Abstract
Lessons Learned.
The combination of axitinib and crizotinib has a manageable safety and tolerability profile, consistent with the profiles of the individual agents when administered as monotherapy.
The antitumor activity reported here for the combination axitinib/crizotinib does not support further study of this combination treatment in metastatic renal cell carcinoma given the current treatment landscape.
Background.
Vascular endothelial growth factor (VEGF) inhibitors have been successfully used to treat metastatic renal cell carcinoma (mRCC); however, resistance eventually develops in most cases. Tyrosine protein kinase Met (MET) expression increases following VEGF inhibition, and inhibition of both has shown additive effects in controlling tumor growth and metastasis. We therefore conducted a study of axitinib plus crizotinib in advanced solid tumors and mRCC.
Methods.
This phase Ib study included a dose‐escalation phase (starting doses: axitinib 3 mg plus crizotinib 200 mg) to estimate maximum tolerated dose (MTD) in patients with solid tumors and a dose‐expansion phase to examine preliminary efficacy in treatment‐naïve patients with mRCC. Safety, pharmacokinetics, and biomarkers were also assessed.
Results.
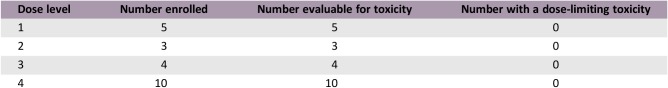
No patients in the dose‐escalation phase (n = 22) experienced dose‐limiting toxicity; MTD was estimated to be axitinib 5 mg plus crizotinib 250 mg. The most common grade ≥3 adverse events were hypertension (18.2%) and fatigue (9.1%). In the dose‐expansion phase, overall response rate was 30% (95% confidence interval [CI], 11.9–54.3), and progression‐free survival was 5.6 months (95% CI, 3.5–not reached).
Conclusion.
The combination of axitinib plus crizotinib, at estimated MTD, had a manageable safety profile and showed evidence of modest antitumor activity in mRCC.
Abstract
经验总结
• 阿西替尼联合克唑替尼具有可控的安全性和耐受性特性,与单一疗法用药时的个体药物特征相一致。
• 鉴于当前的治疗环境,本文中所报告的阿西替尼/克唑替尼联合疗法的抗肿瘤活性不支持将此联合疗法用于转移性肾细胞癌的进一步研究。
摘要
背景。血管内皮生长因子 (VEGF) 抑制剂已成功用于治疗转移性肾细胞癌 (mRCC);然而,大多数情况下最终会产生耐药性。在抑制VEGF后,酪氨酸蛋白激酶 Met (MET) 的表达增加,且两者的抑制作用在控制肿瘤生长和转移方面显现出附加效应。因此,我们对阿西替尼联合克唑替尼治疗晚期实体瘤和转移性肾细胞癌的效果进行了研究。
方法。此项 IB 期研究包括剂量递增阶段(起始剂量:阿西替尼 3 mg,加上克唑替尼 200 mg),以评估实体瘤患者的最大耐受剂量 (MTD);剂量扩展阶段,以检验对mRCC治疗无效患者的初步疗效。此外,还对安全性、药代动力学和生物标志物进行了评估。
结果。在剂量递增阶段(n=22),无患者出现剂量限制性毒性;预估的MTD为:阿西替尼 5 mg,加上克唑替尼 250 mg。最常见的≥ 3 级不良事件为高血压 (18.2%) 和疲劳 (9.1%)。在剂量扩展阶段,总体反应率为 30% [95% 置信区间 (CI),11.9‐54.3],无进展生存期为 5.6 个月(95% CI,3.5‐ 未达到)。
结论。在达到预估的MTD时,阿西替尼联合克唑替尼疗法具有可管理的安全特性,且有证据表明,对治疗mRCC显示出适度的抗肿瘤活性。
Discussion
Despite the success of agents that target VEGF and VEGF receptors (VEGFRs) [1], [2], [3] in mRCC, a subset of patients are refractory to VEGF inhibitor treatment, and most patients who are responsive to treatment will eventually develop resistance [4], [5]. Proposed explanations for resistance include the activation of pathways favoring epithelial‐mesenchymal transition, such as MET [5], [6], [7], [8], [9], [10]. Preclinical in vivo studies have shown that combining MET and VEGFR inhibition has synergistic effects on tumor growth, angiogenesis, invasiveness, and metastasis [6], [7], [8], [9], [10], [11]. Crizotinib is an inhibitor of anaplastic lymphoma kinase (ALK), MET/hepatocyte growth factor receptor, and ROS1 receptor tyrosine kinases and is approved for the treatment of ALK‐positive or ROS1‐positive metastatic non‐small cell lung cancer [12], [13]. Axitinib is a specific tyrosine kinase inhibitor of VEGFRs 1–3 that is approved for the treatment of mRCC after failure of one prior systemic therapy [14], [15]. We hypothesized that combining crizotinib with axitinib would provide greater clinical benefit than VEGF‐directed therapy alone.
