Gastric adenocarcinoma is the third deadliest malignant neoplasm worldwide. This article describes the results of an evaluation of HER2 expression in circulating tumor cells and plakoglobin in circulating tumor microemboli from nonmetastatic gastric adenocarcinoma patients.
Keywords: Circulating tumor cells, Circulating tumor microemboli, Gastric adenocarcinoma, HER2, Plakoglobin
Abstract
Background.
Gastric adenocarcinoma (GAC) is the third deadliest malignant neoplasm worldwide, mostly because of late disease diagnosis, low chemotherapy response rates, and an overall lack of tumor biology understanding. Therefore, tools for prognosis and prediction of treatment response are needed. Quantification of circulating tumor cells (CTCs) and circulating tumor microemboli (CTM) and their expression of biomarkers has potential clinical relevance. Our aim was to evaluate CTCs and CTM and their expression of HER2 and plakoglobin in patients with nonmetastatic GAC, correlating the findings to clinicopathological data.
Materials and Methods.
CTC enrichment was performed with isolation by size of epithelial tumor cells, and the analysis was performed with immunocytochemistry and microscopy. Two collections were made: one at diagnosis (55 samples before neoadjuvant treatment) and one after surgery and before adjuvant therapy (33 samples).
Results.
A high detection rate of CTCs (90%) was observed at baseline. We evaluated HER2 expression in 45/55 biopsy samples and in 42/55 CTC samples, with an overlap of 36 subjects. Besides the good agreement observed for HER2 expression in primary tumors and paired CTCs for 36 cases (69.4%; κ = 0.272), the analysis of HER2 in CTCs showed higher positivity (43%) compared with primary tumors (11%); 3/5 patients with disease progression had HER2‐negative primary tumors but HER2‐positive CTCs. A significant CTC count drop in follow‐up was seen for CTC‐HER2‐positive cases (4.45 to 1.0 CTCs per mL) compared with CTC‐HER2‐negative cases (2.6 to 1.0 CTCs per mL). The same was observed for CTC‐plakoglobin‐positive cases (2.9 to 1.25 CTCs per mL).
Conclusion.
CTC analysis, including their levels, plakoglobin, and HER2 expression, appears to be a promising tool in the understanding the biology and prognosis of GAC.
Implications for Practice.
The analysis of circulating tumor cell levels from the blood of patients with gastric adenocarcinoma, before and after neoadjuvant treatment, is useful to better understand the behavior of the disease as well as the patients more likely to respond to treatment.
摘要
背景。胃腺癌 (GAC) 是全球第三大致命恶性肿瘤,主要原因是疾病诊断较晚、化疗疗效较低以及对肿瘤生物学的整体认识不足。因此,我们需要用于预后及治疗反应预测工具。循环肿瘤细胞 (CTC) 和循环肿瘤微栓 (CTM) 的量化及其生物标志物的表达具有潜在的临床意义。我们的目的是评估 CTC 和 CTM 及其 HER2 和 plakoglobin在非转移性 GAC 患者中的表达,并将该发现与临床病理数据相关联。
材料和方法。使用上皮肿瘤细胞大小分离法进行 CTC 富集,并用免疫细胞化学和电子显微镜进行分析。制作了两个集合:一个在诊断时(新辅助化疗前 55 个样本),一个在手术后和辅助性化疗前(33 个样本)。
结果。在基线观察到 CTC 检出率高 (90%)。我们评估了 45/55 个活检样本和 42/55 个 CTC 样本中的 HER2 的表达情况,其中有 36 名受试者表达重叠。除了在原发性肿瘤和成对 CTC 中观察到 HER2 表达的良好一致性 36 例(69.4%; κ = 0.272),CTC 中 HER2 的分析显示与原发性肿瘤(11%)相比具有更高的阳性(43%);3/5 名疾病进展的患者具有 HER2 阴性原发性肿瘤但为 HER2 阳性 CTC。与 CTC‐HER2 阴性病例(每毫升 2.6 至 1.0 个 CTC)相比,CTC‐HER2 阳性病例(每毫升 4.45 至 1.0 个 CTC)的跟进中 CTC 计数显着下降。在 CTC ‐ plakoglobin阳性病例(每毫升 2.9 至 1.25 个 CTC)中观察到相同情况。
结论。CTC 分析,包括其水平、plakoglobin和 HER2 表达,似乎是理解 GAC 生物学及预后的一种极具前途的工具。
实践意义:在新辅助治疗之前和之后对来自胃腺癌患者的血液中的循环肿瘤细胞水平进行分析有助于更好地理解疾病行为以及更可能对治疗产生反应的患者。
Introduction
Gastric adenocarcinoma (GAC) remains a significant malignancy in most Latin American countries and in Asia, where low survival rates are primarily associated with late disease diagnosis and a higher proportion of proximal and more aggressive lesions [1]. According to the Global Burden of Disease Cancer Collaboration [2], GAC is among the top five most incident tumors, and it stands as the third deadliest malignant neoplasm worldwide.
There are still remarkable differences in clinical management of patients with GAC because of variations in demographic, epidemiological, and biologic aspects of these aggressive tumors. Questions regarding the best time for systemic therapy or radiotherapy, the extent of surgery, the selection of new drugs, and the identification of disease relapse or progression could be better answered with biomarkers of systemic therapy response and are still a matter of debate and a lack of consensus prevails. Therefore, new approaches and technologies for the early diagnosis and the prediction of treatment response are of utmost need [3], [4].
Blood is the preferred biomarker source for the accurate monitoring of disease status and tumor evaluation because of low invasiveness in its collection, good stability, and its comprehensive representation of the physiological state of an individual. Furthermore, blood can usually be collected serially, providing consecutive snapshots of disease status. Blood is particularly useful in the context of liquid biopsies (LBs), where it allows a series of approaches for the detection of informative markers. In oncology, LBs encompasses three main components: (a) the detection and quantification of cell‐free tumor‐derived DNA [5], [6], [7], [8]; (b) the quantification and cargo determination of extracellular vesicles [9], [10], [11]; and (c) the detection, quantification, morphological analysis, and determination of biomarkers in circulating tumor cells (CTCs) [12], [13], [14], [15], [16].
