Current preoperative risk estimation for lymph node metastasis in endometrial carcinoma is inadequate. Optimized use of clinical biomarkers might be a first step toward improved selection for tailored treatment. This review reports on the diagnostic accuracy of preoperative clinical biomarkers for the prediction of lymph node metastasis in patients with endometrial carcinoma.
Keywords: Endometrial carcinoma, Lymph node metastasis, Risk stratification, Meta‐analysis, Biomarkers, Imaging
Abstract
Background.
In endometrial carcinoma (EC), preoperative classification is based on histopathological criteria, with only moderate diagnostic performance for the risk of lymph node metastasis (LNM). So far, existing molecular classification systems have not been evaluated for prediction of LNM. Optimized use of clinical biomarkers as recommended by international guidelines might be a first step to improve tailored treatment, awaiting future molecular biomarkers.
Aim.
To determine the diagnostic accuracy of preoperative clinical biomarkers for the prediction of LNM in endometrial cancer.
Methods.
A systematic review was performed according to the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Studies identified in MEDLINE and EMBASE were selected by two independent reviewers. Included biomarkers were based on recommended guidelines (cancer antigen 125 [Ca‐125], lymphadenopathy on magnetic resonance imaging, computed tomography, and 18F‐fluorodeoxyglucose positron emission tomography/computed tomography [18FDG PET‐CT]) or obtained by physical examination (body mass index, cervical cytology, blood cell counts). Pooled sensitivity, specificity, area under the curve (AUC), and likelihood ratios were calculated with bivariate random‐effects meta‐analysis. Likelihood ratios were classified into small (0.5–1.0 or 1–2.0), moderate (0.2–0.5 or 2.0–5.0) or large (0.1–0.2 or ≥ 5.0) impact.
Results.
Eighty‐three studies, comprising 18,205 patients, were included. Elevated Ca‐125 and thrombocytosis were associated with a moderate increase in risk of LNM; lymphadenopathy on imaging with a large increase. Normal Ca‐125, cytology, and no lymphadenopathy on 18FDG PET‐CT were associated with a moderate decrease. AUCs were above 0.75 for these biomarkers. Other biomarkers had an AUC <0.75 and incurred only small impact.
Conclusion.
Ca‐125, thrombocytosis, and imaging had a large and moderate impact on risk of LNM and could improve preoperative risk stratification.
Implications for Practice.
Routine lymphadenectomy in clinical early‐stage endometrial carcinoma does not improve outcome and is associated with 15%–20% surgery‐related morbidity, underlining the need for improved preoperative risk stratification. New molecular classification systems are emerging but have not yet been evaluated for the prediction of lymph node metastasis. This article provides a robust overview of diagnostic performance of all clinical biomarkers recommended by international guidelines. Based on these, at least measurement of cancer antigen 125 serum level, assessment of thrombocytosis, and imaging focused on lymphadenopathy should complement current preoperative risk stratification in order to better stratify these patients by risk.
Introduction
Endometrial carcinoma (EC) is the most common gynecological malignancy in industrialized countries [1], [2]. Most patients present with early‐stage disease and have a favorable prognosis. However, approximately 10% of clinical early‐stage patients have lymph node metastasis (LNM) [3], [4]. Primary treatment of EC consists of hysterectomy with bilateral salpingo‐oophorectomy. Pelvic and para‐aortic lymphadenectomy can be performed to enable tailored adjuvant therapy based on the presence of LNM. Yet, routine lymphadenectomy in clinical early‐stage EC has not demonstrated to improve outcome and is associated with 15%–20% surgery‐related morbidity, underlining the need for improved risk stratification [5], [6], [7], [8].
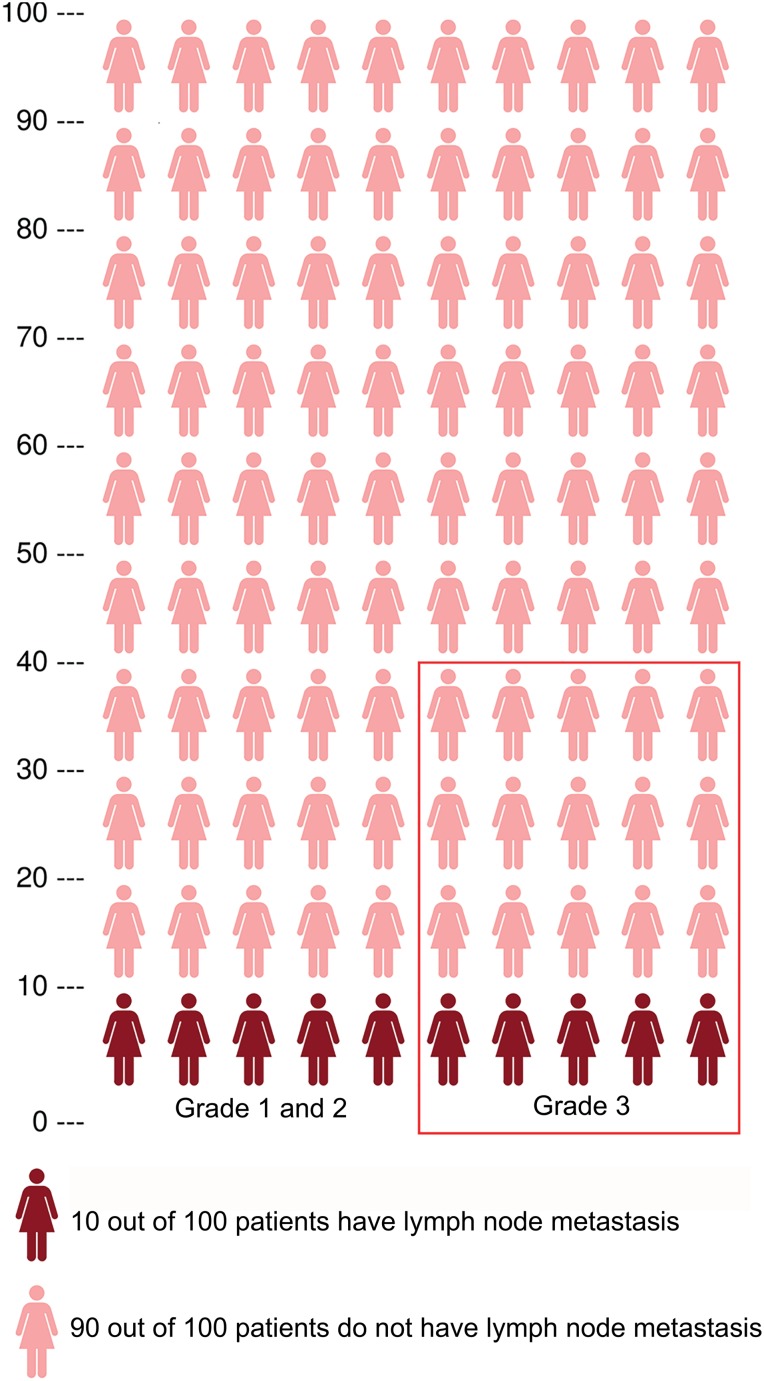
Approximately 80% of patients are preoperatively diagnosed with low‐risk histology, i.e., grade 1 or 2 endometrioid endometrial carcinoma, facing 5%–9% risk of LNM [4], [9]. Around 20% are preoperatively diagnosed with high‐risk histology, i.e., grade 3 or nonendometrioid endometrial carcinoma, facing 18%–24% risk of LNM [4], [9], [10]. When classifying patients as low risk or high risk of LNM solely based on preoperative histology, a substantial number of patients with LNM will be missed (Fig. 1). Furthermore, in up to 33% of all patients, preoperative histology is discordant with postoperative histology, resulting in a subsequent incorrect risk estimation of LNM [11].
Figure 1.
Population of 100 patients with endometrial carcinoma with a population risk of approximately 10% on lymph node metastasis (LNM). Risk stratification based on preoperative tumor histology classifies 80% of patients as low‐risk, with a 5%–9% risk of LNM, and 20% of patients as high‐risk, with an 18%–24% risk of LNM. The patients within the red frame are classified as high‐risk based on preoperative tumor histology. This figure illustrates that patients are being misclassified in both risk groups.