In this study, the combination of axitinib and crizotinib was tolerable in patients with advanced solid tumors, including mRCC. No patient experienced a dose‐limiting toxicity in the dose‐escalation phase, and axitinib 5 mg twice daily (BID) in combination with crizotinib 250 mg BID was selected as the MTD. No new safety issues for the combination were identified. The overall adverse events for the different treatment groups were manageable through medical intervention and/or dose modification, and a low proportion of patients discontinued because of toxicity.
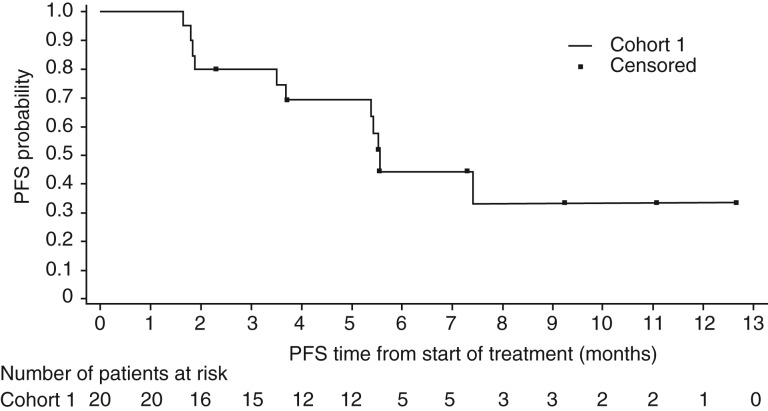
The confirmed objective response rate (ORR) for treatment‐naïve patients with mRCC receiving axitinib and crizotinib (30%) in this trial was similar to results for single‐agent axitinib versus sorafenib as first‐line therapy in the phase III trial (32%) [15]. A randomized phase II trial of cabozantinib versus sunitinib (CABOSUN) reported an ORR of 46% for first‐line poor‐ or intermediate‐risk patients with mRCC treated with cabozantinib [16]. The median progression‐free survival (PFS) observed in the dose‐expansion phase cohort 1 (treatment‐naïve patients) was 5.6 months (95% CI, 3.5–not reached; Fig. 1), which was shorter than the PFS of axitinib alone (10.1 months; 95% CI, 7.2–12.1) as first‐line therapy [15]. In CABOSUN, the reported estimated PFS for patients treated with cabozantinib was 8.2 months (95% CI, 6.2–8.8) [17].
Figure 1.
Progression‐free survival in cohort 1. Date of data cutoff: May 24, 2017. Cohort 1: no prior systemic therapy directed at advanced renal cell carcinoma.
Abbreviation: PFS, progression‐free survival.
In conclusion, the combination of axitinib and crizotinib in patients with solid tumors has a manageable safety profile, consistent with the profiles of the individual agents administered as monotherapy, and demonstrated antitumor activity in treatment‐naïve patients with mRCC. Given the more robust antitumor activity of cabozantinib, and promising trial data for newer immuno‐oncology treatments, the antitumor activity reported here does not support further study of axitinib plus crizotinib in mRCC.
Trial Information
- Disease
Renal cell carcinoma – clear cell
- Disease
Solid tumor
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
None
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
Dose finding and preliminary efficacy
- Primary Endpoint
Maximum tolerated dose
- Primary Endpoint
null
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Efficacy
- Secondary Endpoint
Biomarkers
- Additional Details of Endpoints or Study Design
- Patients in the dose‐expansion phase were enrolled into two cohorts: patients in cohort 1 had received no prior systemic therapy for mRCC, whereas patients in cohort 2 had one or two prior systemic treatment regimens directed at mRCC, with at least one prior therapy being a VEGF pathway inhibitor, and resistance to the most recently received VEGF pathway inhibitor.
- Investigator's Analysis
Active but results overtaken by other developments
Drug Information: Dose Escalation Phase
- Drug 1
- Generic/Working Name
Axitinib
- Trade Name
Inlyta
- Company Name
Pfizer
- Drug Type
Small molecule
- Drug Class
VEGFR
- Dose
Multiple milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
BID
- Drug 2
- Generic/Working Name
Crizotinib
- Trade Name
Xalkori
- Company Name
Pfizer
- Drug Type
Small molecule
- Drug Class
ALK
- Dose
Multiple milligrams (mg) per flat dose
- Route
Oral (po)
Drug Information: Dose Expansion Phase
- Drug 1
- Generic/Working Name
Axitinib
- Trade Name
Inlyta
- Company Name
Pfizer
- Drug Type
Small molecule
- Drug Class
VEGFR
- Dose
5 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
BID
- Drug 2
- Generic/Working Name
Crizotinib
- Trade Name
Xalkori
- Company Name
Pfizer
- Drug Type
Small molecule
- Drug Class
ALK
- Dose
250 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
BID
Dose Escalation Table for Phase I Dose Escalation Phase
See Table 1 for additional details.