Studies on CTCs have been demonstrated to be highly informative but also very heterogeneous. CTCs that undergo epithelial‐mesenchymal transition express distinct molecules, including epithelial, mesenchymal, and stem cell markers [17], [18], [19]. Because of the variability of these markers, some authors postulate that methods based on physical properties of these cells may be more reliable than expression‐dependent markers for CTC enrichment [17], [20], [21]. Marker‐independent enrichment also allows the recovery of clusters of CTCs and other cells, denominated circulating tumor microemboli (CTM). CTM may be more prone to survive bloodstream shear stress compared with CTCs [22] and to overcome cytotoxic treatment, leading to metastasis formation [23], [24]. In particular, in patients with GAC, very few studies have evaluated the presence and clinical value of CTCs and CTM [25], [26], [27].
The clinical value of CTC and CTM analysis can be enhanced if the expression of markers of aggressiveness in these cells are also vetted; examples include the human epidermal growth factor receptor 2 (ERBB2/HER2), an oncogenic transmembrane receptor that plays a relevant role in GAC and breast cancer tumorigenesis. A recent study demonstrated discrepant amplification levels of HER2 between paired CTCs and biopsies from initially HER2‐negative primary GAC tumors, with possible impacts over treatment response and metastatic potential [27].
Plakoglobin, also known as γ‐catenin, is a cytoplasmic protein that alternatively with other catenins, bind to cadherins, allowing an optimal adhesive function [28]. Inactivation of catenins and disruption of the binding cadherin and catenin result in loss of cell‐to‐cell adhesion [29]. A study [30] showed that loss or down‐regulation of E‐cadherin and catenin in primary tumors correlates with poor survival in advanced gastric adenocarcinoma. Another study from the same group [31] with 41 paraffin‐embedded gastrectomy samples showed that abnormal expression of E‐cadherin and catenin was more frequently seen in diffuse than in intestinal type tumors (p < .005). In a pivotal study of CTC markers in breast cancer [24], the authors showed plakoglobin to be markedly up‐regulated in CTM compared with CTCs (219‐fold). Importantly, tumors enriched in plakoglobin appear to produce plakoglobin‐enriched CTM that are held together because of activity of this protein in adherens junctions and desmosomes. As demonstrated by these authors, CTM abundance and high levels of plakoglobin expression in tumors are associated with adverse prognosis. In this sense, plakoglobin may have important potential as a prognostic marker in CTM. As far as we know, no study has evaluated the role of plakoglobin in CTCs and CTM from patients with GAC.
In the article presented here, we evaluated the presence of CTCs and CTM, as well as their HER2 and plakoglobin protein expression, in 88 samples derived from 55 patients diagnosed with GAC. The data were correlated with clinicopathological information in an attempt to evaluate if CTC or CTM analysis could contribute to a better clinical management of GAC.
Materials and Methods
Patient Recruitment
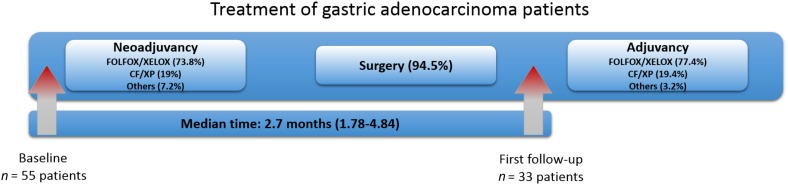
Patients with GAC were recruited at the Department of Abdominal Surgery ‐ Surgical Oncology of the A.C. Camargo Cancer Center, São Paulo, Brazil, from March 2016 to April 2017. Patients with nonmetastatic GAC, above 18 years old, and chemotherapy‐naïve were included. Patients who underwent surgical procedures within 3 weeks before CTC analysis were excluded. Clinicopathological data were obtained from the medical records. A total of 88 blood samples were obtained from 55 patients after they were informed about the study and had signed an informed consent form previously approved by the institutional review board (2134/15). All patients had blood samples collected at diagnosis, before neoadjuvant therapy (initial diagnosis; baseline). Clinical stage (cT, cN, and cM) was defined mainly by abdominal computed tomography or by magnetic resonance imaging and endoscopic ultrasound. For 33 subjects, a second blood collection was also obtained after surgery and before adjuvant treatment (median of 2.7 months’ interval, range 1.78–4.84 months; Fig. 1). Chemotherapy consisted mostly of FOLFOX (folinic acid, 5‐fluorouracil and oxaliplatin) or XELOX (capecitabine and oxaliplatin) in both neoadjuvant and adjuvant treatment, (73.8% and 77.4%, respectively), followed by cisplatin plus 5‐fluorouracil or cisplatin plus capecitabine (19% and 19.4%, respectively).
Figure 1.
Flow chart of patients enrolled in this study. 55 patients with gastric cancer were included before the beginning of neoadjuvancy (baseline); 73.8% of them received FOLFOX or XELOX‐based chemotherapy; 94.5% of all patients was submitted to surgical procedure. The follow‐up was made for 33 patients before the beginning of adjuvant chemotherapy, where FOLFOX and XELOX were also more commonly used (77.4%). The median time between baseline and follow‐up was 2.7 months (minimum 1.78 and maximum 4.84 months).
Abbreviations: CF/XP, cisplatin and 5‐fluorouracil or cisplatin and capecitabine; FOLFOX, folinic acid, 5‐fluorouracil and oxaliplatin; GC, gastric cancer; XELOX, capecitabine and oxaliplatin.
HER2 status information on tumor biopsies was taken from medical records. The immunohistochemistry score for HER2 expression in GAC was adapted from the guidelines described by Rüschoff et al. (2010) for biopsies: 0 score, no membranous staining or staining only in rare cells (less than five cohesive cells); 1+ score, staining is weak or detected in only one part of the membrane of at least five cohesive cells; 2+ score, moderate or weak complete or basolateral membranous staining of at least five cohesive cells; and 3+ score, strong complete or basolateral membranous staining of at least five cohesive cells [32].
