Complex brain metastases are the most challenging forms of brain cancer. This article reports results of a phase II trial of concurrent temozolomide with hypofractionated stereotactic radiotherapy in patients with complex brain metastases.
Keywords: Hypofractionated stereotactic radiotherapy, Temozolomide, Concurrent chemoradiotherapy, Complex brain metastasis
Abstract
Purpose.
Complex brain metastases (BMs), such as large lesions, lesions within or close to eloquent locations, or multiple recurrent/progressive BMs, remain the most challenging forms of brain cancer because of decreased intracranial control rates and poor survival. In the present study, we report the results from a single institutional phase II trial of concurrent temozolomide (TMZ) with hypofractionated stereotactic radiotherapy (HFSRT) in patients with complex brain metastases, including assessment of its feasibility and toxicity.
Patients and Methods.
Fifty‐four patients with histologically proven primary cancer and complex BMs were enrolled between 2010 and 2015. All the patients were treated with concurrent HFSRT and TMZ (administrated orally at a dosage of 75 mg/m2 per day for at least 20 days). The primary endpoint was overall survival (OS).
Results.
The median follow‐up time was 30.6 months. The local control rates at 1 and 2 years were 96% and 82%, respectively. The median OS was 17.4 months (95% confidence interval [CI], 12.6–22.2), and the OS rates at 1 and 2 years were 65% (95% CI, 52%–78%) and 33% (19%–47%). Only six patients (15.8%) died of intracranial disease. The median brain metastasis‐specific survival was 46.9 months (95% CI, 35.5–58.4). Treatment‐related grade 3–4 adverse events were rare and included one grade 3 hematological toxicity and two grade 3 liver dysfunctions.
Conclusion.
Treatment using HFSRT concurrent with TMZ was well tolerated and could significantly extend OS compared with historical controls in complex BMs. Large randomized clinical trials are warranted. Trial registration ID: NCT02654106.
Implications for Practice.
The treatment using hypofractionated stereotactic radiotherapy concurrent with temozolomide appeared to be safe and could significantly extend overall survival compared with historical control in complex brain metastases. Large randomized clinical trials are warranted to verify our results.
Introduction
The incidence of brain metastases (BMs) has increased in recent years, possibly because of better control of the primary tumor, along with improved sensitivity in imaging technology that enable increased detection of small metastases. Despite the fact that selected subgroups of patients with BMs can achieve longer survival and good neurological functions [1], some complex lesions, such as large BMs, recurrent multiple lesions, and BMs within or close to eloquent locations, remain the most challenging, with a median survival of less than 12 months [2], [3], [4], [5], [6].
Hypofractionated stereotactic radiotherapy (HFSRT) provides a radiobiological advantage, especially in terms of lower toxicity over single‐fraction treatment, and thus seems to be more appropriate than stereotactic radiosurgery (SRS) for complex BMs [7]. HFSRT has been demonstrated as a safe and effective treatment for large BMs with an acceptable toxicity. However, more than 60% of patients with complex BMs still eventually died of intracranial disease progression after HFSRT [2], [8].
To better control the intracranial lesions in patients with complex BMs, novel treatment modalities are warranted that do not increase without increasing damage to normal brain tissue. Temozolomide (TMZ) is an oral alkylating agent known to penetrate the blood‐brain barrier. Both in vitro and in vivo studies have demonstrated that TMZ can also act as a radiosensitizing agent in addition to its cytotoxic activity [7], [9]; thus, TMZ and has become the standard of care for high‐grade gliomas. However, the evidence from prospective studies on the impact of the radiosensitizing effect resulting from the combination of TMZ and whole brain radiotherapy (WBRT) in BMs is conflicting. Some evidence showed that the combination achieved a longer higher objective response rate (ORR) without increasing of toxicity [10], [11], [12], [13]. However, the Radiation Therapy Oncology Group (RTOG) 0320 phase III randomized trial, which was closed early because of accrual limitations, failed to demonstrate that the triple modality treatment of WBRT + SRS + TMZ in patients with non‐small cell lung cancer with one to three brain metastases could improve survival [14]. The rates of grade 3–5 toxicity related to WBRT + SRS + TMZ were significantly higher than those related to WBRT + SRS (41% vs. 11%; p < .001) in that study, and the severe toxicities might also potentially have compromised the ability to deliver subsequent systemic therapy for relapse.