Recently, The Cancer Genome Atlas (TCGA) identified four distinct subgroups based on genomic background, including an “ultramutated” subgroup associated with mutations in the exonuclease domain of polymerase‐ε (POLE); a microsatellite‐instable subgroup, with deficiency of one or more mismatch repair proteins; a copy number‐high subgroup with frequent p53 mutations; and a copy number‐low subgroup [12]. These molecular subgroups categorize patients with distinct prognoses, but so far TCGA classification has not been studied in relation to the risk estimation of LNM [13], [14], [15], [16]. Several guidelines, including the European Society of Medical Oncology, European Society of Gynecological Oncology, European Society for Radiotherapy and Oncology (ESMO‐ESGO‐ESTRO) consensus conference guideline, incorporate measurement of cancer antigen 125 (Ca‐125) and/or assessment of lymphadenopathy by imaging as part of preoperative workup [8], [17]. Furthermore, clinical biomarkers obtained during standardized preoperative workup, including assessment of body mass index (BMI), cervical cytology, and analysis of hemocytometric parameters, are demonstrated to carry prognostic information about the risk of LNM and may specifically be valuable because they are widely available as part of the routine workup [18], [19], [20], [21].
In summary, current preoperative risk estimation for LNM in EC is only moderate, and optimized use of clinical biomarkers might be a first step toward improved selection awaiting future molecular biomarkers. Therefore, this systematic review investigates the reported diagnostic accuracy of preoperative clinical biomarkers for the prediction of LNM in patients with EC.
Materials and Methods
Search Strategy and Selection Criteria
The systematic review was performed according to the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines for meta‐analyses of observational studies in medicine [22]. A literature search was performed in MEDLINE and EMBASE from June 1997 to January 14, 2019. The following keywords and all known synonyms for these keywords were used: “endometrial cancer,” “BMI,” “cervical cytology,” “Ca‐125,” “hemoglobin,” “leukocytes,” “thrombocytes,” “MRI,” “CT,” “PET‐CT,” and “lymph node metastases.” Parameters were included when incorporated in the ESMO‐ESGO‐ESTRO or Society of Gynecologic Oncology guidelines (Ca‐125, computed tomography [CT], magnetic resonance imaging [MRI], 18F‐fluorodeoxyglucose positron emission tomography/computed tomography [18FDG PET‐CT]) or when judged as being routinely obtained by physical examination (BMI, cervical cytology, blood cell counts including hemoglobin, leukocytes, and thrombocytes). The search strategy can be found in supplemental online Appendix 1. Additional searches were performed by manual cross‐referencing in included studies and systematic reviews.
Study Selection
Eligible studies investigated the diagnostic accuracy of one of the preoperative biomarkers in patients with EC. The reference standard had to be histological lymph node assessment by lymphadenectomy. Exclusion criteria were conference abstracts, case reports, studies comprising fewer than five patients, case‐control studies, publications older than 20 years or not in English, review articles, outcome measures other than LNM, and (partial) absence of the reference standard. In publications in which diagnostic performance metrics were reported without the raw data to construct a contingency table, the authors were contacted. In case of overlapping patient cohorts, the study with most outcome data were included. Two investigators (C.R., F.S.) independently reviewed each study for eligibility based on title and abstract. The full text of presumably eligible studies was evaluated to decide if the study fulfilled the inclusion criteria. In case of discrepancies, consensus was made after discussion with a third reviewer (J.P.). The agreement on the selection of studies between the investigators was calculated by means of a weighted κ.
Assessment of Study Quality
The revised tool for Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) was used for assessment of methodological quality of the studies by two investigators (C.R., F.S.) [23]. In case of disagreement, consensus was achieved with a third investigator (J.P.). Risk of bias was assessed as “low risk,” “high risk,” or “unclear risk” in four domains: patient selection, index test, reference standard, and flow and timing. The first three domains were also assessed in terms of applicability. Studies were classified as “low risk of bias” or “low risk regarding applicability” in a specific domain, when all subdomains were scored as “low risk.” For bias in “patient selection,” it was assessed whether consecutive inclusion was present and whether patients were inappropriately excluded based on age, histology, or International Federation of Gynecology and Obstetrics (FIGO) stage. For “index test” and “reference standard” it was assessed whether patients received the same reference standard (pelvic and para‐aortic lymphadenectomy). For “flow and timing” it was assessed whether interval was acceptable (<2 weeks).
Data Extraction
A data extraction form was designed with information about study design, patient characteristics (age, menopausal status), preoperative biomarkers, number of patients with and without LNM, and tumor characteristics (grade and histological subtype).
Statistical Analysis
Contingency tables containing the number of patients with LNM as assessed by the preoperative test and by lymphadenectomy were constructed, and the number of true positives, false negatives, false positives, and true negatives were extracted. From these, likelihood ratios (LRs), sensitivity and specificity, and their 95% confidence intervals (CIs) were calculated for each study and visualized by means of forest plots. Pooled estimates for LRs, sensitivity and specificity, and area under the curve (AUC) were calculated by means of bivariate random‐effects meta‐analysis [24]. An AUC above 0.75 was considered clinically relevant [25]. Using sensitivity, specificity, and prevalence, the pooled positive predictive values (PPVs) and negative predictive values (NPVs) were also estimated:
The median LNM prevalence of all included studies (13.4%) was used to estimate PPV and NPV.
To quantify the test impact on the posttest probability, LRs were categorized: “small increase,” “moderate increase,” and “large increase” for positive LRs between 1.0 and 2.0, 2.0 and 5.0, and 5.0 and 10.0, respectively; “small decrease,” “moderate decrease,” and “large decrease” for negative LRs between 0.5 and 1.0, 0.2 and 0.5, and 0.1 and 0.2, respectively [26], [27]. Posttest probabilities were calculated using Fagan's nomogram [28].
The I2 statistic was used for all variables to estimate the amount of heterogeneity between the studies [29]. Heterogeneity was graduated: null for I2 = 0%, minimal for 0% < I2 ≤ 25%, low for 25% < I2 ≤ 50%, moderate for 50% < I2 ≤ 75%, and high for I2 > 75%. In case of high heterogeneity, we investigated whether the heterogeneity could be explained by the use of various definitions of positive test results by performing subgroup analyses based on these definitions (Table 1).
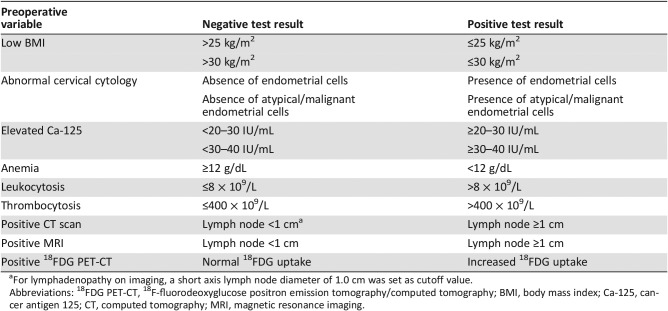
Table 1. Preoperative biomarkers, including the definitions used in the included studies for negative and positive test results.
For lymphadenopathy on imaging, a short axis lymph node diameter of 1.0 cm was set as cutoff value.
Abbreviations: 18FDG PET‐CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; BMI, body mass index; Ca‐125, cancer antigen 125; CT, computed tomography; MRI, magnetic resonance imaging.
Furthermore, separate analyses were performed for studies including low‐risk and high‐risk populations. A study population was considered low risk when the study prevalence of LNM was below the median (13.4%) and high‐risk when the prevalence of LNM was above the median (13.4%). To estimate the corresponding PPVs and NPVs, the median prevalence of all low‐risk and high‐risk population studies, respectively, was used (10.5% and 19.0%). The statistical software R was used for the statistical analysis (version 3.3.2) with the MADA package (1.9‐9) [30].