Patient Characteristics: Dose Escalation Phase
- Number of Patients, Male
12
- Number of Patients, Female
10
- Age
Median (range): 62.0 years (34.0–78.0 years)
- Performance Status: ECOG
-
0 —
1 — 8
2 — 14
3 —
Unknown —
- Cancer Types or Histologic Subtypes
-
Head and neck, 1
Bladder, 2
Non‐small cell lung cancer, 1
Renal cell carcinoma, 7
Hepatocellular, 1
Adrenal, 1
Breast, 1
Pancreas, 2
Colorectal, 4
Other, 2
Patient Characteristics: Dose Expansion Phase
- Number of Patients, Male
22
- Number of Patients, Female
6
- Age
Median (range): 62.5 years (45.0–76.0 years)
- Performance Status: ECOG
-
0 — 12
1 — 15
2 — 1
3 —
Unknown —
- Other
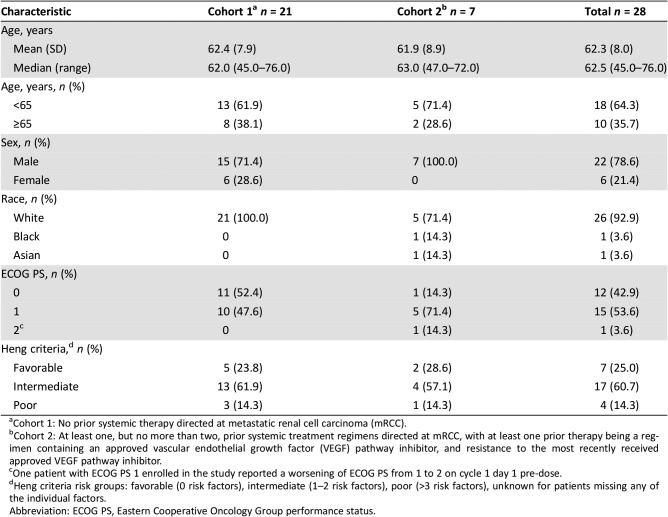
- Patients in the dose expansion phase were divided into two cohorts: cohort 1 (n = 21), no prior systemic therapy toward mRCC, and cohort 2 (n = 7), at least one, but no more than two prior systemic treatment regimens directed at renal cell carcinoma, with at least one prior therapy being a regimen containing an approved VEGF pathway inhibitor, and resistance to the most recently approved VEGF pathway inhibitor. For details of patient characteristics, refer to Tables 2 and 3.
- Cancer Types or Histologic Subtypes
-
Metastatic renal cell carcinoma, cohort 1: 21
Metastatic renal cell carcinoma, cohort 2: 7
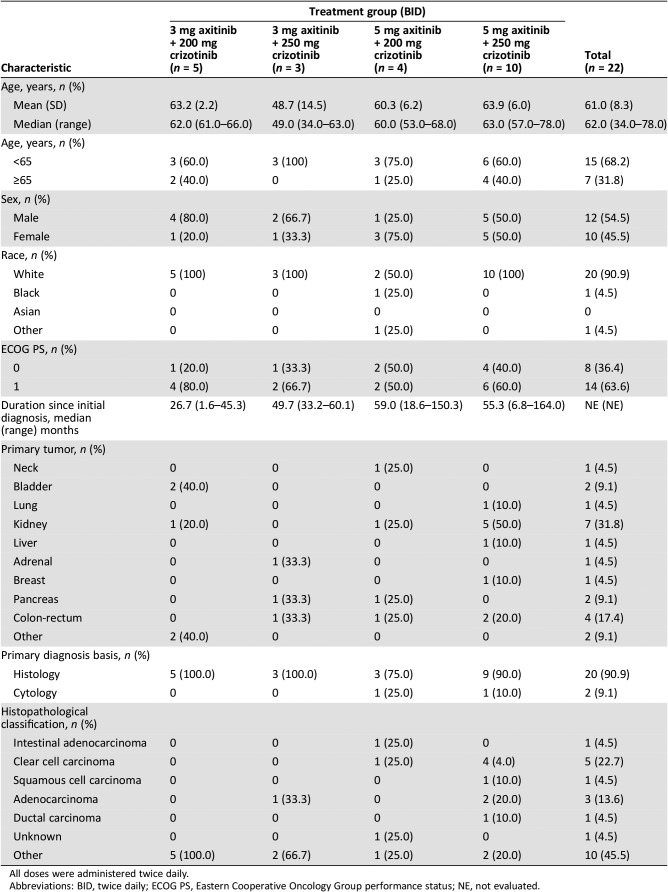
Table 2. Patient characteristics in the dose‐escalation phase by treatment group.
All doses were administered twice daily.
Abbreviations: BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not evaluated.
Table 3. Baseline demographics and characteristics for patients with metastatic renal cell carcinoma in the dose‐expansion phase, by cohort.
Cohort 1: No prior systemic therapy directed at metastatic renal cell carcinoma (mRCC).
Cohort 2: At least one, but no more than two, prior systemic treatment regimens directed at mRCC, with at least one prior therapy being a regimen containing an approved vascular endothelial growth factor (VEGF) pathway inhibitor, and resistance to the most recently received approved VEGF pathway inhibitor.
One patient with ECOG PS 1 enrolled in the study reported a worsening of ECOG PS from 1 to 2 on cycle 1 day 1 pre‐dose.