The determination of histopathological response to neoadjuvant treatment considered the percentage of viable tumor cells relative to therapy‐induced fibrosis. Here we used the Becker tumor regression grading system, which scores samples as follows: 1a, with no residual tumor or tumor bed; 1b, with <10% residual tumor or tumor bed; 2, with 10%–50% residual tumor or tumor bed; or 3, with >50% residual tumor or tumor bed [33]. We defined pathological downstaging as the occurrence of ypT0–2ypN0 tumors in patients previously staged as cT3–4 or cN1–3 (cN+).
CTC Isolation
For each patient, 8 mL of blood were collected on ethylenediamine tetraacetic acid tubes and maintained under gentle homogenization for up to 4 hours at room temperature until filtration using isolation by size of epithelial tumor cells (ISET; Rarecells Diagnostics, Paris, France), according to the instructions provided by the manufacturer. Using ISET, cells with diameters >8 μm were retained, bound to the polycarbonate membranes by negative pressure, and stored at −20°C until further analysis.
Immunocytochemistry
Immunocytochemistry (ICC) assays were performed on ISET membranes, as previously described [34]. Briefly, membranes containing captured CTCs were cut and placed into 24‐well plates for antigenic retrieval, followed by hydration. Cells were permeabilized, and endogenous peroxidase was blocked with 3% hydrogen peroxide in the dark. Membrane spots were submitted to dual color immunocytochemistry (diaminobenzidine [DAB]+/Permanent Red; Agilent Technologies, Santa Clara, CA) and incubated with antibodies diluted in Tris‐buffered saline with 10% fetal calf serum. To amplify the antibody signal, the spots were incubated with Envision G/2 Doublestain System, Rabbit/Mouse (Agilent Technologies) followed by incubation with DAB+/Permanent Red (Agilent Technologies). Cells were stained with hematoxylin and analyzed by light microscope (BX61; Olympus, Tokyo, Japan). The following antibodies were used: anti‐HER2 (1:400 dilution; Cell Signaling Technology, Danvers, MA) and antiplakoglobin (1:200 dilution; Cusabio, Wuhan, China). As positive controls of antibody specificity, we used cell lines that, according to the Human Protein Atlas (http://www.proteinatlas.org/), were positive for HER2 (SKBR3) and plakoglobin (HCT8). For this, ∼100 cells were “spiked” in 8 mL of blood of a healthy donor and filtered on ISET to provide positive controls. The same cell lines were used as negative control of ICC without the use of primary antibodies to exclude cross‐reactivity. We also used cell lines that did not express the tested proteins, according to the Human Protein Atlas (U‐87 MG for both HER2 and plakoglobin). All cell lines were acquired from American Type Culture Collection (Manassas, VA). To confirm that CTCs analyzed were not leucocytes, we used anti‐CD45 antibody (1:100 dilution; CSB‐PA010546, Cusabio). CTCs were characterized based on the following criteria: negative staining for CD45, hyperchromatic and irregular nucleus with a size ≥12 μm, visible presence of cytoplasm, and a high nucleus‐to‐cytoplasm ratio (0.8). CTC counts were determined as the number of CTCs per mL of blood, and each ISET membrane spot was considered as 1 mL of blood [17]. Patients were considered as positive for CTC presence if at least one of the four ISET spots analyzed contained one CTC (at least one CTC in 4 mL of blood or 0.25 CTC in 1 mL of blood) [17]. Cell clusters were considered as CTM if they contained more than two CTCs grouped [35]. In relation to HER2 and plakoglobin expression analysis in CTCs by immunocytochemistry, we considered a CTC to be positive if it expressed these markers, independent of the intensity, and negative if the CTC had no expression. Patient was considered positive for HER2 expression in CTC if had at least one positive CTC and negative if all CTCs were negative. After ICC reactions, cells were stained with hematoxylin and analyzed by light microscopy (Research System Microscope BX61; Olympus).
Statistical Analysis
The baseline patient characteristics are expressed as absolute and relative frequencies for qualitative variables and as the median, minimum, and maximum values for quantitative variables. Associations between qualitative variables were evaluated by chi‐squared test or Fisher's exact test, as appropriate. To compare CTC levels at baseline and follow‐up, the Wilcoxon test for paired samples was applied. To compare CTM presence and absence at baseline and follow‐up, McNemar's test was applied. Survival curves were estimated by the Kaplan‐Meier estimator, and the difference between curves was evaluated by the log‐rank test. Progression‐free survival was measured from baseline (first CTC collection) until the first disease progression, determined by image examinations. Patients not experiencing an event were censored at the last hospital visit. To verify the concordance between HER2 expression in primary tumors and CTCs, the κ test was used. The two‐sided significance level was fixed at 5% for all tests. Statistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corporation, Armonk, NY) and R software version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients with Gastric Cancer
A total of 55 consecutive patients with nonmetastatic GAC were included, the majority of whom were men (n = 33; 60%); the median age at diagnosis was 57 years (37–86 years). Most cases were diagnosed as Lauren's diffuse histological subtype (n = 28; 59.6%). At the time of the inclusion, 59.3% (n = 32) of patients had clinical T3 or T4a, according to the 8th edition of the American Joint Committee on Cancer's cancer staging manual. Most patients presented lymph node metastasis (cN+; 53.7%) and underwent neoadjuvant treatment (n = 42; 76.4%). After neoadjuvant treatment, only seven cases (21.2%) showed tumor downstaging. Further clinicopathological characteristics are listed in Table 1. The median patient follow‐up of all cases was 14.9 months (95% confidence interval, 13.6–16.3).