Results
Study Selection
A total of 7,072 studies were retrieved, of which 6,453 remained after removal of duplicates. Based on title and abstract, 525 studies were relevant. After full‐text screening, 83 studies, comprising 18,205 patients, were included in the systematic review (supplemental online Appendix 2) [18], [19], [20], [21], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109]. Three studies lacked raw data, and because the authors did not respond after being contacted, these publications were excluded [68], [110], [111]. Two studies had overlapping study cohorts, and one of these was excluded [67], [112]. One additional article was identified by extensive cross‐checking of the reference lists [103]. Ten studies were analyzed multiple times because they included two or three biomarkers [53], [59], [68], [75], [76], [77], [78], [80], [87], [104]. The agreement of the two reviewers (C.R. and F.S.) regarding eligibility was 94% (κ = 0.89; 95% CI, 0.85–0.93). Characteristics of the included studies are shown in supplemental online Appendix 3.
Assessment of Study Quality
The risk of bias and the applicability was evaluated by means of the QUADAS‐2 tool (supplemental online Appendices 4 and 5). Ten studies were assessed for two or three biomarkers, so in total 95 evaluations were performed. The risk of bias was low in 4 (4.2%) evaluations, unclear in 81 (85.2%) evaluations, and high in 10 (10.5%) evaluations. Forty‐three (45.3%) evaluations had an unclear risk of bias in patient selection, attributable to the absence of selection criteria or no description of consecutive inclusion. Furthermore, in a majority of studies, the mean number of lymph nodes resected by lymphadenectomy was varying. Applicability concerns were mainly attributable to patient selection: the histological diagnoses were not described properly or only patients with either low‐risk or high‐risk histology were included.
Study Results
I2 for pooled positive LR and negative LR estimates was <50% for all markers, indicating absence of moderate heterogeneity (Table 2). The next section will focus on pooled LR estimates and AUCs; however, information about pooled sensitivity, specificity, and PPVs can be found in Table 2. Individual study data with forest plots for sensitivity, specificity, and predictive values can be found in supplemental online Appendix 6.
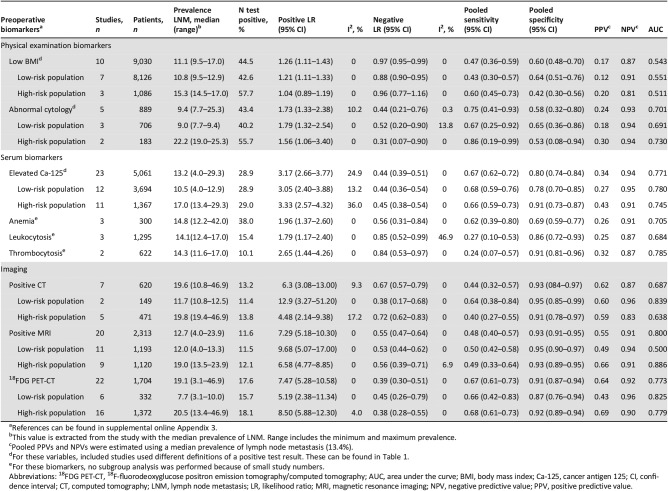
Table 2. Pooled diagnostic test characteristics of preoperative biomarkers for the prediction of LNM.
References can be found in supplemental online Appendix 3.
This value is extracted from the study with the median prevalence of LNM. Range includes the minimum and maximum prevalence.
Pooled PPVs and NPVs were estimated using a median prevalence of lymph node metastasis (13.4%).
For these variables, included studies used different definitions of a positive test result. These can be found in Table 1.
For these biomarkers, no subgroup analysis was performed because of small study numbers.
Abbreviations: 18FDG PET‐CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; AUC, area under the curve; BMI, body mass index; Ca‐125, cancer antigen 125; CI, confidence interval; CT, computed tomography; LNM, lymph node metastasis; LR, likelihood ratio; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value.
BMI
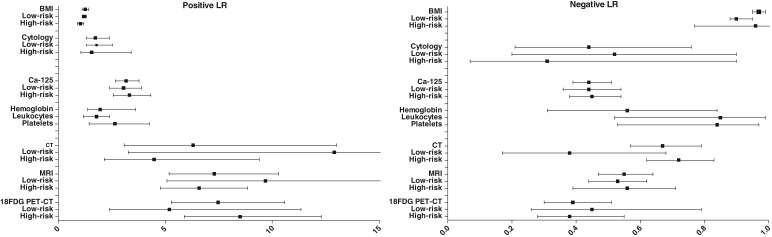
In patients with a low BMI, the pooled positive LR point estimate was 1.26, indicating a small increase in risk of LNM (Table 3). In patients with high BMI, the negative LR estimate was 0.97, indicating a small decrease in risk of LNM. Similar estimates were found in the low‐risk population studies. LR estimates in the high‐risk population studies were not significantly different from 1 (Table 2, Fig. 2). The AUC was found to be 0.543.
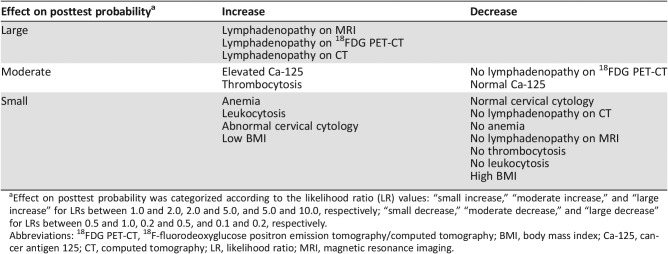
Table 3. Effect on posttest probability of lymph node metastasis categorized according to the likelihood ratio value.
Effect on posttest probability was categorized according to the likelihood ratio (LR) values: “small increase,” “moderate increase,” and “large increase” for LRs between 1.0 and 2.0, 2.0 and 5.0, and 5.0 and 10.0, respectively; “small decrease,” “moderate decrease,” and “large decrease” for LRs between 0.5 and 1.0, 0.2 and 0.5, and 0.1 and 0.2, respectively.
Abbreviations: 18FDG PET‐CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; BMI, body mass index; Ca‐125, cancer antigen 125; CT, computed tomography; LR, likelihood ratio; MRI, magnetic resonance imaging.
Figure 2.
Forest plots displaying pooled likelihood ratios with corresponding 95% confidence intervals.
Abbreviations: 18FDG PET‐CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; BMI, body mass index; Ca‐125, cancer antigen 125; CT, computed tomography; LR, likelihood ratio; MRI, magnetic resonance imaging.
Cervical Cytology
In patients with abnormal cervical cytology, the pooled positive LR estimate was 1.73, and in patients with normal cervical cytology, the pooled negative LR was 0.44. In analysis of low‐risk and high‐risk population studies, similar results were found (Table 2). LRs varied depending on the definition of abnormal cervical cytology. When the presence of endometrial cells was regarded as abnormal, regardless of whether these were atypical, pooled positive LR and negative LR estimates were 1.30 and 0.21, respectively. When the presence of atypical endometrial cells was regarded as abnormal, pooled positive LR and negative LR estimates were 2.06 and 0.66, respectively (supplemental online Appendix 7). The AUC was found to be 0.701.
Serum Biomarkers
Overall, Ca‐125 was elevated in 28.9% of all patients. In patients with elevated Ca‐125, the pooled positive LR estimate was 3.17, and in patients with normal Ca‐125, the pooled negative LR estimate was 0.44, both indicating a moderate effect on the risk of LNM. These results were comparable for low‐risk and high‐risk population studies (Table 2), and predictive values did not vary depending on the chosen threshold (supplemental online Appendix 7). The AUC was found to be 0.771.
In patients with elevated Ca‐125, the pooled positive LR estimate was 3.17 and in patients with normal Ca‐125 the pooled negative LR estimate was 0.44, both indicating a moderate effect on the risk of LNM. These results were comparable for low‐risk and high‐risk population studies and predictive values did not vary depending on the chosen threshold.
In patients with preoperative anemia, leukocytosis, or thrombocytosis, the pooled positive LR estimates were 1.96, 1.79 (both small increase), and 2.66 (moderate increase), respectively. In all three biomarkers the pooled negative LR estimates were above 0.50, indicating a small decrease in risk of LNM. The AUC was found to be above 0.75 for thrombocytosis (0.785) but not for anemia and leukocytosis. No subgroup analysis could be performed because of insufficient numbers of studies.
