This article reports an Italian observational retrospective study conducted on the use of lenalidomide in everyday clinical practice to determine if clinical trial results are confirmed in real‐life situations.
Keywords: Lenalidomide, Diffuse large B‐cell lymphoma, Relapsed, Refractory, Real life
Abstract
Background.
Diffuse large B‐cell lymphoma (DLBCL) is the most common non‐Hodgkin lymphoma subtype, and approximately 50% of the patients are >60 years of age. Patients with relapsed/refractory (rr) disease have a poor prognosis with currently available treatments. Lenalidomide is available in Italy for patients with rrDLBCL based on a local disposition of the Italian Drug Agency.
Subjects, Materials, and Methods.
An observational retrospective study was conducted in 24 Italian hematology centers with the aim to improve information on effectiveness and safety of lenalidomide use for rrDLBCL in real practice.
Results.
One hundred fifty‐three patients received lenalidomide for 21/28 days with a median of four cycles. At the end of therapy, there were 36 complete responses (23.5%) and 9 partial responses with an overall response rate (ORR) of 29.4%. In the elderly (>65 years) subset, the ORR was 33.6%. With a median follow‐up of 36 months, median overall survival was reached at 12 months and median disease‐free survival was not reached at 62 months. At the latest available follow‐up, 29 patients are still in response out of therapy. Median progression‐free survivals differ significantly according to age (2.5 months vs. 9.5 in the younger vs. elderly group, respectively) and to disease status at the latest previous therapy (15 months for relapsed patients vs. 3.5 for refractory subjects). Toxicities were manageable, even if 30 of them led to an early drug discontinuation.
Conclusion.
Lenalidomide therapy for patients with rrDLBCL is effective and tolerable even in a real‐life context, especially for elderly patients.
Implications for Practice.
Diffuse large B‐cell lymphoma (DLBCL) is the most common subtype of non‐Hodgkin lymphoma, and approximately 50% of the patients are >60 years of age. Patients with relapsed/refractory (rr) disease have a poor prognosis, reflected by the remarkably short life expectancy of 12 months with currently available treatments. The rrDLBCL therapeutic algorithm is not so well established because data in the everyday clinical practice are still poor. Lenalidomide for patients with rrDLBCL is effective and tolerable even in a real‐life context, especially for elderly patients.
Introduction
Diffuse large B‐cell lymphoma (DLBCL) is the most common non‐Hodgkin lymphoma (NHL) subtype; DLBCL is a heterogeneous malignancy that comprises multiple subtypes based on cell‐of‐origin (COO) with differences in terms of clinical presentation, prognosis, and treatment response [1], [2]. Germinal center B cell (GCB) and non‐GCB subtypes can be distinguished using immunohistochemistry [3]. The incidence of DLBCL increases with age [4]. Approximately 50% of the patients with DLBCL are older than 60 years. The main characteristic of DLBCL is the abnormal malignant growth of B cells. DLBCL develops either in the lymph nodes or outside the lymphatic system, including bone marrow, spleen, and thymus [4]. Typically, DLBCL requires an immediate therapeutic approach because of the rapid progression of the disease, and treatment is given with curative intent.
Currently, the most common frontline treatment for DLBCL is R‐CHOP, a regimen that consists of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. In fact, clinical trials have shown that the addition of rituximab to the CHOP regimen significantly improves patients’ outcomes. For 50%–60% of patients, R‐CHOP is curative and thus sufficiently effective. Patients with DLBCL who are event free at 24 months generally have a subsequent overall survival (OS) equivalent to that of the age‐ and sex‐matched general population, although few relapses (<10%) can still occur after 24 months [5]. However, the relapsing rate in DLBCL is between 30% and 40% in the first 2 years after the end of first line, and nearly 10% of patients display primary refractory disease [6]. Unfortunately, relapsed and refractory disease remains a major cause of morbidity and mortality in patients with DLBCL [7]. Patients with relapsed/refractory disease have a poor prognosis with currently available treatments, reflected by the remarkably short life expectancy of 12 months [6].
