Cancer can cause an altered physiological environment that puts patients at risk for drug‐drug interactions, drug‐disease interactions, and adverse drug reactions. The prescribing of multiple medications for coexisting conditions can contribute to negative outcomes. This article reports on the prevalence of this concern, as well as the causality, predictability, and preventability of adverse drug reactions causing or contributing to hospitalization.
Keywords: Adverse drug reactions, Hospitalization, Multimorbidity, Polypharmacy, Clinical oncology
Abstract
Background.
Our goal was to determine (a) the prevalence of multimorbidity and polypharmacy in patients with cancer and (b) the prevalence, predictability, and preventability of adverse drug reactions (ADRs) causing/contributing to hospitalization.
Materials and Methods.
We conducted a 12‐month prospective observational study of patients aged ≥16 years admitted to an oncology center. Older adults were aged ≥70 years.
Results.
We enrolled 350 patients: 52.3% (n = 183) female, mean age 63.6 years (SD 12.1), 36.6% (n = 121) aged ≥70 years. Multimorbidity (≥2 conditions) was identified in 96.9%; 68% had ≥5 conditions. The median number of medications was 6 (interquartile range [IQR] 4–8); 47% were prescribed ≥6 medications and 11.4% ≥11 medications. Older adults had higher numbers of comorbid conditions (7 [IQR 5–10] vs. 5 [IQR 3–7]) and were prescribed more medications (median 7 [IQR 4–9] vs. 4 [IQR 2–7]). ADRs caused/contributed to hospitalization in 21.5% (n = 75): 35.8% (n = 72) of emergency admissions and 4.7% (n = 3) of elective admissions. The most common ADRs were neutropenia with infection (25.3%), dyspepsia/nausea/vomiting (20%), and constipation (20%). Causative medications included systemic anticancer therapies (SACTs; 53.3%), opioids (17.3%), corticosteroids (6.7%), and nonsteroidal anti‐inflammatory drugs (5.3%). ADR prevalence was similar in older and younger adults secondary to SACTs (8.3% vs. 13.1%), non‐cancer medications (10.7% vs. 8.3%), and both (0% vs. 1.3%). ADRs were predictable in 89.3% (n = 67), definitely avoidable in 29.3% (n = 22), and possibly avoidable in 33.3% (n = 25). No association was identified between ADRs and age, gender, daily medication number, length of stay, or death. No ADR predictor variables were identified by logistic regression.
Conclusion.
More than 21% of admissions to an oncology service are ADR‐related. ADRs are caused by both SACTs and non‐cancer‐specific medications. The majority are predictable; ≥60% may be preventable. Patients with cancer have high levels of multimorbidity and polypharmacy, which require vigilance for related adverse outcomes.
Implications for Practice.
A diagnosis of cancer often occurs in patients with multimorbidity and polypharmacy. Cancer can cause an altered physiological environment, placing patients at risk of drug‐drug interactions, drug‐disease interactions, and adverse drug reactions (ADRs). This study identified that ADRs caused or contributed to one in five hospital admissions of patients with cancer. ADRs were caused by systemic anticancer therapies (SACTs) in 53.3% of cases and non‐cancer medications in 45.4% of cases, and a combination of both in 1.3%. ADRs occurred in similar frequencies in older and younger patients secondary to SACTs (8.3% vs. 13.1%, p = .295), non‐SACTs (10.7% vs. 8.3%, p = .107), and a combination of both (0% vs. 1.3%, p = .240). The majority of ADRs were predictable (89.3%) and potentially preventable (62.6%). These findings support the need for increased awareness of medication‐related adversity in patients with cancer and interventions to minimize their occurrence, thus supporting the American Society of Clinical Oncology guidelines that recommend adults ≥65 years of age receiving chemotherapy have geriatric assessment to identify medical and medication issues.
Introduction
The incidence of cancer is expected to rise significantly over the next decade [1] principally in older adults because of demographic shifts toward an aging population [2], [3]. With advancing age comes an increase in the prevalence of comorbid illnesses including hypertension [4], postural hypotension [5], falls [6], cardiac failure [7], atrial fibrillation [8], stroke [9], chronic kidney disease [10], and dementia [11]. Aging and comorbidity correlate with increasing numbers of prescribed medications [12]. Although often indicated, there is evidence to show that polypharmacy (the coprescription of ≥6 medications) [13] is associated with inappropriate prescribing and negative outcomes including adverse drug reactions (ADRs) as well as excessive health resource use [14], [15]. ADRs cause or contribute to 7% of hospital admissions in the general adult population [16], [17] and up to 20% of hospital admissions in older patients [17], [18]. The prevalence of ADRs as a cause of hospitalization in patients with cancer is unclear.