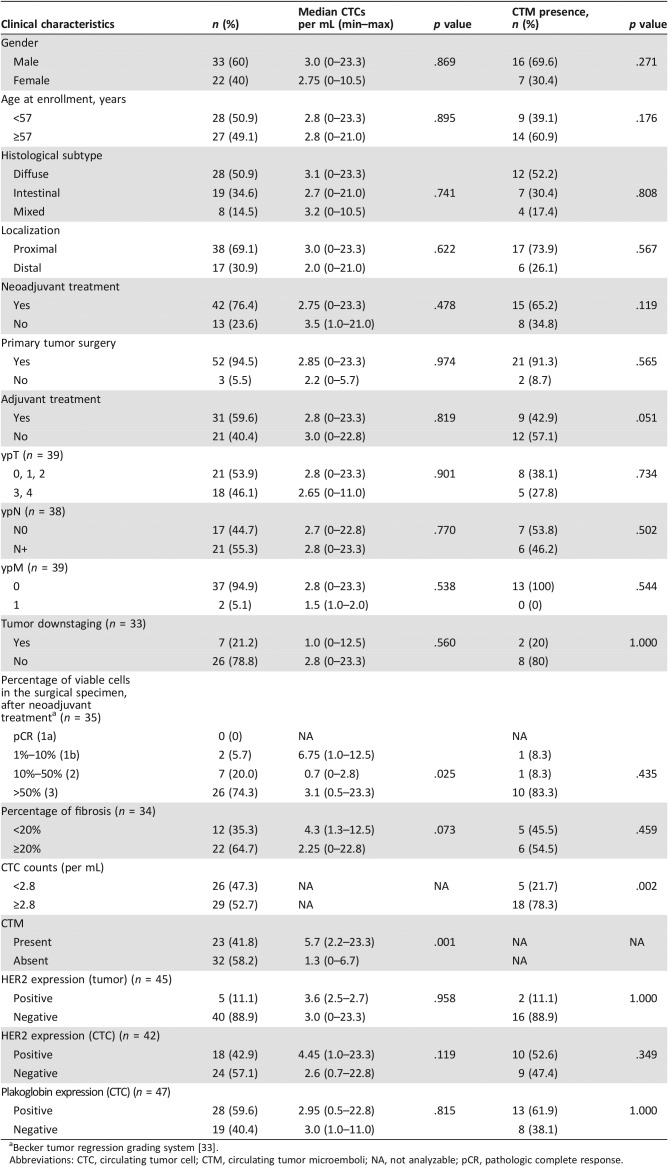
Table 1. Clinicopathological aspects, epidemiology, and pathological association of patients with gastric cancer with the median of CTCs and with presence of CTM at baseline.
Becker tumor regression grading system [33].
Abbreviations: CTC, circulating tumor cell; CTM, circulating tumor microemboli; NA, not analyzable; pCR, pathologic complete response.
Circulating Tumor Cells
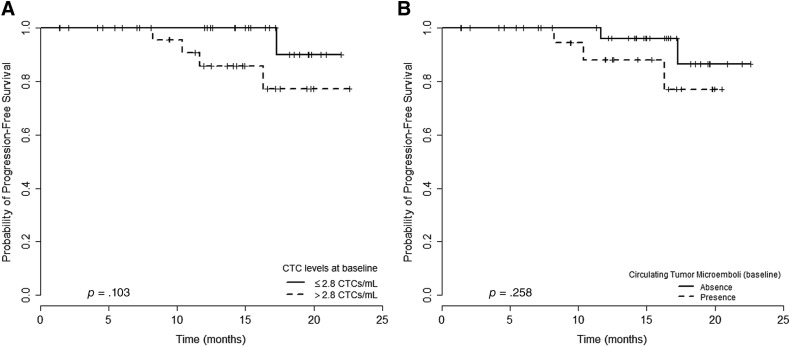
CTCs were observed in 90.9% of the patients (50/55), with a median of 2.8 CTCs per mL (0–23.3 CTCs per mL) at baseline. Patients with CTC counts above the median had a nonsignificant trend for a lower progression‐free survival (PFS; median PFS not reached; p = .103; Fig. 2A).
Figure 2.
PFS analysis of patients with nonmetastatic gastric cancer in relation to isolated CTCs and CTM at baseline. (A): Patients with the count of CTCs above 2.8 per mL versus those with count of CTCs under 2.8 per mL: median PFS not achieved for both (p = .103). (B): Patients with CTM presence had poor mean PFS in relation to those with CTM absence (18.7 months vs. 21.6 months, respectively; p = .258).
Abbreviations: CTCs, circulating tumor cells; CTM, circulating tumor microemboli; PFS, progression‐free survival.
We observed no significant statistical differences between diffuse and intestinal histological Lauren's subtypes concerning median CTC values per mL of blood (Table 1). Regarding postneoadjuvant pathological T (ypT0, ypT1, ypT2 vs. ypT3, ypT4), N (ypN0 vs. ypN+), and M (ypM0 vs. ypM1) status, we found no differences in the median of CTCs between the groups evaluated.
We evaluated HER2 expression in 45/55 biopsy samples and in 42/55 CTCs samples, with an overlap of 36 subjects. At baseline, for biopsies we observed 5/45 (11%) positive for HER2 expression, contrasting with 18/42 (42.9%) HER2‐positive cases for CTCs and a much reduced HER2 positivity for CTM (1/42, 2.4%); moreover, patients presenting HER2‐negative CTCs had a trend for better PFS, although the median PFS was not reached so far (p = .092; Fig. 3A). These results were not observed either in CTM (p = .76) or in primary tumors (p = .41; data not shown).
Figure 3.
PFS analysis of patients with nonmetastatic gastric cancer in relation to the expression of HER2 protein in isolated CTCs, and plakoglobin protein in CTM at baseline. (A): Patients with HER2‐positive CTCs and patients with HER2‐negative CTCs: median PFS not achieved for both (p = .092). (B): Patients with plakoglobin‐positive CTM and patients with plakoglobin‐negative CTM: 16.3 months versus not achieved, respectively (p = .114). (C): Patients with diffuse histological subtype evaluated for HER2 in CTCs and plakoglobin in CTM: patients with both proteins negative had better PFS than patients with one or the other positive (PFS not calculated; p = .027).
Abbreviations: CTCs, circulating tumor cell; CTM, circulating tumor microemboli; HER2, human epidermal growth factor receptor 2; PFS, progression‐free survival.