Second‐line therapy is usually a salvage one with multiagent immunochemotherapy, followed in responder patients by consolidation with high dose chemotherapy and autologous stem cell transplantation (auto‐HSCT). Third and later lines are usually chemo‐salvage or palliative care or allogeneic transplantation (allo‐HSCT). Allo‐HSCT is reserved to a small proportion of high‐risk patients. Typically, complete response (CR) or near CR obtained with salvage chemotherapy is needed for patients before proceeding to the high‐dose chemotherapy. Nevertheless, many patients with relapsed/refractory disease are ineligible for transplantation because of age, advanced disease stage, comorbidities, and/or inadequate response to salvage chemotherapy. The SCHOLAR‐1 study, a multicohort retrospective NHL research conducted in 636 patients with refractory DLBCL, showed an objective response rate of 26% (CR 7%) to the next line of therapy with a median overall survival of 6.3 months [8]. At relapse, for patients who are unable to undergo transplant, there is no accepted standard of care, which may include R‐chemo combinations (such as gemcitabine‐containing regimen or bendamustine), or novel agents in a clinical trial context [9], [10]. Often, the outcome is poor and the chances to achieve disease control are highly unlikely [7], even if encouraging results have been reported in a phase I study with chimeric antigen receptor T cells [11].
Currently, no agents are approved for relapsed/refractory DLBCL by the U.S. Food and Drug Administration, and the European Medicines Agency has granted conditional approval for pixantrone in multiply relapsed/refractory NHL [12].
Lenalidomide is an oral immunomodulator with direct antineoplastic activity and immunologic effects, including blocking tumor cell proliferation and angiogenesis, and stimulating T‐cell‐ and natural killer cell‐mediated cytotoxicity in experimental models [13], [14].
Patients with relapsed/refractory DLBCL have achieved 25%–35% overall response rate (ORR) with lenalidomide monotherapy [15], [16], [17]. A retrospective analysis reported clinical benefit from single‐agent lenalidomide in relapsed/refractory DLBCL (ORR 28%), with preferential activity in patients with non‐GCB versus GCB disease (ORR 53% vs. 9%, respectively, p = .006) [15]. Recently, Czuczman and colleagues designed a randomized study using COO‐based subtyping to elucidate differences in activity of lenalidomide over salvage monotherapy (investigator's choice) in relapsed/refractory DLBCL [18]. Lenalidomide elicited longer progression‐free survival (PFS) in non‐GCB patients (most pronounced in an activated B‐cell–like designated patients).
Since May 2011, lenalidomide has been available in Italy for patients with relapsed/refractory DLBCL managed in the real‐life context, based on a local disposition of the Agenzia Italiana del Farmaco issued according to a national law (Law 648/96: “medicinal products that are provided free of charge on the national health service”). Thus, a large Italian observational retrospective study was conducted on the use of lenalidomide in the everyday clinical practice to check if clinical trial results are confirmed even in a real‐life context.
Subjects, Materials, and Methods
An observational retrospective study was conducted among patients with relapsed/refractory DLBCL treated with lenalidomide in 24 Italian centers outside of a clinical trial context. The study was approved by our institutional board (Azienda Ospedaliera di Bologna, Policlinico S.Orsola‐Malpighi, coordinating center) and by all involved Ethical Committees and registered in the Italian Registry of Observational Studies. All participants gave written informed consent in accordance with the Declaration of Helsinki. A shared database was used after the approval of all the authors, and variables were strictly defined to avoid bias in reporting data.
From May 2011 to January 2015, 153 patients were treated with lenalidomide monotherapy according to the Law 648/96. Patients received a starting dose of 10 or 15 or 20 or 25 mg/day of lenalidomide for 21 days of a 28‐day cycle until disease progression or relapse; the initial dosing and dose adjustments were at physician's discretion.
The primary endpoint of the study was the ORR; the secondary endpoints were OS, PFS, disease‐free survival (DFS), and the safety profile.
Treatment response was assessed by investigators based on international criteria [19].
Safety and tolerability were evaluated by recording incidence, severity, and type of any adverse event (AE) according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Elderly patients were defined as those aged >65 years at the first administration of lenalidomide.
OS was defined as the time from initiation of therapy to death from any cause and was censored at the date of last available follow‐up. PFS was measured from initiation of therapy to progression, relapse, or death from any cause and was censored at the date of last available follow‐up. DFS was calculated for CR patients from the first documentation of response to the date of relapse or death due to lymphoma or acute toxicity of treatment [19].