Heng criteria risk groups: favorable (0 risk factors), intermediate (1–2 risk factors), poor (>3 risk factors), unknown for patients missing any of the individual factors.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Primary Assessment Method: Dose Escalation Phase
- Title
Cohort 1
- Number of Patients Evaluable for Toxicity
22
- Evaluation Method
- Patients were monitored for dose‐limiting toxicity, which was defined as any of the following events: grade 4 neutropenia; febrile neutropenia; grade ≥3 neutropenic infection; grade ≥3 thrombocytopenia with bleeding; grade 4 thrombocytopenia; any nonhematologic grade ≥3 toxicities, except asymptomatic hypophosphatemia, hyperuricemia without signs and symptoms of gout; or persistent (despite maximal medical therapy) grade ≥3 nausea, vomiting, or diarrhea.
Primary Assessment Method: Dose Expansion Phase
- Title
Cohort 1
- Number of Patients Evaluated for Efficacy
20
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 6 (30%)
- Response Assessment SD
n = 10 (50%)
- Response Assessment PD
n = 4 (20%)
- (Median) Duration Assessments PFS
5.6 months
- Title
Cohort 2
- Number of Patients Evaluated for Efficacy
7
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 1 (14.3%)
- Response Assessment SD
n = 3 (42.9%)
- Response Assessment PD
n = 1 (14.3%)
- Response Assessment OTHER
n = 2 (28.6%)
Adverse Events
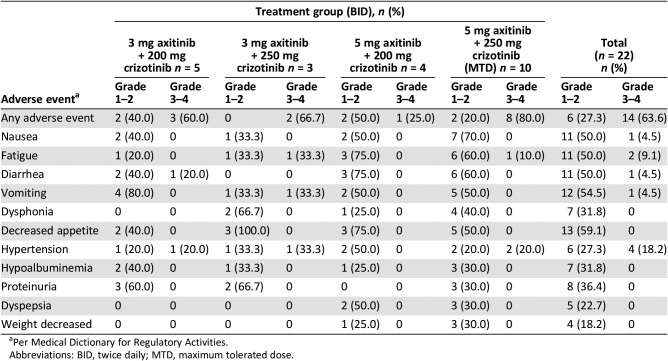
Table 4. Adverse events (all causalities) reported in more than two patients in any cohort during the dose‐escalation phase.
Per Medical Dictionary for Regulatory Activities.
Abbreviations: BID, twice daily; MTD, maximum tolerated dose.
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active but results overtaken by other developments
A subset of patients with metastatic renal cell carcinoma (mRCC) are refractory to vascular endothelial growth factor (VEGF) inhibitor treatment, and most patients who are initially responsive eventually develop resistance [1], [2]. Proposed mechanisms of resistance include activation of pathways favoring epithelial‐mesenchymal transition, such as tyrosine protein kinase Met (MET), also known as hepatocyte growth factor receptor (HGFR), and changes in the tumor vasculature and dominant VEGF isoform [2], [3], [4], [5], [6], [7]. Preclinical in vivo studies have shown that combining MET and VEGF receptor (VEGFR) inhibition has synergistic effects on tumor growth, angiogenesis, invasiveness, and metastasis [3], [4], [5], [6], [7], [8]. Specifically, studies using VEGF‐targeted therapy‐resistant and ‐sensitive animal models showed increased antitumor effect when a VEGF‐targeted and a MET‐targeted agent were used together [8]. Furthermore, the proven clinical activity of cabozantinib in advanced renal cell carcinoma (RCC) supports use of this combination [9].
Crizotinib is an inhibitor of anaplastic lymphoma kinase (ALK), MET/HGFR, and ROS1 receptor tyrosine kinases approved for the treatment of patients with ALK‐positive or ROS1‐positive metastatic non‐small cell lung cancer [10], [11]. Axitinib is a specific tyrosine kinase inhibitor (TKI) of VEGFRs 1–3 with proven benefit in mRCC treatment and is approved for patients with mRCC after failure of one prior systemic therapy [12], [13]. We hypothesized that combining the MET inhibitor, crizotinib, with the VEGFR inhibitor, axitinib, would provide greater clinical benefit than VEGF‐directed therapy alone.
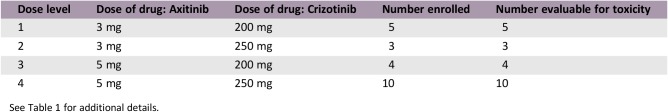
This study was a phase Ib, open‐label, multicenter trial with two phases: a dose‐escalation phase in patients with advanced solid tumors to estimate the maximum tolerated dose (MTD) and a dose‐expansion phase to examine preliminary efficacy in treatment‐naïve patients with mRCC. Safety, pharmacokinetics, and biomarkers were also assessed. In the dose escalation phase, patient de‐escalation and escalation of axitinib and crizotinib followed the modified toxicity probability interval (Table 1) [14].
Table 1. Dose levels in the dose‐escalation phase.
Abbreviations: BID, twice daily; QD, once daily.
In the dose‐expansion phase, patients were enrolled into two cohorts: cohort 1 patients had no prior systemic mRCC‐directed therapies, and cohort 2 patients had one or two prior systemic mRCC‐directed therapies. Recruitment for cohort 2 was stopped at seven patients because of scarcity of qualified patients.
