This article evaluates the prognostic impact of different surgical procedures on patients with ipsilateral breast tumor recurrence after breast conserving surgery under real‐world conditions.
Keywords: Breast cancer; The Surveillance, Epidemiology, and End Results (SEER) database; Ipsilateral recurrence; Surgery; Radiation therapy
Abstract
Background.
The benefit of repeat lumpectomy for ipsilateral breast tumor recurrence (IBTR) after breast conserving surgery is currently inconclusive.
Materials and Methods.
Patients with IBTR with definitive surgery were identified in the Surveillance, Epidemiology, and End Results registry between 1973 and 2013. The effect of different IBTR surgeries on overall and cancer‐specific mortality was assessed using risk‐adjusted Cox proportional hazard regression modeling and stratified propensity score‐matching analysis (PSMA).
Results.
Of the 5,098 patients with IBTR, 4,048 (79.4%) women underwent mastectomy and 1,050 (20.1%) underwent repeat lumpectomy. In multivariable Cox regression analysis, repeat lumpectomy was associated with increased overall mortality (hazard ratio for death [HR], 1.522; 95% confidence interval [CI], 1.317–1.759; p < .001) and cancer‐specific mortality (HR, 1.666; 95% CI, 1.319–2.105; p < .001). Similar HRs were derived from the PSMA cohort. However, we found no significant difference in overall mortality for women who underwent repeat lumpectomy followed by radiation therapy (RT) compared with that for those who underwent mastectomy. Moreover, patients with IBTR with small tumors (≤1 cm) who underwent repeat lumpectomy with RT rather than without had similar overall and cancer‐specific survival rates to those who underwent mastectomy.
Conclusion.
Our investigation suggests that compared with mastectomy, repeat lumpectomy for IBTR is associated with higher overall and cancer‐specific mortality under real‐world observational conditions. Furthermore, repeat lumpectomy with RT is equivalent to mastectomy with respect to overall mortality and may influence treatment decision making for patients with small IBTR.
Implications for Practice.
Although mastectomy has been regarded as the standard treatment for ipsilateral breast tumor recurrence (IBTR) after breast conserving surgery, many patients diagnosed with small and early‐detected recurrent tumor might be technically suitable for a less invasive surgical procedure. However, different studies have drawn inconsistent conclusions. The present study is a population‐based analysis, which demonstrated the overall unfavorable impact of repeat lumpectomy over mastectomy on survival outcomes for patients with IBTR. However, patients with small IBTR (≤1 cm) that can tolerate radiation therapy may be the optimal candidates for repeat lumpectomy.
摘要
背景。乳房保留手术后对同侧乳房肿瘤复发 (IBTR) 行重复乳房肿瘤切除术的好处目前尚无定论。
材料和方法。我们通过 1973 至 2013 年的监测、流行病学和最终结果登记表确定了经根治性手术的IBTR患者。通过风险校正 Cox 比例风险回归模型和分层倾向评分配对分析 (PSMA) 评估了不同的IBTR手术对总死亡率和癌症特异性死亡率的影响。
结果。在 5 098 名IBTR患者中,有 4 048 (79.4%) 位女性接受了乳房切除术,1 050 (20.1%) 位女性接受了重复乳房肿瘤切除术。多变量 Cox 回归分析显示,重复乳房肿瘤切除术与总死亡率增加 [死亡风险比 (HR),1.522;95% 置信区间 (CI),1.317–1.759;p < 0.001]和癌症特异性死亡率(HR,1.666;95% CI,1.319–2.105;p < 0.001)增加有关。类似的HR来自PSMA队列。但是,我们发现,在重复乳房肿瘤切除术后接受放射治疗 (RT) 的女性的总死亡率与接受乳房切除术的女性并无明显差异。另外,接受重复乳房肿瘤切除术与RT的小肿瘤 (≤1 cm) IBTR患者的总生存率和癌症特异性生存率与接受乳房切除术的患者相似。
结论。我们的研究表明,与乳房切除术相比,对IBTR行重复乳房肿瘤切除术与真实世界观察条件下的总死亡率和癌症特异性死亡率高有关。另外,重复乳房肿瘤切除术与RT的总死亡率与乳房切除术的总死亡率相当,并且可影响对小IBTR患者的治疗决定。
实践意义:虽然乳房切除术被认为是乳房保留手术后同侧乳房肿瘤复发 (IBTR) 的标准治疗方法,但许多被诊断为早期发现的小复发肿瘤的患者在技术上可能适合接受侵入性较小的手术。但是,不同的研究得出的结论也不一致。本研究是一项基于人群的分析,证实了重复乳房肿瘤切除术与乳房切除术对IBTR患者的总体不利影响。但是,可以耐受放射治疗的小IBTR (≤1 cm) 患者可能最适合重复乳房肿瘤切除术。
Introduction
Breast cancer is the most common malignancy in women worldwide [1]. In recent years, breast conserving surgery (BCS) has been the first choice for more than 60% of primary early‐stage breast cancer cases; compared with mastectomy, BCS provides better cosmetic outcomes and similar long‐term survival rates [2], [3], [4]. Nevertheless, approximately 5%–10% of patients will encounter ipsilateral breast tumor recurrence (IBTR) 10 years after BCS [5].