Imaging
In most studies, a 1.5T MRI scanner was used, except in three studies that used both 1.5T and 3.0T scanners. Two studies did not provide specifications on scanner type (supplemental online Appendix 3). The pooled positive LR point estimates for CT, MRI, and 18FDG PET‐CT were 6.30, 7.29, and 7.47, respectively, indicating a large increase. The pooled negative LR point estimates were 0.67 for CT, 0.55 for MRI, and 0.39 for 18FDG PET‐CT. Subgroup analyses resulted in comparable estimates in low‐ and high‐risk population studies. Overall, the presence of LNM increased the odds of positive findings on imaging approximately seven times, whereas the absence of LNM decreased the odds approximately two times. The AUC was 0.800 for MRI, 0.773 for 18FDG PET‐CT, and 0.687 for CT.
Translation to Clinical Practice
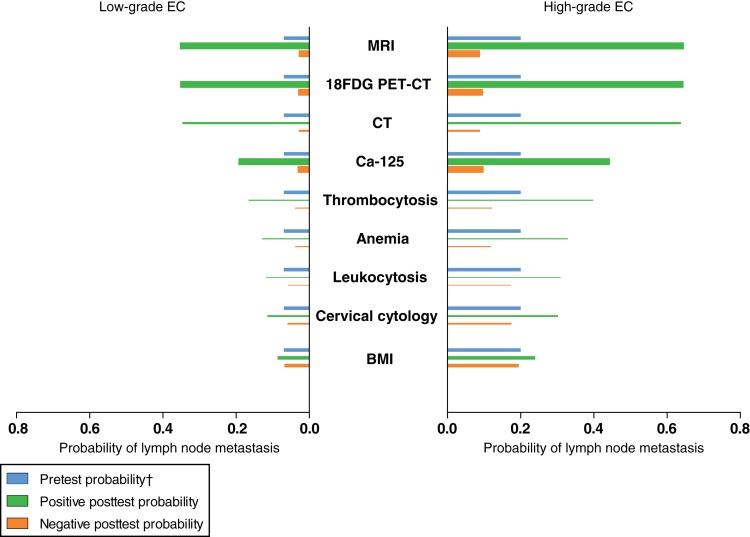
As preoperative tumor grade currently is one of the most important preoperative predictors for LNM, the contributive value of all evaluated clinical biomarkers to preoperative tumor grade is summarized in Figure 3. Results are shown for two clinical settings based on patients presenting with low‐grade EC and an estimated 7% a priori probability on LNM, and patients presenting with high‐grade EC with an estimated 20% a priori probability. Results are classified according their increase or decrease on the odds of LNM, i.e., small, moderate, and large.
Figure 3.
Summarizing results of pre‐ and posttest probabilities for evaluated clinical biomarkers in low‐ and high risk‐risk preoperative setting. Estimates based on pooled likelihood ratios (Table 2). Thickness of bars represents the number of studies performed on each marker; range, 2–20.
†, Based on prevalence of lymph node metastasis in patients preoperatively diagnosed with low‐grade (7%, left panel) and high‐grade endometrial cancer (20%, right panel), respectively.
Abbreviations: 18FDG PET‐CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; BMI, body mass index; Ca‐125, cancer antigen 125; CT, computed tomography; EC, endometrial carcinoma; MRI, magnetic resonance imaging.
Discussion
In this review we have demonstrated that several clinical biomarkers are significantly associated with LNM and thus may improve current preoperative risk stratification of patients with EC. As expected, the presence of lymphadenopathy on imaging had a large impact on the risk of LNM. Elevated Ca‐125 and thrombocytosis were associated with a moderate increased risk of LNM, whereas normal cervical cytology, Ca‐125, and absence of lymphadenopathy on 18FDG PET‐CT were associated with a moderate decreased risk of LNM. All markers having a moderate or large impact were found to have clinically relevant AUCs (0.75), whereas markers with small impact did not.
Interestingly, we found that high BMI (>25/30 kg/m2) was associated with reduced risk of LNM. This could be explained by the higher prevalence of low‐grade endometrioid ECs in obese patients who overall have a low risk of LNM [34]. Yet, we could not rule out whether lymphadenectomy was less frequently performed in obese patients with EC because of comorbidity and/or surgical difficulties, because this could underestimate the risk of LNM in obese patients [34]. However, once lymphadenectomy is performed, numbers of lymph nodes are reported to be equal in obese and nonobese patients [34].
Abnormal cervical cytology was a predictor of LNM. The presence of atypical endometrial cells in cervical cytology could be a consequence of EC cells detaching and exfoliating, as in patients with serous histology, who present with abnormal cervical cytology in 66%–88% [113], [114]. Because it is often obtained by general practitioner prior to referral for postmenopausal bleeding and can easily be added in the workup by gynecologists, further validation is worthwhile [115].
The association between Ca‐125 level and LNM has already been demonstrated in a large number of studies, and Ca‐125 has also been shown to predict the high‐risk features deep myometrial invasion (MI) and lymphovascular space invasion [48], [51], [60], [116]. Nevertheless, Ca‐125 has not yet been implemented in standardized preoperative workup, possibly because of controversies regarding the appropriate cutoff value. In this meta‐analysis we found that the diagnostic accuracy was similar using varying cutoff values.
We found that preoperative anemia, leukocytosis, and thrombocytosis were associated with a small (anemia, leukocytosis) or moderate (thrombocytosis) increased risk of LNM. Interestingly, abnormal blood cell counts are reportedly associated with poor prognosis in several malignancies [117], [118]. Whereas anemia and leukocytosis only have a small impact on risk of LNM, thrombocytosis has a moderate impact and has also been proposed as a marker to be included into a preoperative scoring system for advanced disease in EC [119]. Because of limited study numbers, further validation is needed.
Whereas anemia and leukocytosis only have a small impact on risk of LNM, thrombocytosis has a moderate impact and has also been proposed as a marker to be included into a pre‐operative scoring system for advanced disease in EC.
Interestingly, for imaging, the presence of LNM increased the odds of positive findings on imaging approximately seven times, whereas the absence of LNM decreased the odds only two times. The different imaging techniques rely on pelvic or para‐aortic lymph node enlargement (CT and MRI), or increased glucose metabolism (18FDG PET‐CT), which is only detectible when there are sufficient tumor cells present to discriminate them from physiological glucose metabolism from surrounding nonmalignant cells. Micrometastases leading to minimally increased avidity being barely detectible would typically be missed. The relatively low negative LR estimates suggest that omitting lymphadenectomy in patients with negative findings on imaging may thus lead to potential surgical undertreatment in some patients, underlining the importance of incorporating multiple predictors into risk‐stratification models. On the other hand, the very high positive LR and PPV estimates of CT, MRI, and 18FDG PET‐CT justify lymphadenectomy in patients with imaging findings indicating lymph node metastases. The ability of MRI to assess MI and cervical involvement as well supports the use of MRI in addition to 18FDG PET‐CT or CT for preoperative risk stratification [120]. Diagnostic superiority was also demonstrated in the AUC values, which were shown to be higher than 0.75 for MRI and 18FDG PET‐CT but lower than 0.75 for CT.
To our knowledge, this is the first systematic review comprehensively investigating the diagnostic accuracy of preoperative clinical biomarkers in EC for the prediction of LNM. We have performed an in‐depth analysis including subgroup analyses to improve clinical applicability. However, some limitations need to be addressed. Most studies documented a study design with only moderate quality, being retrospective and lacking a consecutive research design because of selective performance of lymphadenectomy. Although I2 was <50% for all biomarkers, some clinical heterogeneity could exist between the studies. With study population and the employed thresholds being important sources of heterogeneity, separate analyses were performed based on these two.
Although we have shown the impact of several biomarkers on risk of LNM, multivariable analysis is impossible in this setting, and therefore the combined value of markers cannot be concluded from this review and requires further analysis of individual patient data.