HFSRT concurrent with TMZ might be a promising strategy because of its lower toxicity compared with WBRT + SRS, especially for patients with complex BMs. In the present study, we report the first prospective phase II study evaluating concurrent TMZ with HFSRT for patients with complex BMs, assessing its feasibility, toxicity, and efficacy.
Methods and Materials
Eligibility Criteria
Patients were eligible for inclusion if they had histologically proven primary cancer and complex BMs, which were defined as large lesions (a tumor volume ≥ 6 cc or a maximum diameter ≥3 cm), lesions within or close to eloquent locations (such as the brainstem, optic apparatus, and the internal capsule), or multiple recurrent/progressive BMs (at least three). Other eligibility criteria included Karnofsky performance score (KPS) ≥60 or KPS ≥40 but simply caused by BMs, age of 18–75 years, adequate function of liver and kidney function (defined as liver function test results more than 1.5 times the institutional upper limit of normal and both blood urea nitrogen and creatinine within the normal range), and adequate bone marrow reserve (defined as white blood cell count ≥4.0 × 109/L, neutrophils ≥1.5 × 109/L, hemoglobin ≥110 g/L, and platelets ≥100 × 109/L). Enrollment of patients treated using other treatments before HFSRT, e.g., surgery, SRS, or WBRT, was allowed. Patients with severe systemic diseases (e.g., myocardial infarction within the past 6 months, severe arrhythmia), those who were unable or unwilling to comply with the study protocol, those with a survival expectancy less than 3 months, pregnant patients, or those unsuitable to participate in the study (in the opinion of the treating physician) were excluded.
The protocol of this trial was approved by the institutional ethics review board. All patients provided written informed consent. The trial has been registered on clinicaltrials.gov with the number NCT02654106.
Treatments
All the patients were treated with concurrent HFSRT and TMZ. HFSRT was performed using a Brainlab Planning System and Varian linear accelerator. Enhanced computed tomography (CT) images and magnetic resonance imaging (MRI) images of the 2 mm in thickness were fused. The gross tumor volume (GTV) was defined by the contrast‐enhanced tumor on the fused images.
HFSRT was performed as previously described [2]. In brief, patients were immobilized in the supine position in a tight thermoplastic stereotactic head mask. The clinical tumor volume (CTV) was a zero‐margin expansion of the GTV, and the planning target volume (PTV) was defined by adding a margin of 2 mm to the GTV and CTV [2], [15]. The PTV was enclosed by a 90% isodose curve of the prescribed dose in HFSRT. The optimized fraction schedule was determined based on tumor volumes and locations, as follows: 52 Gy/13–15f for large lesions; 32–42 Gy/4–7f for lesions in close to functional area; 39–45 Gy/13–15f for lesions within the brainstem; 40–45 Gy/10–15f for reirradiated large lesions; and 20–24 Gy/1–2f for other concurrent small lesions. Supplemental online Table 1 summarizes the dose prescriptions and their corresponding biologically effective dose and equivalent dose in 2 Gy/f.
TMZ was administrated orally at a dosage of 75 mg/m2 per day during the HFSRT treatment for at least 20 days. Adjuvant TMZ was started one month after HFSRT at 150 mg/ m2 per day for 5 days every 28 days until the patient could not tolerate the drug or the tumor progressed, and for no more than six cycles. There is no strong evidence showing that adjuvant TMZ could significantly improve survival; therefore, adjuvant TMZ was allowed but not mandated in this trial.
Evaluation and Follow‐Up
Pretreatment evaluation was performed within 1 week before treatment and included a full medical history, physical and neurologic examinations, laboratory investigations, and brain MRI or CTs.
All patients underwent weekly physical and neurologic examinations, as well as complete blood count and blood chemistry examinations during concurrent treatment. A complete clinical evaluation, laboratory tests, and MRI or CT were performed 2 months after HFSRT. The patients were then followed up every 3 months. The follow‐up evaluations consisted of clinical evaluation, enhanced brain MRI or CT, and imaging examinations of the original tumor and other metastases.
Toxicity was recorded according to the NRG‐RTOG Acute and Late Central Nervous System (CNS) Toxicity Criteria and the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Endpoints
The primary endpoint was overall survival (OS). The secondary endpoints included brain metastasis‐specific survival (BMSS), local control rate (LC), intracranial disease control rate (IDLC), intracranial progression‐free survival (IPFS), and toxicities.