Demographics and patients’ characteristics were summarized by descriptive statistics.
Survival functions were estimated by using the Kaplan‐Meier method and were compared using log‐rank test.
Statistical analyses were performed with Stata 11 (StataCorp, College Station, TX), and p values were set at .05.
Results
Patients underwent baseline assessments including physical examination and routine hematology and biochemistry evaluations as well as imaging prior to therapy.
The characteristics of the 153 patients are summarized in Table 1. The median age at lenalidomide was 72 years (range, 25–93 years), and 110 (71.9%) were elderly patients; 75 were males, and 78 were females. Thirty‐five (22.9%) patients had systemic symptoms at baseline.
Table 1. Patient demographics and characteristics at baseline.
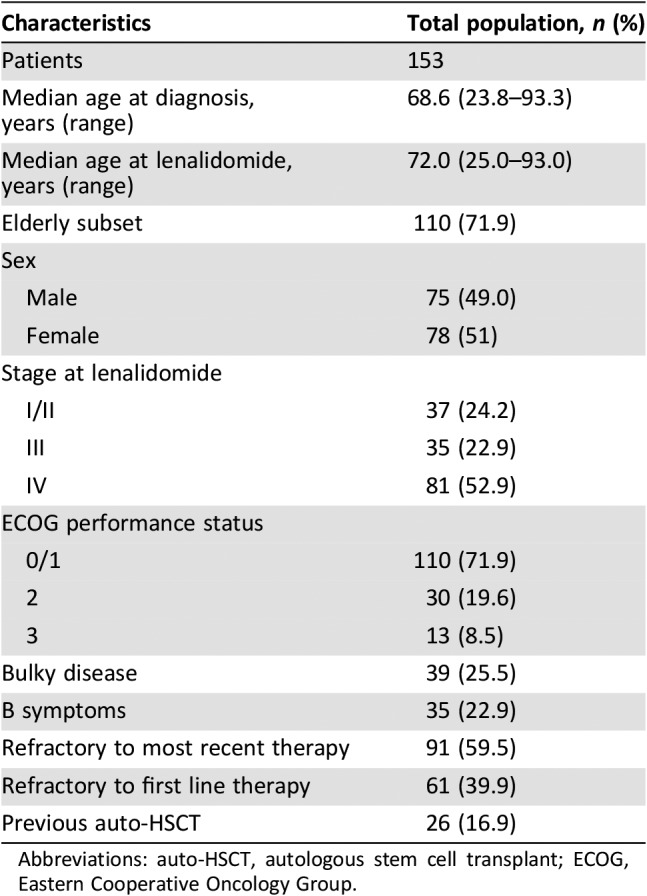
Abbreviations: auto‐HSCT, autologous stem cell transplant; ECOG, Eastern Cooperative Oncology Group.
The median number of prior lymphoma‐related systemic regimens was 2 (range, 1–6) including high‐dose chemotherapy and auto‐HSCT (n = 26, 16.9%). Ninety‐one (59.5%) patients had disease that was refractory to last therapy, and 61 patients (39.9%) were considered refractory to first‐line therapy.
Response to Treatment (Table 2)
Table 2. Response to lenalidomide.
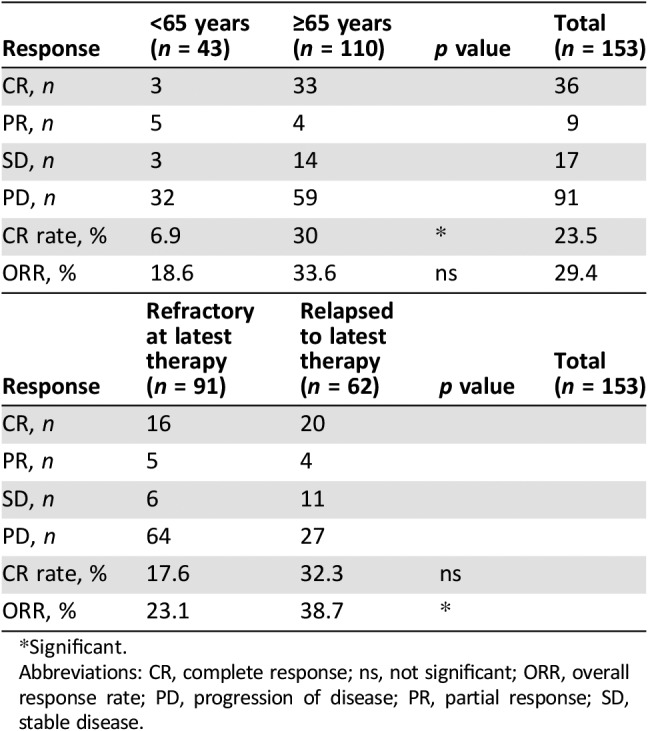
Significant.