Enrolled patients were ≥18 years old and had histologically and/or cytologically confirmed diagnosis of advanced solid tumor refractory to standard therapy (dose‐escalation phase) or confirmed clear‐cell mRCC (dose‐expansion phase). Patient demographics and characteristics are presented in Tables 2 and 3.
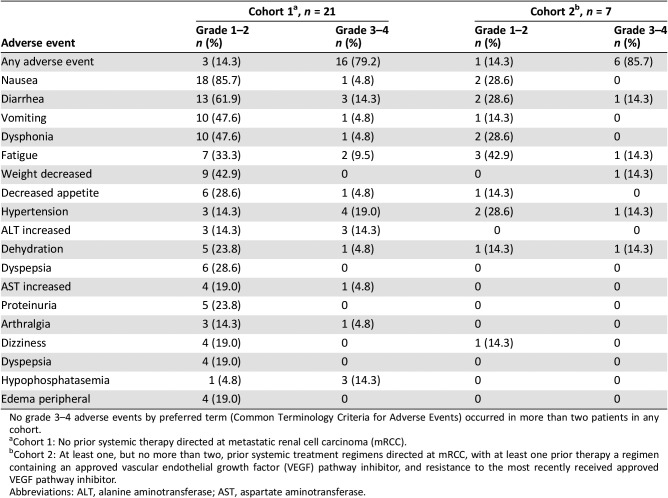
This study demonstrated that the combination of axitinib and crizotinib is tolerable in patients with advanced solid tumors, including mRCC. No patient experienced a dose‐limiting toxicity in the dose‐escalation phase, and axitinib 5 mg twice daily (BID) in combination with crizotinib 250 mg BID was selected as the MTD. No new safety issues were identified given the known safety profile of both drugs, and a low proportion of patients had to discontinue therapy due to toxicity. Adverse event profiles are presented in Tables 4 and 5.
Table 5. Adverse events by Common Terminology Criteria for Adverse Events grade reported in more than three patients during the dose‐expansion phase (patients with metastatic renal cell carcinoma).
No grade 3–4 adverse events by preferred term (Common Terminology Criteria for Adverse Events) occurred in more than two patients in any cohort.
Cohort 1: No prior systemic therapy directed at metastatic renal cell carcinoma (mRCC).
Cohort 2: At least one, but no more than two, prior systemic treatment regimens directed at mRCC, with at least one prior therapy a regimen containing an approved vascular endothelial growth factor (VEGF) pathway inhibitor, and resistance to the most recently received approved VEGF pathway inhibitor.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
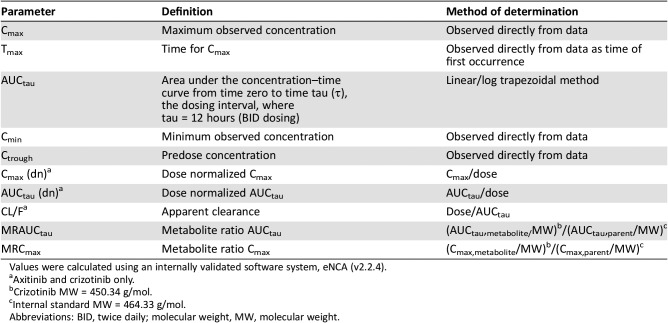
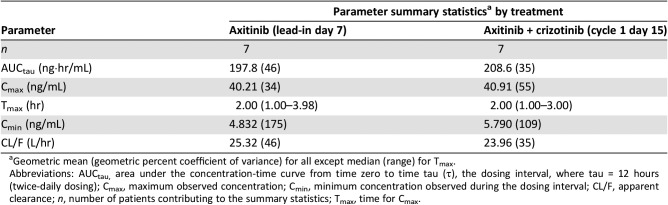
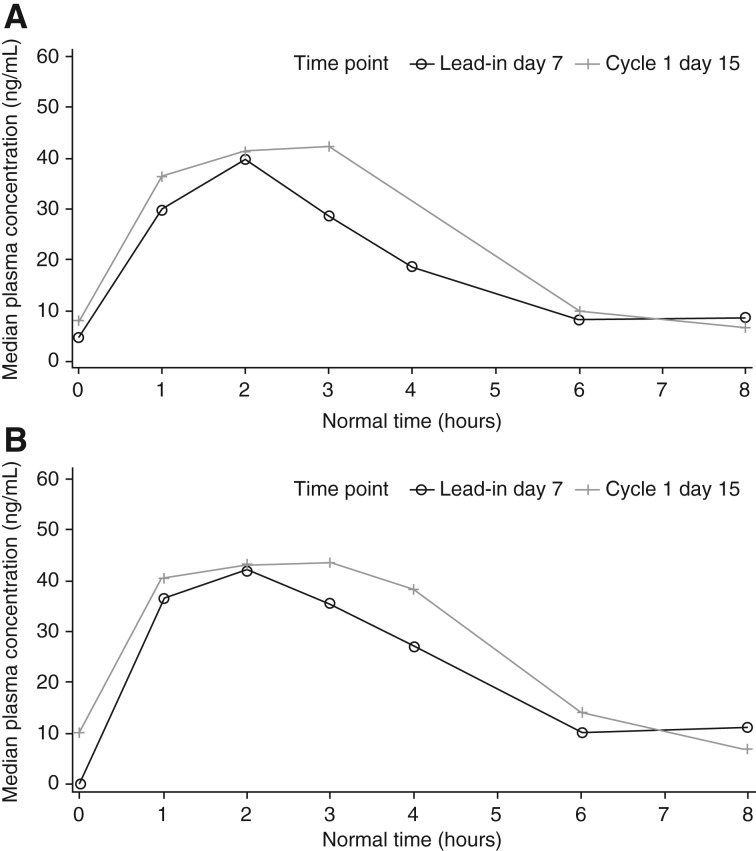
The potential drug‐drug interaction with combined use of axitinib and crizotinib was evaluated. Clinical data indicates that crizotinib is a moderate time‐dependent CYP3A4/5 inhibitor whereas axitinib is primarily metabolized by CYP3A4/5 [15], [16]. Pharmacokinetic parameters for axitinib were calculated for each patient and treatment, as applicable, using noncompartmental analysis of concentration‐time data (Table 6). Details of pharmacokinetic effects of crizotinib on axitinib when coadministered are provided in Table 7 and Figure 2. Coadministration of axitinib with crizotinib had no clinically meaningful effect on the pharmacokinetics of axitinib. Therefore, the potential efficacy of the axitinib‐crizotinib combination was not compromised by reduced axitinib exposure in patients with mRCC.