Among the strongest prognosticators for LNM are lymphovascular space invasion, tumor grade and histology, and deep MI [121], [122], [123], [124], [125]. However, preoperative assessment of these markers is accompanied with some challenges. Lymphovascular space invasion is based on postoperative histological examination of the surgical specimen and cannot be reliably assessed preoperatively. MI can be assessed preoperatively by MRI, transvaginal ultrasound, or intraoperatively by frozen section with varying diagnostic accuracies. For transvaginal ultrasound, sensitivity and specificity for deep MI are reported to be 71%–85% and 72%–90%, respectively [126]. For MRI, sensitivity and specificity are 63%–100% and 56%–100% [120], [127].
Several models have been developed and validated for prediction of LNM in order to improve existing risk stratification [58], [128], [129]. Among these, one model has an AUC of 0.75, indicating good diagnostic accuracy [130]. This model has included serum Ca‐125, together with MRI parameters (MI, lymphadenopathy, and extension beyond uterine corpus), adequately identifying 43% of patients as low risk for LNM (<4%), with a false‐negative rate of only 1.3% [128].
For future implementation, it is important to acknowledge of the increasing impact of sentinel node (SN) procedures. These procedures create the possibility to reduce treatment‐related morbidity and might lead to more adequate staging [131]. Still, it is questionable whether SN procedures should be performed in all patients with EC, with a population‐based risk of 10% for LNM. Sentinel node procedures require sufficient expertise and are generally available in large oncology centers, and thus are demanding for public health care facilities and costs. It could therefore be imagined that preoperative risk stratification will remain a crucial part in EC care in order to expose only those patients at risk to more extensive surgical procedures. In what perspective the investigated biomarkers could complement the recent TCGA classification remains to be elucidated. Even though this classification identifies four subgroups with distinct prognoses, it is unknown whether these subgroups are associated with the risk of LNM. Recent TCGA classification has raised debate about how to relate prognostic molecular classification systems to traditional histopathological criteria, and an integrated genomic‐pathologic classification was proposed as a superior classification [13]. This discussion is also relevant for implementing these clinical biomarkers complementary to molecular classification.
Conclusion
We identified clinical biomarkers that could contribute to an improved preoperative risk stratification and more individualized treatment strategy in patients with EC. Lymphadenopathy identified at preoperative imaging incurred a large risk of LNM, and as such should be incorporated into future preoperative risk‐stratification models. Furthermore, clinical biomarkers with moderate impact on risk of LNM, i.e., Ca‐125 serum levels and thrombocytosis are candidates for future preoperative risk‐stratification models in addition to the established markers. In what way they could complement molecular classification should be studied.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We acknowledge Onying Chan, librarian at the Medical Library of Radboud University, Nijmegen, for her help on developing the search strategy. This research was funded by the Dutch Cancer Society (grant 10616/2016‐2).
Author Contributions
Conception/design: Casper Reijnen, Joanna IntHout, Leon F.A.G. Massuger, Fleur Strobbe, Heidi V.N. Küsters‐Vandevelde, Ingfrid S. Haldorsen, Marc P.L.M. Snijders, Johanna M.A. Pijnenborg
Collection and/or assembly of data: Casper Reijnen, Joanna IntHout, Leon F.A.G. Massuger, Fleur Strobbe, Heidi V.N. Küsters‐Vandevelde, Ingfrid S. Haldorsen, Marc P.L.M. Snijders, Johanna M.A. Pijnenborg
Data analysis and interpretation: Casper Reijnen, Fleur Strobbe, Johanna M.A. Pijnenborg
Manuscript writing: Casper Reijnen, Joanna IntHout, Leon F.A.G. Massuger, Fleur Strobbe, Heidi V.N. Küsters‐Vandevelde, Ingfrid S. Haldorsen, Marc P.L.M. Snijders, Johanna M.A. Pijnenborg
Final approval of manuscript: Casper Reijnen, Joanna IntHout, Leon F.A.G. Massuger, Fleur Strobbe, Heidi V.N. Küsters‐Vandevelde, Ingfrid S. Haldorsen, Marc P.L.M. Snijders, Johanna M.A. Pijnenborg
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Ma J, Zou Z et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983;15:10–17. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Morrow CP, Bundy BN et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group study. Cancer 1987;60:2035–2041. [DOI] [PubMed] [Google Scholar]
- 5.Vargas R, Rauh‐Hain JA, Clemmer J et al. Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: A SEER analysis. Gynecol Oncol 2014;133:216–220. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti Panici P, Basile S, Maneschi F et al. Systematic pelvic lymphadenectomy vs. No lymphadenectomy in early‐stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst 2008;100:1707–1716. [DOI] [PubMed] [Google Scholar]
- 7.ASTEC Study Group ; Kitchener H, Swart AM, Qian Q et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009;373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo N, Creutzberg C, Amant F et al. ESMO‐ESGO‐ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow‐up. Ann Oncol 2016;27:16–41. [DOI] [PubMed] [Google Scholar]
- 9.Trovik J, Wik E, Werner HM et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer 2013;49:3431–3441. [DOI] [PubMed] [Google Scholar]
- 10.Morrow CP, Bundy BN, Kurman RJ et al. Relationship between surgical‐pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol 1991;40:55–65. [DOI] [PubMed] [Google Scholar]
- 11.Visser NCM, Reijnen C, Massuger L et al. Accuracy of endometrial sampling in endometrial carcinoma: A systematic review and meta‐analysis. Obstet Gynecol 2017;130:803–813. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network ; Kandoth C, Schultz N, Cherniack AD et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stelloo E, Nout RA, Osse EM et al. Improved risk assessment by integrating molecular and clinicopathological factors in early‐stage endometrial cancer‐combined analysis of the PORTEC cohorts. Clin Cancer Res 2016;22:4215–4224. [DOI] [PubMed] [Google Scholar]
- 14.Kommoss S, McConechy MK, Kommoss F et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population‐based case series. Ann Oncol 2018;29:1180–1188. [DOI] [PubMed] [Google Scholar]
- 15.Talhouk A, McConechy MK, Leung S et al. Confirmation of ProMisE: A simple, genomics‐based clinical classifier for endometrial cancer. Cancer 2017;123:802–813. [DOI] [PubMed] [Google Scholar]
- 16.Talhouk A, McConechy MK, Leung S et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SGO Clinical Practice Endometrial Cancer Working Group ; Burke WM, Orr J, Leitao M et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol Oncol 2014;134:385–392. [DOI] [PubMed] [Google Scholar]
- 18.Gunderson CC, Java J, Moore KN et al. The impact of obesity on surgical staging, complications, and survival with uterine cancer: A gynecologic Oncology Group LAP2 ancillary data study. Gynecol Oncol 2014;133:23–27. [DOI] [PubMed] [Google Scholar]
- 19.Brown AK, Gillis S, Deuel C et al. Abnormal cervical cytology: A risk factor for endometrial cancer recurrence. Int J Gynecol Cancer 2005;15:517–522. [DOI] [PubMed] [Google Scholar]
- 20.Antonsen SL, Høgdall E, Christensen IJ et al. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: A prospective multicenter study (ENDOMET). Acta Obstet Gynecol Scand 2013;92:1313–1322. [DOI] [PubMed] [Google Scholar]