Abbreviations: CR, complete response; ns, not significant; ORR, overall response rate; PD, progression of disease; PR, partial response; SD, stable disease.
Median dose for patients was 15 mg/day. The wide range of dose depends, at the beginning, on patients’ characteristics, comorbidity, and age in line with what has been decided by the treating physician. During treatment, dose was de‐escalated or escalated because of toxicities/AEs or resolution of toxicities/AEs, respectively. Escalation was also based on the fact that, despite the factors that initially determined the option for a lower dose, the patient tolerated well the first drug administrations. Patients received lenalidomide for a median of 4 cycles (range, 1–60); a median of 12 cycles (range, 5–60) was registered in patients who obtained a CR. Among the 153 patients, 36 (23.5%) achieved a CR and 9 (5.9%) obtained a partial response (PR) with an ORR of 29.4%; among the remaining patients, 17 (11.1%) had stable disease and 91 (59.5%) showed progression of disease, respectively.
The best response rate was higher in the elderly subset: 33 (30%) CRs and 4 (3.6%) PRs. We checked if baseline characteristics were different between elderly and younger patients. The only variable that was statistically significant was the outcome (relapsed or refractory) after the latest therapy before lenalidomide. Among elderly patients, 50.1% were refractory, versus 81.4% in younger ones (p < .001).
Among the 61 patients who were refractory to first line, 15 (24.6%) achieved CR and 1 (1.6%) had PR, with an ORR of 26.2%; in the subset of 91 patients refractory to the last line prior to lenalidomide, we observed 16 (17.6%) CRs and 5 (5.5%) PRs, leading to an ORR of 23.1%. At the latest available follow‐up, no patients were still under lenalidomide treatment, but 29 patients showed continuous CR (CCR). This subset (Table 3) of patients is represented by 14 patients who were refractory to first‐line treatment and by 15 multirelapsed patients (12 of them were refractory to last previous therapy); among these 29 patients, the median number of previous treatments was 2 (range, 1–5) and, in particular, 17 out of 29 displayed stage IV disease at the start of therapy with lenalidomide. A statistically significant correlation between dose and responses occurred for doses ≥15 mg/day.
Table 3. Demographics and characteristics of the 29 patients in continuous complete response after lenalidomide.