Table 6. Pharmacokinetic parameters determined in study.
Values were calculated using an internally validated software system, eNCA (v2.2.4).
Axitinib and crizotinib only.
Crizotinib MW = 450.34 g/mol.
Internal standard MW = 464.33 g/mol.
Abbreviations: BID, twice daily; molecular weight, MW, molecular weight.
Table 7. Summary of plasma axitinib pharmacokinetic parameters following multiple oral doses of axitinib alone and in combination with multiple oral doses of crizotinib (dose‐expansion cohort 1).
Geometric mean (geometric percent coefficient of variance) for all except median (range) for Tmax.
Abbreviations: AUCtau, area under the concentration‐time curve from time zero to time tau (τ), the dosing interval, where tau = 12 hours (twice‐daily dosing); Cmax, maximum observed concentration; Cmin, minimum concentration observed during the dosing interval; CL/F, apparent clearance; n, number of patients contributing to the summary statistics; Tmax, time for Cmax.
Figure 2.
Median plasma axitinib concentration‐time profiles following multiple oral doses of axitinib alone and in combination with multiple oral doses of crizotinib for dose‐expansion cohort 1. Linear (A) and semilogarithmic (B) scales. Lead‐in day 7, axitinib only; cycle 1 day 15, axitinib + crizotinib.
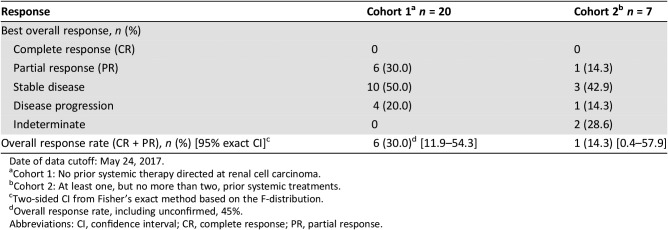
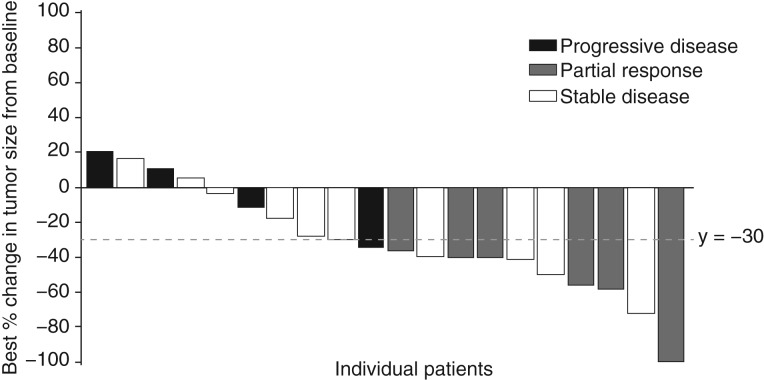
The confirmed objective response rate (ORR) for treatment‐naïve patients with RCC receiving axitinib in combination with crizotinib (30%; Table 8) was similar to results for single‐agent axitinib versus sorafenib as first‐line therapy in the phase III trial (32%) [13]. In all, 80% of patients in cohort 1 experienced some degree of tumor response (Fig. 3). A randomized phase II trial of cabozantinib versus sunitinib (CABOSUN) reported an ORR of 46% for first‐line poor‐ or intermediate‐risk patients with mRCC treated with cabozantinib [17]. The median progression‐free survival (PFS) observed in the dose‐expansion phase cohort 1 (treatment‐naïve patients) was 5.6 months (95% confidence interval [CI], 3.5–not reached), which was shorter than the PFS of axitinib single agent (10.1 months; 95% CI, 7.2–12.1) as first‐line therapy versus sorafenib [13]. In CABOSUN, the reported estimated PFS for patients treated with cabozantinib was 8.2 months (95% CI, 6.2–8.8) [9].