We were able to evaluate HER2 expression in 36 matched cases (biopsies and CTCs for the same subject) and found 69.4% agreement (κ = .272; p = .04): 4/36 cases (11.1%) positive for both primary tumor and CTCs, 21/36 cases (58.3%) negative in both, 1/36 cases (2.8%) positive only in primary tumors, and 10/36 cases (27.8%) positive only in CTCs.
For 31 HER2‐negative primary tumors, we found 10 cases (32.2%) with HER2‐positive CTCs, and among the 5 HER2‐positive primary tumors, most (n = 4, 80%) also had paired CTC expressing HER2. Interestingly, 3/5 patients who experienced disease progression had HER2‐negative primary tumors but showed HER2 expression in their CTCs. All five patients with progressive disease had HER2‐negative primary tumors.
Plakoglobin expression was evaluated in 47/55 patients and was found positive in 59.6% of CTCs (baseline). We found no correlation between plakoglobin expression in isolated CTCs (baseline) and PFS (p = .962; data not shown).
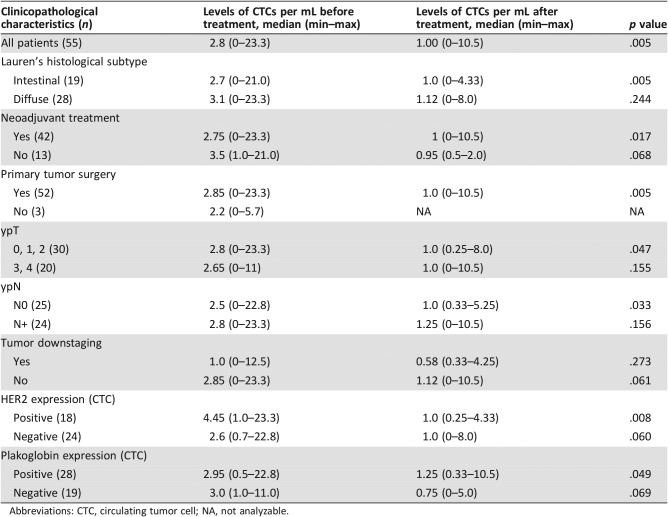
Follow‐up blood collection was performed in 33 patients (all were submitted to surgery; 29 underwent neoadjuvant treatment), at a median of 41 days after surgery (20–116 days). Most patients (61.5%) had a second blood collection at the starting day of the adjuvant treatment (38.5% of remaining cases had the second blood collection between 1 and 18 days before beginning the adjuvant treatment). The detection rate of CTCs (Fig. 4A and B) was also high at this time point (93.9%), but there was a smaller median count compared with baseline (1.0 vs. 2.8 CTCs per mL; p = .005; Table 2). The comparison of these time points also showed significant reduction in Lauren's intestinal subtype (2.7 CTCs per mL vs. 1.0 CTC per mL; p = .005), for those who underwent neoadjuvant treatment (2.75 CTCs per mL vs. 1.0 CTC per mL; p = .017), and revealed a similar trend for those who had no neoadjuvant treatment (3.5 CTCs per mL to 0.95 CTCs per mL; p = .068) and in the group submitted to surgery (2.85 CTCs per mL to 1.0 CTC per mL; p = .005). According to postneoadjuvant pathological classification, the ypT 0, 1, or 2 group (2.9 CTCs per mL to 1.0 CTC per mL; p = .011) and the yN0 group (3.0 CTCs per mL to 1.0 CTC per mL; p = .019) presented a decreased CTC levels. According to downstaging evaluation, neither group (downstaging vs. no downstaging) had difference in CTC decreasing (p = .27 and p = .06, respectively).
Figure 4.
Immunostaining of CTCs and CTM from patients with gastric cancer. (A, B): CTCs visualized with hematoxylin‐eosin (×40). (C): CTM visualized with hematoxylin‐eosin (×40). (D): CTC stained with anti‐HER2 antibody, visualized with DAB, and counterstained with hematoxylin‐eosin (×40). (E): CTC stained with antiplakoglobin antibody, visualized with DAB, and counterstained with hematoxylin‐eosin (×40). (F): CTM stained with antiplakoglobin antibody, visualized with DAB, and counterstained with hematoxylin‐eosin (×20). All images were analyzed on Research System Microscope BX61 (Olympus) coupled to a digital camera (SC100; Olympus).
Abbreviations: CTCs, circulating tumor cells; CTM, circulating tumor microemboli; DAB, diaminobenzidine; HER2, human epidermal growth factor receptor.
Table 2. Clinicopathological aspects and CTC counts before and after treatment.
Abbreviations: CTC, circulating tumor cell; NA, not analyzable.
The expression of HER2 and plakoglobin in CTCs appeared to be informative (Fig. 4D and E). A more significant CTC count drop in the follow‐up was seen for baseline HER2‐positive cases (4.45 CTCs per mL at baseline to 1.0 CTC per mL at follow‐up; p = .008) compared with baseline‐HER2 negative cases (2.6 CTCs per mL to 1.0 CTC per mL; p = .06). A reduced CTC count between baseline and follow‐up was also seen for plakoglobin‐positive cases (2.9 CTCs per mL to 1.25 CTCs per mL; p = .04) with a similar trend being observed for plakoglobin‐negative cases (median CTCs per mL dropping from 3.0 to 0.75; p = .06).
At follow‐up, HER2 CTC expression was evaluated in 33/33 cases, showing that no case presented either CTC or CTM as positive. Plakoglobin expression at follow‐up (n = 33) was positive in CTC in 18 cases (54.5%), and no plakoglobin expression was found in CTM. All detailed information from all patients is available in supplemental online Table 1.
No differences in CTC counts were seen for positive versus negative tumors for Helicobacter pylori (3.0 CTCs per mL vs. 2.4 CTCs per mL, respectively; p = .13).
