOI] [PubMed] [Google Scholar]
- 6.Shinnick JK, Kumar N, Beffa L et al. Management of low‐grade cervical Mullerian adenosarcoma in a 14‐year‐old girl. J Pediatr Adolesc Gynecol 2017;30:652–654. [DOI] [PubMed] [Google Scholar]
- 7.Kanayama S, Nakamura M, Oi H et al. Case report of successful childbearing after conservative surgery for cervical Mullerian adenosarcoma. Case Rep Obstet Gynecol 2017;2017:4187416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanamandra SK, Leong MY, and Fortier MV. Vaginal mass in a 13‐year‐old girl. Ann Acad Med Singapore 2014;43:127–129. [PubMed] [Google Scholar]
- 9.Buyukkurt S, Guzel AB, Gumurdulu D et al. Mullerian adenosarcoma of the uterine cervix in an adolescent girl. J Pediatr Adolesc Gynecol 2010;23:e13–e15. [DOI] [PubMed] [Google Scholar]
- 10.Jones MW, Lefkowitz M. Adenosarcoma of the uterine cervix: A clinicopathological study of 12 cases. Int J Gynecol Pathol 1995;14:223–229. [DOI] [PubMed] [Google Scholar]
- 11.Zaloudek CJ, Norris HJ. Adenofibroma and adenosarcoma of the uterus: A clinicopathologic study of 35 cases. Cancer 1981;48:354–366. [DOI] [PubMed] [Google Scholar]
- 12.Geisler JP, Orr CJ, Manahan KJ. Robotically assisted total laparoscopic radical trachelectomy for fertility sparing in stage IB1 adenosarcoma of the cervix. J Laparoendosc Adv Surg Tech A 2008;18:727–729. [DOI] [PubMed] [Google Scholar]
- 13.Togami S, Kawamura T, Fukuda M et al. Clinical management of uterine cervical mullerian adenosarcoma: A clinicopathological study of six cases and review of the literature. Taiwan J Obstet Gynecol 2018;57:479–482. [DOI] [PubMed] [Google Scholar]
- 14.Chin PS, Chia YN, Lim YK et al. Diagnosis and management of Mullerian adenosarcoma of the uterine cervix. Int J Gynaecol Obstet 2013;121:229–232. [DOI] [PubMed] [Google Scholar]
- 15.Seagle BL, Falter KJ, Lee SJ et al. Mullerian adenosarcoma of the cervix: Report of two large tumors with sarcomatous overgrowth or heterologous elements. Gynecol Oncol Case Rep 2014;9:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales F DA, Medina R ML, Trujillo LM et al. Müllerian adenosarcoma of the uterine cervix with sarcomatous overgrowth: A case report of aggressive disease in a young patient. Int J Surg Case Rep 2016;27:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrelli TS, Gizzo S, Di Gangi S et al. Cervical Mullerian adenosarcoma with heterologous sarcomatous overgrowth: A fourth case and review of literature. BMC Cancer 2011;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedlander ML, Covens A, Glasspool RM et al. Gynecologic Cancer InterGroup (GCIG) consensus review for mullerian adenosarcoma of the female genital tract. Int J Gynecol Cancer 2014;24(9 suppl 3):S78–S82. [DOI] [PubMed] [Google Scholar]
- 19.Machida H, Nathenson MJ, Takiuchi T et al. Significance of lymph node metastasis on survival of women with uterine adenosarcoma. Gynecol Oncol 2017;144:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner EJ, Toussaint T, Leitao MM Jr et al. Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol Oncol 2013;129:140–144. [DOI] [PubMed] [Google Scholar]
- 21.Chen KT. Rhabdomyosarcomatous uterine adenosarcoma. Int J Gynecol Pathol 1985;4:146–152. [DOI] [PubMed] [Google Scholar]
- 22.Carroll A, Ramirez PT, Westin SN et al. Uterine adenosarcoma: An analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol 2014;135:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benito V, Lubrano A, Arencibia O et al. Clinicopathologic analysis of uterine sarcomas from a single institution in the Canary Islands. Int J Gynaecol Obstet 2009;107:44–49. [DOI] [PubMed] [Google Scholar]
- 24.Lee YJ, Kim DY, Suh DS et al. Feasibility of uterine preservation in the management of early‐stage uterine adenosarcomas: A single institute experience. World J Surg Oncol 2017;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin PS, Chia YN, Lim YK et al. Diagnosis and management of Mullerian adenosarcoma of the uterine cervix. Int J Gynecol Obstet 2013;121:229–232. [DOI] [PubMed] [Google Scholar]
- 26.Goh C, Lin XH, Chin PS et al. Uterine preservation in a young patient with adenosarcoma of the uterus ‐ Case report and review of literature. Gynecol Oncol Rep 2018;25:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]