- 21.Ekici H, Malatyalioglu E, Kokcu A et al. Do leukocyte and platelet counts have benefit for preoperative evaluation of endometrial cancer? Asian Pac J Cancer Prev 2015;16:5305–5310. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: A proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 23.Whiting PF, Rutjes AW, Westwood ME et al.; QUADAS‐2 Group. QUADAS‐2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 24.Eusebi P, Reitsma JB, Vermunt JK. Latent class bivariate model for the meta‐analysis of diagnostic test accuracy studies. BMC Med Res Methodol 2014;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM 2006;8:19–20. [DOI] [PubMed] [Google Scholar]
- 26.Henderson MC, Tierney LM Jr, Smetana GW. The Patient History: An Evidence‐Based Approach to Differential Diagnosis. 2nd edition. New York: McGraw‐Hill; 2012:30. [Google Scholar]
- 27.McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002;17:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med 1975;293:257. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 30.Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 31.Akbayır O, Corbacıoglu Esmer A, Numanoglu C et al. Influence of body mass index on clinicopathologic features, surgical morbidity and outcome in patients with endometrial cancer. Arch Gynecol Obstet 2012;286:1269–1276. [DOI] [PubMed] [Google Scholar]
- 32.Canlorbe G, Bendifallah S, Raimond E et al. Severe obesity impacts recurrence‐free survival of women with high‐risk endometrial cancer: Results of a French multicenter study. Ann Surg Oncol 2015;22:2714–2721. [DOI] [PubMed] [Google Scholar]
- 33.Crosbie EJ, Roberts C, Qian W et al. Body mass index does not influence post‐treatment survival in early stage endometrial cancer: Results from the MRC ASTEC trial. Eur J Cancer 2012;48:853–864. [DOI] [PubMed] [Google Scholar]
- 34.Everett E, Tamimi H, Greer B et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol 2003;90:150–157. [DOI] [PubMed] [Google Scholar]
- 35.Gates EJ, Hirschfield L, Matthews RP et al. Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium. J Natl Med Assoc 2006;98:1814–1822. [PMC free article] [PubMed] [Google Scholar]
- 36.Hachisuga T, Kawarabayashi T, Hirakawa T et al. The effect of being overweight on survival in endometrioid carcinoma of the endometrium at different ages. Int J Gynecol Cancer 2000;10:228–232. [DOI] [PubMed] [Google Scholar]
- 37.Jeong NH, Lee JM, Lee JK et al. Role of body mass index as a risk and prognostic factor of endometrioid uterine cancer in Korean women. Gynecol Oncol 2010;118:24–28. [DOI] [PubMed] [Google Scholar]
- 38.Lino‐Silva LS, de León DC, Salcedo‐Hernández RA et al. A high body mass index is not a worse prognostic factor for endometrial carcinoma in a predominantly obese population. Clin Transl Oncol 2013;15:243–247. [DOI] [PubMed] [Google Scholar]
- 39.Martra F, Kunos C, Gibbons H et al. Adjuvant treatment and survival in obese women with endometrial cancer: An international collaborative study. Am J Obstet Gynecol 2008;198:89.e81–89.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuBeshter B, Deuel C, Gillis S et al. Endometrial cancer: The potential role of cervical cytology in current surgical staging. Obstet Gynecol 2003;101:445–450. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda K, Mori M, Uchiyama M et al. Preoperative cervical cytology in endometrial carcinoma and its clinicopathologic relevance. Gynecol Oncol 1999;72:273–277. [DOI] [PubMed] [Google Scholar]
- 42.Guy MS, Cheng G, Post MD et al. Outcomes of women with atypical glandular cells on preoperative cytology and endometrial cancer. Int J Gynecol Cancer 2014;24:266–271. [DOI] [PubMed] [Google Scholar]
- 43.Patsner B, Morgan SA. The potential value of preoperative cytobrush papanicolaou smear in clinical stage I endometrial carcinoma. J Low Genit Tract Dis 1999;3:4–6. [DOI] [PubMed] [Google Scholar]
- 44.Baek MH, Lee SW, Park JY et al. Identification of a preoperative predictive factor for lymph node metastasis in uterine papillary serous carcinoma long‐term results from a single institution. Int J Gynecol Cancer 2015;25:69–74. [DOI] [PubMed] [Google Scholar]
- 45.Chao A, Tang YH, Lai CH et al. Potential of an age‐stratified CA125 cut‐off value to improve the prognostic classification of patients with endometrial cancer. Gynecol Oncol 2013;129:500–504. [DOI] [PubMed] [Google Scholar]
- 46.Chen YL, Huang CY, Chien TY et al. Value of pre‐operative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust N Z J Obstet Gynaecol 2011;51:397–402. [DOI] [PubMed] [Google Scholar]
- 47.Chung HH, Kim JW, Park NH et al. Use of preoperative serum CA‐125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet Gynecol Scand 2006;85:1501–1505. [DOI] [PubMed] [Google Scholar]
- 48.Pinar Cilesiz Goksedef B, Gorgen H, Baran SY et al. Preoperative serum CA 125 level as a predictor for metastasis and survival in endometrioid endometrial cancer. J Obstet Gynaecol Can 2011;33:844–850. [DOI] [PubMed] [Google Scholar]
- 49.Han SS, Cho JY, Park IA et al. Feasibility of routine lymphadenectomy in clinical stage‐I endometrial cancer. Med Sci Monit 2008;14:CR183–CR189. [PubMed] [Google Scholar]
- 50.Han SS, Lee SH, Kim DH et al. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand 2010;89:168–174. [DOI] [PubMed] [Google Scholar]
- 51.Jiang T, Huang L, Zhang S. Preoperative serum CA125: A useful marker for surgical management of endometrial cancer. BMC Cancer 2015;15:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Kong TW, Paek J et al. Predicting model of lymph node metastasis using preoperative tumor grade, transvaginal ultrasound, and serum CA‐125 level in patients with endometrial cancer. Int J Gynecol Cancer 2016;26:1630–1635. [DOI] [PubMed] [Google Scholar]
- 53.Sadowski EA, Robbins JB, Guite K et al. Preoperative pelvic MRI and serum cancer antigen‐125: Selecting women with grade 1 endometrial cancer for lymphadenectomy. AJR Am J Roentgenol 2015;205:W556–W564. [DOI] [PubMed] [Google Scholar]
- 54.Sebastianelli A, Renaud MC, Grégoire J et al. Preoperative CA 125 tumour marker in endometrial cancer: Correlation with advanced stage disease. J Obstet Gynaecol Can 2010;32:856–860. [DOI] [PubMed] [Google Scholar]
- 55.Sood AK, Buller RE, Burger RA et al. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstet Gyencol 1997;90:441–447. [DOI] [PubMed] [Google Scholar]
- 56.Suh DH, Kim HS, Chung HH et al. Pre‐operative systemic inflammatory response markers in predicting lymph node metastasis in endometrioid endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol 2012;162:206–210. [DOI] [PubMed] [Google Scholar]
- 57.Todo Y, Okamoto K, Hayashi M et al. A validation study of a scoring system to estimate the risk of lymph node metastasis for patients with endometrial cancer for tailoring the indication of lymphadenectomy. Gynecol Oncol 2007;104:623–628. [DOI] [PubMed] [Google Scholar]
- 58.Todo Y, Sakuragi N, Nishida R et al. Combined use of magnetic resonance imaging, CA 125 assay, histologic type, and histologic grade in the prediction of lymph node metastasis in endometrial carcinoma. Am J Obstet Gynecol 2003;188:1265–1272. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Han C, Teng F et al. Predictive value of serum HE4 and CA125 concentrations for lymphatic metastasis of endometrial cancer. Int J Gynaecol Obstet 2017;136:58–63. [DOI] [PubMed] [Google Scholar]
- 60.Yoon JH, Yoo SC, Kim WY et al. Para‐aortic lymphadenectomy in the management of preoperative grade 1 endometrial cancer confined to the uterine corpus. Ann Surg Oncol 2010;17:3234–3240. [DOI] [PubMed] [Google Scholar]
- 61.Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac J Cancer Prev 2014;15:10231–10236. [DOI] [PubMed] [Google Scholar]