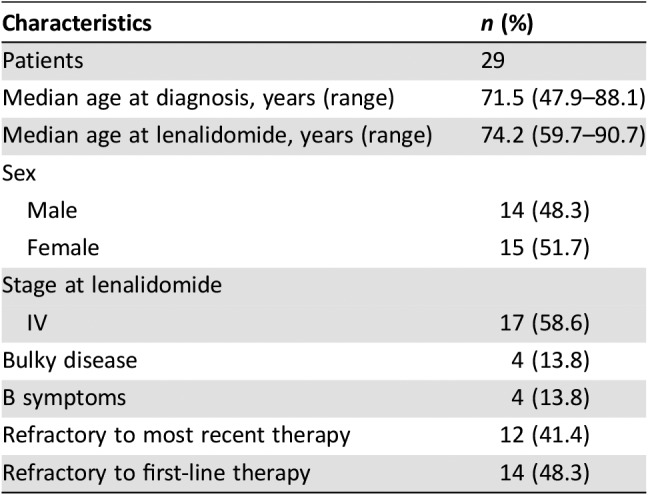
Outcome
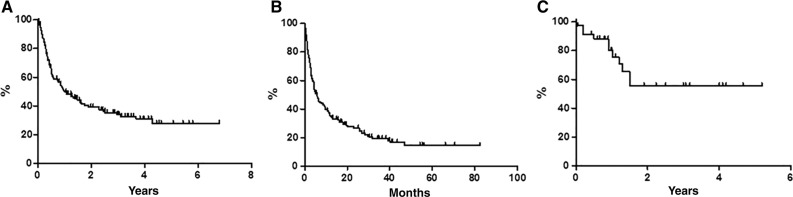
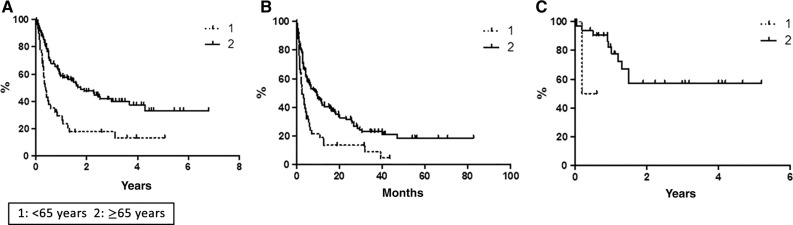
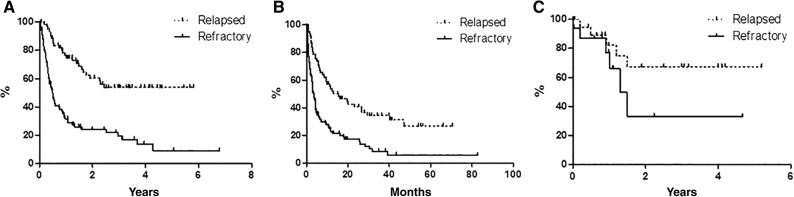
With a median follow‐up of 36 months, global OS was 27.7% at 80 months (Fig. 1A), with median reached at 12 months. Global PFS at 80 months was 14.6%, with median achieved at 6 months (Fig. 1B). Global DFS was 55.5% at 62 months (Fig. 1C): only 7 out of 36 (19.4%) CR patients relapsed, and 29 patients were in CCR, with a median duration of response of 12 months. According to age, median OS was 4 months in younger versus 20 months in elderly patients (p < .0001; Fig. 2A); median PFS was 2.5 months in younger versus 9.5 months in elderly patients (p < .0007; Fig. 2B); median DFS was not statistically significant because there were only three young patients (50.0% vs. 57.0%; Fig. 2C). On the basis of the disease status in respect to last previous therapy (relapsed vs. refractory), at the time of the analysis, the median OS has not been reached in the relapsed subset, whereas it was 5 months in refractory patients (p < .0001; Fig. 3A). The median PFS was 15 months for relapsed patients versus 3.5 months for refractory patients (p < .0001; Fig. 3B); the median DFS has not been reached in the relapsed subset, whereas it was 1.5 months in refractory patients (p = .16; Fig. 3C).
Figure 1.
Survivals in the whole sample (n = 153), estimated by the Kaplan‐Meier method. (A): Overall survival. (B): Progression‐free survival. (C): Disease‐free survival.
Figure 2.
Survival comparison (estimated by the Kaplan‐Meier method and compared by log‐rank test) between elderly (n = 110) and nonelderly (n = 43) patients. (A): Overall survival. (B): Progression‐free survival. (C): Disease‐free survival.
Figure 3.
Survival comparison (estimated by the Kaplan‐Meier method and compared by log‐rank test) between relapsed (n = 62) and refractory (n = 91) patients. (A): Overall survival. (B): Progression‐free survival. (C): Disease‐free survival.
At the latest follow‐up, 61 (39.9%) patients were alive and 92 deceased: 89 of lymphoma, 2 of secondary malignancies (namely, acute myeloid leukemia, 12 and 15 months after lenalidomide, respectively), and 1 of a car accident.