OS was defined as the interval from the start of HFSRT to death of any cause. BMSS was defined as the interval from start of HFSRT to death resulting from BM [16]. Local control was defined as no evidence of disease progression at the site of HFSRT. IDLC was defined as no evidence of an increase in the bidimensional tumor area for any of the tracked BMs compared with their size at the time of HFSRT, or the appearance of any new BMs on a follow‐up MRI. IPFS was defined as the interval from the start of HFSRT to any intracranial progression, or to death of any cause. Intracranial progression was defined as any increase in the bidimensional tumor area for any of the tracked BMs, or the appearance of any new BMs on a follow‐up MRI.
Statistical Analysis
Historically, the 1‐year overall survival rate in patients with complex BMs treated with SRS or HFSRT alone has been approximately 45% (supplemental online Table 6). The study was designed to detect a 15% absolute improvement in the 1‐year OS rate (60% vs. 45%) at a significance level of .05 (two‐sided). Assuming an exponential distribution of survival times, this primary hypothesis is equivalent to a 44% reduction in the monthly hazard ratio (from 0.0665 to 0.0426). Based on a Z‐test comparing the logarithm of the hazard adjusting for single‐arm trials [17], the study required 37 deaths of 54 patients to ensure 85% power for the primary hypothesis.
OS, BMSS, and IPFS were calculated using Kaplan‐Meier plots. LC and IDLC were calculated using the cumulative incidence. Hazard ratios (unadjusted and adjusted) were estimated using Cox proportional hazards models. A two‐sided p < .05 was considered statistically significant.
All the analyses were perfomed using the SPSS software package (version 20.0, IBM Corporation, Armonk, NY).
Results
Patients
Between January 2010 and May 2015, 60 patients were enrolled initially. Six patients did not receive the protocol treatment because of ineligibility in two patients, refusal in three patients, and an unknown reason in one patient. Therefore, a total of 54 patients, 250 lesions (45 large lesions), were eventually treated and were evaluable for efficacy. No patients were lost to follow‐up. The treatment schema and consort diagram are shown in Figure 1. The median follow‐up time of the patients was 30.6 months (range, 6.2–70.8 months; 95% confidence interval [CI], 21.5–31.6 months). Patients’ characteristics are shown in Table 1.
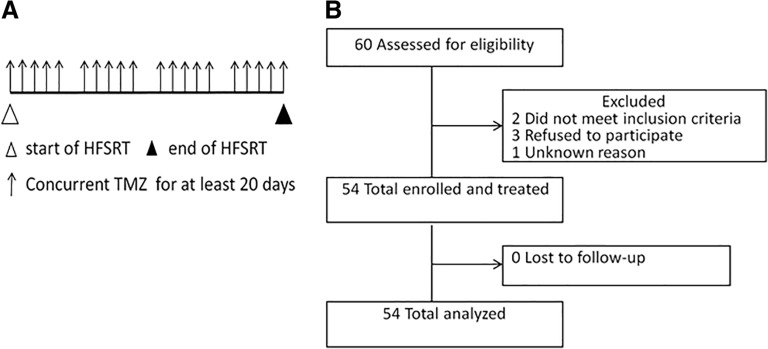
Figure 1.
Trial profile. (A): Treatment schema. (B): Consort diagram.
Abbreviations: HFSRT, hypofractionated stereotactic radiotherapy; TMZ, temozolomide.
Table 1. Pretreatment characteristics of patients (n = 54).