Several small studies have shown that the majority of older patients presenting to oncological services are prescribed multiple medications for treatment of coexisting conditions as well as primary and secondary prevention of disease. A British study of 100 older adults with metastatic cancer reported that patients were prescribed a median of 7 (interquartile range [IQR] 1–17) daily medications [19]. A Canadian study of 112 older adults with newly diagnosed cancer found that patients were prescribed five medications before initiation of systemic anticancer therapy (SACT) [20]. Cancer can result in an altered physiological status that influences the pharmacokinetic handling and pharmacodynamic sensitivity to several drugs, often with requirement for dose adjustment, particularly in the context of renal or hepatic dysfunction. Furthermore, SACTs contribute to additional drug burden in the form of chemotherapy and supportive drugs placing patients at increased risk of additive toxicity, drug‐drug interactions, drug‐disease interactions, and ADRs.
Comprehensive Geriatric Assessment (CGA) is a multidimensional interdisciplinary diagnosis process focused on determining an older person's medical, psychological, and functional capability in order to develop a coordinated and integrated plan for treatment and long‐term follow‐up [21]. A systematic review investigating the role of geriatric assessment in oncology patients found that geriatric assessment in this setting is feasible, takes about 10–45 minutes, and can influence treatment decisions [22]. The American Society of Clinical Oncology (ASCO) now recommends that patients ≥65 years of age receiving chemotherapy have specialist geriatric assessment to identify medical and medication issues that are not captured by regular oncology assessments. In addition, ASCO recommends that geriatric assessment interventions be implemented to manage nononcological problems [23].
Specific chemotherapy‐related ADRs are well described, classified, and risk‐stratified to enable vigilance among patients and clinicians prior to and during treatment [24]. However, the prevalence and impact of multimorbidity, polypharmacy, and non‐SACT‐related ADRs in patients with cancer are less well studied, particularly as contributory factors to acute hospitalization. Therefore, our goal was to determine (a) the prevalence of multimorbidity and medication use in patients attending a specialist cancer center and (b) the prevalence, causality, predictability, and preventability of ADRs causing or contributing to hospitalization.
Materials and Methods
Study Design and Setting
This prospective observational study was conducted over a 12‐month period in two academic teaching hospitals in the Republic of Ireland: Cork University Hospital and Mercy University Hospital. Together, these make up one of eight regional cancer centers in the Republic of Ireland and serve a population of 800,000 people. The study protocol was approved by the local Clinical Research Ethics Committee.
Participants and Eligibility
All patients aged ≥16 years, admitted under the medical or radiation oncology services, were eligible for inclusion. Older adults were defined as ≥70 years of age, and younger adults were defined as <70 years of age. Participants were enrolled from three admission pathways: emergency, elective, and medical oncology day unit attendances. Emergency and elective admissions were reviewed within 72 hours of presentation to hospital, thus enabling inclusion of patients admitted over the weekend. Patients attending the day unit received repeated cycles of SACT over several weeks, with the same patients attending weekly over a defined period. A representative sample of patients attending the day units in each hospital were studied for a 1‐week period to avoid repetition. Patients were not enrolled if they were deemed to be actively dying by the treating physician or if they had participated in the study on a prior admission. Written informed consent to participate was sought from each participant. Consent was obtained from the patient's legal representative in cases in which patients were unable to give consent because of reduced decision‐making ability (e.g., cognitive impairment).
Sample Size Calculation
A sample of 333 patients was required to detect an ADR rate of 7% with a margin of error of 2% at 95% confidence.
Data Collection and Measurements
All participants were interviewed by a single physician researcher. The following data were collected: (a) demographic details, (b) medical comorbidities with associated Cumulative Illness Rating Scale (CIRS) [25], (c) detailed list of medications, (d) functional ability using the Barthel Index [26], (e) cognitive status using the mini‐mental state examination [27], the 4 A's test (4‐AT) [28], and the Diagnostic and Statistical Manual of Mental Disorders V for delirium [29], (f) hematological and biochemical laboratory values, and (g) electrocardiogram. Medication reconciliation was completed using the Structured History taking of Medication use [30] to ensure accurate identification of all medications being consumed by patients. The World Health Organization (WHO) definition of multimorbidity was employed (i.e., the co‐occurrence of two or more chronic medical conditions in one person) [31].
Adverse Drug Reactions: Definition and Process of Identification
The primary outcome measure was the proportion of patients experiencing one or more nontrivial, probable, or certain ADRs causing or contributing significantly to hospital admission. Edwards and Aronson's definition of an ADR was used (i.e., “An appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product”) [32].