Table 8. Best confirmed overall response and objective response rate from patients during the dose‐expansion phase.
Date of data cutoff: May 24, 2017.
Cohort 1: No prior systemic therapy directed at renal cell carcinoma.
Cohort 2: At least one, but no more than two, prior systemic treatments.
Two‐sided CI from Fisher's exact method based on the F‐distribution.
Overall response rate, including unconfirmed, 45%.
Abbreviations: CI, confidence interval; CR, complete response; PR, partial response.
Figure 3.
Change in tumor size in patients in cohort 1 of dose‐expansion phase. Patients in cohort 1 had no prior systemic therapy directed at advanced renal cell carcinoma. Partial responses are confirmed (tumor reduction ≥30%). Date of data cutoff: May 24, 2017.
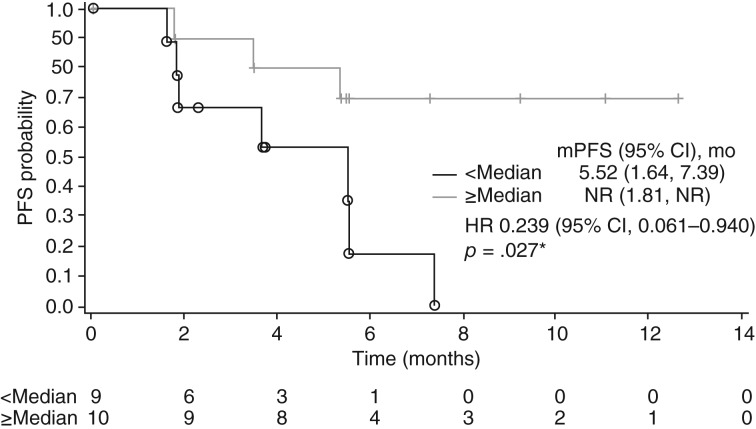
Biomarker analyses in the present study showed a trend toward lower baseline levels of HGF, IL‐8, NGAL, TIMP1, and VEGFR3 associating with better radiographic responses. This result aligns with previous studies in patients with mRCC receiving VEGFR TKIs [18], [19]. Additionally, lower soluble MET levels following treatment (cycle 1 day 15 and cycle 5 day 1) were associated with longer PFS, which is consistent with a correlation between MET expression and poor prognosis [20]. Patients with mRCC whose tumors had a higher percentage of CD8+ cells (greater than or equal to the median for the cohort) at baseline experienced prolonged PFS (hazard ratio, 0.239; 95% CI, 0.061–0.940; p = .027; Fig. 4). The prognostic value of CD8+ cells in patients with RCC treated with VEGFR TKIs, is controversial as higher amounts or density of infiltrating CD8+ T cells in tumor tissues was associated with both shorter survival and conversely longer disease‐free survival [18], [20], [21], [22], [23]. Overall, the results of this study suggest that the prognostic value of these biomarkers in mRCC, in particular CD8 expression, warrant further exploration.
Figure 4.
Progression‐free survival for patients in cohort 1 by percent of CD8‐positive cells greater than or equal to (≥Median) or less than (<Median) the median percent of CD8‐positive cells for all patients in cohort 1. Cohort 1, no prior systematic therapy. *, log‐rank p value.
Abbreviations: CI, confidence interval; HR, hazard ratio; mPFS, median progression‐free survival; NR, not reached; PFS, progression‐free survival.
A limitation of this trial was the single‐arm design with no monotherapy comparator groups, which precluded direct comparison of the combination treatment with the respective drugs used alone in the mRCC population. An additional limitation was halting of accrual in the dose‐expansion phase cohort 2, precluding characterization of the combination in previously treated patients with mRCC.
In conclusion, the combination of axitinib and crizotinib in patients with solid tumors has a manageable safety and tolerability profile, consistent with the profiles of the individual agents when administered as monotherapy. The combination demonstrated antitumor activity in treatment‐naïve patients with RCC. Given the more robust antitumor activity of cabozantinib, as well as promising trial data for newer immuno‐oncology treatments, the antitumor activity reported here does not support further study of the combination of axitinib and crizotinib in mRCC.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (a) for indications that have been approved in the U.S. and/or European Union or (b) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Figures and Tables
Acknowledgments
This study was sponsored by Pfizer Inc. Medical writing support was provided by Charles Cheng of Engage Scientific Solutions and was funded by Pfizer Inc.