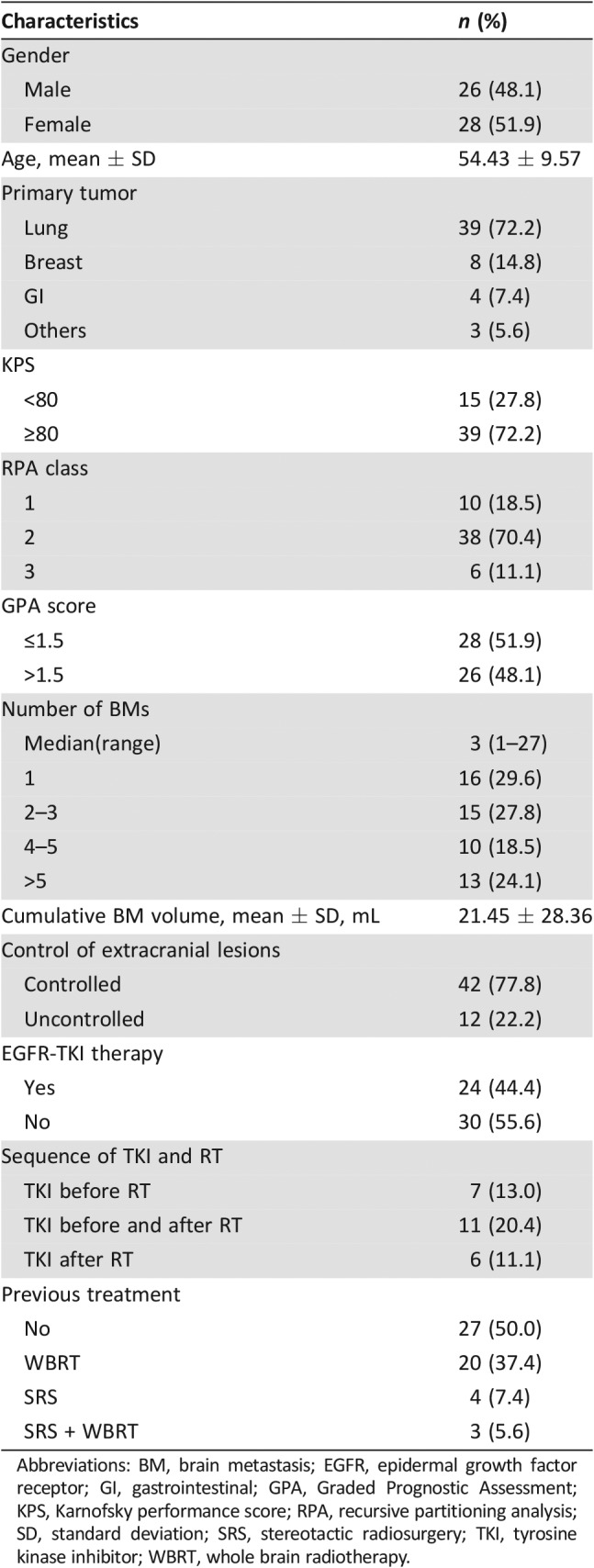
Abbreviations: BM, brain metastasis; EGFR, epidermal growth factor receptor; GI, gastrointestinal; GPA, Graded Prognostic Assessment; KPS, Karnofsky performance score; RPA, recursive partitioning analysis; SD, standard deviation; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiotherapy.
Treatment
The median interval between diagnosis of the primary tumor and BMs was 12.4 months (0–166.1 months), and the median interval between diagnosis of BMs and HFSRT was 1.7 months (0.1–43.3 months). All the patients finished the planned concurrent chemoradiotherapy (CCRT). The KPS immediately after CCRT was equal to or higher than that before treatment in all patients. Thus, 88.9% of the patients had a KPS of no less than 80%, compared with 72.2% before treatment. The proportion of patients who received adjuvant TMZ was 33.3%. The reasons for not finishing adjuvant TMZ included patients’ intolerance (28.5%), economical reasons (17.2%), disease progression (11.4%), and patient rejection (42.9%). As shown in Table 1, 24 patients received epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) treatment. Gene mutations were detected in 15 (62.5%) of them, and 12 harbored EGFR mutation.
Intracranial Tumor Control
Seven patients experienced local progression and required additional treatment. Only two patients had local progression within one year. The median total tumor volume was 11.27 cc (0.62–142.81 cc), and the median volume of the largest tumor was 6.99 cc (0.22–142.81 cc). There was no difference in local control between the groups with different tumor volumes. Among these seven patients, three patients had large lesions (tumor volumes: 8.89 cc, 8.99 cc, and 27.29 cc, respectively), and six patients (85.71%) with previous history of WBRT were reirradiated. The detailed characteristics of all these patients are summarized in supplemental online Table 2. Among three patients with large lesions who had local failure, two were treated with WBRT previously.