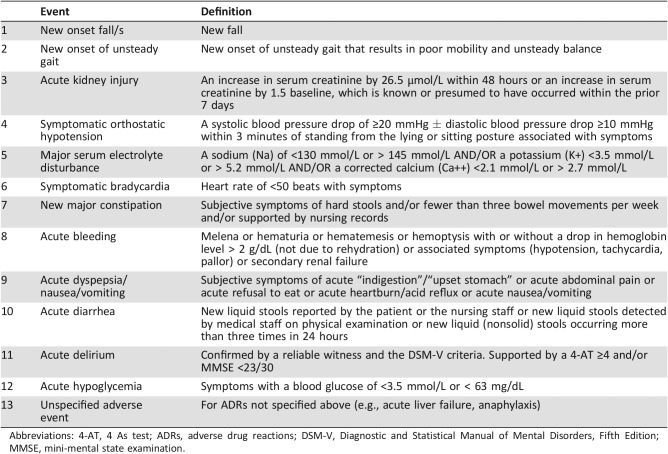
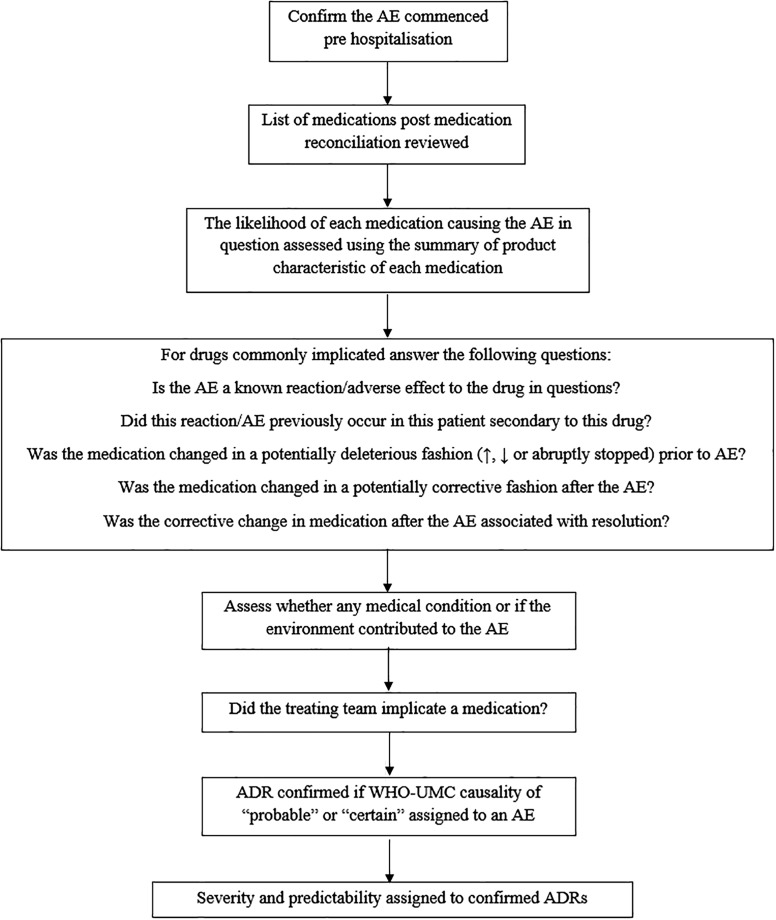
To limit potential bias from selective ADR reporting and to ensure ADRs were not missed, a “trigger list” comprising the 12 most common drug‐related adverse events (AEs) was used [33]. This list was derived from previous studies within our research group and aims to standardize the identification and classification of ADRs [34]. Each potential ADR on this trigger list is initially identified by the occurrence of an AE represented by a clearly defined clinical symptom or syndrome (e.g., a fall, bleeding, delirium, or metabolic disturbance such as hyponatremia or hypoglycemia; Table 1). The identification of such AEs triggers a standardized process of medication review that investigates the precise sequence of clinical events to determine whether a medication caused or contributed to the AE. Figure 1 outlines this process. ADRs not listed in the trigger list were also studied (e.g., anaphylaxis).
Table 1. Adverse event trigger list.
Abbreviations: 4‐AT, 4 As test; ADRs, adverse drug reactions; DSM‐V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; MMSE, mini‐mental state examination.
Figure 1.
Standardized process to determine whether a medication caused or contributed to an AE.
Abbreviations: ADR, adverse drug reaction; AE, adverse event; WHO‐UMC, World Health Organization‐Uppsala Monitoring Centre.
ADR Causality, Severity, Predictability, and Preventability
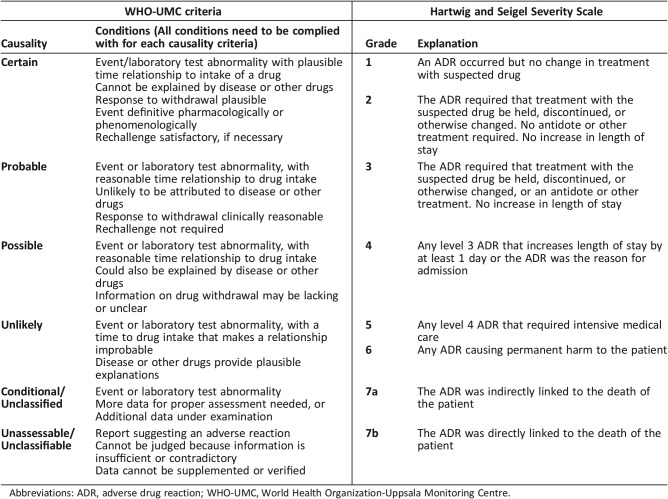
Causality was assessed using the WHO‐Uppsala Monitoring Centre criteria, whereby the likelihood of a drug causing the AE is certain, probable, possible, unlikely, or unrelated [35] (Table 2). ADR severity was assessed using the Hartwig & Seigel scale, in which severity is graded according to clinical consequence on a scale of one to seven [36] (Table 2). A grade 1 ADR requires no medical intervention; a grade 7 ADR represents a fatal event. Preventability was evaluated using Hallas criteria (i.e., definitely avoidable, possibly avoidable, and unavoidable) [37]. ADRs were deemed predictable if they were listed in the relevant summary of product characteristics (SmPCs) as common (≥1% and < 10%) or very common (≥10%) occurrences. The SmPCs of medications were accessed on an online up‐to‐date repository of medications information (www.medicines.ie).