Safety
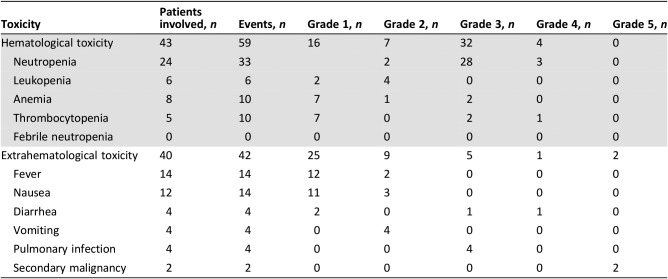
All patients were included in the safety analysis. In general, the treatment was well tolerated and the toxicity profile was very similar to previously published data. Forty‐three patients had hematologic toxicity, and 40 patients showed extrahematologic toxicity (both toxicities were observed in 12 patients). Among hematologic side effects, grade 3/4 was reported in 36 patients: 31 neutropenia, 3 thrombocytopenia, and 2 anemia, all related to lenalidomide; no febrile neutropenia was observed. On the other hand, grade 3/4 extrahematologic toxicity was observed in six patients: four pulmonary infections and two episodes of diarrhea.
Thirty patients had early discontinuation and 44 patients had dose reduction due to AEs. Globally, 17 severe AEs were reported, and 3 of them resulted in death as stated above (acute myeloid leukemia, 12 and 15 months after lenalidomide, respectively, and one car accident). For the complete AEs list, refer to Table 4.
Table 4. Toxicities.
Discussion
Patients with relapsed DLBCL not eligible for auto‐HSCT or having relapse after auto‐HSCT have a low likelihood of cure. Lenalidomide is an oral immunomodulatory agent that exerts anticancer effects through multiple mechanisms, including the inhibition of angiogenesis, recruitment of natural killer cells, upregulation of CD80 and CD40, impairment of inflammatory cytokines, and effects on the tumor microenvironment [20]. Lenalidomide as single agent showed activity in multiple NHL subpopulations, including heavily pretreated relapsed/refractory DLBCL [15], [16], [17]. In a phase II trial investigating lenalidomide monotherapy, patients with DLBCL achieved an ORR of 28% and a median PFS of 2.7 months [16].
Recently, we reported a retrospective multicenter study conducted in patients with relapsed/refractory NHL treated with lenalidomide monotherapy through a Named Patient Program in Italy; in the 19 evaluable patients with DLBCL, ORR was 42.1%, with a 31.6% CR rate. The ORR was higher in patients who responded to last previous therapy compared with those who were refractory; mean duration of response in patients receiving any lenalidomide dose was 10.5 months [21].
In addition, Mondello et al. published a retrospective real‐life analysis assessing the efficacy and toxicity of lenalidomide in 123 cases of relapsed/refractory DLBCL. During a median follow‐up period of 4.5 years, CR was achieved in 21% of cases, with an ORR of 37% and a median PFS of 34 months [22].
Our retrospective analysis on 153 patients reports that ORR and CR rate were comparable to those observed in clinical trials investigating similar DLBCL populations treated with lenalidomide as single agent [13], [14], [15], [16], [17]. In fact, 23.5% of patients treated in monotherapy with lenalidomide achieved a CR, and 5.9% obtained a PR, with an ORR of 29.4%. In addition, the best response rate was observed in elderly (>65 years) patients, probably because of a higher incidence of patients refractory to the last therapy in the younger group.
The strengths of this work are represented by the large sample size (to our knowledge, this is the largest report on lenalidomide for DLBCL used in daily clinical practice ever published) and the long follow‐up (median of 3 years). Of note are the interesting data in terms of CR rate in the elderly patients for whom, according to the therapeutic algorithm of DLBCL, there is a real unmet clinical need. In addition, our study describes a subset of 29 patients in CCR with a median DFS that was not reached at 5 years (55.5% at 62 months). Correlations between dose and responses were calculated, but analyses were possible only on the average dose; thus, no incisive conclusions can be drawn.
The main limitation of this study is a lack of a centralized histological review of the tissue samples: this situation did not allow comparing the response to treatment based on the COO.
Mondello and coworkers observed ORR and CR rates (37% and 21%, respectively) similar to the ones we reported but with a higher median PFS (34 months) [22]. However, that study included a different population because the median age was 64 years (vs. 72 years in our series) and the median number of prior treatment regimens was 1 (range, 1–3 [vs. 2, range 1–6 in our study population]); in addition, data on patients in CCR or DFS estimation are lacking.