Mastectomy has been regarded as the standard treatment for IBTR after BCS. However, many patients diagnosed with small and early‐detected IBTR might be technically suitable for a repeat lumpectomy procedure [6]. Therefore, it is important to evaluate whether repeat lumpectomy for IBTR in some cases may provide a prognosis similar to that of mastectomy. However, there has been no published or ongoing randomized clinical trials comparing mastectomy to repeat lumpectomy in patients with IBTR. Thus far, several small‐sample, single institution, retrospective reports concerning the different prognosis between repeat lumpectomy and mastectomy have been published, but they have led to controversial conclusions [7], [8], [9], [10], [11], [12], [13]. Inevitable selection bias may have affected the conclusions of these reports.
For this study, we used the population‐based Surveillance, Epidemiology, and End Results (SEER) database to retrospectively evaluate the prognostic impact of different surgical procedures on patients with IBTR under real‐world conditions. We also sought to study the synergistic effects of radiation therapy (RT) and surgery on local treatment of IBTR and identify patients who may be optimal candidates for repeat lumpectomy.
Materials and Methods
Data Acquisition and Patient Selection
The SEER program is the largest population‐based, publicly available cancer data set that covers approximately 26% of the U.S. population. The SEER registries collect all data on patient demographics, primary tumor site, tumor histopathology, stage at diagnosis, first course of treatment, and follow‐up patients for vital status. Our study cohort was derived from the SEER database (November 2015 submission) by using SEER*Stat software provided by the National Cancer Institute.
Between 1973 and 2013, a total of 1,455,282 female patients (>18 years) were diagnosed as having primary breast cancer. Of these patients, 440,985 with histologically confirmed invasive breast cancer who underwent BCS were included in the initial patient population (Fig. 1). After merging patient‐unique identification numbers, 6,920 patients with same breast affected after primary surgery were included (Fig. 1). Patients with IBTR were excluded if they had skin or chest wall infiltration or metastatic disease or did not undergo recurrent tumor surgery. Overall, we identified 5,098 patients for further analysis. Patients were stratified into a mastectomy subgroup or repeat lumpectomy subgroup (Fig. 1). Baseline characteristics of both subgroups are presented in Table 1. The recurrent interval time was measured from the date of initial surgery to the date of IBTR diagnosis.
Figure 1.
Flow chart for creation of the Surveillance, Epidemiology and End Results (SEER) patient data set.
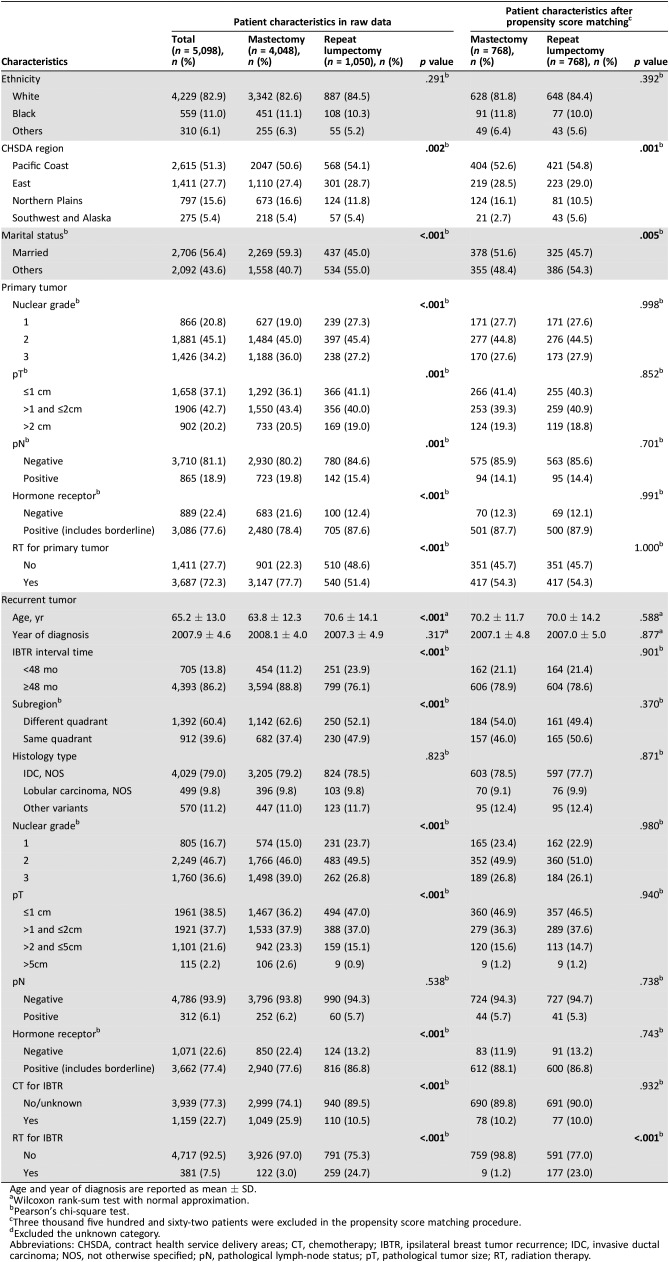
Table 1. Patient characteristics and bias for IBTR surgery.