Six of the seven patients (85.7%) who experienced local progression underwent salvage treatments (one with surgery, four with reirrediation, and one with chemotherapy alone). The local control rates at 1 and 2 years were 96% (95% CI, 95%–100%) and 82% (95% CI, 67%–97%), respectively. Seventeen patients developed new BMs, with a median progression time of 5.5 months. The IDLC at 1 and 2 years were 70% (95% CI 57%–83%) and 59% (95% CI 43%–75%), respectively. Fifteen (88.2%) patients received radiation therapy again (10 with HSRT alone and 5 with HSRT plus WBRT), and the other two patients underwent salvage surgery. Two patients developed local progressions and new BMs simultaneously.
Survivals
Thirty‐eight patients in this cohort have died. Only 6 patients (15.8%) died of intracranial disease, 14 (36.8%) died of progression of primary tumors, 3 (7.9%) died of extracranial metastases, and the other 15 (39.5%) died of cachexia, severe infection, and other non‐cancer‐related reasons. Of the six patients who died of CNS progression, the causes of death included progression of treated lesions (n = 4), new BMs (n = 1), and meningeal metastasis (n = 1).
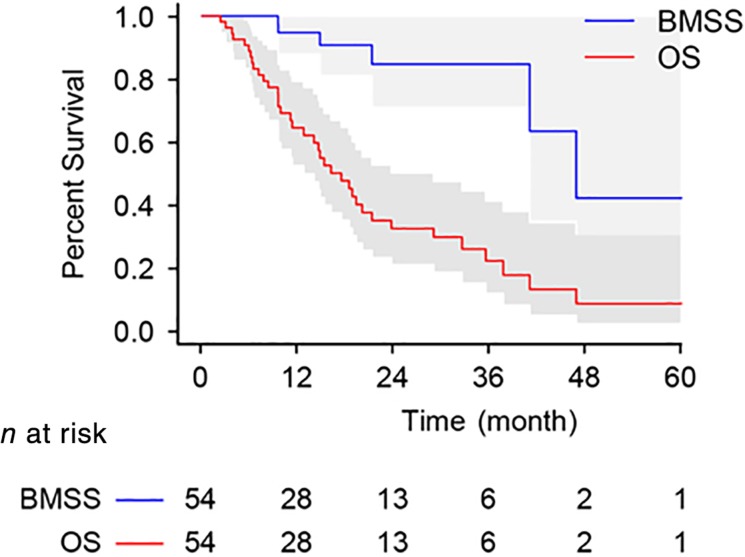
As shown in Figure 2, the median OS was 17.4 months (95% CI, 12.6–22.2), and the OS at 1 and 2 years was 65% (95% CI, 52%–78%) and 33% (19%–47%), respectively. The 1‐year OS was significantly higher than the historical control OS rate of 45% (1‐sided p < .001). The median BMSS was 46.9 months (95% CI, 35.5–58.4). The BMSS at 1 and 2 years was 95% (95% CI, 88%–100%) and 85% (95% CI, 70%–100%), respectively. The median IPFS was 12.7 months (95% CI, 6.8–18.7; supplemental online Fig. 2A).
Figure 2.
Kaplan‐Meier curves for OS and BMSS.
Abbreviations: BMSS, brain metastasis‐specific survival; OS, overall survival.
We also investigated the effect of adjuvant TMZ. As shown in supplemental online Figure 2B, there was a favorable trend of OS for patients who received adjuvant TMZ (n = 18) compared with those who did not (median survival time [MST], 20.1 months versus 14.0 months; 1 year OS, 82% versus 58%), although no statistically significant difference was observed (log‐rank test p = .263).
The sample size was relatively small; therefore, only univariate analyses by Cox regression were performed. As shown in supplemental online Table 3, no factors were identified as a significant predictor of OS, including gender, age, KPS, graded prognostic assessment score, treatment character (primary, salvage), status of primary tumor, status of extracranial metastases, target agent (EGFR‐TKI or trastuzumab) usage, and status of adjuvant TMZ.