Footnotes
ClinicalTrials.gov Identifier: NCT01999972
Sponsor(s): Pfizer
Principal Investigator: M. Dror Michaelson
IRB Approved: Yes
Disclosures
M. Dror Michaelson: Exelixis, Eisai, Novartis, Pfizer (C/A); Shilpa Gupta: Merck, Bristol‐Meyers Squibb, Seattle Genetics (C/A), Janssen, Exelixis (H), Astellas, Bristol‐Myers Squibb (RF); Neeraj Agarwal: Astellas, Astra Zeneca, Argos, Bristol‐Meyers Squibb, Bayer, Clovis, Eisai, Exelixis, EMD Serono, Ely Lilly, Foundation One, Genentech, Merck, Medivation, Novartis, Nektar, Pfizer, Pharmacyclics (C/A); Thomas Powles: Pfizer, AstraZeneca, Roche, Bristol‐Myers Squibb (C/A), Bristol‐Myers Squibb, Ipsen, Exelexis, Roche, Merck, Pfizer, Novartis, AstraZeneca, Incyte, Seattle Genetics (H), AstraZeneca, Roche (RF); Ulka Vaishampayan: Bayer, Bristol‐Myers Squibb, Exelixis, Pfizer, Astellas (C/A), Sanofi, Bristol‐Myers Squibb, Exelixis, Bayer, Pfizer, Astellas. (H), Bayer, Bristol‐Myers Squibb, Novartis, Pfizer, Exelixis, Astellas (RF); James Larkin: Merck, Bristol‐Meyers Squibb, Incyte, Dynavax, Novartis, Ivax, Vista, Roche, Pfizer, Syneos Health, Bicycletx Ltd., Ultimovacs, Covance (C/A, H), Bristol‐Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Achilles, Roche, Nektom, Covance, Immunocor, Avro (RF); Brad Rosbrook: Pfizer (E); Erjian Wang: Pfizer (E, OI); Danielle Murphy: Pfizer (E); Panpan Wang: Pfizer (E); Maria Josè Lechuga: Pfizer (E); Olga Valota: Pfizer (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Grépin R, Pagès G. Molecular mechanisms of resistance to tumour anti‐angiogenic strategies. J Oncol 2010;2010:835680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahangiri A, De Lay M, Miller LM et al. Gene expression profile identifies tyrosine kinase c‐Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 2013;19:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 2012;12:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shojaei F, Lee JH, Simmons BH et al. HGF/c‐Met acts as an alternative angiogenic pathway in sunitinib‐resistant tumors. Cancer Res 2010;70:10090–10100. [DOI] [PubMed] [Google Scholar]
- 6.Shojaei F, Simmons BH, Lee JH et al. HGF/c‐Met pathway is one of the mediators of sunitinib‐induced tumor cell type‐dependent metastasis. Cancer Lett 2012;320:48–55. [DOI] [PubMed] [Google Scholar]
- 7.You WK, Sennino B, Williamson CW et al. VEGF and c‐Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 2011;71:4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciamporcero E, Miles KM, Adelaiye R et al. Combination strategy targeting VEGF and HGF/c‐met in human renal cell carcinoma models. Mol Cancer Ther 2015;14:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou SH, Bartlett CH, Mino‐Kenudson M et al. Crizotinib for the treatment of ALK‐rearranged non‐small cell lung cancer: A success story to usher in the second decade of molecular targeted therapy in oncology. The Oncologist 2012;17:1351–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw AT, Ou SH, Bang YJ et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 13.Hutson TE, Lesovoy V, Al‐Shukri S et al. Axitinib versus sorafenib as first‐line therapy in patients with metastatic renal‐cell carcinoma: A randomised open‐label phase 3 trial. Lancet Oncol 2013;14:1287–1294. [DOI] [PubMed] [Google Scholar]
- 14.Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose‐finding trials. Clin Trials 2010;7:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xalkori (crizotinib) prescribing information. New York, NY: Pfizer; 2011; last update, 2018. Available at http://labeling.pfizer.com/showlabeling.aspx?id=676. Accessed October 5, 2018. [Google Scholar]
- 16.Yamazaki S, Johnson TR, Smith BJ. Prediction of drug‐drug interactions with crizotinib as the CYP3A substrate using a physiologically based pharmacokinetic model. Drug Metab Dispos 2015;43:1417–1429. [DOI] [PubMed] [Google Scholar]
- 17.Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 18.Tran HT, Liu Y, Zurita AJ et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal‐cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012;13:827–837. [DOI] [PubMed] [Google Scholar]
- 19.Porta C, Paglino C, De Amici M et al. Predictive value of baseline serum vascular endothelial growth factor and neutrophil gelatinase‐associated lipocalin in advanced kidney cancer patients receiving sunitinib. Kidney Int 2010;77:809–815. [DOI] [PubMed] [Google Scholar]
- 20.Gibney GT, Aziz SA, Camp RL et al. c‐Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 2013;24:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano O, Sato M, Naito Y et al. Proliferative activity of intratumoral CD8(+) T‐lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res 2001;61:5132–5136. [PubMed] [Google Scholar]
- 22.Choueiri TK, Figueroa DJ, Fay AP et al. Correlation of PD‐L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071–1077. [DOI] [PubMed] [Google Scholar]
- 23.George DJ, Martini JF, Staehler M et al. Immune biomarkers predictive for disease‐free survival with adjuvant sunitinib in high‐risk locoregional renal cell carcinoma: From randomized phase III S‐TRAC study. Clin Cancer Res 2018;24:1554–1561. [DOI] [PubMed] [Google Scholar]