Age and year of diagnosis are reported as mean ± SD.
Wilcoxon rank‐sum test with normal approximation.
Pearson's chi‐square test.
Three thousand five hundred and sixty‐two patients were excluded in the propensity score matching procedure.
Excluded the unknown category.
Abbreviations: CHSDA, contract health service delivery areas; CT, chemotherapy; IBTR, ipsilateral breast tumor recurrence; IDC, invasive ductal carcinoma; NOS, not otherwise specified; pN, pathological lymph‐node status; pT, pathological tumor size; RT, radiation therapy.
Statistical Analysis
The Wilcoxon rank‐sum test with normal approximation was used to compare continuous factors, such as age and year, between mastectomy and repeat lumpectomy subgroups. Pearson's chi‐square test was used to compare categorical factors.
Overall survival (OS) and breast cancer‐specific survival (BCSS) were plotted using the Kaplan‐Meier method. Cox proportional hazard models were used to compare OS and BCSS between the mastectomy and repeat lumpectomy groups. The p values based on likelihood ratio tests were used to compare patient mortality. The proportional hazard assumption was tested by inspection of hazard ratio (HR) plots.
To further adjust for potential baseline confounders, a propensity score‐matching analysis (PSMA) was carried out using multivariable logistic regression. The adjusted covariates were as follows: ethnicity, primary tumor characteristics (nuclear grade, pathological tumor size [pT], pathological lymph‐node status [pN], hormone receptor status, and RT), and IBTR characteristics (age and year of diagnosis, time interval, subregion, histology, nuclear grade, pT, pN, hormone receptor status, and chemotherapy [CT]). Those selected covariates were considered associated with surgical selection and the prognosis of IBTR, even though some did not show significance in the crude model. The differences in propensity score in each pair were no more than 0.001. After PSMA adjusting, differences in clinical and tumor characteristics between the two groups were compared with a chi‐square test. Cox regression was used to analyze the prognostic value of IBTR surgery types.
A level of p < .05 was used to indicate significance. All statistical tests were two‐tailed. Statistical analysis was performed using SPSS version 23.0 (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics and PSMA
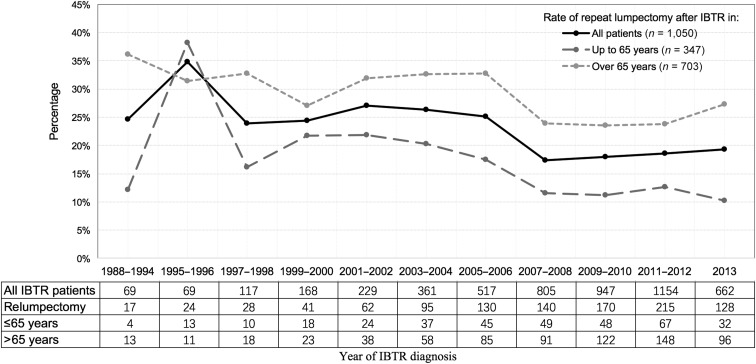
Among the 5,098 women eligible for analysis (Fig. 1), 4,048 (79.4%) patients underwent mastectomy as surgical therapy for IBTR, whereas 1,050 (20.6%) underwent repeat lumpectomy. Although IBTR cases have increased over the years in SEER registries, the percentage of patients undergoing repeat lumpectomy has decreased dramatically (from 34.8% in 1995–1996 to 19.3% in 2013). The patterns were similar between patients ≤65 years of age (from 38.2% in 1995–1996 to 10.3% in 2013) and those >65 years of age (from 31.4% in 1995–1996 to 27.4% in 2013; Fig. 2). Clinicopathological features are listed in Table 1. The median age of the repeat lumpectomy group was 70.6 ± 14.1 years, significantly older than that of the mastectomy group (median age 63.8 ± 12.3 years; p < .001). Compared with patients who underwent a mastectomy, patients who underwent a repeat lumpectomy were more likely to live in the Pacific Coast region (54.1% vs. 50.6%), be unmarried (55.0% vs. 40.7%), have a smaller primary tumor size (41.2% vs. 36.2% for ≤1 cm), and have a higher rate of primary RT omission (48.6% vs. 22.3%; Table 1). In addition, patients with IBTR who received repeat lumpectomy had earlier (23.9% vs. 11.2% for interval times <48 months) and more same quadrant (47.9% vs. 37.4%) recurrence, better differentiation (23.7% vs. 15% for well‐differentiated), and smaller recurrent tumor (47% vs. 36.2% for ≤1 cm) but less chemotherapy (10.5% vs. 25.9%) than did those who underwent mastectomy (Table 1). More repeat lumpectomy procedures were performed in patients with hormone receptor‐positive IBTR (Table 1). A minority of each group (24.7% of those undergoing repeat lumpectomy and 3% of the mastectomy group) underwent RT after surgery (Table 1).