Circulating Tumor Microemboli
CTM were detected at baseline for 22/55 patients (41.8%; Fig. 4C and F) and declined at follow‐up (2/33; 6.1%; p = .06). The only two positive cases for CTM detection at follow‐up showed no tumor downstaging after the neoadjuvant treatment. CTM were more frequently observed in patients with CTC counts above the median (p = .002; Table 1). Additionally, there was no difference in PFS between CTM‐positive patients compared with CTM‐negative patients (18.7 months vs. 21.6 months, respectively; p = .258; Fig. 2B).
Most patients had absence of CTM in both baseline and follow‐up analysis (n = 18; 62%). An evaluation of CTM before and after neoadjuvant treatment (n = 29) showed nine cases (31%) to be CTM positive at baseline but negative at follow‐up collection. Conversely, only two cases lacked CTM at baseline but presented CTM at follow‐up collection (7%), and none presented CTM at both moments (0%; p = .065). Despite the absence of statistical significance, we observed that the group of viable cells scored 3, according to the Becker tumor regression grading system, had more patients with CTM presence at baseline (10/12 with CTM presence; Table 1).
From the 47 patients with samples tested for plakoglobin expression, 9 had this protein detected in CTM, resulting in a trend of worse median PFS (15.9 months vs. 21.3 months; p = .114; Fig. 3B). This trend could not be observed for isolated CTCs (18.6 months vs. 21.0 months; p = .962), where the plakoglobin expression was much lower. When the presence of HER2 in CTCs and the presence of plakoglobin in CTM were considered together in all patients included, we observed no statistical difference in PFS (p = .061; data not shown). However, after division into histological subgroups, this PFS analysis was better reflected in diffuse histological subtype (Fig. 3C; p = .027). Patients with negativity for both proteins had better PFS than those with presence of HER2 in CTCs or plakoglobin in CTM.
Discussion
We evaluated CTCs and CTM in the blood of 55 patients diagnosed with nonmetastatic GAC, as well as their expression of HER2 and plakoglobin. In general, the median CTC count was high (2.8 CTCs per mL), compared with other publications that have employed the same ISET approach, such as metastatic breast, lung, and pancreatic cancers (respectively with 0.27, 2.3, and 0.67 CTCs per mL) [36], but similar compared with other tumors such as pancreatic cancer (1.2 CTCs per mL) [21], colon cancer (median of 2.0 CTCs per mL and 2.3 CTCs per mL) [34], [37], and sarcomas (median of 2.0 CTCs per mL and 2.6 CTCs per mL) [12], [38]. For head and neck cancer and lung cancer the median was very similar to our data for patients with GAC (3.0 CTCs per mL for both) [13], [39]. We should also note that this high CTC count also paralleled a high frequency of patients (90%) presenting detectable CTCs. To the best of our knowledge, the study presented here is only the second one made in patients with GAC using ISET. The pioneer ISET‐GAC study evaluated patients from China (45 nonmetastatic and 41 metastatic cases) and, perhaps because of differences in study design and ethnicity, the authors report a much lower CTC detection rate in nonmetastatic cases (52%) versus the 90% detection rate in our study. However, despite this difference, these authors conclude (and our findings give support to this concept) that for most GAC cases, cell invasion and entry of cancer cells into the bloodstream occurs in the early stages of the disease, which probably justifies the high rates of distant recurrence [25] and the overall poor prognosis of this disease.
This finding is intriguing and deserves some considerations. Most CTC‐based studies have evaluated samples in the metastatic setting, when much higher CTC counts and detection rates are expected. However, when CTCs are released from primary tumors in the early stage of disease, as postulated by some authors and reviewed by Klein [40], the detection, quantification, and evaluation of putative markers in early‐stage disease may be instrumental for the development of diagnostic strategies and alternative therapeutics for the early management of more aggressive disease. This finding also supports the self‐seeding hypothesis, postulated by Norton and Massagué [41] and successfully tested by Kim et al. [42], in which CTCs released by the original lesions reinfiltrate the primary tumors, contributing to their growth after a period of tumor cell priming in circulation. We hope our study can encourage the use of CTCs in early disease stages of all tumor types, in an attempt to identify effective diagnostic and therapeutic tools.
The second collection point was performed for 33 subjects. As expected, we observed a reduction in the CTC counts. This reflects the reduction of the tumor mass during surgery and reinforces the notion that CTCs can be used in follow‐up even in the absence of clinically detectable metastasis. Moreover, the CTC count drop was more evident in groups of better prognosis [ypT 0, 1, 2 group (2.9 CTCs per mL to 1.0 CTC per mL; p = .011), and yN0 group (3.0 CTCs per mL to 1.0 CTC per mL; p = .019)], demonstrating that CTCs can be a valuable indicator in monitoring treatment response. This result opens a new perspective in the decision about which patients with ypT 3, 4 or N+ tumors are more likely to benefit from postoperative chemotherapy, based on CTC counts.
Mishima et al. [27] evaluated HER2 amplification by the three‐dimensional‐immunofluorescence‐fluorescent in situ hybridization (3D‐IF‐FISH) method and compared their results with the U.S. Food and Drug Administration‐approved CellSearch system (Menarini Silicon Biosystems, Huntington Valley, PA). Besides the higher CTC detection rates obtained by the 3D‐IF‐FISH approach (88.3%), it also showed more HER2‐positive CTCs in patients with HER2‐negative primary tumors (13/50; 26%) than the CellSearch system (detection rate, 52.3%; 3/27, 11.1% HER2‐positive CTCs in HER2‐negative primary tumors). The 3D‐IF‐FISH and ISET approaches showed similar results in terms of detection rate (88.3% vs. 90.9%) and HER2‐positivity in CTCs being released by HER2‐negative primary tumor cases (26% vs. 32.2%). Here, we observed a much higher HER2 positivity in CTCs (43%) compared with primary tumors (11%). Although HER2 positivity has been associated with more aggressive tumor behaviors in breast cancer, its prognostic value in gastric cancer is still controversial [43], [44]. We are tempted to speculate that evaluation of HER2 in CTCs can contribute to determining which patients with GACs will have higher malignancy and mortality rates.