Safety
Treatment‐emergent adverse events (AEs) possibly related to the study treatment are listed in Table 2. Acute/late CNS toxicities associated with exposure to radiation therapy or to chemotherapy were reported. Overall, concurrent TMZ and HFSRT were well tolerated. Four patients (7.4%) reported an acute neurological AE, which appeared as various degrees of headache during treatment and could be relieved by using steroids. Three patients had grade (G) 1 AEs, and one had a G2 AE. The rates of severe (≥G3) TMZ‐related systemic AEs was 5.6%, including one G3 hematological toxicity and two G3 liver dysfunctions. Two patients developed a late CNS toxicity (one G1 appeared as a slight headache, and one G3 appeared as dyskinesia of the right side limbs). The patient with G3 late CNS toxicity had a large tumor volume of 126 cm3 and dyskinesia before treatment. After CCRT, the dyskinesia was markedly relieved but reappeared 3 years after treatment. There were no G4 or higher AEs. Three patients with large size lesions (tumor volumes: 126 cc, 9 cc, and 6 cc, respectively) developed both sypmptom and edema deterioration 10 to 15 months after HFSRT. The symtoms improved after steroid therapy. All the three patients are still alive with no evidence of recurrence at last follow‐up. Therefore, radiation necrosis (RN) was clinically diagnosed.
Table 2. Chemoradiation‐related toxic effects.
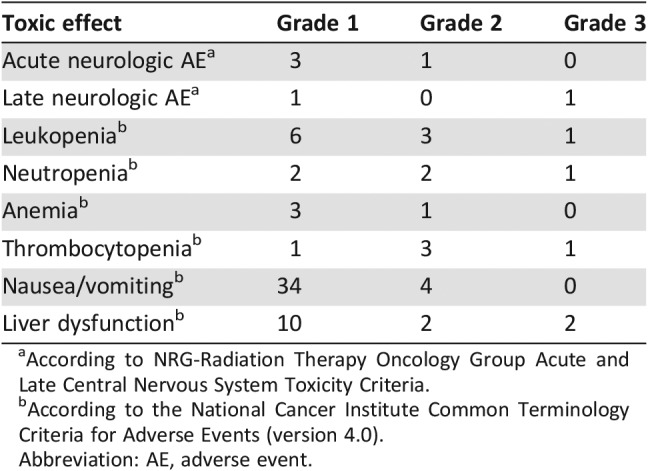
According to NRG‐Radiation Therapy Oncology Group Acute and Late Central Nervous System Toxicity Criteria.
According to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Abbreviation: AE, adverse event.
No difference in toxicities was observed between groups of patients with different tumor volumes. As shown in the supplemental online Table 4, there was no difference in AEs between first irradiation and reirradiation. Only seven patients had lesions within or close to eloquent locations (such as the brainstem, optic apparatus, and the internal capsule). There were no statistically significant differences among them in terms of acute or late CNS toxicities.
Discussion
The survival benefit of concurrent HFSRT and TMZ might be caused by an increased local control rate resulting from chemoradiotherapy. In addition, the ability to control subclinical lesions might contribute to reducing the distant failure to treat brain metastases.
Supplemental online Table 5 lists several recent randomized controlled trials (RCTs) with small sample sizes that compared TMZ + WBRT with WBRT alone. The results showed that adding TMZ might increase the ORR, but no significant improvements in OS were observed. However, the combination of WBRT and TMZ increased toxicities of grade ≥ 3, including in RTOG 0320, which was a multicenter phase III RCT closed prematurely because of slow accrual and poor results [11], [12], [13], [14].
The results of the present study demonstrated a MST of 17.4 months and a grade 3 to 5 toxicity rate of 7%, which compares favorably with the results of RTOG 0320. This might be because our study used HFSRT instead of SRS + WBRT, which made it possible for large tumors to receive higher doses than the single‐dose SRS, which may have resulted in large tumors achieving similar local control rates as small ones. Kim et al. also reported similar results [18]. Second, patients in our study did not receive WBRT concurrently with TMZ, or the interval between TMZ and previous WBRT was longer than 6 months, which may have contributed to the reduction in toxicities. Third, in RTOG 0320, patients in the WBRT + SRS + TMZ arm received less standard chemotherapy than those in the WBRT + SRS arm, which may have resulted in poor control of systemic disease, as well as a poor OS.