Figure 2.
Trend for repeat lumpectomy after IBTR from 1988 to 2013.
Abbreviation: IBTR, ipsilateral breast tumor recurrence.
PSMA was performed as described previously to account for potential bias due to an imbalance between the repeat lumpectomy and mastectomy groups. After the matching procedure, 3,562 patients (282 patients in the repeat lumpectomy group, 3,280 in the mastectomy group) who lacked a propensity score match were excluded. By PSMA, imbalance across the two patient groups could be avoided for most parameters, except contract health service delivery areas region, marital status, and RT for IBTR (Table 1).
Different IBTR Surgical Types and Other Risk Factors for IBTR Survival
Median follow‐up duration of repeat lumpectomy and mastectomy subcohorts was 3.5 years and 3.6 years, respectively, with maximums of 21.9 years and 25 years. The prognostic impact based on different types of surgery for IBTR is outlined in Fig. 3. Kaplan‐Meier analysis demonstrated that patients who underwent a repeat lumpectomy tended to have worse OS (p < .001; Fig. 3A) and BCSS (p = .001; Fig. 3B). After adjusting for clinical and tumor characteristics, compared with the mastectomy group, the repeat lumpectomy group had significantly decreased OS (hazard ratio for death [HR], 1.522; 95% CI, 1.317–1.759; p < .001) and BCSS (HR, 1.666; 95% CI, 1.319–2.105; p < .001; Table 2).
Figure 3.
Unadjusted overall and cancer‐specific survival analysis in patients with IBTR. Repeat lumpectomy compared with mastectomy in the whole cohort in terms of (A) overall survival and (B) cancer‐specific survival. Repeat lumpectomy compared with mastectomy in the propensity score‐matching cohort in terms of (C) overall survival and (D) cancer‐specific survival.
Abbreviation: IBTR, ipsilateral breast tumor recurrence.
Table 2. Prognostic factors for overall and cancer‐specific mortality of patients with IBTR.
Hazard ratios (HR) with 95% confidence intervals.
Univariable Cox regression analysis.
Multivariable Cox regression with adjustment for ethnicity, primary tumor characteristics (nuclear grade, pT, hormone receptor and RT) and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, CT and different surgical types).
Multivariable Cox regression with adjustment for ethnicity, primary tumor characteristics (nuclear grade, pT, pN, hormone receptor and RT) and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, CT, RT and different surgical types).
Likelihood ratio tests.
Abbreviations: CI, confidence interval; CT, chemotherapy; HR, hazard ratio; IBTR, ipsilateral breast tumor recurrence; IBTR, ipsilateral breast tumor recurrence; IDC, invasive ductal carcinoma; NOS, not otherwise specified; pN, pathological lymph‐node status; pT, pathological tumor size; RT, radiation therapy.
For the PSMA patient cohort, both OS and BCSS were worse in the repeat lumpectomy group than in the mastectomy group (p = .007 and p = .004, respectively; Fig. 3C, 3D). In the Cox regression model after PSMA, the repeat lumpectomy group was still associated with poorer OS (HR, 1.505; 95% CI, 1.233–1.838; p < .001) and BCSS (HR, 1.787; 95% CI, 1.250–2.555; p = .001; Table 2) relative to the mastectomy group.
Other than the use of repeat lumpectomy, poor prognostic factors in multivariable Cox regression and the PSMA cohort included older age, early diagnosis year, larger primary or recurrent tumor, higher nuclear grade, and hormone receptor‐negative IBTR (Table 2). Shorter interval time is associated with worse OS and BCSS in multivariable Cox regression cohort (p = .001 for both, Table 2). However, the recurrent subregion, CT and RT for IBTR are not associated with patients’ survival (Table 2).