Conclusion
Despite the known potential bias of all observational studies, the present study, which represents the largest report on lenalidomide in patients with relapsed/refractory DLBCL in the standard daily clinical practice outside a trial setting, shows that lenalidomide is a feasible treatment option for patients with relapsed/refractory DLBCL in real life. For this reason, it must be considered in the therapeutic algorithm of relapsed/refractory DLBCL as a targeted approach, even for elderly subjects.
Footnotes
For Further Reading: Patrizia Mondello, Normann Steiner, Wolfgang Willenbacher et al. Lenalidomide in Relapsed or Refractory Diffuse Large B‐Cell Lymphoma: Is It a Valid Treatment Option? The Oncologist 2016;21:1107–1112.
Implications for Practice: Despite the advent of new treatment strategies, many patients with diffuse large B‐cell lymphoma (DLBCL) relapse or die of the disease; hence, novel therapeutic approaches are urgently needed. This study confirms that lenalidomide is a valid and well‐tolerated treatment option for relapsed/refractory (R/R) DLBCL. Superior outcomes were observed in non‐germinal center B‐cell (GCB) DLBCL, probably because of inhibition of the nuclear factor‐κB pathway. Similarly, high drug doses resulted in greater clinical benefits. Overall, lenalidomide is a suitable therapeutic option for R/R DLBCL, especially in non‐GCB DLBCL, and 25 mg/day dosing should be preferred.
Author Contributions
Conception/design: Alessandro Broccoli, Lisa Argnani, Pier Luigi Zinzani
Provision of study material or patients: Alessandro Broccoli, Beatrice Casadei, Annalisa Chiappella, Carlo Visco, Monica Tani, Nicola Cascavilla, Annarita Conconi, Monica Balzarotti, Maria Christina Cox, Dario Marino, Maria Cecilia Goldaniga, Roberto Marasca, Cristina Tecchio, Caterina Patti, Gerrado Musuraca, Liliana Devizzi, Federico Monaco, Alessandra Romano, Angelo Fama, Michelle Zancanella, Rossella Paolini, Luigi Rigacci, Claudia Castellino, Pier Luigi Zinzani
Collection and/or assembly of data: Alessandro Broccoli, Beatrice Casadei, Annalisa Chiappella, Carlo Visco, Monica Tani, Nicola Cascavilla, Annarita Conconi, Monica Balzarotti, Maria Christina Cox, Dario Marino, Maria Cecilia Goldaniga, Roberto Marasca, Cristina Tecchio, Caterina Patti, Gerrado Musuraca, Liliana Devizzi, Federico Monaco, Alessandra Romano, Angelo Fama, Michelle Zancanella, Rossella Paolini, Luigi Rigacci, Claudia Castellino, Lisa Argnani, Pier Luigi Zinzani
Data analysis and interpretation: Alessandro Broccoli, Lisa Argnani, Pier Luigi Zinzani
Manuscript writing: Alessandro Broccoli, Lisa Argnani, Pier Luigi Zinzani
Final approval of manuscript: Alessandro Broccoli, Beatrice Casadei, Annalisa Chiappella, Carlo Visco, Monica Tani, Nicola Cascavilla, Annarita Conconi, Monica Balzarotti, Maria Christina Cox, Dario Marino, Maria Cecilia Goldaniga, Roberto Marasca, Cristina Tecchio, Caterina Patti, Gerrado Musuraca, Liliana Devizzi, Federico Monaco, Alessandra Romano, Angelo Fama, Michelle Zancanella, Rossella Paolini, Luigi Rigacci, Claudia Castellino, Lisa Argnani, Pier Luigi Zinzani
Disclosures
Annalisa Chiappella: Celgene, Janssen (SAB), Amgen, Roche, Celgene, Teva, Nanostring, Janssen (H); Roberto Marasca: Jannsen, Roche, Gilead, Abbvie, Shire (C/A), Jannsen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lenz G, Wright G, Dave SS et al. Stromal gene signatures in large‐B‐cell lymphomas. N Engl J Med 2008;359:2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenwald A, Wright G, Chan WC et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. N Engl J Med 2002;346:1937–1947. [DOI] [PubMed] [Google Scholar]
- 3.Hans CP, Weisenburger DD, Greiner TC et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282. [DOI] [PubMed] [Google Scholar]
- 4.Cultrera JL, Dalia SM. Diffuse large B‐cell lymphoma: Current strategies and future directions. Cancer Control 2012;19:204–213. [DOI] [PubMed] [Google Scholar]