Table 2. WHO‐UMC causality criteria and Hartwig and Siegel Severity Scale.
Abbreviations: ADR, adverse drug reaction; WHO‐UMC, World Health Organization‐Uppsala Monitoring Centre.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics version 22 (IBM, Armonk, NY). Descriptive data included mean and SD for normally distributed variables and median and IQR for nonparametric variables. Differences in the distribution of categorical variables were compared using the Pearson's chi‐square test and continuous variables using the independent t test. The Mann‐Whitney U and Kruskal‐Wallis tests were employed to determine independence of two or more nonparametric variables, respectively. Logistic regression was used to determine the influence of gender, age, medication number, and burden of comorbidity on ADRs. A probability value of <.05 was considered statistically significant.
Results
Baseline Demographics
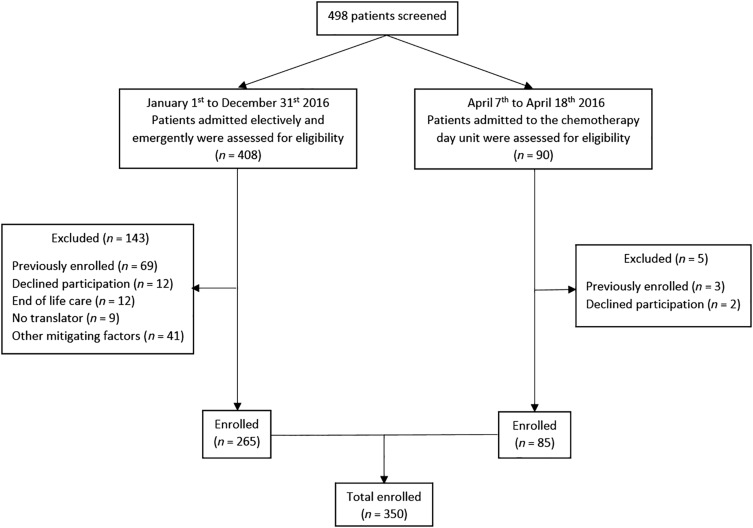
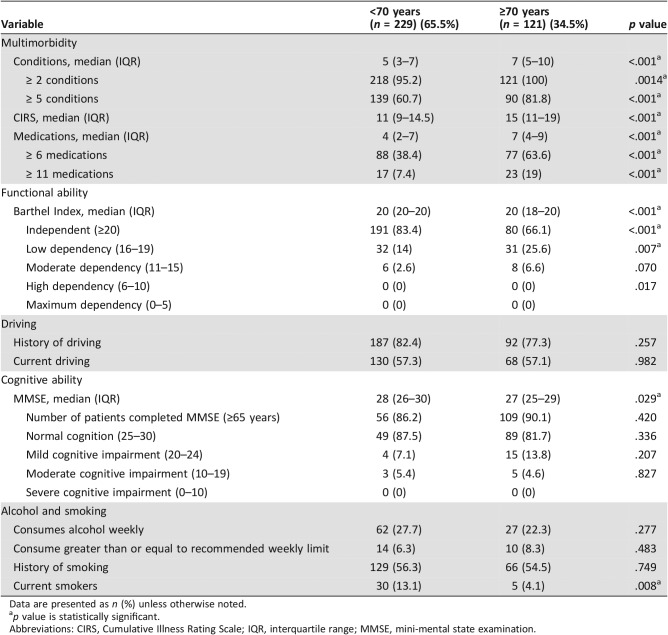
Four hundred and eight oncology inpatients and 90 oncology day unit attendees were screened for study inclusion. In total, 350 patients, comprising 265 hospital inpatients and 85 oncology day unit admissions, enrolled in this study. Of the hospital inpatients, 201 were admitted emergently (57.4% of study population) and 64 electively (18.3% of study sample). Reasons for exclusion are outlined in Figure 2. Baseline characteristics are presented in Table 3. In brief, 183 patients (52.3%) were female, the mean age was 63.6 (SD 12.1) years, range 16–90, with 36.6% aged 70 years or older.
Figure 2.
Participant screening, exclusion, and enrollment.