RT Impacts the Survival of Patients with IBTR
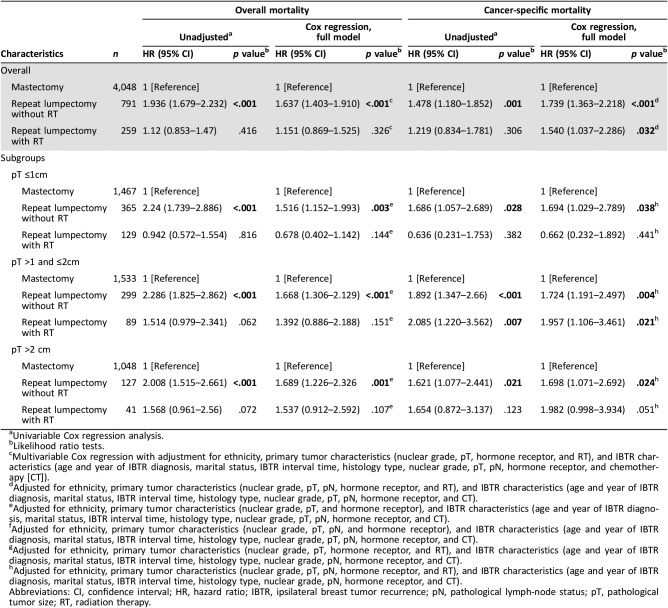
To clarify the potential role of RT in patients with IBTR, we divided our study cohort into three groups: mastectomy (4,048 patients, 79.4%), repeat lumpectomy without RT (791 patients, 15.5%), and repeat lumpectomy with RT (259 patients, 5.1%). In multivariable Cox regression analysis, the omission of RT after repeat lumpectomy demonstrated worse OS (HR, 1.637; 95% CI, 1.403–1.91; p < .001) and BCSS (HR, 1.739; 95% CI, 1.363–2.218; p < .001; Table 3) than did mastectomy. Interestingly, compared with mastectomy, repeat lumpectomy with RT was slightly associated with worse BCSS (HR, 1.54; 95% CI, 1.037–2.286; p = .032; Table 3). In contrast, no significant difference of OS was found between the repeat lumpectomy with RT and mastectomy groups (p = .326; Table 3). Moreover, irrespective of whether RT was performed for primary BCS, repeat lumpectomy with RT achieved similar OS and BCSS to those of mastectomy in patients with IBTR (Table 3).
Table 3. The impact of different local treatments after IBTR on overall and cancer‐specific mortality of patients with IBTR.
Univariable Cox regression analysis.
Likelihood ratio tests.
Multivariable Cox regression with adjustment for ethnicity, primary tumor characteristics (nuclear grade, pT, hormone receptor, and RT), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, and chemotherapy [CT]).
Adjusted for ethnicity, primary tumor characteristics (nuclear grade, pT, pN, hormone receptor, and RT), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, and CT).
Adjusted for ethnicity, primary tumor characteristics (nuclear grade, pT, and hormone receptor), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, and CT).
Adjusted for ethnicity, primary tumor characteristics (nuclear grade, pT, pN, and hormone receptor), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pT, pN, hormone receptor, and CT).
Adjusted for ethnicity, primary tumor characteristics (nuclear grade, pT, hormone receptor, and RT), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pN, hormone receptor, and CT).
Adjusted for ethnicity, primary tumor characteristics (nuclear grade, pT, pN, hormone receptor, and RT), and IBTR characteristics (age and year of IBTR diagnosis, marital status, IBTR interval time, histology type, nuclear grade, pN, hormone receptor, and CT).
Abbreviations: CI, confidence interval; HR, hazard ratio; IBTR, ipsilateral breast tumor recurrence; pN, pathological lymph‐node status; pT, pathological tumor size; RT, radiation therapy.
Generally, recurrent tumors (79.9%) were smaller than 2 cm in our study cohort, with 1,961 (38.5%) of IBTRs being ≤1 cm. Subgroup analysis was carried out according to pT of IBTR, with the aim of finding patients who could benefit more from repeat lumpectomy rather than mastectomy. Multivariable Cox regression analysis among all pT subgroups revealed that patients who underwent repeat lumpectomy without RT had higher overall and cancer‐specific mortality than did those who underwent mastectomy (Table 3). However, there was no significant difference in terms of overall and cancer‐specific mortality between repeat lumpectomy with RT and mastectomy in patients with recurrent tumors ≤1 cm (p = .144 and p = .441, respectively; Table 3).
Discussion
The present study is the first population‐based analysis using both multivariable Cox regression and PSMA models to assess the impact of different types of surgery (repeat lumpectomy and mastectomy) for IBTR patients. Our investigation suggests that repeat lumpectomy for IBTR would increase overall and cancer‐specific mortality in both models under real‐world observational conditions.