Regarding the expression of plakoglobin in isolated CTCs and CTM, as expected, our findings clearly reinforce the importance of plakoglobin only in CTM, probably acting to maintain the tight clustering of cells in the microemboli. A promising trend was observed for plakoglobin‐positive CTM as a marker of poor PFS (15.9 months vs. 21.3 months; p = .114; Fig. 3B). Even in this scenario of a small sample size and a reduced follow‐up, our results suggest a potential role of plakoglobin in maintaining cell adherence that constitutes the CTM and in promoting tumor spread, as clusters are more prone to survive and to lead to a faster disease progression [24]. Additionally, the combined analysis of plakoglobin in CTM and HER2 expression in single CTCs, even acting in distinct signaling pathways, showed that these two proteins could predict worse PFS in patients with GAC when one or the other is expressed, especially in diffuse histological subtypes (Fig. 3C).
Conclusion
The multidisciplinary treatment for resectable tumors, which today mainly includes a combination of chemotherapy and surgery, is one of the most promising strategies to deliver patients the benefit of different treatment modalities [45], [46], [47], [48]. So far, there are no trustworthy markers to evaluate the in vivo risk of metastatic disease or progression risk in this multidisciplinary scenario. However, the data presented by us and others suggest that the analysis of biomarker expression and relative CTC counts contribute to evaluate response and determination of prognosis.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by grants from São Paulo Research Foundation (FAPESP; grant 14/26897‐0). E.A.A. is a Ph.D. candidate supported by a fellowship from FAPESP (grant 2015/16952‐6); A.C.B. is a Ph.D. candidate supported by a fellowship from CAPES (Coordination of Superior Level Staff Improvement; grant 1756356); B.C.T.C.P.F. is a PhD candidate supported by a fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq; Grant: 141822/2016‐3); E.D.‐N. is a research fellow from CNPq. The authors acknowledge the support given by the institutional biobank of the A.C. Camargo Cancer Center.
Author Contributions
Conception/design: Diana Noronha Nunes, Emmanuel Dias‐Neto, Ludmilla T. Domingos Chinen
Provision of study material or patients: Laís Senda, Maria Fernanda Arruda Almeida, Maria Dirlei Begnami, Felipe J.F. Coimbra, Wilson Luiz da Costa Jr.
Collection and/or assembly of data: Emne A. Abdallah, Alexcia C. Braun, Bianca C.T.C.P. Flores, Ana Cláudia Urvanegia, Maria Fernanda Arruda Almeida, Maria Dirlei Begnami, Felipe J.F. Coimbra, Wilson Luiz da Costa Jr., Diana Noronha Nunes, Emmanuel Dias‐Neto, Ludmilla T. Domingos Chinen
Data analysis and interpretation: Emne A. Abdallah, Vinicius Calsavara, Victor Hugo Fonseca de Jesus, Maria Fernanda Arruda Almeida, Felipe J.F. Coimbra, Wilson Luiz da Costa Jr., Diana Noronha Nunes, Emmanuel Dias‐Neto, Ludmilla T. Domingos Chinen
Manuscript writing: Emne A. Abdallah, Alexcia C. Braun, Bianca C.T.C.P. Flores, Laís Senda, Ana Cláudia Urvanegia, Vinicius Calsavara, Victor Hugo Fonseca de Jesus, Maria Fernanda Arruda Almeida, Maria Dirlei Begnami, Felipe J.F. Coimbra, Wilson Luiz da Costa Jr., Diana Noronha Nunes, Emmanuel Dias‐Neto, Ludmilla T. Domingos Chinen
Final approval of manuscript: Emne A. Abdallah, Alexcia C. Braun, Bianca C.T.C.P. Flores, Laís Senda, Ana Cláudia Urvanegia, Vinicius Calsavara, Victor Hugo Fonseca de Jesus, Maria Fernanda Arruda Almeida, Maria Dirlei Begnami, Felipe J.F. Coimbra, Wilson Luiz da Costa Jr., Diana Noronha Nunes, Emmanuel Dias‐Neto, Ludmilla T. Domingos Chinen
Disclosures
The authors indicated no financial relationships.
References
- 1.GE4GAC Group . Genomics and epidemiology for gastric adenocarcinomas. Appl Cancer Res 2017;37:7. [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration ; Fitzmaurice C, Allen C, Barber RM et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coimbra FJ, Costa WL Jr, Montagnini AL et al. The interaction between N‐category and N‐ratio as a new tool to improve lymph node metastasis staging in gastric cancer: Results of a single cancer center in Brazil. Eur J Surg Oncol 2011;37:47–54. [DOI] [PubMed] [Google Scholar]
- 4.Costa WL Jr, Coimbra FJ, Fogaroli RC et al. Adjuvant chemoradiotherapy after d2‐lymphadenectomy for gastric cancer: The role of n‐ratio in patient selection. Results of a single cancer center. Radiat Oncol 2012;7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes DN, Kowalski LP, Simpson AJ. Detection of oral and oropharyngeal cancer by microsatellite analysis in mouth washes and lesion brushings. Oral Oncol 2000;36:525–528. [DOI] [PubMed] [Google Scholar]
- 6.Nunes DN, Kowalski LP, Simpson AJ. Circulating tumor‐derived DNA may permit the early diagnosis of head and neck squamous cell carcinomas. Int J Cancer 2001;92:214–219. [DOI] [PubMed] [Google Scholar]
- 7.Sausen M, Phallen J, Adleff V et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun 2015;6:7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todenhöfer T, Struss WJ, Seiler R et al. Liquid biopsy‐analysis of circulating tumor DNA (ctDNA) in bladder cancer. Bladder Cancer 2018;4:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar GM, Balaj L, Stott SL et al. Liquid biopsy for brain tumors. Expert Rev Mol Diagn 2017;17:943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amorim MG, Valieris R, Drummond RD et al. A total transcriptome profiling method for plasma‐derived extracellular vesicles: Applications for liquid biopsies. Sci Rep 2017;7:14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong D, Wildman DE. Extracellular vesicles and the promise of continuous liquid biopsies. J Pathol Transl Med 2018;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun AC, de Mello CAL, Corassa M et al. EGFR expression in circulating tumor cells from high‐grade metastatic soft tissue sarcomas. Cancer Biol Ther 2018;19:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanelli MF, Oliveira TB, Braun AC et al. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor‐β receptor I in head and neck cancer. Head Neck 2017;39:2283–2292. [DOI] [PubMed] [Google Scholar]