Table 3. Characteristics of study population according to age category (n = 350).
Data are presented as n (%) unless otherwise noted.
p value is statistically significant.
Abbreviations: CIRS, Cumulative Illness Rating Scale; IQR, interquartile range; MMSE, mini‐mental state examination.
Prevalence of Multimorbidity
Multimorbidity (≥2 chronic conditions) was identified in 96.9% of patients, with 68% having ≥5 conditions. The median number of comorbid conditions in older adults was 7 (IQR 5–10), versus 5 (IQR 3–7) in younger adults. Older adults also had a higher median CIRS score than younger adults (15 [IQR 11–19] vs. 11 [IQR 9–14.5], p < .001).
The most common cancer sites were breast (18.3%), lung (16.6%), and colorectal (13.1%), with lung cancer being more prevalent in older patients (22.3% vs. 13.5%, p = .036), and breast cancer being more prevalent in younger patients (21.4% vs 12.4%, p = .038). The most common non‐cancer diagnoses were hypertension (39.7%), dyslipidemia (38%), and gastroesophageal reflux disease (27.1%). Older adults had a higher prevalence of hypertension (62.8% vs. 27.5%, p < .001), dyslipidemia (51.2% vs. 31%, p < .001), ischemic heart disease (26.4% vs. 8.7%, p < .001), atrial fibrillation (22.3% vs. 4.8%, p < .001), diabetes mellitus (19.8% vs. 10%, p = .011), cardiac failure (5.1% vs. 0.9%, p = .015), osteoarthritis (24% vs. 12.2%, p = .005), and hypothyroidism (18.2% vs. 10.5%, p = .043).
Prescribed Medications
A total of 2,575 medications were prescribed regularly to 326 (93.1%) of 350 patients, and a further 491 medications were prescribed “as required” to 280 (80%) patients. The median number of medications was 5 (IQR 3–8), range 0–24. Polypharmacy (≥6 medications) was identified in 47.1% of patients and major polypharmacy (≥11 medications) in 11.4%. Older patients were prescribed more medications than younger patients (median 7 [IQR 4–9] vs. 4 [IQR 2–7], p < .001). Older patients had higher rates of polypharmacy (63% vs. 38.4%, p < .001) and major polypharmacy (19% vs. 7.4%, p = .001).
Proton pump inhibitors (58.3%), opioids (30.9%), and statins (29.1%) were the most common non‐cancer prescriptions. Older patients were more commonly prescribed antiplatelet therapy (32.2% vs. 11.8%, p < .001), statins (43.8% vs. 21.4%, p < .001), beta blockers (37.2% vs. 16.2%, p < .001), and angiotensin receptor blockers (23.1% vs. 7.9%, p < .001). 5‐fluorouracil (18.2%), doxorubicin (12.4%), and oxaliplatin (12%) were the most commonly prescribed SACTs, with older patients less likely to receive SACTs than younger patients (56.2% vs. 75%, p < .001).
Adverse Events and Adverse Drug Reactions
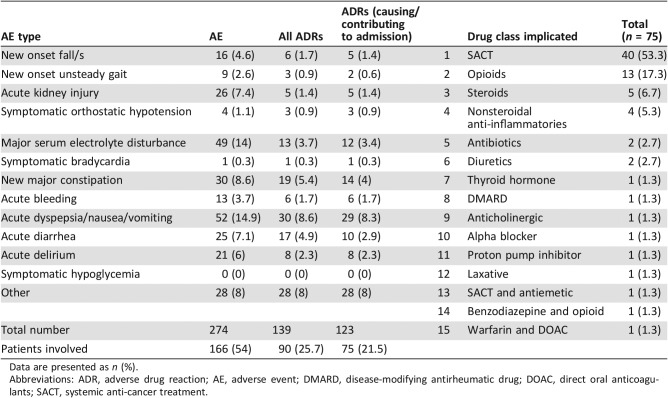
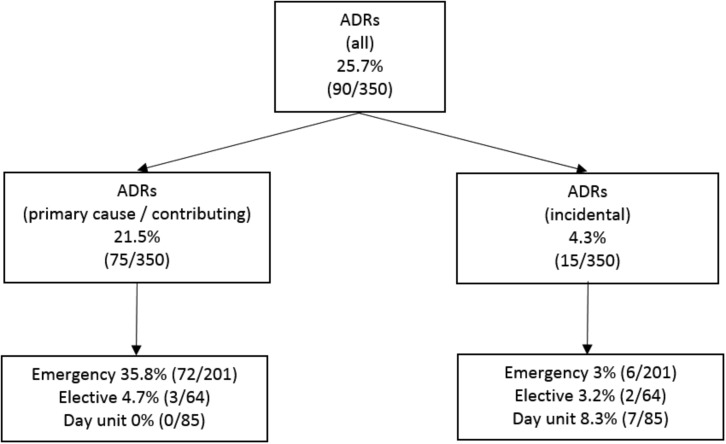
A total of 274 AEs were identified in 166 (47.4%) patients, of which 139 were specifically categorized as ADRs in 90 (25.7%) patients (Table 4). Of these, an ADR caused or contributed significantly to hospitalization in 75 patients (21.5% of the study sample and 28.3% of oncology patients requiring hospitalization); 72 of 201 emergency admissions (35.8%) and 3 of 64 (4.7%) elective admissions were related to ADRs (Fig. 3). ADRs did not cause or contribute significantly to admission in 15 patients. Rather, these were incidental and were categorized as trivial. Clinically significant ADRs were not identified in patients attending the oncology day unit.