- 5.Maurer MJ, Ghesquières H, Jais JP et al. Event‐free survival at 24 months is a robust end point for disease‐related outcome in diffuse large B‐cell lymphoma treated with immunochemotherapy. J Clin Oncol 2014;32,1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raut LS, Chakrabarti PP. Management of relapsed‐refractory diffuse large B cell lymphoma. South Asian J Cancer 2014;3:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg JW. Relapsed/refractory diffuse large B‐cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
- 8.Crump M, Neelapu SS, Farooq U et al. Outcomes in refractory diffuse large B‐cell lymphoma: Results from the international SCHOLAR‐1 study. Blood 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Gnaoui T, Dupuis J, Belhadj K et al. Rituximab, gemcitabine and oxaliplatin: An effective salvage regimen for patients with relapsed or refractory B‐cell lymphoma not candidates for high‐dose therapy. Ann Oncol 2007;18:1363–1368. [DOI] [PubMed] [Google Scholar]
- 10.Vacirca JL, Acs PI, Tabbara IA et al. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol 2014;93:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke FL, Neelapu SS, Bartlett NL et al. Phase 1 Results of ZUMA‐1: A multicenter study of KTE‐C19 Anti‐CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 2017;25:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pean E, Flores B, Hudson I et al. The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non‐Hodgkin's B‐cell lymphomas: Summary of the scientific assessment of the committee for medicinal products for human use. The Oncologist 2013;18:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghandi AK, Kang I, Havens CG et al. Immunomodulatory agents lenalidomide and pomalidomide co‐stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4 (CRBN.). Br. J Haematol 2014;164:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu I, Adams M, Carter T et al. Lenalidomide enhances natural killer cell and monocyte‐mediated antibody‐dependent cellular cytotoxicity of rituximab‐treated Cd20+ tumor cells. Clin Cancer Res 2008;14:4650–4657. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez‐Ilizaliturri FJ, Deeb G, Zinzani PL et al. Higher response to lenalidomide in relapsed/refractory diffuse large B‐cell lymphoma in nongerminal center B‐cell‐like than in germinal center B‐cell‐like phenotype. Cancer 2011;117:5058–5066. [DOI] [PubMed] [Google Scholar]
- 16.Witzig TE, Vose JM, Zinzani PL et al. An international phase II trial of single‐agent lenalidomide for relapsed or refractory aggressive B‐cell non‐Hodgkin's lymphoma. Ann Oncol 2011;22:1622–1627. [DOI] [PubMed] [Google Scholar]
- 17.Wiernik PH, Lossos IS, Tuscano JM et al. Lenalidomide monotherapy in relapsed or refractory aggressive non‐ Hodgkin's lymphoma. J Clin Oncol 2008;26:4952–4957. [DOI] [PubMed] [Google Scholar]
- 18.Czuczman MS, Trněný M, Davies A et al. A phase 2/3 multicenter, randomized, open‐label study to compare the efficacy and safety of lenalidomide versus investigator's choice in patients with relapsed or refractory diffuse large B‐cell lymphoma. Clin Cancer Res 2017;23:4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 20.Tageja N. Lenalidomide ‐ Current understanding of mechanistic properties. Anticancer Agents Med Chem 2011;11:315–326. [DOI] [PubMed] [Google Scholar]
- 21.Zinzani PL, Rigacci L, Cox MC et al. Lenalidomide monotherapy in heavily pretreated patients with non‐Hodgkin lymphoma: An Italian observational multicenter retrospective study in daily clinical practice. Leuk Lymphoma 2015;56:1671–1676. [DOI] [PubMed] [Google Scholar]
- 22.Mondello P, Steiner N, Willenbacher W et al. Lenalidomide in relapsed or refractory diffuse large B‐cell lymphoma: Is it a valid treatment option? The Oncologist 2016;21:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]