Because of widespread use of BCS, advancements in systemic therapy, and standardized postoperative follow‐up, the opportunity for small and isolated IBTR diagnosis have increased [14]. The question then arises as to whether repeat lumpectomy can achieve the same prognosis as mastectomy. Indeed, the incidence of IBTR is still not high enough to conduct a randomized comparison of repeat lumpectomy and mastectomy for IBTR, and only limited research concerning the outcomes of different surgical approaches has been published [7], [8], [9], [10], [11], [12], [13]. Some studies showed no significant difference in prognosis between repeat lumpectomy and mastectomy for IBTR, which was inconsistent with our investigation [7], [8], [9], [10], [11], [15]. The reasons for controversial conclusions vary among the different studies. The most important reason may be the lack of adjustment for potential confounders in these studies to reduce selection bias between the repeat lumpectomy and mastectomy groups. For example, patients treated with repeat lumpectomy procedures tend to have better prognostic variables than patients that undergo mastectomy. To precluded selection bias, Yoshida et al. [11] first introduced PSMA in their study. Based on a small sample, they found no difference in terms of survival between repeat lumpectomy and mastectomy after IBTR. However, our analysis of a large survival cohort of over 5,000 patients with IBTR demonstrated an unfavorable prognostic impact when comparing repeat lumpectomy with mastectomy, which is in concordance with several published retrospective studies [12], [13]. Although patients with IBTR who underwent repeat lumpectomy had smaller recurrent tumors (47% vs. 36.2% for ≤1 cm IBTR) and better differentiation (23.7% vs. 15% for well differentiated) than did those who underwent mastectomy, unfavorable effects on prognosis were found in both unadjusted and multivariable Cox regression models. Moreover, we carried out the PSMA to further adjust for potential baseline confounders and found that the repeat lumpectomy group was associated with worse OS and BCSS relative to the mastectomy group (Table 2).
Many studies have suggested that an additional course of RT is required for patients with IBTR who undergo a repeat lumpectomy to achieve better local disease control [16], [17], [18], [19], [20], [21], [22], [23]. Recently, the GEC‐ESTRO working group published a retrospective study of 217 patients with IBTR treated with repeat lumpectomy plus RT in eight European institutions between 2000 and 2009 [23]. The study demonstrated that patients who received repeat lumpectomy plus RT achieved comparable 5‐ and 10‐year DFS and OS to those who underwent a mastectomy. It is commonly accepted that a second full‐dose course of whole breast radiotherapy therapy after repeat lumpectomy cannot be tolerated by the tissues and would lead to unacceptable levels of toxicities and poor cosmetic result [6]. However, several published articles demonstrate that accelerated partial breast irradiation after repeat lumpectomy is safe and feasible and can achieve acceptable skin, fibrosis, and breast pain toxicity, as well as satisfactory cosmetic results [18], [24], [25]. Despite the importance and safety of RT for IBTR, we were surprised that the majority of patients (75.3%) did not receive RT after repeat lumpectomy in our real‐world observational study. Thus, we divided the repeat lumpectomy group into two subgroups based upon whether they received RT for IBTR. The omission of RT after repeat lumpectomy was associated with worse survival in both unadjusted and multivariable Cox regression models. In contrast, compared with those underwent mastectomy, patients with IBTR who underwent repeat lumpectomy with RT achieved worse BCSS but similar OS. Furthermore, irrespective of whether RT has been performed after primary BCS or not, repeat lumpectomy with RT can achieve similar OS and BCSS to mastectomy for patients with IBTR. Therefore, we considered that the RT approach may be an important impact factor in different survival outcomes for the mastectomy and repeat lumpectomy groups. Thus, additional RT is recommended with repeat lumpectomy, regardless of whether patients have been treated with RT before.
It is well established that patients with IBTR can benefit from mastectomy; however, several studies have tried to identify suitable patients for repeat lumpectomy [26], [27], [28]. Recurrence interval after primary BCS is one of the most important factors that affect surgical decision making for IBTR. In 2012, the German Guideline Program in Oncology suggested that repeat lumpectomy may be considered in patients with ductal carcinoma in situ or invasive breast cancer with a long recurrence‐free interval and no skin infiltration [29]. The reason for the guideline is that IBTR with a long recurrence‐free interval (late recurrence) is usually considered as a new primary tumor, which is more likely to appear away from previous lumpectomy site and implies a favorable prognosis [30]. In our study, late recurrence (interval time ≥48 months) is indeed an independent factor for predicting outcomes for patients with IBTR in both multivariate Cox regression analysis and PSMA models, except for BCSS in the PSMA cohort. However, patients with IBTR with early or tumor‐bed (same quadrant) recurrence were more likely to receive a repeat lumpectomy in our real‐world observational study. The cause of this phenomenon may be that the omission of RT for primary BCS or previous lumpectomy site recurrence provides surgical conditions for repeat lumpectomy in patients with earlier recurrence.