Table 4. Adverse events, adverse drug reactions, and causative drug classes.
Data are presented as n (%).
Abbreviations: ADR, adverse drug reaction; AE, adverse event; DMARD, disease‐modifying antirheumatic drug; DOAC, direct oral anticoagulants; SACT, systemic anti‐cancer treatment.
Figure 3.
Distribution and classification of ADRs.
Abbreviation: ADRs, adverse drug reactions.
ADR Causality, Severity, Predictability, and Preventability
The most common ADRs were neutropenia with infection (25.3%), dyspepsia/nausea/vomiting (20%), and constipation (20%). The 12‐point ADR trigger list identified 64% (n = 48) of all ADRs that caused or contributed to hospitalization. Older and younger adults were equally likely to experience the same ADR type (p = .493), as were men and women (p = .142).
An ADR probably or certainly caused or contributed significantly to hospitalization in 75 patients (21.5%). ADRs occurred in similar frequencies in older and younger patients secondary to SACTs (8.3% vs. 13.1%, p = .295), non‐SACTs (10.7% vs. 8.3%, p = .107), and a combination of both (0% vs. 1.3%, p = .240). Females were more likely to experience an ADR secondary to SACT (15.8 vs. 6.6%, p = .005). Males were more likely to experience ADRs secondary to non‐cancer‐specific treatment (10.8 vs. 7.7%, p = .040). Drugs implicated in ADRs are listed in Table 3; the most common were SACTs (n = 40), opioids (n = 13), corticosteroids (n = 5), and nonsteroidal anti‐inflammatories (n = 4).
Of the 75 ADRs, 73 (97.3%) were categorized as the principal reason for admission using the Hartwig and Siegel severity scale (grade 4). Two ADRs (2.7%) required intensive medical input (grade 5). Sixty‐seven ADRs (89.3%) were categorized as predictable. Twenty‐two ADRs (29.3%) were definitely avoidable, 25 (33.3%) possibly avoidable, and 28 (37.4%) unavoidable using Hallas criteria.
ADR Predictor Variables
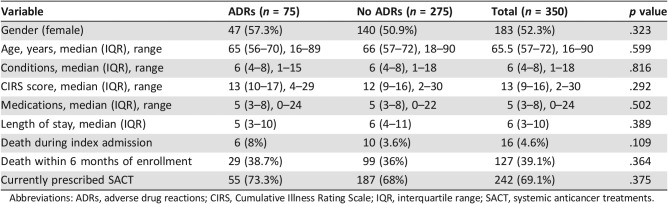
When day unit admissions were removed from the analysis, all of whom had no clinically significant ADR, it was identified that ADRs were more common in patients prescribed SACT than those not (73.3% vs. 54.7%, p = .005). Otherwise, there was no difference between those who experienced ADRs and those who did not (Table 5). No variables were significantly associated with ADRs. Logistic regression examined the influence of age, gender, SACT, number of comorbidities, and number of medications on the risk of ADR. No predictor variables were identified.
Table 5. Univariate analysis of association.
Abbreviations: ADRs, adverse drug reactions; CIRS, Cumulative Illness Rating Scale; IQR, interquartile range; SACT, systemic anticancer treatments.
Discussion
This is the first study to rigorously assess ADRs as a cause of hospitalization in an oncological population using a standardized methodology of evaluating adverse clinical events to determine ADRs.
Cancer is often one of several diagnoses in patients. Accordingly, many experience high levels of polypharmacy and major polypharmacy, putting them at an increased risk of developing ADRs, as identified in this study. Many medications involved in cancer treatment regimens have the potential to worsen other conditions (e.g., corticosteroid use in diabetes mellitus). In addition, some commonly prescribed drugs have the potential to interact with chemotherapy (e.g., 5‐fluorouracil increases the anticoagulant effect of warfarin). During treatment with many chemotherapeutic agents, up to 30% will experience nausea, vomiting, or diarrhea. This can have implications for other medications, specifically antihypertensive agents, which when volume depleted could provoke dizziness and falls. Additionally, ongoing diuretic use in this context could increase the risk of a patient developing an acute kidney injury. Older adults in this study were more likely to have a diagnosis of diabetes mellitus, atrial fibrillation, and hypertension, putting them at a higher risk of such medication‐related adversity.