As shown in supplemental online Table 6, previous studies using SRT in large‐volume BMs reported that the 1‐year LC and OS rates were 61%–94.2% and 20%–55.3%, respectively [2], [19], [20], [21]. Concurrent HFSRT and TMZ suggested an increased survival, with an MST of 17.4 months (95% CI, 12.6–22.2), and the 1‐year LC and OS rates were 96% and 65%, respectively. We previously reported the results of using HFSRT alone to treat BMs larger than 3 cm from 2003 to 2009 [2]. The BMSS at 1 year in the present study was 95%, which was better than that achieved previously (76.8%). This suggested that concurrent chemoradiotherapy may decrease the intracranial failure rate, whereas the increase in OS depends more on better control of systemic disease. In addition, using 13–15 fractions of HFSRT seems to have resulted in better 1‐year LC and 1‐year OS than those gained in studies using 1–5 fractions.
According to supplemental online Table 2, the schedules of 52 Gy in 13–15 fractions appeared to have a higher local failure rate. However, there were other factors, such as previous treatment, which were related to local control. Therefore, we cannot draw the conclusion that this specific schedule is at higher risk for local failure or toxicitiy. Thus, the best fraction mode and dose need to be investigated in future studies.
It is a clinical challenge to distinguish tumor recurrance from RN, because biopsy, the gold standard of diagnosis, is invasive and only applicable to accessible lesions. The incidence of RN lies between 5% and 25% for patients treated with SRS [22]. The radiation dose, the fraction size, and the administration of chemotherapy are the most important risk factors. In our study, the incidence of RN diagnosed by convincing imaging was 5.6%, which is similar to that of SRS alone. However, the exact number is not known because of a lack of pathologic confirmation.
Most of our patients had lung cancer and the use of TKIs might play an important role in the treatment of brain metastases; therefore, the patients’ treatment sequences of EGFR‐TKI and radiotherapy were further analyzed (Table 1). Seven patients progressed after TKI therapy and then received radiotherapy. Six of them continued to receive TKI after RT. None of the patients had an anaplastic lymphoma kinase gene rearrangement. No patients received osimertinib or alectinib either. In future studies, we will restrict the pathology of the primary tumor and investigate the effect of TKIs and other small‐molecule agents to treat BMs.
There were several limitations of the present study. First, because this is a single‐arm phase II trial from a single center, our analysis results may not be as generalizable as larger, multicenter trials. Second, although most of the patients had primary tumors of lung cancer (72.3%), BMs from different kinds of primary tumors may have varied biological behaviors, which may have added potential confounding factors to the analysis. Lastly, we have not provided the quantitative data of the patients’ quality of life and neurocognitive function, which should be included in the designing of future studies.
Conclusion
Treatment with HFSRT concurrent with TMZ was well tolerated and could significantly extend OS compared with historical controls in complex BMs. This study is part of the necessary next step evaluation to assess the appropriateness of our approach. Large randomized clinical trials are warranted to verify our results.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the National Key Projects of Research and Development of China (grant 2016YFC0904600), Beijing Hope Marathon Special Fund (grant LC2011A07), Chinese Anticancer Association Professional Committee of Neuro‐Oncology (grant CSNO‐2015‐MSD01), and Nonprofit Central Research Institue Fund of Chinese Academy of Medical Sciences (grant 2018PT32011).
We thank all the patients and their families.
Author Contributions
Conception/design: Jianping Xiao, Yexiong Li
Provision of study material or patients: Nan Bi, Yuchao Ma, Jianping Xiao, Junling Li, Kai Wang, Xiaodong Huang, Junlin Yi
Collection and/or assembly of data: Nan Bi, Yuchao Ma, Hongmei Zhang, Yingjie Xu, Ye Zhang, Qingfeng Liu, Lei Deng, Wenqing Wang, Xuesong Chen, Feng Liu, Ruizhi Zhao, Siran Yang
Data analysis and interpretation: Nan Bi, Yuchao Ma, Jianping Xiao, Hongmei Zhang, Chen Hu
Manuscript writing: Nan Bi, Yuchao Ma
Final approval of manuscript: Jianping Xiao, Chen Hu, Yexiong Li
Nan Bi, Yuchao Ma, Jianping Xiao, Hongmei Zhang, Yingjie Xu, Yuan Tian, Junling Li, Ye Zhang, Qingfeng Liu, Kai Wang, Lei Deng, Wenqing Wang, Xuesong Chen, Feng Liu, Ruizhi Zhao, Siran Yang, Xiaodong Huang, Junlin Yi, Chen Hu, Yexiong Li
Disclosures
The authors indicated no financial relationships.