Tumor size may be another important factor that affects which type of surgery is performed [27]. In our study, recurrent tumor size is associated with outcome in patients with IBTR, and as the tumor size increases, HRs of both overall and cancer‐specific mortality increase in the multivariate Cox analysis model and PSMA cohort. To identify patients with IBTR who are suitable for repeat lumpectomy, we performed subgroup analysis based on IBTR tumor size. Among patients with IBTR tumor size <1 cm, there was no significant difference in overall and cancer‐specific mortality between repeat lumpectomy in the RT and mastectomy groups. However, repeat lumpectomy without RT had increases in both overall and cancer‐specific mortality in subgroups of patients with different IBTR tumor sizes even in pT <1 cm (HR, 1.516 and 1.694, respectively).
We would like to acknowledge some limitations of our study. A major limitation of this study is that the SEER database does not include definite information about recurrence. To identify IBTR events, we choose patients with two or more registered entries and with the same breast affected after primary surgery [31], [32]. Moreover, given the limited resolution of the SEER registry database, a bias due to the imbalance of the repeat lumpectomy group compared with the mastectomy group cannot be excluded. For instance, patients who received repeat lumpectomy (70.6 years) were significantly older than those who received mastectomy (63.8 years) in our population‐based observational study. In addition, patients with IBTR who underwent a repeat lumpectomy were more likely to be hormone receptor positive (86.8% vs. 77.6%). However, we carried out both multivariable Cox regression analysis and additional PSMA to reduce potential confounding. Furthermore, only known prognostic factors could be included in multivariable analysis. Some unidentified clinical and treatment‐level variables, including physical status, single or multifocal tumors, and receipt of endocrine or HER2‐targeted therapy were missing, which might have contributed to the observed results.
Conclusion
The present study supports the unfavorable impact of repeat lumpectomy over mastectomy on survival outcomes of patients with IBTR under real‐world observational conditions. Most importantly, we emphasize the importance of RT after repeat lumpectomy for patients with IBTR. Patients with small (≤1 cm) IBTR that can tolerate RT are the optimal candidates for repeat lumpectomy. Although further results of randomized studies are needed, patients should be fully informed about the benefits and risks of different surgical approaches according to the currently available evidence, and treatment decision making may require the multidisciplinary input of clinicians from different specialties.
Acknowledgments
This work was supported by the Shenkang center city hospital emerging frontier technology joint research project (SHDC12015119).
Contributed equally.
Author Contributions
Conception/design: Yonghui Su, Rong Guo, Jiong Wu
Provision of study material or patients: Yonghui Su, Rong Guo, Jingyan Xue
Collection and/or assembly of data: Yonghui Su, Weiru Chi, Jia Wang
Data analysis and interpretation: Jingyan Xue, Yayun Chi, Benlong Yang
Manuscript writing: Yonghui Su
Final approval of manuscript: Yonghui Su, Rong Guo, Jingyan Xue, Yayun Chi, Weiru Chi, Jia Wang, Benglong Yang, and Jiong Wu
Disclosures
The authors indicated no financial relationships.
References
- 1.International Agency For Research On Cancer . Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed February 1, 2017.
- 2.Veronesi U, Cascinelli N, Mariani L et al. Twenty‐year follow‐up of a randomized study comparing breast‐conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 3.van Maaren MC, de Munck L, de Bock GH et al. 10 year survival after breast‐conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the netherlands: A population‐based study. Lancet Oncol 2016;17:1158–1170. [DOI] [PubMed] [Google Scholar]
- 4.Kummerow KL, Du L, Penson DF et al. Nationwide trends in mastectomy for early‐stage breast cancer. JAMA Surg 2015;150:9–16. [DOI] [PubMed] [Google Scholar]
- 5.Wapnir IL, Anderson SJ, Mamounas EP et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast And Bowel Project node‐positive adjuvant breast cancer trials. J Clin Oncol 2006;24:2028–2037. [DOI] [PubMed] [Google Scholar]
- 6.Burger AE, Pain SJ, Peley G. Treatment of recurrent breast cancer following breast conserving surgery. Breast J 2013;19:310–318. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz JM, Amalric R, Brandone H et al. Local recurrence after breast‐conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer 1989;63:1912–1917. [DOI] [PubMed] [Google Scholar]
- 8.Fodor J, Major T, Polgár C et al. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early‐stage invasive breast cancer. Breast 2008;17:302–308. [DOI] [PubMed] [Google Scholar]
- 9.Alpert TE, Kuerer HM, Arthur DW et al. Ipsilateral breast tumor recurrence after breast conservation therapy: Outcomes of salvage mastectomy vs. salvage breast‐conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys 2005;63:845–851. [DOI] [PubMed] [Google Scholar]