- 14.Chinen LTD, Abdallah EA, Braun AC et al. Circulating tumor cells as cancer biomarkers in the clinic. Adv Exp Med Biol 2017;994:1–41. [DOI] [PubMed] [Google Scholar]
- 15.Massari F, Di Nunno V, Comito F et al. Circulating tumor cells in genitourinary tumors. Ther Adv Urol 2018;10:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geethadevi A, Parashar D, Bishop E et al. ERBB signaling in CTCs of ovarian cancer and glioblastoma. Genes Cancer 2017;8:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs MG, Hou JM, Sloane R et al. Analysis of circulating tumor cells in patients with non‐small cell lung cancer using epithelial marker‐dependent and ‐independent approaches. J Thorac Oncol 2012;7:306–315. [DOI] [PubMed] [Google Scholar]
- 18.Javaid S, Zhang J, Smolen GA et al. MAPK7 regulates EMT features and modulates the generation of CTCs. Mol Cancer Res 2015;13:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow CJ, Trapani F, Metcalf RL et al. Tumourigenic non‐small‐cell lung cancer mesenchymal circulating tumour cells: A clinical case study. Ann Oncol 2016;27:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poruk KE, Valero V 3rd, Saunders T et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg 2016;264:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoja L, Backen A, Sloane R et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012;106:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regmi S, Fu A, Luo KQ. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep 2017;7:39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou JM, Krebs MG, Lancashire L et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small‐cell lung cancer. J Clin Oncol 2012;30:525–532. [DOI] [PubMed] [Google Scholar]
- 24.Aceto N, Bardia A, Miyamoto DT et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Fan L, Zhou P et al. Detection of circulating tumor cells and circulating tumor microemboli in gastric cancer. Transl Oncol 2017;10:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Ling Y, Qi Q et al. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol. 2017;6:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishima Y, Matsusaka S, Chin K et al. Detection of HER2 amplification in circulating tumor cells of HER2‐negative gastric cancer patients. Target Oncol 2017;12:341–351. [DOI] [PubMed] [Google Scholar]
- 28.Vestweber D. VE‐cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol 2008;28:223–232. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz JM, Leach S, Kaurah P et al. Catenin family genes are not commonly mutated in hereditary diffuse gastric cancer. Cancer Epidemiol Biomark Prev 2012;21:2272–2274. [DOI] [PubMed] [Google Scholar]
- 30.Jawhari A, Jordan S, Poole S et al. Abnormal immunoreactivity of the E‐cadherin‐catenin complex in gastric carcinoma: Relationship with patient survival. Gastroenterology 1997;112:46–54. [DOI] [PubMed] [Google Scholar]
- 31.Ohene‐Abuakwa Y, Noda M, Perenyi M et al. Expression of the E‐cadherin/catenin (alpha‐, beta‐, and gamma‐) complex correlates with the macroscopic appearance of early gastric cancer. J Pathol 2000;192:433–439. [DOI] [PubMed] [Google Scholar]
- 32.Rüschoff J, Dietel M, Baretton G et al. HER2 diagnostics in gastric cancer‐guideline validation and development of standardized immunohistochemical testing. Virchows Arch 2010;457:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker K, Mueller JD, Schulmacher C et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–1530. [DOI] [PubMed] [Google Scholar]
- 34.Abdallah EA, Fanelli MF, Souza E Silva V et al. MRP1 expression in CTCs confers resistance to irinotecan‐based chemotherapy in metastatic colorectal cancer. Int J Cancer 2016;139:890–898. [DOI] [PubMed] [Google Scholar]
- 35.Khoja L, Shenjere P, Hodgson C et al. Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res 2014;24:40–46. [DOI] [PubMed] [Google Scholar]
- 36.Farace F, Massard C, Vimond N et al. A direct comparison of CellSearch and ISET for circulating tumour‐cell detection in patients with metastatic carcinomas. Br J Cancer 2011;105:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buim ME, Fanelli MF, Souza VS et al. Detection of KRAS mutations in circulating tumor cells from patients with metastatic colorectal cancer. Cancer Biol Ther 2015;16:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinen L, Mello C, Abdallah E et al. Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: A window on sarcoma‐cell invasion. OncoTargets Ther 2014;7:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz MT de M, Abdallah EA, Tariki MS et al. Circulating tumor cells as marker of poor prognosis in metastatic lung cancer: A pilot study. Appl Cancer Res 2018;38:3. [Google Scholar]
- 40.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer 2009;9:302–312. [DOI] [PubMed] [Google Scholar]
- 41.Norton L, Massagué J. Is cancer a disease of self‐seeding? Nat Med 2006;12:875–878. [DOI] [PubMed] [Google Scholar]
- 42.Kim MY, Oskarsson T, Acharyya S et al. Tumor self‐seeding by circulating cancer cells. Cell 2009;139:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer ‐ A systematic analysis of data from the literature. J Cancer 2012;3:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pazo Cid RA, Antón A. Advanced HER2‐positive gastric cancer: Current and future targeted therapies. Crit Rev Oncol Hematol 2013;85:350–362. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 46.Ajani JA, Winter K, Okawara GS et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): Quality of combined modality therapy and pathologic response. J Clin Oncol 2006;24:3953–3958. [DOI] [PubMed] [Google Scholar]
- 47.Yoshikawa T, Sasako M, Yamamoto S et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015–1022. [DOI] [PubMed] [Google Scholar]
- 48.Fava BEC, da Costa WL Jr, Medeiros MLL et al. Neoadjuvant intraperitoneal chemotherapy followed by radical surgery and HIPEC in patients with very advanced gastric cancer and peritoneal metastases: Report of an initial experience in a western single center. World J Surg Oncol 2018;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]