- 62.Metindir J, Bilir Dilek G. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol 2009;135:125–129. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi R, Mabuchi S, Kawano M et al. Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: A monoinstitutional study. Gynecol Oncol 2015;137:112–118. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi R, Mabuchi S, Kuroda H et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial cancer. Int J Gynecol Cancer 2017;27:1399–1407. [DOI] [PubMed] [Google Scholar]
- 65.Tamussino KF, Gücer F, Reich O et al. Pretreatment hemoglobin, platelet count, and prognosis in endometrial carcinoma. Int J Gynecol Cancer 2001;11:236–240. [DOI] [PubMed] [Google Scholar]
- 66.Wilairat W, Benjapibal M. Presence of anemia and poor prognostic factors in patients with endometrial carcinoma. Asian Pac J Cancer Prev 2012;13:3187–3190. [DOI] [PubMed] [Google Scholar]
- 67.Worley MJ Jr, Nitschmann CC, Shoni M et al. The significance of preoperative leukocytosis in endometrial carcinoma. Gynecol Oncol 2012;125:561–565. [DOI] [PubMed] [Google Scholar]
- 68.Antonsen SL, Jensen LN, Loft A et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer ‐ A multicenter prospective comparative study. Gynecol Oncol 2013;128:300–308. [DOI] [PubMed] [Google Scholar]
- 69.Body N, Lavoué V, De Kerdaniel O et al. Are preoperative histology and MRI useful for classification of endometrial cancer risk? BMC Cancer 2016;16:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabrita S, Rodrigues H, Abreu R et al. Magnetic resonance imaging in the preoperative staging of endometrial carcinoma. Eur J Gynaecol Oncol 2008;29:135–137. [PubMed] [Google Scholar]
- 71.Chan CYH, Shek SKW, Kwok SKY et al. Diagnostic accuracy of preoperative magnetic resonance imaging in staging endometrial cancer: A five‐year experience. Hong Kong J Radiol 2016;19:249–255. [Google Scholar]
- 72.Cho H, Kim YT, Kim JH. Accuracy of preoperative tests in clinical stage I endometrial cancer: The importance of lymphadenectomy. Acta Obstet Gynecol Scand 2010;89:175–181. [DOI] [PubMed] [Google Scholar]
- 73.Foti PV, Farina R, Coronella M et al. Endometrial carcinoma: MR staging and causes of error. Radiol Med 2013;118:487–503. [DOI] [PubMed] [Google Scholar]
- 74.Hahn HS, Song HS, Lee IH et al. Magnetic resonance imaging and intraoperative frozen sectioning for the evaluation of risk factors associated with lymph node metastasis in endometrial cancer. Int J Gynecol Cancer 2013;23:1411–1416. [DOI] [PubMed] [Google Scholar]
- 75.Inubashiri E, Hata K, Kanenishi K et al. Positron emission tomography with the glucose analog [F]‐fluoro‐2‐deoxy‐D‐glucose for evaluating pelvic lymph node metastasis in uterine corpus cancer: Comparison with CT and MRI findings. J Obstet Gynaecol Res 2009;35:26–34. [DOI] [PubMed] [Google Scholar]
- 76.Kim HJ, Cho A, Yun M et al. Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann Nucl Med 2016;30:104–113. [DOI] [PubMed] [Google Scholar]
- 77.Lee HJ, Park JY, Lee JJ et al. Comparison of MRI and 18F‐FDG PET/CT in the preoperative evaluation of uterine carcinosarcoma. Gynecol Oncol 2016;140:409–414. [DOI] [PubMed] [Google Scholar]
- 78.Lu R, Guixia S. Research into the value of B‐mode ultrasound, CT and MRI examinations in the diagnosis of preoperative myometrial infiltration of endometrial cancer and lymph node metastasis. Open Med (Warsaw) 2015;10:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manfredi R, Mirk P, Maresca G et al. Local‐regional staging of endometrial carcinoma: Role of MR imaging in surgical planning. Radiology 2004;231:372–378. [DOI] [PubMed] [Google Scholar]
- 80.Park JY, Kim EN, Kim DY et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol 2008;108:486–492. [DOI] [PubMed] [Google Scholar]
- 81.Rockall AG, Meroni R, Sohaib SA et al. Evaluation of endometrial carcinoma on magnetic resonance imaging. Int J Gynecol Cancer 2007;17:724–728. [DOI] [PubMed] [Google Scholar]
- 82.Ryoo UN, Choi CH, Yoon JY et al. MR imaging in endometrial carcinoma as a diagnostic tool for the prediction of myometrial invasion and lymph node metastasis. Cancer Res Treat 2007;39:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin KE, Park BK, Kim CK et al. MR staging accuracy for endometrial cancer based on the new FIGO stage. Acta Radiol 2011;52:818–824. [DOI] [PubMed] [Google Scholar]
- 84.Teng F, Zhang YF, Wang YM et al. Contrast‐enhanced MRI in preoperative assessment of myometrial and cervical invasion, and lymph node metastasis: Diagnostic value and error analysis in endometrial carcinoma. Acta Obstet Gynecol Scand 2015;94:266–273. [DOI] [PubMed] [Google Scholar]
- 85.Todo Y, Choi HJ, Kang S et al. Clinical significance of tumor volume in endometrial cancer: A Japan‐Korea cooperative study. Gynecol Oncol 2013;131:294–298. [DOI] [PubMed] [Google Scholar]
- 86.Ytre‐Hauge S, Husby JA, Magnussen IJ et al. Preoperative tumor size at MRI predicts deep myometrial invasion, lymph node metastases, and patient outcome in endometrial carcinomas. Int J Gynecol Cancer 2015;25:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atri M, Zhang Z, Dehdashti F et al. Utility of PET/CT to evaluate retroperitoneal lymph node metastasis in high‐risk endometrial cancer: Results of ACRIN 6671/GOG 0233 trial. Radiology 2017;283:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Connor JP, Andrews JI, Anderson B et al. Computed tomography in endometrial carcinoma. Obstet Gynecol 2000;95:692–696. [DOI] [PubMed] [Google Scholar]
- 89.Ryo E, Yasugi T, Mizutani K et al. Diagnostic usefulness of intraoperative ultrasonography in avoiding unnecessary para‐aortic lymphadenectomy in women with endometrial carcinoma. Int J Gynecol Cancer 2011;21:859–863. [DOI] [PubMed] [Google Scholar]
- 90.Zerbe MJ, Bristow R, Grumbine FC et al. Inability of preoperative computed tomography scans to accurately predict the extent of myometrial invasion and extracorporal spread in endometrial cancer. Gynecol Oncol 2000;78:67–70. [DOI] [PubMed] [Google Scholar]
- 91.Bese T, Sal V, Demirkiran F et al. The combination of preoperative fluorodeoxyglucose positron emission tomography/computed tomography and sentinel lymph node mapping in the surgical management of endometrioid endometrial cancer. Int J Gynecol Cancer 2016;26:1228–1238. [DOI] [PubMed] [Google Scholar]
- 92.Crivellaro C, Signorelli M, Guerra L et al. Tailoring systematic lymphadenectomy in high‐risk clinical early stage endometrial cancer: The role of 18F‐FDG PET/CT. Gynecol Oncol 2013;130:306–311. [DOI] [PubMed] [Google Scholar]
- 93.Gholkar NS, Saha SC, Prasad G et al. The accuracy of integrated [(18)F] fluorodeoxyglucose‐positron emission tomography/computed tomography in detection of pelvic and para‐aortic nodal metastasis in patients with high risk endometrial cancer. World J Nucl Med 2014;13:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horowitz NS, Dehdashti F, Herzog TJ et al. Prospective evaluation of FDG‐PET for detecting pelvic and para‐aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol 2004;95:588–592. [DOI] [PubMed] [Google Scholar]
- 95.Husby JA, Reitan BC, Biermann M et al. Metabolic tumor volume on 18F‐FDG PET/CT improves preoperative identification of high‐risk endometrial carcinoma patients. J Nucl Med 2015;56:1191–1198. [DOI] [PubMed] [Google Scholar]
- 96.Klar M, Meyer PT, Hancke K et al. Evaluation of FDG‐PET for detecting lymph node metastasis in uterine corpus cancer. Anticancer Res 2010;30:3787–3790. [PubMed] [Google Scholar]
- 97.Nayot D, Kwon JS, Carey MS et al. Does preoperative positron emission tomography with computed tomography predict nodal status in endometrial cancer? A pilot study. Curr Oncol 2008;15:123–125. [PMC free article] [PubMed] [Google Scholar]