The vast majority of cancer drugs, as expected, are prescribed by oncologists, but the majority of other drugs are prescribed by patients’ general practitioners (GPs) or other hospital specialists. An extensive knowledge of chemotherapeutic agents and medications required to treat non‐cancer conditions is required to assess risk and adjust medications accordingly. At present, this does not appear to be the sole responsibility of any one doctor. Once a cancer diagnosis is made, patients and other doctors often defer to the treating oncologist for treatment of all ailments, despite many patients having complex non‐cancer multimorbidity. This highlights the need for specialist geriatric assessment, as recommended by ASCO, as it has the potential to identify and manage complex multimorbidity and associated polypharmacy in older patients with cancer, thus impacting treatment decisions and potentially improving outcomes.
Interestingly, many ADRs in patients with cancer in this study were not caused by toxic SACTs, with the most commonly implicated non‐SACT medications being opioids, corticosteroids and NSAIDs, medications that commonly cause ADRs in the general population [9], [24], [28]. Both older and younger adults experienced ADRs at similar frequencies secondary to both SACTs and non‐SACTs, highlighting the need for vigilance for potential ADRs in oncological patients of all ages. The adverse event trigger list used in this study may prove useful in this regard (i.e., if a patient presents with a new clinical event such as a fall, worsening confusion, major bleeding, metabolic disturbance, or major constipation, then this should prompt the treating clinician to evaluate all prescribed medications as being potentially causative). This offers an opportunity to intervene in prescribing practices early and thus either avoid ADRs or improve their recognition so that they can be identified early and adverse consequences minimized or avoided. This could be achieved through specialist geriatric assessment, which encompasses (a) dose adjustment or deprescribing where appropriate because of age‐related and cancer‐related changes in pharmacokinetic and pharmacodynamic responses to commonly prescribed medications and (b) therapeutic goal setting where stringent targets for primary or secondary prevention of illness may no longer be a priority. Deprescribing of potentially futile medications could reduce the risk of potential ADRs in such cases. In the present study, 39.1% of patients died within 6 months of their hospital admission, many of whom continued to receive preventive therapies such as statins during their final months. Deprescribing of such medications could be negatively viewed by patients and clinicians. However, discussions regarding appropriate deprescribing are required so that medication cessation or dose adjustment is not viewed as withdrawal of care but rather prescription optimization to minimize ADRs, drug‐drug, and drug‐disease interactions.
There were some limitations to this study. First, patients with cancer not under the care of an oncology service were excluded. Such patients may be ineligible for SACT or radiotherapy but may also have complex multimorbidity, polypharmacy, and ADRs. Prescribing optimization and review of therapeutic goals in these patients is also warranted. Second, patients could only enroll once in this study. Therefore, prevalence rates of multimorbidity and polypharmacy are accurate; however, it is possible that ADRs on repeat admissions were missed because of this exclusion.
This study highlights the importance of an integrated care approach for patients with cancer, particularly older adults. Older adults may benefit from CGA at the time of cancer diagnosis and during treatment when the risk of drug‐drug and drug‐disease interactions is likely to be at its peak. Such an approach may result in optimization of cognitive and functional abilities and appropriate optimization of medications to include dose adjustment and deprescribing where appropriate. The application of prescribing tools such as STOPP [38], [39] and OncPal [40] could assist in reducing medication burden in a structured fashion and thus prevent ADRs. Medication reviews are an important part of the treatment of cancer, and oncologists, GPs, and other treating physicians need to be educated regarding the potential for medication‐related harm during this high‐risk time.
Conclusion
This study shows that multimorbidity, polypharmacy, and ADRs are highly prevalent in patients with cancer and that ADRs caused by SACT and non‐cancer medications are a significant cause of acute hospitalization in oncology patients, accounting for 35.8% of emergency admissions and affecting 21.5% of the study population. Such patients may benefit from Comprehensive Geriatric Assessment and specialist medication evaluation at this time.
Acknowledgments
The authors wish to acknowledge the support of the Health Research Board Clinical Research Facilities at University College Cork (HRB CRF‐C). The authors also wish to thank the two hospitals, Cork University Hospital and Mercy University Hospital, where this study was undertaken. The study protocol was approved by the Cork Clinical Research Ethics Committee. The authors would also like to thank the funding received from the European Union's Seventh Framework Project (EU FP7, grant number 305930).
Author Contributions
Conception/design: Amanda Hanora Lavan, Deirdre O'Mahony, Denis O'Mahony, Paul Gallagher
Collection and/or assembly of data: Amanda Hanora Lavan
Data analysis and interpretation: Amanda Hanora Lavan, Mary Buckley, Paul Gallagher
Manuscript writing: Amanda Hanora Lavan, Deirdre O'Mahony, Mary Buckley, Denis O'Mahony, Paul Gallagher
Final approval of manuscript: Amanda Hanora Lavan, Deirdre O'Mahony, Mary Buckley, Denis O'Mahony, Paul Gallagher
Disclosures
The authors indicated no financial relationships.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Registry . Cancer projections for Ireland 2015‐2040. Cork, Ireland: National Cancer Registry, 2014.