References
- 1.Pinkham MB, Whitfield GA, Brada M. New developments in intracranial stereotactic radiotherapy for metastases. Clin Oncol (R Coll Radiol) 2015;27:316–323. [DOI] [PubMed] [Google Scholar]
- 2.Jiang XS, Xiao JP, Zhang Y et al. Hypofractionated stereotactic radiotherapy for brain metastases larger than three centimeters. Radiat Oncol 2012;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Xiao J, Li X et al. Fifty percent patients avoid whole brain radiotherapy: Stereotactic radiotherapy for multiple brain metastases: A retrospective analysis of a single center. Clin Transl Oncol 2012;14:599–605. [DOI] [PubMed] [Google Scholar]
- 4.Raldow AC, Chiang VL, Knisely JP et al. Survival and intracranial control of patients with 5 or more brain metastases treated with gamma knife stereotactic radiosurgery. Am J Clin Oncol 2013;36:486–490. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Serizawa T, Shuto T et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi‐institutional prospective observational study. Lancet Oncol 2014;15:387–395. [DOI] [PubMed] [Google Scholar]
- 6.Hunter GK, Suh JH, Reuther AM et al. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:1394–1398. [DOI] [PubMed] [Google Scholar]
- 7.van Rijn J, Heimans JJ, van den Berg J et al. Survival of human glioma cells treated with various combination of temozolomide and X‐rays. Int J Radiat Oncol Biol Phys 2000;47:779–784. [DOI] [PubMed] [Google Scholar]
- 8.Inoue HK, Sato H, Suzuki Y et al. Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: A single‐institutional prospective study. Radiat Oncol 2014;9:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Nifterik KA, van den Berg J, Stalpers LJ et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys 2007;69:1246–1253. [DOI] [PubMed] [Google Scholar]
- 10.Chua D, Krzakowski M, Chouaid C et al. Whole‐brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non‐small‐cell lung cancer: A randomized, open‐label phase II Study. Clinical Lung Cancer 2010;11:176–181. [DOI] [PubMed] [Google Scholar]
- 11.Cao KI, Lebas N, Gerber S et al. Phase II randomized study of whole‐brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann Oncol 2015;26:89–94. [DOI] [PubMed] [Google Scholar]
- 12.Gamboa‐Vignolle C, Ferrari‐Carballo T, Arrieta Ó et al. Whole‐brain irradiation with concomitant daily fixed‐dose Temozolomide for brain metastases treatment: A randomised phase II trial. Radiother Oncol 2012;102:187–191. [DOI] [PubMed] [Google Scholar]
- 13.Hassler MR, Pfeifer W, Knocke‐Abulesz TH et al. Temozolomide added to whole brain radiotherapy in patients with multiple brain metastases of non‐small‐cell lung cancer: A multicentric Austrian phase II study. Wien Klin Wochenschr 2013;125:481–486. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto PW, Wang M, Robins HI et al. A phase 3 trial of whole brain radiation therapy and strerotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non‐small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J RadiatOncol Bio Phys 2013;85:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minniti G, Scaringi C, Paolini S et al. Single‐fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation‐induced brain necrosis. Int J Radiat Oncol Biol Phys 2016;95:1142–1148. [DOI] [PubMed] [Google Scholar]
- 16.Lischalk JW, Oermann E, Collins SP et al. Five‐fraction stereotactic radiosurgery (SRS) for single inoperable high‐risk non‐small cell lung cancer (NSCLC) brain metastases. Radiat Oncol 2015;10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics 1982;38:63–170. [PubMed] [Google Scholar]
- 18.Kim YJ, Cho KH, Kim JY et al. Single‐dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 2011;81:483–489. [DOI] [PubMed] [Google Scholar]
- 19.Yomo S, Hayashi M, Nicholson C. A prospective pilot study of two‐session Gamma Knife surgery for large metastatic brain tumors. J Neurooncol 2012;109:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JH, Kim DG, Chung HT et al. Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 2012;83:113–120. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi Y, Serizawa T, Nagano O et al. Three‐staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 2009;74:1543–1548. [DOI] [PubMed] [Google Scholar]
- 22.Vellayappan B, Tan CL, Yong C et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol 2018;8:395. [DOI] [PMC free article] [PubMed] [Google Scholar]