- 98.Nogami Y, Banno K, Irie H et al. The efficacy of preoperative positron emission tomography‐computed tomography (PET‐CT) for detection of lymph node metastasis in cervical and endometrial cancer: Clinical and pathological factors influencing it. Jpn J Clin Oncol 2015;45:26–34. [DOI] [PubMed] [Google Scholar]
- 99.Picchio M, Mangili G, Samanes Gajate AM et al. High‐grade endometrial cancer: Value of [18F]FDG PET/CT in preoperative staging. Nucl Med Commun 2010;31:506–512. [DOI] [PubMed] [Google Scholar]
- 100.Signorelli M, Crivellaro C, Buda A et al. Staging of high‐risk endometrial cancer with PET/CT and sentinel lymph node mapping. Clin Nucl Med 2015;40:780–785. [DOI] [PubMed] [Google Scholar]
- 101.Signorelli M, Guerra L, Buda A et al. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer: Detection of pelvic nodal metastases. Gynecol Oncol 2009;115:231–235. [DOI] [PubMed] [Google Scholar]
- 102.Suga T, Nakamoto Y, Saga T et al. Clinical value of FDG‐PET for preoperative evaluation of endometrial cancer. Ann Nucl Med 2011;25:269–275. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki R, Miyagi E, Takahashi N et al. Validity of positron emission tomography using fluoro‐2‐deoxyglucose for the preoperative evaluation of endometrial cancer. Int J Gynecol Cancer 2007;17:890–896. [DOI] [PubMed] [Google Scholar]
- 104.Aşicıoğlu O, Gungorduk K, Ozdemir A et al. A novel preoperative scoring system based on 18‐FDG PET‐CT for predicting lymph node metastases in patients with high‐risk endometrial cancer. J Obstet Gynaecol 2019;39:105–109. [DOI] [PubMed] [Google Scholar]
- 105.Konuralp Atakul BK, Taşkın S, Soydal C et al. Preoperative 18F‐fluorodeoxyglucose positron emission tomography/CT in prediction of uterine risk factors and lymph node metastasis: An analysis of 111 endometrioid endometrial cancer patients. Gynecol Obstet Invest 2017;82:340–348. [DOI] [PubMed] [Google Scholar]
- 106.Bogani G, Gostout BS, Dowdy SC et al. Clinical utility of preoperative computed tomography in patients with endometrial cancer. Int J Gynecol Cancer 2017;27:1685–1693. [DOI] [PubMed] [Google Scholar]
- 107.Erdemoğlu E, Çerçi SS, Erdemoğlu E et al. Role of positron emission tomography‐computed tomography in endometrial cancer. Turk J Obstet Gynecol 2017;14:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Momtahan M, Hosseini M, Robati M et al. Predictive value of Kanagawa Cancer Center scoring system for lymph node metastasis and need for lymphadenectomy in patients with endometrial cancer: A validation study. Int J Gynecol Cancer 2018;28:1290–1296. [DOI] [PubMed] [Google Scholar]
- 109.Taşkın S, Şükür YE, Varlı B et al. Nomogram with potential clinical use to predict lymph node metastasis in endometrial cancer patients diagnosed incidentally by postoperative pathological assessment. Arch Gynecol Obstet 2017;296:803–809. [DOI] [PubMed] [Google Scholar]
- 110.Kitajima K, Suenaga Y, Ueno Y et al. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with 18F‐FDG contrast‐enhanced PET/CT and dynamic contrast‐enhanced pelvic MRI. Eur J Radiol 2013;82:1672–1676. [DOI] [PubMed] [Google Scholar]
- 111.Baker W, Pelkofski E, Te Paske J et al. Preoperative imaging of uterine malignancy: A low‐value service. Gynecol Oncol 2015;137:285–290. [DOI] [PubMed] [Google Scholar]
- 112.Worley MJ Jr, Nitschmann CC, Shoni M et al. Preoperative leukocytosis imposes an increased risk of recurrence and death among patients with nonendometrioid endometrial carcinoma. Int J Gynecol Cancer 2013;23:312–317. [DOI] [PubMed] [Google Scholar]
- 113.Roelofsen T, Geels YP, Pijnenborg JM et al. Cervical cytology in serous and endometrioid endometrial cancer. Int J Gynecol Pathol 2013;32:390–398. [DOI] [PubMed] [Google Scholar]
- 114.Amkreutz LCM, Pijnenborg JMA, Joosten DWL et al. Contribution of cervical cytology in the diagnostic work‐up of patients with endometrial cancer. Cytopathology 2018;29:63–70. [DOI] [PubMed] [Google Scholar]
- 115.Meijer LJ, Bruinsma ACA, Pameijer AS et al. NHG‐Standaard Vaginaal bloedverlies (derde herziening). Huisarts Wet 2014;57:406–414. https://www.nhg.org/?tmp‐no‐mobile=1&q=node/1781. Accessed June 6, 2019. [Google Scholar]
- 116.Hsieh CH, ChangChien CC, Lin H et al. Can a preoperative CA 125 level be a criterion for full pelvic lymphadenectomy in surgical staging of endometrial cancer? Gynecol Oncol 2002;86:28–33. [DOI] [PubMed] [Google Scholar]
- 117.Hefler L, Mayerhofer K, Leibman B et al. Tumor anemia and thrombocytosis in patients with vulvar cancer. Tumour Biol 2000;21:309–314. [DOI] [PubMed] [Google Scholar]
- 118.Pedersen LM, Milman N. Diagnostic significance of platelet count and other blood analyses in patients with lung cancer. Oncol Rep 2003;10:213–216. [PubMed] [Google Scholar]
- 119.Tuomi T, Pasanen A, Luomaranta A et al. Risk‐stratification of endometrial carcinomas revisited: A combined preoperative and intraoperative scoring system for a reliable prediction of an advanced disease. Gynecol Oncol 2015;137:23–27. [DOI] [PubMed] [Google Scholar]
- 120.Horváth K, Pete I, Vereczkey I et al. Evaluation of the accuracy of preoperative MRI in measuring myometrial infiltration in endometrial carcinoma. Pathol Oncol Res 2014;20:327–333. [DOI] [PubMed] [Google Scholar]
- 121.Gadducci A, Cavazzana A, Cosio S et al. Lymph‐vascular space involvement and outer one‐third myometrial invasion are strong predictors of distant haematogeneous failures in patients with stage I‐II endometrioid‐type endometrial cancer. Anticancer Res 2009;29:1715–1720. [PubMed] [Google Scholar]
- 122.Vaizoglu F, Yuce K, Salman MC et al. Lymphovascular space involvement is the sole independent predictor of lymph node metastasis in clinical early stage endometrial cancer. Arch Gynecol Obstet 2013;288:1391–1397. [DOI] [PubMed] [Google Scholar]
- 123.Mariani A, Webb MJ, Keeney GL et al. Predictors of lymphatic failure in endometrial cancer. Gynecol Oncol 2002;84:437–442. [DOI] [PubMed] [Google Scholar]
- 124.Briët JM, Hollema H, Reesink N et al. Lymphvascular space involvement: An independent prognostic factor in endometrial cancer. Gynecol Oncol 2005;96:799–804. [DOI] [PubMed] [Google Scholar]
- 125.Carey MS, O'Connell GJ, Johanson CR et al. Good outcome associated with a standardized treatment protocol using selective postoperative radiation in patients with clinical stage I adenocarcinoma of the endometrium. Gynecol Oncol 1995;57:138–144. [DOI] [PubMed] [Google Scholar]
- 126.Alcázar JL, Orozco R, Martinez‐Astorquiza Corral T et al. Transvaginal ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: A systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2015;46:405–413. [DOI] [PubMed] [Google Scholar]
- 127.Haldorsen IS, Salvesen HB. What is the best preoperative imaging for endometrial cancer? Curr Oncol Rep 2016;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang S, Kang WD, Chung HH et al. Preoperative identification of a low‐risk group for lymph node metastasis in endometrial cancer: A Korean gynecologic oncology group study. J Clin Oncol 2012;30:1329–1334. [DOI] [PubMed] [Google Scholar]
- 129.Lee JY, Jung DC, Park SH et al. Preoperative prediction model of lymph node metastasis in endometrial cancer. Int J Gynecol Cancer 2010;20:1350–1355. [DOI] [PubMed] [Google Scholar]
- 130.Koskas M, Fournier M, Vanderstraeten A et al. Evaluation of models to predict lymph node metastasis in endometrial cancer: A multicentre study. Eur J Cancer 2016;61:52–60. [DOI] [PubMed] [Google Scholar]
- 131.Bogani G, Murgia F, Ditto A et al. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta‐analysis. Gynecol Oncol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]