- 4.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics‐‐2015 update: A report from the American Heart Association. Circulation 2015;131:E29–E322. [DOI] [PubMed] [Google Scholar]
- 5.Finucane C, O'Connell MD, Fan CW et al. Age‐related normative changes in phasic orthostatic blood pressure in a large population study: Findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 2014;130:1780–1796. [DOI] [PubMed] [Google Scholar]
- 6.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med 2002;18:141–158. [DOI] [PubMed] [Google Scholar]
- 7.Dharmarajan K, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin 2017;13:417–426. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel RB, Yin X, Gona P et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd‐Jones D, Adams RJ, Brown TM et al. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 10.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population‐based studies: Systematic review. BMC Public Health 2008;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo Ilaria, Mayer Flavia, Lacorte Eleonora et al. A systematic review and meta‐analysis on the prevalence of dementia in Europe: Estimates from the highest‐quality studies adopting the DSM IV Diagnostic Criteria. J Alzheimers Dis 2018;66:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannucci PM, Nobili A; REPOSE Investigators . Multimorbidity and polypharmacy in the elderly: Lessons from REPOSI. Intern Emerg Med 2014;9:723–734. [DOI] [PubMed] [Google Scholar]
- 13.Bushardt RL, Massey EB, Simpson TW et al. Polypharmacy: Misleading, but manageable. Clin Interv Aging 2008;3:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottenkolber D, Schmiedl S, Rottenkolber M et al. Drug‐induced blood consumption: The impact of adverse drug reactions on demand for blood components in German departments of internal medicine. Basis Clin Pharmacol Toxicol 2012;111:240–247. [DOI] [PubMed] [Google Scholar]
- 15.Cahir C, Fahey T, Teeling M et al. Potentially inappropriate prescribing and cost outcomes for older people: A national population study. Br J Clin Pharmacol 2010;69:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirmohamed M, James S, Meakin S et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004;329:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhawassi TM, Krass I, Bajorek B et al. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging 2014;9:2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta‐analysis of observational studies. Pharm World Sci 2002;24:46–54. [DOI] [PubMed] [Google Scholar]
- 19.Cashman J, Wright J, Ring A. The treatment of co‐morbidities in older patients with metastatic cancer. Support Care Cancer 2010;18:651–655. [DOI] [PubMed] [Google Scholar]
- 20.Puts MT, Costa‐Lima B, Monette J et al. Medication problems in older, newly diagnosed cancer patients in Canada: How common are they? A prospective pilot study. Drugs Aging 2009;26:519–536. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein LZ, Stuck AE, Siu AL et al. Impacts of geriatric evaluation and management programs on defined outcomes: Overview of the evidence. J Am Geriatr Soc 1991;39:8S–16S. [DOI] [PubMed] [Google Scholar]
- 22.Puts MT, Hardt J, Monette J et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst 2012;104:1133–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohile SG, Dale W, Somerfield MR et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 2018;36:2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.British National Formulary . BNF 67: The Authority on the Selection and Use of Medicines, March ‐ September 2014. London, U.K.: Pharmaceutical Press, 2014. [Google Scholar]
- 25.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 28.4AT Rapid Clinical Test for Delirium. Available at www.the4at.com. Accessed January 1, 2018.
- 29.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing, 2013:596. [Google Scholar]
- 30.Drenth‐van Maanen AC, Spee J, van Hensbergen L et al. Structured history taking of medication use reveals iatrogenic harm due to discrepancies in medication histories in hospital and pharmacy records. J Am Geriatr Soc 2011;59:1976–1977. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organisation. The World Health Report 2008 Primary Health care (now more than ever). New York, NY: World Health Organization, 2008:148. [Google Scholar]
- 32.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000;356:1255–1259. [DOI] [PubMed] [Google Scholar]
- 33.Lavan A, Eustace J, Dahly D et al. Incident adverse drug reactions in geriatric inpatients: A multicentred observational study. Ther Adv Drug Saf 2018;9:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor MN, Gallagher P, Byrne S et al. Adverse drug reactions in older patients during hospitalisation: Are they predictable? Age Ageing 2012;41:771–776. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO) , Uppsala Monitoring Centre. The use of the WHO‐UMC system for standardized case causality assessment. Available at https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. Accessed January 1, 2018.
- 36.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229–2232. [PubMed] [Google Scholar]
- 37.Hallas J, Harvald B, Gram LF et al. Drug related hospital admissions: The role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990;228:83–90. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher P, Ryan C, Byrne S et al. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008;46:72–83. [DOI] [PubMed] [Google Scholar]
- 39.O'Mahony D, O'Sullivan D, Byrne S et al. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015;44:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindsay J, Dooley M, Martin J et al. The development and evaluation of an oncological palliative care deprescribing guideline: The ‘OncPal deprescribing guideline’. Support Care Cancer 2015;23:71–78. [DOI] [PubMed] [Google Scholar]