- 10.Salvadori B, Marubini E, Miceli R et al. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg 1999;86:84–87. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida A, Takahashi O, Okumura Y et al. Prognosis after mastectomy versus repeat lumpectomy in patients with ipsilateral breast cancer recurrence: A propensity score analysis. Eur J Surg Oncol 2016;42:474–480. [DOI] [PubMed] [Google Scholar]
- 12.Galper S, Blood E, Gelman R et al. Prognosis after local recurrence after conservative surgery and radiation for early‐stage breast cancer. Int J Radiat Oncol Biol Phys 2005;61:348–357. [DOI] [PubMed] [Google Scholar]
- 13.Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg 2008;196:495–499. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative G , Peto R, Davies C et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100 000 women in 123 randomised trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komoike Y, Akiyama F, Iino Y et al. Analysis of ipsilateral breast tumor recurrences after breast‐conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer 2005;12:104–111. [DOI] [PubMed] [Google Scholar]
- 16.Mullen EE, Deutsch M, Bloomer WD. Salvage radiotherapy for local failures of lumpectomy and breast irradiation. Radiother Oncol 1997;42:25–29. [DOI] [PubMed] [Google Scholar]
- 17.Deutsch M. Repeat high‐dose external beam irradiation for in‐breast tumor recurrence after previous lumpectomy and whole breast irradiation. Int J Radiat Oncol Biol Phys 2002;53:687–691. [DOI] [PubMed] [Google Scholar]
- 18.Guix B, Lejárcegui JA, Tello JI et al. Exeresis and brachytherapy as salvage treatment for local recurrence after conservative treatment for breast cancer: Results of a ten‐year pilot study. Int J Radiat Oncol Biol Phys 2010;78:804–810. [DOI] [PubMed] [Google Scholar]
- 19.Njeh CF, Saunders MW, Langton CM. Accelerated partial breast irradiation (APBI): A review of available techniques. Radiat Oncol 2010;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannoun‐Levi JM, Castelli J, Plesu A et al. Second conservative treatment for ipsilateral breast cancer recurrence using high‐dose rate interstitial brachytherapy: Preliminary clinical results and evaluation of patient satisfaction. Brachytherapy 2011;10:171–177. [DOI] [PubMed] [Google Scholar]
- 21.Kauer‐Dorner D, Pötter R, Resch A et al. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: Alternative to mastectomy? Results from a prospective trial. Radiother Oncol 2012;102:96–101. [DOI] [PubMed] [Google Scholar]
- 22.Shah C, Wilkinson JB, Jawad M et al. Outcome after ipsilateral breast tumor recurrence in patients with early‐stage breast cancer treated with accelerated partial breast irradiation. Clin Breast Cancer 2012;12:392–397. [DOI] [PubMed] [Google Scholar]
- 23.Hannoun‐Levi JM, Resch A, Gal J et al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: Multicentric study of the GEC‐ESTRO Breast Cancer Working Group. Radiother Oncol 2013;108:226–231. [DOI] [PubMed] [Google Scholar]
- 24.Arthur DW, Winter KA, Kuerer HM et al. NRG oncology‐radiation therapy oncology group study 1014: 1‐year toxicity report from a phase 2 study of repeat breast‐preserving surgery and 3‐dimensional conformal partial‐breast reirradiation for in‐breast recurrence. Int J Radiat Oncol Biol Phys 2017;98:1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chadha M, Feldman S, Boolbol S et al. The feasibility of a second lumpectomy and breast brachytherapy for localized cancer in a breast previously treated with lumpectomy and radiation therapy for breast cancer. Brachytherapy 2008;7:22–28. [DOI] [PubMed] [Google Scholar]
- 26.Gentilini O, Botteri E, Rotmensz N et al. When can a second conservative approach be considered for ipsilateral breast tumour recurrence? Ann Oncol 2007;18:468–472. [DOI] [PubMed] [Google Scholar]
- 27.Gentilini O, Botteri E, Veronesi P et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: Criteria for selecting the best candidates. Ann Surg Oncol 2012;19:3771–3776. [DOI] [PubMed] [Google Scholar]
- 28.Vila J, Garcia‐Etienne CA, Vavassori A et al. Conservative surgery for ipsilateral breast tumor recurrence. J Surg Oncol 2014;110:62–67. [DOI] [PubMed] [Google Scholar]
- 29.Harms W, Budach W, Dunst J et al. DEGRO practical guidelines for radiotherapy of breast cancer VI: Therapy of locoregional breast cancer recurrences. Strahlenther Onkol 2016;192:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panet‐Raymond V, Truong PT, McDonald RE et al. True recurrence versus new primary: An analysis of ipsilateral breast tumor recurrences after breast‐conserving therapy. Int J Radiat Oncol Biol Phys 2011;81:409–417. [DOI] [PubMed] [Google Scholar]
- 31.Yi M, Giordano SH, Meric‐Bernstam F et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node‐positive breast cancer patients: Experience from the SEER database. Ann Surg Oncol 2010;17(suppl 3):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poleszczuk J, Luddy K, Chen L et al. Neoadjuvant radiotherapy of early‐stage breast cancer and long‐term disease‐free survival. Breast Cancer Res 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]